Abstract
Candida albicans is the most frequent cause of fungal keratitis in temperate regions. Caspofungin has potent activity against Candida spp. in a variety of clinical settings. Little is known, however, about its activity against fungal keratitis. We compared the efficacy of topical caspofungin with that of topical amphotericin B (AMB) in a rabbit model of experimental keratomycosis. Keratitis was induced with a standardized inoculum of Candida albicans (SC 5314) placed on the debrided cornea. Twenty-four hours after infection, animals were randomly assigned to treatment with 0.15% caspofungin, 0.5% caspofungin, 0.15% AMB, and a saline control (n = 12 rabbits in each group). For the first 12 h, treatment was repeated every 30 min and, after a 12-h pause, was resumed at hourly intervals for another 12 h. The animals were examined and killed 12 h after administration of the last dose. Treatment effects were evaluated by clinical assessment, fungal culture, and histopathology. Drug treatment significantly reduced corneal fungal recovery from 3.78 log10 CFU in saline-treated animals to 2.97, 1.76, and 1.18 log10 CFU in animals treated with 0.15% caspofungin, 0.5% caspofungin, and 0.15% AMB, respectively. By histopathology, the mean hyphal density was significantly lower in the corneas of treated animals than in those of the controls; there was no difference in hyphal densities between the different treatment groups. The depth of corneal invasion was not significantly reduced by the antifungal treatments. By clinical assessment, keratitis progressed in animals treated with saline, whereas disease progression was inhibited by all drug treatment regimens. In our rabbit model, 0.5% caspofungin was as effective as 0.15% AMB for the topical treatment of Candida keratitis. The potential clinical efficacy of caspofungin awaits further investigation.
Candida albicans is the most frequently isolated etiologic agent of fungal keratitis in temperate regions (12). Chronic ocular surface disease and prolonged corticosteroid use, either systemic or topical, appear to be the main predisposing factors for Candida keratitis. Topical 0.15% amphotericin B (AMB) is usually favored over topical 5% natamycin and 1% miconazole for treatment, but controlled clinical trials are lacking (13). The response to treatment is generally favorable, even in the presence of deep stromal lesions.
Caspofungin (CAS) is a recently introduced echinocandin antifungal with fungicidal activity in vitro against all Candida spp., including strains resistant to fluconazole. The drug also has potent activity against C. albicans biofilms (2). In randomized clinical trials, CAS has been shown to be as effective as AMB for the treatment of oropharyngeal, esophageal, and invasive candidiasis (1, 6, 14). As a large and highly protein-bound molecule, it does not penetrate well into the central nervous system and, by inference, into the eye. This may explain in part why CAS has not been studied for the treatment of fungal eye diseases. We are also unaware of case reports on its use for the treatment of keratomycosis.
The aim of this study was to determine the efficacy of topical CAS in an experimental model of Candida keratitis and to compare it with the efficacy of AMB.
MATERIALS AND METHODS
Animals.
Forty-eight adult (24 male) Burgundy fawn rabbits (weight, 2.6 to 4.4 kg) were used in this study. They were provided by an authorized breeding center and were kept in individual cages under well-defined and standardized conditions (in a humidity- and temperature-controlled room with a cycle of 13 h of light and 11 h of dark). They received standard dry food and water ad libitum. All eyes were initially examined by an ophthalmologist with a hand-held slit lamp. Only animals without any signs of ocular pathology were included. All experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Swiss federal and local ethical and agricultural committees.
Yeast.
C. albicans strain SC 5314 (a kind gift from D. Sanglard, Centre Hospitalier Universitaire Vaudois [CHUV], Lausanne, Switzerland) was used for all experiments. This well-characterized strain has been used in a rabbit keratitis model, in which it proved to be highly invasive for the corneal stroma after surface inoculation (7, 9). The strain was maintained in yeast extract-peptone-glucose (YPG) broth supplemented with 16.7% glycerol at −20°C.
The fungal inoculum was prepared as described by O'Day et al. (9). Briefly, an aliquot of Candida (0.5 ml) containing approximately 108 blastoconidia was thawed, and 10-μl samples were cultured overnight in 25 ml of YPG broth at 30°C in a rotary shaker at 200 rpm. A sample of the overnight culture was counted in a hemacytometer to determine the inoculum size. This was confirmed by counting of the CFU. Typically, each culture flask yielded 3.8 × 109 CFU. For each experiment, the contents of two to four flasks were pooled. Immediately preceding the inoculation, the pooled overnight culture was pelleted by centrifugation at 2,500 × g for 3 min in a 50-ml sterile polypropylene tube, the supernatant was discarded, and the tube was inverted to further drain the fungal pellet.
Induction of keratitis.
Keratitis was established by using a contact lens-induced model (7, 9). Rabbits in groups of 6 to 12 each were anesthetized with intramuscular ketamine (35 mg/kg of body weight; Ketalar; Parke-Davis, Ann Arbor, Mich.) and xylazine (5 mg/kg; Xylapan; Chassot, Bern, Switzerland). Corneal anesthesia was obtained with topical 0.2% novocaine (Inselspital Pharmacy, Bern, Switzerland). The nictitating membrane of the right eye was removed by sharp dissection. A 7-mm filter paper disk moistened with 99% 1-heptanol (Sigma-Aldrich, Lausanne, Switzerland) was placed on the center of the cornea for 30 s. The corneal epithelium was then atraumatically removed by gentle wiping it with a K sponge (Katena Products, Denville, N.J.). After the eye was rinsed with balanced salt solution (Alcon, Fort Worth, Tex.) to remove any remaining traces of 1-heptanol, the fungal inoculum (40 μl of fungal paste containing approximately 109 CFU) was transferred to the denuded cornea with a large-bore pipette tip and covered with a flexible contact lens (diameter, 14.2 mm) made of etafilcon A (Acuvue; Johnson & Johnson Vision Products, Jacksonville, Fla.). The lids were closed with 5-0 silk tarsorrhaphy sutures to prevent contact lens extrusion.
Antifungal agents.
CAS (caspofungin diacetate, Cancidas; Merck & Co., Inc., Whitehouse Station, N.J.) was diluted in sterile normal saline (0.9% NaCl) to the desired concentrations of 1.5 and 5 mg/ml. Amphotericin B deoxycholate (Fungizone; Bristol-Myers Squibb, Stamford, Conn.) was prepared in sterile water to a concentration of 1.5 mg/ml. The choice of drug concentrations used in the experiments was based on the results of in vitro toxicology studies (5; D. Goldblum, D. Zuercher, S. Zimmerli, and B. E. Frueh, Abstr. Assoc. Res. Vision Ophthalmol. Annu. Meet. 2002, abstr. 1612, 2002). All drops were freshly made daily and were kept at 4°C and protected from light between uses.
Treatment protocol.
The treatment protocol was adapted from a study of AMB (10). Twenty-four hours after infection, the rabbits were again anesthetized as described above. The tarsorrhaphy sutures and contact lens were removed, and an ophthalmologist examined the eye with a hand-held slit lamp to evaluate and grade the extent of keratitis, as described previously (10). Photographs were taken before the induction of keratitis, before treatment, and at the end of treatment. Animals were randomly assigned to one of four treatments (0.15% CAS, 0.5% CAS, 0.15% AMB, and saline control). Each treatment was studied in 12 animals. Eye drops (1 drop of 20 μl per time point) were administered to both eyes, with the uninfected contralateral eyes with intact corneal epithelium serving as treatment controls. Topical application was repeated every 5 min during the first hour and every 30 min during the following 9 h. After a 12-h pause, the treatment was resumed at hourly intervals for another 12 h. Twelve hours after the last drug administration, the animals were reevaluated clinically by the same ophthalmologist (who was blinded to the treatment) and the animals were killed by cervical dislocation and subsequent exsanguination.
Postmortem sample collection.
The eyes were enucleated promptly, and the infected corneas were excised at the limbus and bisected. One half was used for quantitative fungal recovery and the other half was fixed in 10% paraformaldehyde for histopathology.
Analysis.
Data were collected for determination of three independent outcome measures: clinical assessment, quantitative fungal recovery, and histology.
Clinical grading and scoring.
The clinical features of keratitis were graded by an ophthalmologist (who was blinded to the treatment) with a hand-held slit lamp prior to the start of treatment and at the end of treatment. The following grading scheme was used: for density of infiltration, 0, no disease present; 1, light stromal infiltration; 2, dense stromal infiltration; and 3, large infiltration; for depth of corneal infiltration, 0, no disease present; 1, superficial infiltration; 2, ulceration with stromal infiltration; and 3, deep ulceration with or without hypopyon.
The means of the two grading scores for extent and depth of infiltration were calculated to result in a composite clinical score.
Quantitative fungal recovery.
The corneal halves were cut into 8 to 10 small pieces of approximately equal size with scissors, transferred to 3 ml of sterile normal saline in a 15-ml test tube, and ground on ice for three pulses of 10 s each with 20-s intervals with a Labsonic U ultrasound tissue grinder equipped with a needle probe (40 T; B. Braun Diessel Biotech, Melsungen, Germany) (8). Aliquots (10 and 100 μl) of serial dilutions were plated in triplicate on Sabouraud agar plates, and the plates were incubated for 24 h at 37°C before the colonies were counted. The numbers of CFU recovered were expressed as the numbers of CFU per cornea.
Histopathology.
The fixed corneal halves were embedded in paraffin; sectioned to a thickness of 7 μm; and stained with hematoxylin-eosin, Grocott's methenamine silver, and periodic acid-Schiff reagents by standard protocols. To determine the extent of stromal infiltration by fungal elements, the central corneal sections of each eye were examined by light microscopy (DMRBE; Leica, Glattbrugg, Switzerland). Digital photographs (DC 500; Leica) of the area with the deepest stromal infiltration were captured (original magnification, ×200) and analyzed with imaging software (Image Pro Plus, version 4.0; Media Cybernetic, Silver Spring, Md.) calibrated with a micrometer graticule (Pyser-SGI Limited, Edenbridge, United Kingdom). We evaluated the maximal depth of penetration (in micrometers) and the total hyphal area (in square micrometers) in a predefined area measuring 350 by 443 μm of the corneal center. For the section with the deepest corneal penetration by hyphal elements, we preferred to measure the absolute depth of penetration instead of the relative thickness of penetration compared to the total corneal thickness (9). We were concerned that the degree of corneal edema introduced by the inflammation might vary between treatment groups. A proinflammatory effect that might influence corneal edema has been attributed to AMB, in particular. Throughout the experiment, the observer was blinded to the treatment regimen.
Statistical analysis.
Values are expressed as means ± standard deviations (SDs). Clinical grading was analyzed by the Mann-Whitney U test. Quantitative isolate recovery and histology data were first analyzed by the Kruskal-Wallis one-way analysis of variance to compare the four groups. If the Kruskal-Wallis one-way analysis of variance was significant, the Mann-Whitney U test was subsequently used to compare each treatment group with the control (saline-treated) group for significance. To account for multiple comparisons, Bonferroni corrections were used to adjust the P values obtained from the Mann-Whitney U tests. Differences with a first-order error of P < 0.05 were considered statistically significant. All data were analyzed by using the computer statistical analysis software package NCSS 2000 (NCSS, Kaysville, Utah).
RESULTS
Clinical evaluation.
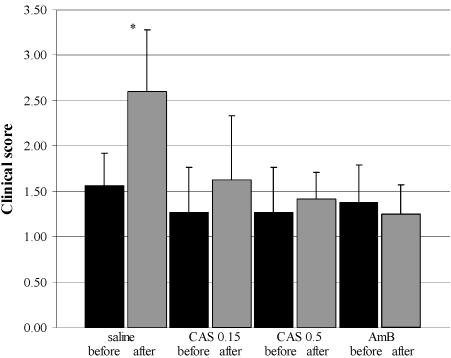
Before and after treatment all animals were clinically evaluated by an ophthalmologist who graded both the extension and the depths of the corneal infiltrates. The pretreatment grade for all animals was 1.4 (mean clinical slit lamp score). In control animals receiving saline eye drops, the combined score for the extension and the depth of the corneal infiltrate increased significantly during treatment to a score of 2.6. In contrast, no significant change was observed in animals treated with 0.15% CAS, 0.5% CAS, or 0.15% AMB (slit lamp scores, 1.6, 1.4, and 1.3, respectively) (Fig. 1).
FIG. 1.
Composite of mean clinical slit lamp scores (±SD) for extension and depth of infiltrative keratitis before and after treatment with saline, 0.15% CAS (CAS 0.15), 0.5% CAS (CAS 0.5), and 0.15% AMB showing a significant increase in the score for saline-treated animals (P = 0.0016) and an arrest of clinical progression by 0.15% CAS, 0.5% CAS, and AMB.
Surprisingly, none of the animals in any treatment group, including the controls, appeared to be in pain or to suffer discomfort. No evidence of drug-related adverse effects in the uninfected drug-treated contralateral eyes with intact corneal epithelium was found. There was also no difference between the treatment groups with respect to chemosis or conjunctival injection in the infected eyes, indicating the absence of any clinically apparent toxicity of the antifungals used.
Isolate recovery.
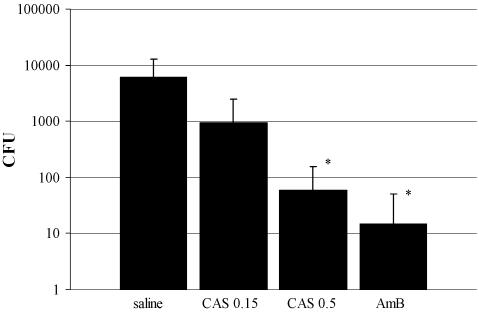
C. albicans was recovered by culture from 10 of 12 corneas from animals in the control group and 11 of 12 corneas from animals treated with 0.15% CAS. In contrast, 7 of 12 and 10 of 12 corneas from animals treated with 0.5% CAS and 0.15% AMB, respectively, were sterile. Compared to the corneas of the saline-treated controls, which grew 3.78 ± 3.82 log10 CFU of C. albicans, fungal recovery was lower in all active treatment groups: 2.97 ± 3.19, 1.76 ± 1.99, and 1.18 ± 1.56 log10 CFU were recovered from corneas of animals treated with 0.15% CAS, 0.5% CAS, and 0.15% AMB, respectively. The difference was significant for the last two treatment groups (Fig. 2).
FIG. 2.
Mean fungal recovery (±SD) from corneas after 2 days of treatment. Compared to the recovery from the saline-treated corneas, significantly fewer numbers of CFU were recovered from corneas treated with 0.5% caspofungin (CAS 0.5; P = 0.015) and 0.15% AMB (P = 0.003). CAS 0.15, 0.15% CAS.
Histopathology.
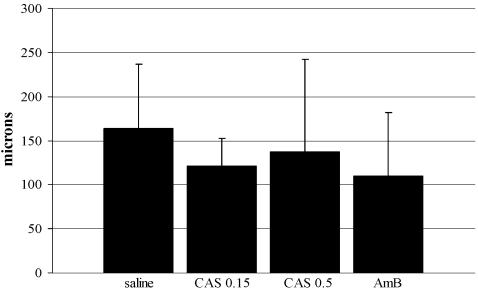
The depth of fungal invasion was 163.6 ± 73.5 μm for the saline-treated control animals, 121.6 ± 30.9 μm for animals treated with 0.15% CAS, 137.3 ± 105.2 μm for animals treated with 0.5% CAS, and 109.7 ± 72.4 μm for animals treated with AMB. Compared to the hyphal depth in the controls, all antifungals appeared to reduce the maximal depth of hyphal invasion into the corneas, but the difference was not statistically significant (Fig. 3).
FIG. 3.
Maximal depth (±SD) of corneal invasion by hyphae after 2 days of treatment measured by histopathology. CAS 0.15, 0.15% CAS; CAS 0.5, 0.5% CAS.
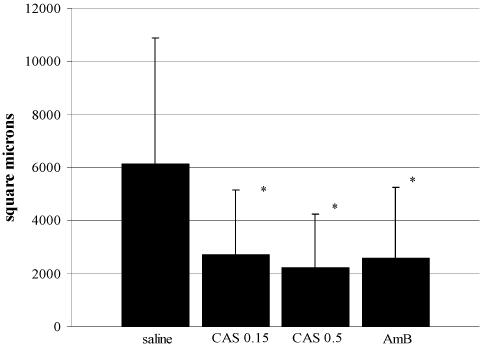
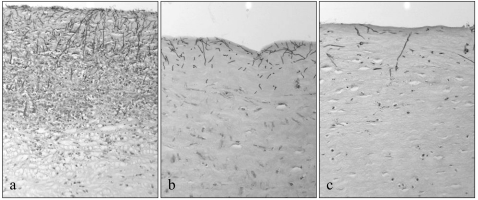
The fungal areas were 6,127 ± 4,767 μm2 for the saline-treated control animals, 2,718 ± 2,444 μm2 for animals treated with 0.15% CAS, 2,223 ± 2,019 μm2 for animals treated with 0.5% CAS, and 2,589 ± 2,663 μm2 for animals treated with AMB. Compared to the hyphal biomass in the saline-treated control animals, antifungal treatment significantly reduced the hyphal biomass, as measured by the total surface area of hyphae in the central areas of the infected corneas. There was no difference between treatment regimens (Fig. 4). Representative examples of the histopathologies of the corneas from the control animals and the animals treated with AMB and 0.5% CAS are shown in Fig. 5.
FIG. 4.
Fungal area as the mean hyphal area (±SD) on histological sections of the centers of infected corneas. *, P < 0.005. CAS 0.15, 0.15% CAS; CAS 0.5, 0.5% CAS.
FIG. 5.
Representative examples of the histopathologies of corneas demonstrating infiltrate density in control animals (a), animals after treatment with AMB (b), and animals after treatment with 0.5% CAS (c). Periodic acid-Schiff stain. Magnification, ×200.
DISCUSSION
The etiology of keratomycosis varies with the geographical location. In tropical and subtropical climate zones with agriculture-based economies, molds, particularly Aspergillus spp., are the predominant causative organisms; and ocular trauma involving organic material is the most common predisposing factor (13). In temperate climates, in contrast, C. albicans is the most frequently isolated pathogen. Risk factors (resulting in impaired local defense mechanisms) include epithelial abrasions as a consequence of, e.g., contact lens wear, systemic or topical use of corticosteroids, and atopic diseases (12).
The most commonly used treatment for Candida keratitis is 0.15% AMB administered topically every 30 to 60 min. There have been well-substantiated concerns, illustrated by our toxicity tests in an established cell culture model, about this polyene's potential corneal toxicity. In clinical practice, however, the 0.15% AMB solution appears to be well tolerated (15). Alternative treatment regimens include topical 5% natamycin and 1% miconazole. In addition, topically administered 0.2% fluconazole has been shown in a rabbit model to reach potentially therapeutic levels in the cornea (16). Its topical use for the treatment of human keratomycosis, however, has not been reported. No randomized clinical trials comparing the efficacies of the various topical antifungal drugs have been conducted, and the limited number of patients for whom results have been reported in case series make it difficult to evaluate the relative effect of treatment with the chosen drug. Nevertheless, the response to treatment for fungal keratitis is usually favorable, even in the presence of deep stromal lesions (11, 13).
CAS is a first-in-class echinocandin with potent activity against Candida and Aspergillus, the dominant human fungal pathogens. In contrast to all other antifungal drugs (that target the cell membrane), echinocandins act on the fungal cell wall by inhibiting the synthesis of an essential component, the (1,3)-d-glucan. In vitro and in vivo CAS is fungicidal against essentially all Candida spp., including fluconazole-resistant strains. Randomized clinical trials with CAS in patients with candidemia, invasive candidiasis, and Candida esophagitis demonstrate that its efficacy is equivalent to that of AMB, with substantially fewer toxic effects (1, 3, 6, 14). The ocular disposition of CAS after systemic treatment and its use as a topical agent for the treatment of fungal infections of the eye have not been reported.
In the present study, we compared the efficacies of two concentrations of CAS to the clinical standard, 0.15% AMB, for the topical treatment of experimental Candida keratitis. Three independent measures were used to evaluate the treatment outcome after 2 days: clinical evaluation, fungal culture, and histological studies. While 0.15% CAS was inferior to AMB, topical 0.5% CAS was consistently as effective as 0.15% AMB in halting the clinical progression of keratomycosis, decreasing the fungal biomass infiltrating the cornea, and reducing the fungal CFU recoverable from the corneas by more than 100-fold. Quantitative recovery of fungal hyphal elements from tissue may be fraught with some methodological limitations. The colony-forming potential of hyphal elements may be very sensitive to the structural damage induced by the effort to separate them from corneal tissue by sonication. Indeed, quantitative fungal recovery has poorly reflected the clinical severity of disease in some studies of Candida keratitis (7, 9). In our hands, by using a well-characterized strain of C. albicans and a standardized sample processing procedure, the level of fungal recovery closely matched the clinical and histological findings.
We did not find evidence of ocular toxicity of topical 0.5% CAS in eyes with large defects in the corneal epithelium or in intact uninfected eyes. It thus appears that the well-established in vitro cell culture system used to evaluate ocular toxicity by topical drugs may be exceedingly sensitive (4). In that system we observed that CAS has substantial toxicity at concentrations exceeding 0.3 mg/ml. In the same in vitro system, however, AMB elicited toxic effects at a concentration 300-fold lower than the clinically well tolerated concentration of 0.15%.
While there are no published results of the use of topical CAS, other echinocandins have been used to treat ocular fungal infections, with results similar to ours. Trujillo et al. (F. Trujillo, G. Paris, L. Woodward, J. Graybill, M. Pena, L. Najvar, Y. Trigo, and W. E. Sponsels, Abstr. Assoc. Res. Vision Ophthalmol. Annu. Meet. 2004, abstr. 113, 2004) conducted a comparative study of 0.15% micafungin and 0.5% natamycin for the treatment of experimental Aspergillus keratitis in a rabbit model. Compared to the effects in saline-treated control animals, both drugs were equally effective in significantly reducing the extent of fungal infiltration. As in our study, there were no drug-related toxic effects.
Matsumoto et al. (Y. Matsumoto, M. Dogru, E. Goto, H. Fujishima, and K. Tsubota, Abstr. Assoc. Res. Vision Ophthalmol. Annu. Meet. 2004, abstr. 4966, 2004) reported on three patients with corneal ulcers due to yeast after keratoplasty that were unresponsive to conventional antifungal therapy. All ulcers healed within 2 weeks of topical treatment with 0.1% micafungin.
Our study with a rabbit model suggests that topical 0.5% CAS is safe and as efficacious as topical 0.15% AMB, the most commonly used agent for the treatment of Candida keratitis. Its potential role in human keratomycosis awaits further investigation.
Acknowledgments
This work was supported in part by an independent research grant from MSD, Merck Sharp & Dohme-Chibret AG, Glattbrugg, Switzerland.
We have no personal financial interests in any of the mentioned drugs, companies, or competing companies.
We thank Dominique Sanglard (CHUV) for generously providing C. albicans (SC 5314) and Hans Liechti, Urs Keller, Jürg Kummer, Aniela Olac, Daniela Zürcher, Monika Kilchenmann, Kathrin Fausch, and Dominic Schulz, members of the laboratory, for expert technical assistance.
REFERENCES
- 1.Arathoon, E. G., E. Gotuzzo, L. M. Noriega, R. S. Berman, M. J. DiNubile, and C. A. Sable. 2002. Randomized, double-blind, multicenter study of caspofungin versus amphotericin B for treatment of oropharyngeal and esophageal candidiases. Antimicrob. Agents Chemother. 46:451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann, S. P., K. VandeWalle, G. Ramage, T. F. Patterson, B. L. Wickes, J. R. Graybill, and J. L. Lopez-Ribot. 2002. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 46:3591-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denning, D. W. 2003. Echinocandin antifungal drugs. Lancet 362:1142-1151. [DOI] [PubMed] [Google Scholar]
- 4.De Saint Jean, M., F. Brignole, A. F. Bringuier, A. Bauchet, G. Feldmann, and C. Baudouin. 1999. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Investig. Ophthalmol. Vis. Sci. 40:619-630. [PubMed] [Google Scholar]
- 5.Goldblum, D., M. Gygax, M. Bohnke, and J. G. Garweg. 2002. In vitro toxicity of rivastigmine and donepezil in cells of epithelial origin. Ophthalmol. Res. 34:97-103. [DOI] [PubMed] [Google Scholar]
- 6.Mora-Duarte, J., R. Betts, C. Rotstein, A. L. Colombo, L. Thompson-Moya, J. Smietana, R. Lupinacci, C. Sable, N. Kartsonis, and J. Perfect. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020-2029. [DOI] [PubMed] [Google Scholar]
- 7.O'Day, D., W. Head, R. Robinson, R. Yang, D. Shetlar, and M. Wang. 1999. Contact lens-induced infection—a new model of Candida albicans keratitis. Investig. Ophthalmol. Vis. Sci. 40:1607-1611. [PubMed] [Google Scholar]
- 8.O'Day, D. M. 1990. Orally administered antifungal therapy for experimental keratomycosis. Trans. Am. Ophthalmol. Soc. 88:685-725. [PMC free article] [PubMed] [Google Scholar]
- 9.O'Day, D. M., W. S. Head, C. Csank, D. J. Shetlar, R. D. Robinson, G. W. McCollum, R. Yang, T. L. Zhu, and M. X. Wang. 2000. Differences in virulence between two Candida albicans strains in experimental keratitis. Investig. Ophthalmol. Vis. Sci. 41:1116-1121. [PubMed] [Google Scholar]
- 10.O'Day, D. M., W. S. Head, R. D. Robinson, and J. A. Clanton. 1986. Bioavailability and penetration of topical amphotericin B in the anterior segment of the rabbit eye. J. Ocul. Pharmacol. 2:371-378. [DOI] [PubMed] [Google Scholar]
- 11.Rosa, R. H., Jr., D. Miller, and E. C. Alfonso. 1994. The changing spectrum of fungal keratitis in south Florida. Ophthalmology 101:1005-1013. [DOI] [PubMed] [Google Scholar]
- 12.Tanure, M. A., E. J. Cohen, S. Sudesh, C. J. Rapuano, and P. R. Laibson. 2000. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea 19:307-312. [DOI] [PubMed] [Google Scholar]
- 13.Thomas, P. A. 2003. Current perspectives on ophthalmic mycoses. Clin. Microbiol. Rev. 16:730-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villanueva, A., E. G. Arathoon, E. Gotuzzo, R. S. Berman, M. J. DiNubile, and C. A. Sable. 2001. A randomized double-blind study of caspofungin versus amphotericin for the treatment of candidal esophagitis. Clin. Infect. Dis. 33:1529-1535. [DOI] [PubMed] [Google Scholar]
- 15.Wong, T. Y., T. P. Ng, K. S. Fong, and D. T. Tan. 1997. Risk factors and clinical outcomes between fungal and bacterial keratitis: a comparative study. CLAO J. 23:275-281. [PubMed] [Google Scholar]
- 16.Yee, R. W., C. J. Cheng, S. Meenakshi, T. M. Ludden, J. E. Wallace, and M. G. Rinaldi. 1997. Ocular penetration and pharmacokinetics of topical fluconazole. Cornea 16:64-71. [PubMed] [Google Scholar]