Abstract
Background and hypothesis
Several studies suggest that raloxifene, a selective estrogen receptor modulator, improves symptoms and cognition in post-menopausal women with Schizophrenia-Spectrum Disorders (SSD). We aimed to assess the effects of adjunctive raloxifene in women and men with SSD.
Study design
This parallel, randomized, double-blind, placebo-controlled trial included adult SSD patients across the Netherlands and Belgium. Participants were stratified by age, sex, and global functioning and randomly assigned 1:1 to 12-week add-on raloxifene or placebo. Primary outcomes were symptom severity at 6, 12, and 38 weeks and cognition at 12 and 38 weeks, as measured with the Positive and Negative Syndrome Scale and the Brief Assessment of Cognition in Schizophrenia, respectively. Intention-to-treat analyses were performed using linear mixed-effect models.
Study results
We assessed 261 patients for eligibility, of which 102 (28% female) were assigned to raloxifene (n = 52) or placebo (n = 48). Although we found no main effect of raloxifene, secondary sex-specific analysis showed that in women, raloxifene had beneficial effects on negative symptoms at week 6 (LSM −2.92; adjusted P = 0.020) and week 12 (LSM −3.12; adjusted P = 0.030), and on working memory at week 38 (LSM 0.73; adjusted P = 0.040), while having negative effects on working memory at week 38 in men (LSM −0.53; adjusted P = 0.026). The number of adverse events was similar between groups.
Conclusions
Our results do not support the use of raloxifene in patients with SSD in general, but suggest female-specific beneficial effects of raloxifene on negative symptoms and working memory. Our findings encourage further research on sex-specific pharmacotherapeutic treatment.
Keywords: RCT, schizophrenia, estrogen, SERM, raloxifene, antipsychotic medication, sex differences
Introduction
Schizophrenia-Spectrum Disorders (SSDs) affect approximately 24 million people worldwide and are associated with considerable morbidity and mortality.1 The treatment of SSD remains challenging as a substantial group of patients remain symptomatic despite adequate treatment.2 Especially negative and cognitive symptoms often persist, severely hampering functional outcomes.2–5 In recent times, reviews and large register studies have brought the spotlight back on sex differences in the diagnosis, treatment and course of SSD. In premenopausal women, negative and cognitive symptoms tend to be milder, which is attributed to the protective effects of estrogens, whereas after menopause, symptoms tend to worsen considerably.6,7 Moreover, in premenopausal women, symptoms ameliorate at times when estrogen levels are high, for example during ovulation and pregnancy, while they tend to exacerbate during the premenstrual phase and after delivery, when levels of estrogen are low.6,7 Despite their protective effects in women with SSD,8–10 estrogens are unsuitable for long-term augmentation therapy, given their carcinogenic side effects.11 The addition of raloxifene, a selective estrogen receptor modulator, has been opted a safe alternative, as it has estrogen-like influences on brain tissue, without the increased risk of breast and uterine cancer.11
A meta-analysis, predominantly based on post-menopausal women, revealed medium to large effects of raloxifene compared to placebo on psychotic symptoms in SSD.12 The exact mechanism by which raloxifene exerts these beneficial effects is not entirely understood but may be explained by its effects on the pre- and post-synaptic modulation of different neurotransmitter systems involved in SSD, such as glutamatergic and dopaminergic neurotransmission.13,14 Although several imaging studies have shown that raloxifene may increase hippocampal and prefrontal brain activity during cognitive tasks,15–17 the clinical evidence of raloxifene’s effects on cognition remains inconclusive, as some studies found beneficial effects,18–21 while others have not.22–24 In premenopausal women and in men, studies on raloxifene efficacy are limited and yielded mixed results.20,21,25 In addition, the long-term effects of raloxifene addition for SSD have not been studied yet. In this study, we aimed to investigate the effects of adjunctive raloxifene in a large sample of patients with SSD after 12 weeks of treatment, as well as its long-term effects at 6, 12, and 24 months post-treatment. As a secondary analysis, we also investigated whether these effects differed between men and women since sex differences in brain dopaminergic activity and estrogen levels imply that responses to estrogen-like therapies may be sex-specific.14,24,26
Methods
Study Design
This study was conducted in full accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and with approval by the Dutch competent authority for human-related research and the local ethics committees. The trial was registered with ClinicalTrials.gov, NCT03043820, and the study protocol has previously been published elsewhere.27 During the study, there were 10 protocol amendments, which are described in Supplementary Material p. 3–4. This study followed Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.28
Participants
We recruited patients from 5 in and outpatient clinics (4 in The Netherlands and 1 in Belgium). All patients had to be willing and capable to provide written informed consent prior to participation. Eligible participants were adults aged ≥18 years with a DSM-IV diagnosis of schizophrenia, schizoaffective, schizophreniform disorder (295.x), or psychotic disorder not otherwise specified (298.9), who were on a fixed dose of antipsychotics for at least 2 weeks. The Mini International Neuropsychiatric Interview 5.0.029 was conducted at the screening to confirm a diagnosis of SSD. As raloxifene can cause fetal harm when administered to pregnant women, female patients of childbearing age were required to consent to six-weekly pregnancy tests during the treatment phase. The exclusion criteria were preexisting cardiovascular disease; a history of thromboembolic events or breast cancer; a familial tendency to form blood clots; hypertriglyceridemia; liver failure; severe kidney failure; and the use of estrogenic or androgenic hormonal therapy (including the use of estrogenic contraception) within 3 months preceding trial participation. Women above the age of 52 and/or with an increased risk of uterine or breast cancer were only included if they could provide a negative result on their regular national cancer screening test. Criteria for study withdrawal were at the request of the patient, pregnancy, or a decision made at the discretion of the responsible physician or trial investigator.
Intervention, Randomization, and Masking
We performed a parallel-design, double-blind, randomized, placebo-controlled trial (RCT). Patients were randomly assigned (1:1) to receive 120 mg/day of raloxifene (two 60 mg tablets) or placebo (two 60 mg tablets containing lactose, identical in size, shape, smell, and color) for 12 weeks, using minimization.30 We chose this dosing as there is some evidence that a higher dose of raloxifene is more effective than a lower dose (120 mg/day vs 60 mg/day).31,32 Patients were stratified by sex, age (above/below 45 years), and personal and social performance (PSP) (Personal and Social Performance scale score above/below 40).33 Randomization sequences were determined by an off-site statistician. The treatment options were shuffled in different blocks of 4 (e.g., AABB, ABAB, BBAA) to avoid reporting detection bias, as there was no detectable pattern in the order of treatment assignment. A single pharmacy was responsible for dispensing the study medication. Patients, informal caregivers, treating physicians, and the researchers carrying out the assessments were masked to the treatment condition until study completion. Study medication was dispensed in 2 batches of 92 tablets at baseline and week 6. Treatment adherence was assessed by counting the number of pills returned at each visit following dispensation. Changes in concomitant medication were monitored throughout the study.
Measures and Procedures
The treatment phase consisted of a baseline visit (week 0), a midterm visit (week 6), and an end-of-treatment visit (week 12), followed by a follow-up phase of 2 years, which consisted of 1 follow-up visit 6 months post-treatment (week 38), and 2 telephone interviews at 12 and 24 months post-treatment (week 64 and 116).
For the schedule of assessments for visits and calls, see Supplementary Table 1. For patients who discontinued trial participation within 12 weeks, end-of-treatment assessments were made when possible. Blood was drawn for analysis of sex hormones (17β-estradiol and testosterone in men; 17β-estradiol and FSH in women), lipids and C-reactive protein (CRP) at baseline and week 12 (Supplementary Material p. 3 and Supplementary Table 2). For safety reasons, triglycerides, renal function, and liver function tests were obligatory at baseline and repeated at week 6 and 12 if these markers were abnormal at baseline. Any new physical or mental complaint was recorded as an adverse event (AE), adverse reaction, or serious adverse event (SAE) at each visit using standard reporting procedures. SAEs were reported between baseline and week 38 because we did not expect any relevant SAEs related to the study medication after this period. The Greene Climacteric Scale31 was used to assess hormone-related complaints.
Outcomes
To evaluate treatment efficacy, we measured the change in positive, negative, and total symptoms at week 6, 12, and 38 with the Positive and Negative Syndrome Scale (PANSS)34 and the change in cognitive functioning at week 12 and 38, based on 4 cognitive domains (processing speed, reasoning/problem solving, verbal memory, and working memory) assessed with the Brief Assessment of Cognition in Schizophrenia (BACS).35 The BACS includes assessments of four neurocognitive domains designated as important by the MATRICS: processing speed (Token Motor Task, Symbol coding, and Verbal Fluency), reasoning and problem solving (Tower of London Test), verbal memory (List learning), and working memory (Digit sequencing). Scores were converted into age- and sex-corrected Z-scores for the four cognitive domains. Secondary outcomes included depression (Beck’s Depression Inventory, BDI),36 quality of life (EQ-5D-5L)37 personal and social performance (PSP)33 and thought and language disorder (TALD)38 (Supplementary Material p. 3 and Supplementary Table 3). To establish interrater reliability, training videos were coded by 2 investigators independently. For premenopausal women with regular menses, the baseline visit was scheduled during days 1–4 of menstruation.
Statistical Analysis
The study was designed to produce clinical data for 100 patients (n = 50 per group), resulting in 80% statistical power with an α level of.05 (two-sided) to detect a standardized effect size of 0.57 in the primary clinical outcomes, based on a previous meta-analysis.12 This sample size had been adjusted for a 10% attrition rate due to drop-out up to week 38. Treatment differences between groups were estimated using least-squares means with 95% CIs supplemented by P values. The treatment effect is the difference between group means (raloxifene minus placebo) attributable to treatment after controlling for baseline scores, reported as an average outcome across time points (6, 12, or 38 weeks). Analyses were carried out following the intention-to-treat principle. Given that there was statistical dependency between repeated measures, linear mixed-effect models were used to assess the 2 experimental conditions with a random effect for the patient. Fixed effects consisted of time (categorical variable), treatment condition, sex, and their two-/three-way interactions to allow the treatment effect to be calculated per sex since current literature implies that responses to estrogen-like therapies may be sex-specific.14,24,26 When there was no significant interaction of treatment-by-sex and/or treatment-by-sex-by-time, as assessed with likelihood-ratio (LR) tests, we removed the interaction from the model and estimated treatment efficacy in women and men together. By this way, we only evaluated potential sex-specific effects of raloxifene versus placebo when there was a clear indication to do this (i.e., when adding the interaction led to a significant improvement of the overall model). In addition, significant P values were adjusted using the Benjamini–Hochberg method controlling for the False Discovery Rate.39 Estimates were adjusted for baseline scores, study site, and for smoking status (continuous variable), since smoking influences raloxifene pharmacokinetics,40 and for age and education (years) for cognition. For exploratory purposes, we examined whether the effect of raloxifene was modified by high/low 17β-estradiol (in men and women separately), testosterone (in men), or menopausal status (in women), using LR tests assessing the effect of the subgroup and its interaction with treatment. For 17β-estradiol and testosterone, subgroup categories were defined as close to the median value (Supplementary Material p. 4). A cutoff of 25 IU/L for FSH was used to differentiate between pre- and post-menopausal women.
Mixed model assumptions were assessed by visual inspection of residuals for normality and by looking for influential observations using Cook’s distance. Cohen’s d effect size was computed to estimate the standardized effect size. All analyses were performed using SPSS version 26.0.41
Data Monitoring Committee
A Data Safety Monitoring Committee, consisting of an independent gynecologist, psychiatrist, and statistician evaluated the study’s progress and safety and advised the sponsor on an ongoing basis.
Results
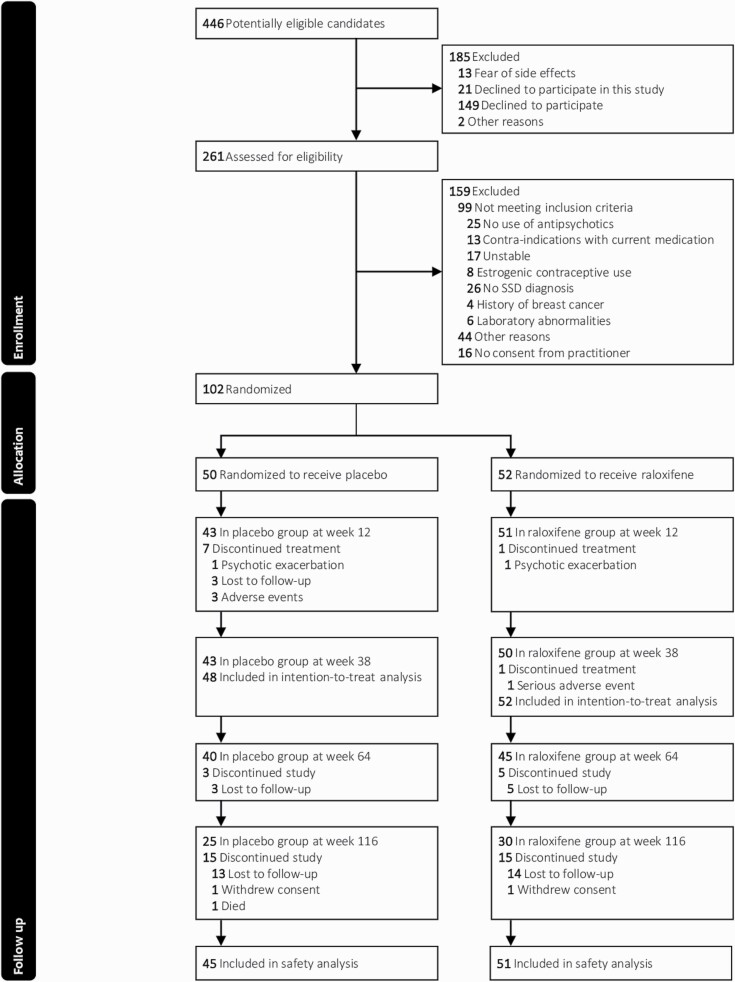
From November 1, 2016, to October 8, 2020, 261 patients were screened, of which 102 were randomized (Figure 1). The last follow-up call was made on January 14, 2022. Eight patients (3 female) dropped out before week 12, of whom 2 (1 female) could not be included in the intention-to-treat analysis due to missing end-of-treatment measures. One male patient dropped out between week 12 and 38. Baseline demographic and clinical characteristics were similar between treatment groups (Table 1, for statistical tests, see Supplementary Table 4). Mean total PANSS scores of 58.9 ± 15.2 (raloxifene group) and 56.6 ± 14.6 (placebo group) indicated a mild level of symptom severity.42 Mean positive and negative PANSS scores were respectively 15.9 ± 5.3 and 13.8 ± 4.8 for the raloxifene group and 14.0 ± 4.2 and 13.4 ± 5.5 for the placebo group. Treatment adherence was high and antipsychotic prescription patterns changes were similar across treatment groups (Supplementary Tables 5–8). The number of pre-menopausal and post-menopausal women was equal across treatment groups (Supplementary Table 9).
Fig. 1.
CONSORT diagram. Note: SSD, Schizophrenia-Spectrum Disorder.
Table 1.
Baseline demographic and clinical characteristics
| Total (N = 100) | Men (N = 72) | Women (N = 28) | ||||
|---|---|---|---|---|---|---|
| Placebo (n = 48) |
Raloxifene (n = 52) |
Placebo (n = 34) |
Raloxifene (n = 38) |
Placebo (n = 14) |
Raloxifene (n = 14) |
|
| Schizophrenia, n (%) | 33 (69) | 43 (83) | 26 (76) | 32 (84) | 7 (50) | 11 (79) |
| Schizoaffective, n (%) | 14 (29) | 9 (17) | 7 (21) | 6 (16) | 7 (50) | 3 (21) |
| Schizophreniform, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Psychosis NOS, n (%) | 1 (2) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| DOI, mean (SD), mo | 185 (116) | 216 (124) | 180 (110) | 190 (101) | 195 (135) | 285 (157) |
| Age, mean (SD), y | 39.3 (11.2) | 42.0 (11.9) | 38.6 (9.7) | 38.8 (10.0) | 41.1 (14.4) | 50.9 (12.6) |
| BMI, mean (SD) | 27.0 (5.1) | 26.3 (4.4) | 28.3 (4.9) | 26.6 (4.8) | 27.0 (5.8) | 25.3 (3.1) |
| Olanzapine Eq. mean (SD), mg/day | 17.6 (11.6) | 17.7 (15.5) | 17.5 (11.5) | 19.2 (17.1) | 17.8 (12.3) | 13.8 (9.2) |
| Adherence, mean (SD), no. of pills | 152 (34.4) | 162 (25.7) | 153 (34.1) | 159 (29.1) | 150 (36.5) | 171 (7.5) |
| Smoking behavior, mean (SD), cigarettes/day | 9.4 (9.7) | 10.0 (12.9) | 10.4 (10.0) | 10.2 (12.4) | 7.1 (8.8) | 9.4 (14.5) |
| Education, mean (SD), y | 12.2 (2.3) | 12.5 (2.4) | 12.2 (2.0) | 12.4 (2.7) | 12.3 (2.9) | 12.6 (1.8) |
| PANSS total score, mean (SD) | 56.6 (14.6) | 58.9 (15.2) | 58.0 (15.1) | 60.7 (15.0) | 53.1 (13.1) | 54.0 (15.0) |
| Positive scale, mean (SD) | 14.0 (4.2) | 15.9 (5.3) | 14.6 (4.2) | 16.5 (5.2) | 12.5 (4.0) | 14.2 (5.2) |
| Negative scale, mean (SD) | 13.4 (5.5) | 13.8 (4.8) | 14.2 (6.0) | 13.9 (5.2) | 11.4 (3.6) | 12.1 (3.4) |
| BACS working memory, mean (SD) | −1.3 (1.1) | −1.2 (1.1) | −1.1 (1.0) | −0.8 (1.0) | −1.7 (1.4) | −0.9 (1.1) |
| BACS verbal memory, mean (SD) | −1.2 (1.1) | −0.8 (1.0) | −1.4 (1.2) | −1.2 (1.2) | −1.2 (0.9) | −1.3 (1.0) |
| BACS processing speed, mean (SD) | −1.1 (0.9) | −1.1 (0.9) | −1.1 (1.1) | −1.1 (0.9) | −1.2 (0.6) | −1.2 (0.9) |
| BACS reasoning/problem solving, mean (SD) | −0.3 (1.3) | −0.4 (1.1) | −0.1 (1.2) | −0.3 (1.1) | −0.6 (1.3) | −0.8 (1.0) |
| BNSS, mean (SD) | 18.4 (13.4) | 15.4 (12.0) | 19.4 (14.2) | 16.2 (12.5) | 15.9 (11.4) | 13.1 (10.3) |
| BDI, mean (SD) | 15.1 (11.7) | 12.4 (9.6) | 13.4 (8.9) | 11.6 (8.2) | 19.4 (16.3) | 14.6 (12.8) |
| PSP, mean (SD) | 56.3 (12.3) | 57.0 (12.4) | 56.3 (13.1) | 55.7 (12.4) | 56.2 (10.6) | 60.5 (11.9) |
| EuroQol-5, mean (SD) | 70.4 (15.1) | 69.3 (13.2) | 71.6 (13.6) | 68.8 (13.4) | 67.6 (18.6) | 70.3 (13.1) |
| TALD total score, mean (SD) | 19.4 (8.1) | 22.1 (10.8) | 18.8 (7.7) | 22.6 (10.0) | 20.8 (9.2) | 20.7 (12.8) |
| Positive scale, mean (SD) | 8.0 (5.0) | 10.5 (8.0) | 8.6 (5.4) | 11.3 (8.0) | 6.5 (3.9) | 8.5 (7.8) |
| Negative scale, mean (SD) | 11.4 (6.2) | 11.5 (6.6) | 10.2 (5.4) | 11.3 (6.5) | 14.3 (7.2) | 12.2 (7.1) |
| 17β-Estradiol, median (IQR), pmol/l | 96.7 (59.8) | 89.9 (54.4) | 83.0 (56.5) | 95.2 (46.8) | 125 (98.0) | 80.0 (102.7) |
| Prolactin, median (IQR), μg/l | 8.93 (13.2) | 8.93 (15.0) | 6.58 (8.93) | 7.05 (12.7) | 10.3 (26.3) | 15.5 (32.9) |
| Testosterone, median (IQR), nmol/l | 15.0 (7.0) | 16.5 (13.5) | ||||
| Premenopausal women, n (%) | 9 (64) | 6 (43) | ||||
Note: DSM-IV, Diagnostic and Statistical Manual of Mental Disorders; NOS, Not Otherwise Specified; y, years; BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); Eq., equivalent; DOI, duration of illness; mo, months; PANSS, Positive And Negative Syndrome Scale; BACS, Brief Assessment of Cognition in Schizophrenia; BNSS, Brief Negative Symptom Scale; BDI, Beck Depression Inventory score; PSP, Personal and Social Performance Score; EuroQol-5, EuroQol-5 dimensions, 5 levels; TALD, Thought and Language Disorder Scale.
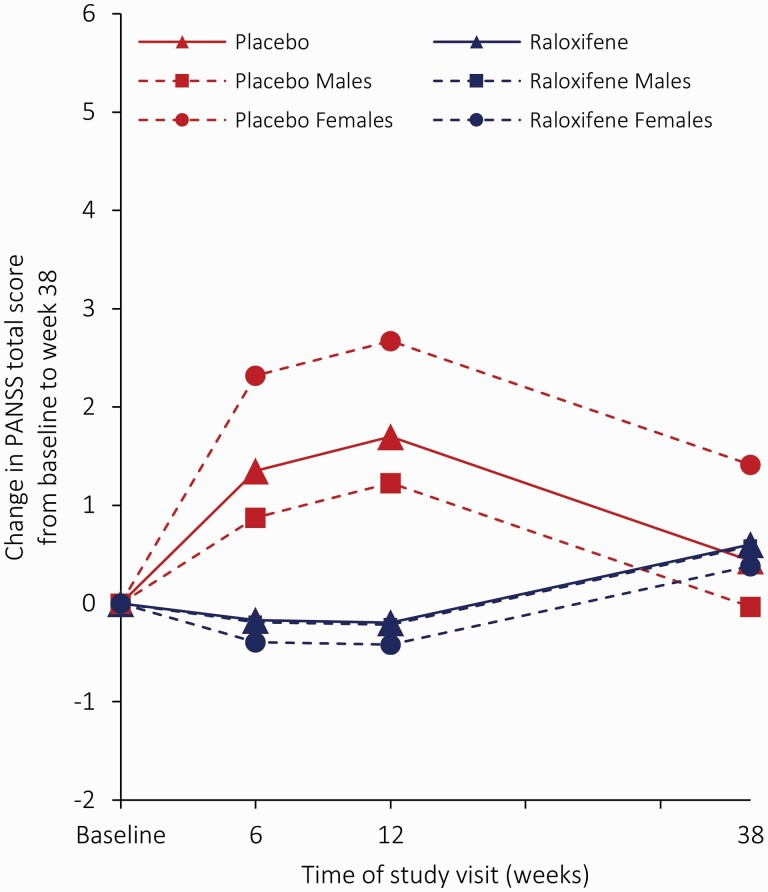
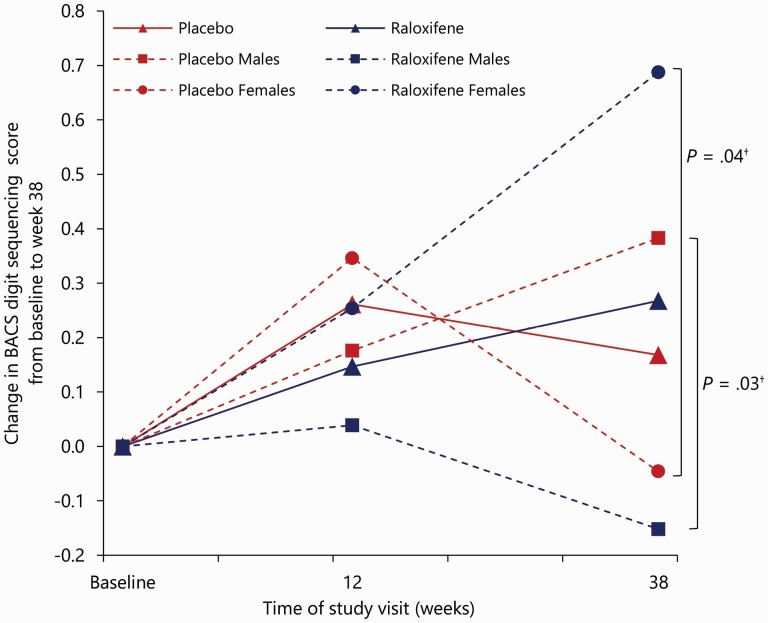
The raloxifene group was not significantly different from the placebo group in PANSS total scores, and for this measure, we found no significant interaction of treatment-by-sex or treatment-by-time (Figure 2; Table 2; and Supplementary Table 10). For negative PANSS scores, an interaction of treatment-by-sex was found (χ2(1) = 4.11; P = 0.04), indicating that treatment effects differed between men and women. Mean change in negative PANSS scores was significantly greater with raloxifene versus placebo in women at week 6 (LSM −2.92; 95% CI −5.26 to −0.57; adjusted P = 0.030) and at week 12 (LSM −3.12; 95% CI −5.49 to −0.74; adjusted P = 0.020), but not at week 38 and not in men (Fig. 3). No effects were found for positive symptoms. Regarding the four cognitive domains, three-way interactions (treatment-by-time-by-sex) were found for the mean change in scores for working memory (χ2(1) = 8.67; P = 0.003) and processing speed (χ2(1) = 5.98; P = 0.014), indicating that treatment effects differed between week 12 and 38 and between men and women (Supplementary Table 10). The sex- and time-specific estimates for verbal memory scores did not reach significance (Table 2). At week 38, improvements in working memory scores were found in women with raloxifene versus women with placebo (LSM 0.73; 95% CI, 0.04 to 1.43; adjusted P = 0.040; Supplementary Fig. 1), in contrast to men, where working memory scores deteriorate with raloxifene as compared to placebo at week 38 (LSM −0.53; 95% CI −0.95 to −0.12; adjusted P = 0.026). We found no significant treatment effects on other secondary outcomes (Table 2 and Supplementary Table 11). Although we found a significant interaction effect of treatment-by-sex for the negative subscale of the Thought and Language Disorder scale (TALD) (χ2(1) = 4.37; P = 0.024), sex-specific estimated effects did not survive multiple testing adjustments (Table 2). Means with SDs of all outcome variables and sex-specific estimates for outcome measures without significant interaction of treatment-by-sex are provided respectively in Supplementary Tables 12 and 13.
Fig. 2.
Least squares mean change in PANSS total score from baseline to week 38 on the full analysis set. The solid lines represent the least squares mean from MMRM with fixed effects of site, sex, age, smoking behavior, years of education, baseline score, treatment condition, study visit (weeks 6, 12, and 38), and visit-by-condition interaction. The dotted lines represent the least squares mean from MMRM with fixed effects of site, sex, age, smoking behavior, years of education, baseline score, treatment condition, study visit (weeks 6, 12, and 38), visit-by-condition, and sex-by-condition interaction. Note: MMRM, mixed-effect model repeated measures; PANSS, positive and negative syndrome scale.
Table 2.
Summary of best estimates of treatment effects of raloxifene versus placebo across time points
| Sex | Time (wks) | n | Estimate (95% CI); P value† | Cohen’s d | SE | |
|---|---|---|---|---|---|---|
| Primary outcomes | ||||||
| PANSS total score | Both (N = 100) | 6 | 100 | −1.52 (−5.03 to 1.99), P = 0.40 | 0.11 | 1.78 |
| 12 | 100 | −1.89 (−5.50 to 1.71), P = 0.30 | 0.13 | 1.83 | ||
| 38 | 93 | 0.16 (−3.47 to 1.99), P = 0.93 | 0.01 | 1.84 | ||
| PANSS negative score | Women (N = 28) | 6 | 28 | −2.92 (−5.26 to −0.57), P = 0.030 | 0.59 | 0.84 |
| 12 | 28 | −3.12 (−5.49 to −0.74), P = 0.020 | 0.65 | 0.86 | ||
| 38 | 26 | −2.31 (−4.70 to 0.07), P = 0.11 | 0.49 | 0.86 | ||
| Men (N = 72) | 6 | 72 | −0.33 (−1.98 to 1.32), P = 0.69 | 0.06 | 1.19 | |
| 12 | 72 | −0.52 (−2.22 to 1.16), P = 0.54 | 0.09 | 1.20 | ||
| 38 | 67 | 0.27 (−1.43 to 1.98), P = 0.75 | 0.05 | 1.21 | ||
| PANSS positive score | Both (N = 100) | 6 | 100 | −0.72 (−1.07 to 0.52), P = 0.25 | 0.15 | 0.63 |
| 12 | 100 | −0.86 (−2.15 to 0.42), P = 0.19 | 0.17 | 0.65 | ||
| 38 | 93 | 0.10 (−1.19 to 1.39), P = 0.88 | 0.02 | 0.65 | ||
| BACS working memory | Women (N = 28) | 12 | 28 | −0.09 (−0.77 to 0.59), P = 0.78 | 0.09 | 0.34 |
| 38 | 23 | 0.73 (0.04 to 1.43), P = 0.040 | 0.62 | 0.35 | ||
| Men (N = 64) | 12 | 64 | −0.14 (−0.57 to 0.30), P = 0.54 | 0.11 | 0.22 | |
| 38 | 60 | −0.53 (−0.95 to −0.12), P = 0.026 | 0.46 | 0.21 | ||
| BACS processing speed | Women (N = 28) | 12 | 28 | 0.11 (−0.31 to 0.53); P = 0.88 | 0.12 | 0.21 |
| 38 | 23 | 0.10 (−0.30 to 0.51); P = 0.93 | 0.11 | 0.21 | ||
| Men (N = 64) | 12 | 64 | 0.02 (−0.26 to 0.30); P = 0.88 | 0.02 | 0.14 | |
| 38 | 60 | 0.01 (−0.26 to 0.28); P = 0.93 | 0.01 | 0.14 | ||
| BACS verbal memory | Both (N = 92) | 12 | 92 | −0.01 (−0.35 to 0.34), P = 0.98 | 0.01 | 0.17 |
| 38 | 83 | −0.37 (−0.82 to 0.09), P = 0.12 | 0.28 | 0.23 | ||
| BACS reasoning/problem solving | Both (N = 92) | 12 | 92 | −0.10 (−0.47 to 0.28), P = 0.61 | 0.08 | 0.19 |
| 38 | 81 | −0.12 (−0.59 to 0.34), P = 0.60 | 0.09 | 0.23 | ||
| Secondary outcomes | ||||||
| BNSS | Both (N = 99) | 6 | 98 | 0.71 (−2.69 to 4.12), P = 0.68 | 0.06 | 1.73 |
| 12 | 93 | −0.41 (−3.88 to 3.05), P = 0.82 | 0.03 | 1.76 | ||
| 38 | 90 | −1.82 (−5.35 to 1.72), P = 0.31 | 0.16 | 1.80 | ||
| BDI | Both (N = 92) | 6 | 92 | 0.16 (−2.33 to 2.65), P = 0.90 | 0.01 | 1.26 |
| 12 | 90 | 0.51 (−2.01 to 3.03), P = 0.69 | 0.05 | 1.28 | ||
| 38 | 89 | 0.52 (−2.02 to 3.07), P = 0.69 | 0.05 | 1.29 | ||
| PSP | Both (N = 100) | 6 | 99 | 1.31 (−2.13 to 4.76), P = 0.45 | 0.10 | 1.75 |
| 12 | 94 | 0.76 (−2.74 to 4.26), P = 0.67 | 0.06 | 1.77 | ||
| 38 | 92 | 1.87 (−1.71 to 5.44), P = 0.32 | 0.15 | 1.81 | ||
| EuroQol-5 dimensions | Both (N = 90) | 12 | 90 | −2.78 (−7.95 to 2.40), P = 0.29 | 0.19 | 2.62 |
| 38 | 88 | 3.44 (−1.89 to 8.77), P = 0.21 | 0.22 | 2.70 | ||
| TALD | Both (N = 99) | 6 | 99 | −0.68 (−3.41 to 2.05), P = 0.62 | 0.07 | 1.38 |
| 12 | 94 | 0.39 (−2.40 to 3.19), P = 0.78 | 0.04 | 1.42 | ||
| 38 | 93 | 0.82 (−1.98 to 3.63), P = 0.56 | 0.09 | 1.42 | ||
| TALD positive score | Both (N = 99) | 6 | 99 | −0.21 (−1.98 to 1.56), P = 0.82 | 0.03 | 0.89 |
| 12 | 94 | 0.57 (−1.39 to 2.54), P = 0.57 | 0.10 | 0.99 | ||
| 38 | 93 | 0.46 (−1.11 to 2.04), P = 0.56 | 0.08 | 0.79 | ||
| TALD negative score | Women (N = 28) | 6 | 28 | −4.20 (−8.24 to 0.16), P = 0.12 | 0.71 | 2.12 |
| 12 | 26 | −4.20 (−8.37 to −0.04), P = 0.10 | 0.67 | 2.10 | ||
| 38 | 26 | −3.33 (−7.54 to 0.87), P = 0.13 | 0.47 | 2.12 | ||
| Men (N = 71) | 6 | 71 | 1.43 (−1.34 to 4.19), P = 0.31 | 0.24 | 1.40 | |
| 12 | 68 | 1.26 (−1.49 to 4.02), P = 0.37 | 0.20 | 1.39 | ||
| 38 | 67 | 2.13 (−0.65 to 4.915), P = 0.13 | 0.34 | 1.41 | ||
Significant results are presented in bold (P < 0.05 after False Discovery Rate correction). Interactions with sex were included when log-likelihood tests were significant. Covariates are study site, time, baseline score, smoking behavior, and condition-by-time. For BACS scores, covariates are study site, time, baseline score, smoking behavior, age, condition-by-time, and years of education. Week 12 was the end-of-treatment visit and week 38 was the follow-up visit (6 months post-treatment). Cohen’s d effect size was calculated as (mean difference)/(pooled standard deviation). Note: wks, Weeks; SE, Standard Error; PANSS, Positive and Negative Symptom Scale; BACS, Brief Assessment of Cognition in Schizophrenia; BNSS, Brief Negative Symptom Scale; BDI, Beck’s Depression Inventory; PSP, Personal and Social Performance scale; EuroQol-5, EuroQol-5 dimensions, 5 levels; TALD, Thought and Language Disorder Scale; †, P value after False Discovery Rate Correction.
Fig. 3.
Least squares mean change in BACS working memory (digit sequencing) from baseline to week 38 on the full analysis set. The dotted lines represent the least squares mean from MMRM with fixed effects of site, sex, age, years of education, smoking status, baseline score, treatment condition, study visit (weeks 12 and 38), and treatment-by-visit-by-sex interaction. The solid lines represent the least squares mean from MMRM with fixed effects of site, sex, age, years of education, smoking status, baseline score, treatment condition, study visit (weeks 12 and 38), and treatment-by-visit interaction. Note: †, P value after False Discovery Rate correction; MMRM, mixed-effect model repeated measures; BACS, Brief Assessment of Cognition in Schizophrenia Negative Symptom Assessment.
For exploratory purposes, we examined whether the effect of raloxifene on primary outcomes differed for subgroups of patients. We examined whether treatment effects differed for pre-menopausal and post-menopausal women, for men and women with low 17β-estradiol levels and high 17β-estradiol levels, and for men with low testosterone levels and high testosterone levels (Supplementary Material p. 4). In women, we found no effect of menopause status or 17β-estradiol levels on symptoms or cognition (Supplementary Table 14). In men, we found an interaction of treatment-by-testosterone for verbal memory (χ2(1) = 3.92; P = 0.047), and of treatment-by-17β-estradiol for processing speed (χ2(1) = 4.93; P = 0.027; Supplementary Table 15). In men with higher testosterone levels, raloxifene had a negative effect on verbal memory compared to placebo at week 38 (LSM −0.88; 95% CI −1.51 to −0.21; adjusted P = 0.044; Supplementary Table 16 and Supplementary Fig. 1). The effects of treatment-by-17β-estradiol in men did not survive multiple testing corrections (Supplementary Table 16).
Safety
Between baseline and week 38, admission to the hospital was classified as SAE. Some were repeated admissions such that 7 admissions occurred in 5 patients in the placebo group, all for incremental psychotic symptoms. In the raloxifene group, 5 admissions occurred in 5 patients. Two were for incremental psychotic symptoms, one was possibly related to raloxifene addition (obstipation), one was for SARS-CoV-2 infection and one was for drug misuse. The prevalence of SAEs, AEs, and ARs was low and similar between groups (Supplementary Tables 17–20). The incidence of hormone-related complaints was low and similar in both groups. We found an interaction of treatment-by-time for physical hormone-related complaints (χ2(4) = 10.87; P = 0.030) driven by a lower score in the raloxifene group at week 116 (LSM −1.87; 95% CI, −3.22 to −0.53; P = 0.006; Supplementary Tables 21 and 22 and Supplementary Figs. 2 and 3).
Discussion
Our results do not support the use of raloxifene in patients with SSD in general. Yet, we showed that the effects of the daily addition of 120 mg raloxifene to regular antipsychotic treatment may be dependent on sex, which warrants further exploration of the potential sex-specific effects of raloxifene. Additional sex-specific analyses suggest a beneficial effect of raloxifene on negative symptoms only in women. At 6 months after treatment discontinuation, we also found evidence for a beneficial effect of raloxifene on working memory in women, while we found negative effects of raloxifene on working memory in men on this time point. No significant differences were observed regarding secondary outcomes in both sexes. Although the effect of raloxifene on symptoms of negative thought disorder differed between men and women, sex-specific treatment effects were not significant.
Importantly, we were not able to replicate previously reported beneficial effects of raloxifene on total and positive symptom severity.12,43,44 This may be explained by the high proportion of men in our study (70%), in which no beneficial effects were observed. In addition, ceiling effects could have played a role as positive symptom severity at baseline was relatively low. Furthermore, we could not replicate the beneficial effects of raloxifene on symptom severity in men, as previously reported by an Iranian study.25 Because our study was well-powered, the absence of any positive effects on symptom severity or cognitive functioning provides no indication for raloxifene addition in men.
Our findings suggest a beneficial effect of raloxifene on negative symptoms in women, which corroborates with previous meta-analyses showing the potential of adjunctive raloxifene in post-menopausal women,10,44 and indicate that these effects can be extended to premenopausal women. In line with our hypothesis that raloxifene has neuroprotective effects, raloxifene seemed to prevent deteriorating effects that were observed in the placebo group. Noteworthy, these beneficial effects of raloxifene were not confirmed by the BNSS, our secondary measure for negative symptoms. Although the BNSS is considered to have a greater sensitivity than the PANSS,45 this discrepancy may be explained by the large variation in negative symptom scores as measured with the BNSS in our sample.
Although one study reported beneficial effects of raloxifene on cognition in a mixed-sex sample of men and premenopausal women,16 this is the first study to report beneficial effects of raloxifene on negative symptoms in a mixed group of pre- and post-menopausal women with SSD. This is a promising first result which is particularly valuable given the persistent nature of negative symptoms and their strong association with functional outcome,2,4,5,46 although replication is essential in premenopausal women. Our finding of a latent beneficial effect of adjunctive raloxifene on working memory in women adds up to the available evidence of a positive direct effect of raloxifene on cognition. If replicated, this could be of considerable interest, taking into account the relative safety of raloxifene and considering the limited treatment options for cognitive impairment in SSD.3,47
This is the first RCT that investigated the long-term effects of adjunctive raloxifene on symptoms and cognition. While our sex-specific findings were based on secondary analyses, they indicate a latent opposing effect of raloxifene on working memory in women and in men. In line with our female-specific beneficial effects, current literature provides evidence that short-term administration of estrogens increases estrogen receptor alpha (ERα) levels in the hippocampus, resulting in sustained neuroprotective effects.48 A recent study derived pluripotent stem cells from SSD patients and differentiated them into neurons, and showed that both estrogens and raloxifene increase synapse density, which may underlie their positive effects on cognition.49 Since the process of synaptogenesis takes time, this could match our findings of a latent beneficial effect on cognition in women.50 Also, this would provide an argument why we found specific effects on working memory because this domain appears to benefit most from higher hippocampal synaptic density.50 Taken together, our results encourage further research on the latent beneficial effects of raloxifene on cognitive functioning in women with SSD.
Our domain-specific results also correspond with other studies reporting beneficial effects of raloxifene only on specific cognitive domains.18–21 It has been suggested that estrogens and estrogen-like compounds may specifically affect brain areas involved in learning and memory,50–53 which is substantiated by our findings and those of other clinical trials and imaging studies.15,16,19,20,51
While our positive findings in female patients may be explained by biological mechanisms in the brain, our negative findings in male patients suggest that raloxifene may have a different effect on the brain in men. Indeed, a growing body of literature has reported clear sex differences in how estrogen modulates hippocampal neurotransmission on a molecular level, indicating that the effect of estrogens on cognitive functioning may be sexually dimorphic (for a review, see Ref. 54). For example, the sexes differ in estrogen receptor subtypes that mediate neural excitation,55 and in how potentiation of excitatory post-synaptic currents is facilitated.56 Estrogen-like compounds may thus have sex-specific effects on brain regions where estrogen receptors are expressed.
We found no evidence for an effect of menopausal status on the efficacy of raloxifene, although this may be due to a lack of power. In addition, our sample size was too small to distinguish a third group of perimenopausal women; a group which we hypothesize to benefit most from raloxifene addition, as they have diminished endogenous production of estrogens, but still have functional estrogen receptors in the brain.57 Long-term estrogen deprivation after menopause transition eventually leads to permanent depletion in ERα levels, while maintaining a sufficient pool of hippocampal ERα is essential for the neuroprotective effects of estrogens on cognition.48 Indeed, in women with Alzheimer’s disease, a therapeutic window has been suggested during which estrogen-like therapy is effective for cognition, which starts during the menopause transition and ends shortly after menopause.58 Such a therapeutic window may also apply for raloxifene addition in women with SSD.18
An important strength of this study is that we reveal through sex-specific analyses that raloxifene may have different effects in women and in men, which encourages further research on sex-specific pharmacotherapeutic treatment strategies.59–61 Our sample contained almost twice as many males as compared to previous RCTs on raloxifene addition in male patients. This can be considered a strength, as it increases the reliability of our null findings regarding symptoms and negative findings on cognition in male patients. The broad inclusion criteria of the current RCT, for example regarding the range of allowed background antipsychotics and the absence of restrictions regarding age, are another strength of our study and allow generalizability and translatability of our findings. On average, RCTs represent only a fifth of the real-world individuals with SSD as a consequence of strict eligibility criteria, which renders their generalizability and translatability low.62
Limitations
A limitation of this study is the relatively small proportion of women in our sample. We expected the use of estrogen-like medication to be more appealing to women than to men and therefore did not make an extra effort to recruit them. In hindsight, we should have made this extra effort. Consequently, the evidence regarding raloxifene’s effect on symptoms in premenopausal women remains limited and requires replication. Our findings also encourage further research into the long-term effects of raloxifene on cognition in women with SSD. Another putative limitation is the relatively mild symptom severity of our sample.42 We are aware of several cases with a minimum score on the PANSS positive or negative scale at baseline, leaving no room for further reduction in symptoms. Nevertheless, our findings do show that women were protected against deterioration on the negative symptom scale, a protection that was absent in men. A further limitation is that patients were permitted to change their regular medications under the supervision of their physician. Although such changes are inevitable in pragmatic-oriented trials of longer duration, this may have added noise to the treatment signal.
Conclusions
In summary, our results do not support the use of raloxifene in patients with SSD in general but indicate that the beneficial effects of raloxifene are females specific. Raloxifene addition has a significant beneficial effect on negative symptoms in women, which confirms and extends findings from previous RCTs in postmenopausal women. Raloxifene also improved working memory in women 6 months after treatment discontinuation. We encourage further exploration of the sex-specific pharmacotherapeutic effects of raloxifene in patients with SSD. These findings support neuroprotective qualities of raloxifene for women with SSD and encourage further exploration of the sex-specific pharmacotherapeutic effects of raloxifene.
Supplementary Material
Acknowledgments
All authors declare to have no competing interest related to this study. IES supervised the study and obtained funding. JNB and IES did the study design and wrote the protocol. BAB and JNB coordinated the study, had access to the raw data, and performed the data analysis. BAB, JNB, and IES interpreted the data, BAB wrote, and JNB and IES revised the manuscript. BAB, JNB, KG, JL, and MM contributed to the recruitment of participants. BAB and JNB collected the data. IES conceived the idea for the study and was involved in the training and supervision of research assistants. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
Bodyl A Brand, Department of Psychiatry, UMC Utrecht Brain Center, University Medical Center Utrecht (UMCU), Utrecht University, Utrecht, The Netherlands; Department of Biomedical Sciences and Systems, Cognitive Neurosciences, University of Groningen, University Medical Center Groningen (UMCG), Groningen, The Netherlands.
Janna N de Boer, Department of Psychiatry, UMC Utrecht Brain Center, University Medical Center Utrecht (UMCU), Utrecht University, Utrecht, The Netherlands; Department of Biomedical Sciences and Systems, Cognitive Neurosciences, University of Groningen, University Medical Center Groningen (UMCG), Groningen, The Netherlands.
Machteld C Marcelis, Department of Psychiatry & Neuropsychology, School for Mental Health and Neuroscience, Maastricht University, Maastricht, The Netherlands; Institute for Mental Health Care Eindhoven (GGzE), Eindhoven, The Netherlands.
Koen P Grootens, Reinier van Arkel Institute for Mental Health Care (RvA), ‘s Hertogenbosch, The Netherlands; Tranzo, Tilburg School of Social and Behavioral Sciences, Tilburg University, Tilburg, The Netherlands.
Jurjen J Luykx, Department of Psychiatry, UMC Utrecht Brain Center, University Medical Center Utrecht (UMCU), Utrecht University, Utrecht, The Netherlands; Department of Psychiatry, Hospital Network Antwerp (ZNA), Antwerp, Belgium; Department of Translational Neuroscience, UMC Utrecht Brain Center, University Medical Center Utrecht, Utrecht University, Utrecht, The Netherlands; Outpatient Second Opinion Clinic, GGNet Mental Health, Warnsveld, The Netherlands.
Iris E Sommer, Department of Psychiatry, UMC Utrecht Brain Center, University Medical Center Utrecht (UMCU), Utrecht University, Utrecht, The Netherlands; Department of Biomedical Sciences and Systems, Cognitive Neurosciences, University of Groningen, University Medical Center Groningen (UMCG), Groningen, The Netherlands.
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Author Contributions
A special thanks to all patients who consented to the study. In addition, we would like to thank Bob Oranje, PhD; P. Roberto Bakker, PhD; Jantina Brummelman, MSc; Sophie Heringa, PhD; Thomas Weickert, PhD; and Bieke de Wilde, MSc; for helping with the study coordination, and Marieke Aarninkhof, MD; Mehmet Acun, MD; Kirsten Catthoor, MD; Shiral Gangadin, MSc; Angelique Goverde, MD; Reinier Koers, MD; and Sebastianus Oude Ophuis, MD conducting patient visits, and/or referring patients to the study team. We also thank all research interns for their daily involvement in the study.
Funding
This work was supported by ZonMW, the Dutch Organization for Health and Research Development (grant number 80-83600-98-40120), as part of the research program Rational Pharmacotherapy (Goed Gebruik Geneesmiddelen), project number 836041008. The funders have no role in the study design, collection, management, analysis and interpretation of data, writing the report, or the decision to submit the report for publication.
References
- 1. WHO. Schizophrenia. Published 2022. https://www.who.int/news-room/fact-sheets/detail/schizophrenia.
- 2. Galderisi S, Mucci A, Buchanan RW, Arango C.. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry. 2018;5(8):664–677. doi: 10.1016/S2215-0366(18)30050-6. [DOI] [PubMed] [Google Scholar]
- 3. Mucci A, Galderisi S, Gibertoni D, et al. Factors associated with real-life functioning in persons with schizophrenia in a 4-year follow-up study of the italian network for research on psychoses. JAMA Psychiatry. 2021;78(5):550. doi: 10.1001/jamapsychiatry.2020.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strassnig MT, Raykov T, O’Gorman C, et al. Determinants of different aspects of everyday outcome in schizophrenia: the roles of negative symptoms, cognition, and functional capacity. Schizophr Res. 2015;165(1):76–82. doi: 10.1016/j.schres.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bobes J, Arango C, Garcia-Garcia M, Rejas J; CLAMORS Study Collaborative Group. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice. J Clin Psychiatry. 2010;71(03):280–286. doi: 10.4088/JCP.08m04250yel. [DOI] [PubMed] [Google Scholar]
- 6. Brand BA, de Boer JN, Dazzan P, Sommer IE.. Towards better care for women with schizophrenia-spectrum disorders. Lancet Psychiatry. 2022;9(4):330–336. doi: 10.1016/S2215-0366(21)00383-7. [DOI] [PubMed] [Google Scholar]
- 7. Riecher-Rössler A. Oestrogens, prolactin, hypothalamic-pituitary-gonadal axis, and schizophrenic psychoses. Lancet Psychiatry. 2017;4(1):63–72. doi: 10.1016/S2215-0366(16)30379-0. [DOI] [PubMed] [Google Scholar]
- 8. Heringa SM, Begemann MJH, Goverde AJ, Sommer IEC.. Sex hormones and oxytocin augmentation strategies in schizophrenia: a quantitative review. Schizophr Res. 2015;168(3):603–613. doi: 10.1016/j.schres.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 9. Begemann MJH, Dekker CF, van Lunenburg M, Sommer IE.. Estrogen augmentation in schizophrenia: a quantitative review of current evidence. Schizophr Res. 2012. doi: 10.1016/j.schres.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 10. Li Z, Wang Y, Wang Z, et al. Estradiol and raloxifene as adjunctive treatment for women with schizophrenia: a meta-analysis of randomized, double-blind, placebo-controlled trials Data Availability Statement: funding: conflicts of interest: ethics approval statement: permission to reproduce material from other sources. Acta Psychiatr Scand. 2022. doi: 10.1111/acps.13530 [DOI] [PubMed] [Google Scholar]
- 11. Ellis AJ, Hendrick VM, Williams R, Komm BS.. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf. 2015;14(6):921–934. doi: 10.1517/14740338.2015.1014799. [DOI] [PubMed] [Google Scholar]
- 12. de Boer J, Prikken M, Lei WU, Begemann M, Sommer I.. The effect of raloxifene augmentation in men and women with a schizophrenia spectrum disorder: a systematic review and meta-analysis. NPJ Schizophr. 2018;4(1):1. doi: 10.1038/s41537-017-0043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gogos A, Sbisa AM, Sun J, Gibbons A, Udawela M, Dean B.. A role for estrogen in schizophrenia: clinical and preclinical findings. Int J Endocrinol. 2015;2015:1–16. doi: 10.1155/2015/615356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kulkarni J, Butler S, Riecher-Rössler A.. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol. 2019;53:100743. doi: 10.1016/j.yfrne.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 15. Ji E, Weickert CS, Lenroot R, et al. Adjunctive selective estrogen receptor modulator increases neural activity in the hippocampus and inferior frontal gyrus during emotional face recognition in schizophrenia. Transl Psychiatry. 2016;6(5):e795–e795. doi: 10.1038/tp.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kindler J, Weickert CS, Skilleter AJ, Catts SV, Lenroot R, Weickert TW.. Selective estrogen receptor modulation increases hippocampal activity during probabilistic association learning in schizophrenia. Neuropsychopharmacology. 2015;40(10):2388–2397. doi: 10.1038/npp.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kindler J, Weickert CS, Schofield PR, Lenroot R, Weickert TW.. Raloxifene increases prefrontal activity during emotional inhibition in schizophrenia based on estrogen receptor genotype. Eur Neuropsychopharmacol. 2016;26(12):1930–1940. doi: 10.1016/j.euroneuro.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 18. Gurvich C, Hudaib A, Gavrilidis E, Worsley R, Thomas N, Kulkarni J.. Raloxifene as a treatment for cognition in women with schizophrenia: the influence of menopause status. Psychoneuroendocrinology. 2019;100:113–119. doi: 10.1016/j.psyneuen.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 19. Huerta-Ramos E, Iniesta R, Ochoa S, et al. Effects of raloxifene on cognition in postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. Eur Neuropsychopharmacol. 2014;24(2):223–231. [DOI] [PubMed] [Google Scholar]
- 20. Weickert TW, Weinberg D, Lenroot R, et al. Adjunctive raloxifene treatment improves attention and memory in men and women with schizophrenia. Mol Psychiatry. 2015;20(6):685–694. doi: 10.1038/mp.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vahdani B, Armani Kian A, Esmaeilzadeh A, Zenoozian S, Yousefi V, Mazloomzadeh S.. Adjunctive raloxifene and isradipine improve cognitive functioning in patients with schizophrenia. J Clin Psychopharmacol. 2020;40(5):457–463. doi: 10.1097/JCP.0000000000001274. [DOI] [PubMed] [Google Scholar]
- 22. Huerta-Ramos E, Labad J, Cobo J, et al. ; RALOPSYCAT Group. Effects of raloxifene on cognition in postmenopausal women with schizophrenia: a 24-week double-blind, randomized, parallel, placebo-controlled trial. Eur Arch Psychiatry Clin Neurosci. 2020;270(6):729–737. doi: 10.1007/s00406-019-01079-w. [DOI] [PubMed] [Google Scholar]
- 23. Weiser M, Levi L, Burshtein S, et al. Raloxifene plus antipsychotics versus placebo plus antipsychotics in severely ill decompensated postmenopausal women with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2017;78(7):e758–e765. doi: 10.4088/JCP.15m10498. [DOI] [PubMed] [Google Scholar]
- 24. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women. JAMA Psychiatry. 2016;73(9):947947. doi: 10.1001/jamapsychiatry.2016.1383. [DOI] [PubMed] [Google Scholar]
- 25. Khodaie-Ardakani MR, Khosravi M, Zarinfard R, et al. A placebo-controlled study of raloxifene added to risperidone in men with chronic schizophrenia. Acta Med Iran. 2015;53(6):337–345. http://www.ncbi.nlm.nih.gov/pubmed/26069170. [PubMed] [Google Scholar]
- 26. Cyr M, Calon F, Morissette M, Grandbois M, Callier S, Paolo T.. Drugs with estrogen-like potency and brain activity potential therapeutic application for the CNS. Curr Pharm Des. 2000;6(12):1287–1312. doi: 10.2174/1381612003399725. [DOI] [PubMed] [Google Scholar]
- 27. Brand BA, de Boer JN, Oude Ophuis SBJ, et al. Raloxifene augmentation in men and women with a schizophrenia spectrum disorder: a study protocol. Contemp Clin Trials Commun. 2020;20:100681. doi: 10.1016/j.conctc.2020.100681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schulz KF, Altman DG, Moher D, CONSORT G. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Overbeek I, Schruers K, Griez E.. Mini international neuropsychiatric interview: Nederlandse versie 5.0. 0. DSM-IV [Dutch version] . Maastricht, The Netherlands. 1999. [Google Scholar]
- 30. Scott NW, McPherson GC, Ramsay CR, Campbell MK.. The method of minimization for allocation to clinical trials. Control Clin Trials. 2002;23(6):662–674. doi: 10.1016/S0197-2456(02)00242-8. [DOI] [PubMed] [Google Scholar]
- 31. Kulkarni J, Gurvich C, Lee SJ, et al. Piloting the effective therapeutic dose of adjunctive selective estrogen receptor modulator treatment in postmenopausal women with schizophrenia. Psychoneuroendocrinology. 2010;35(8):1142–1147. doi: 10.1016/j.psyneuen.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 32. Yaffe K, Krueger K, Cummings SR, et al. Effect of raloxifene on prevention of dementia and cognitive impairment in older women: the Multiple Outcomes of Raloxifene Evaluation (MORE) randomized trial. Am J Psychiatry. 2005. doi: 10.1176/appi.ajp.162.4.683. [DOI] [PubMed] [Google Scholar]
- 33. Nasrallah H, Morosini P, Gagnon DD.. Reliability, validity and ability to detect change of the Personal and Social Performance scale in patients with stable schizophrenia. Psychiatry Res. 2008;161(2):213–224. [DOI] [PubMed] [Google Scholar]
- 34. Kay SR, Fiszbein A, Opler LA.. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 35. Keefe R. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2–3):283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 36. Beck AT, Steer RA, Brown GK.. Manual for the Beck Depression Inventory-II, Vol 1. San Antonio, TX: Psychological Corporation; 1996:82. [Google Scholar]
- 37. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kircher T, Krug A, Stratmann M, et al. A rating scale for the assessment of objective and subjective formal Thought and Language Disorder (TALD). Schizophr Res. 2014;160(1):216–221. [DOI] [PubMed] [Google Scholar]
- 39. Haynes W. Benjamini–Hochberg method. In: Encyclopedia of Systems Biology. Springer, New York, NY. 2013:78. doi: 10.1007/978-1-4419-9863-7_1215 [DOI] [Google Scholar]
- 40. Hochner-Celnikier D. Pharmacokinetics of raloxifene and its clinical application. Eur J Obstet Gynecol Reprod Biol. 1999;85(1):23–29. doi: 10.1016/s0301-2115(98)00278-4. [DOI] [PubMed] [Google Scholar]
- 41. IBM Corp. IBM SPSS Statistics for Windows, Version 26.0. 2019.
- 42. Leucht S, Kane J, Kissling W, Hamann J, Etschel E, Engel R.. What does the PANSS mean? Schizophr Res. 2005;79(2-3):231–238. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 43. Zhu XM, Zheng W, Li XH, et al. Adjunctive raloxifene for postmenopausal women with schizophrenia: a meta-analysis of randomized, double-blind, placebo-controlled trials. Schizophr Res. 2018;197:288–293. doi: 10.1016/j.schres.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 44. Wang Q, Dong X, Wang Y, Li X.. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a meta-analysis of randomized controlled trials. Arch Womens Ment Health. 2018;21(1):31–41. doi: 10.1007/s00737-017-0773-2. [DOI] [PubMed] [Google Scholar]
- 45. Mucci A, Vignapiano A, Bitter I, et al. A large European, multicenter, multinational validation study of the Brief Negative Symptom Scale. Eur Neuropsychopharmacol. 2019;29(8):947–959. doi: 10.1016/J.EURONEURO.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 46. Bucci P, Mucci A, van Rossum IW, et al. Persistent negative symptoms in recent-onset psychosis: relationship to treatment response and psychosocial functioning. Eur Neuropsychopharmacol. 2020;34:76–86. doi: 10.1016/j.euroneuro.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 47. Sinkeviciute I, Begemann M, Prikken M, et al. Efficacy of different types of cognitive enhancers for patients with schizophrenia: a meta-analysis. NPJ Schizophr. 2018;4(1):22. doi: 10.1038/s41537-018-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Daniel JM, Witty CF, Rodgers SP.. Long-term consequences of estrogens administered in midlife on female cognitive aging. Horm Behav. 2015;74:77–85. doi: 10.1016/j.yhbeh.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Michael Deans PJ, Shum C, Perfect L, et al. O10.7. Investigating the mechanisms underlying the beneficial effects of estrogens in schizophrenia. Schizophr Bull. 2018;44(suppl_1):S105–S105. doi: 10.1093/schbul/sby015.259. [DOI] [Google Scholar]
- 50. Zhang HL, Zhao B, Yang P, et al. Steroid receptor coactivator 3 regulates synaptic plasticity and hippocampus-dependent memory. Neurosci Bull. 2021;37(12):1645–1657. doi: 10.1007/s12264-021-00741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khan MM. Neurocognitive, neuroprotective, and cardiometabolic effects of raloxifene: potential for improving therapeutic outcomes in schizophrenia. CNS Drugs. 2016;30(7):589–601. doi: 10.1007/s40263-016-0343-6. [DOI] [PubMed] [Google Scholar]
- 52. Fink G, Sumner BEH, Rosie R, Grace O, Quinn JP.. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325–344. doi: 10.1007/BF02088099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schroeder A, Nakamura JP, Hudson M, et al. Raloxifene recovers effects of prenatal immune activation on cognitive task-induced gamma power. Psychoneuroendocrinology. 2019;110:104448. doi: 10.1016/j.psyneuen.2019.104448 [DOI] [PubMed] [Google Scholar]
- 54. Taxier LR, Gross KS, Frick KM.. Oestradiol as a neuromodulator of learning and memory. Nat Rev Neurosci. 2020. doi: 10.1038/s41583-020-0362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Oberlander JG, Woolley CS.. 17β-Estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J Neurosci. 2017. doi: 10.1523/jneurosci.3011-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jain A, Huang GZ, Woolley CS.. Latent sex differences in molecular signaling that underlies excitatory synaptic potentiation in the hippocampus. J Neurosci. 2019. doi: 10.1523/JNEUROSCI.1897-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ycaza Herrera A, Mather M.. Actions and interactions of estradiol and glucocorticoids in cognition and the brain: implications for aging women. Neurosci Biobehav Rev. 2015;55:36–52. doi: 10.1016/j.neubiorev.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Henderson VW. Alzheimer’s disease: review of hormone therapy trials and implications for treatment and prevention after menopause. J Steroid Biochem Mol Biol. 2014;142:99–106. doi: 10.1016/j.jsbmb.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miguel-Aliaga I. Let’s talk about (biological) sex. Nat Rev Mol Cell Biol. 2022;23(4):227–228. doi: 10.1038/s41580-022-00467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Goldstein JM, Langer A, Lesser JA.. Sex differences in disorders of the brain and heart-a global crisis of multimorbidity and novel opportunity. JAMA Psychiatry. 2021;78(1):7–8. doi: 10.1001/jamapsychiatry.2020.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tannenbaum C, Ellis RP, Eyssel F, Zou J, Schiebinger L.. Sex and gender analysis improves science and engineering. Nature. 2019;575(7781):137–146. doi: 10.1038/s41586-019-1657-6 [DOI] [PubMed] [Google Scholar]
- 62. Taipale H, Schneider-Thoma J, Pinzón-Espinosa J, et al. Representation and outcomes of individuals with schizophrenia seen in everyday practice who are ineligible for randomized clinical trials. JAMA Psychiatry. 2022;79(3):210–218. doi: 10.1001/jamapsychiatry.2021.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.