Abstract
In this dose escalation study, 74 adult cancer patients undergoing bone marrow or peripheral blood stem cell transplantation received fluconazole (400 mg/day) and either normal saline (control) (12 subjects) or micafungin (12.5 to 200 mg/day) (62 subjects) for up to 4 weeks. The maximum tolerated dose (MTD) of micafungin was not reached, based on the development of Southwest Oncology Group criteria for grade 3 toxicity; drug-related toxicities were rare. Commonly occurring adverse events considered related to micafungin were headache (6.8%), arthralgia (6.8%), hypophosphatemia (4.1%), insomnia (4.1%), maculopapular rash (4.1%), and rash (4.1%). Pharmacokinetic profiles for micafungin on days 1 and 7 were similar. The mean half-life was approximately 13 h, with little variance after repeated or increasing doses. Mean maximum concentrations of the drug in serum and areas under the concentration-time curve from 0 to 24 h were approximately proportional to dose. There was no clinical or kinetic evidence of interaction between micafungin and fluconazole. Five of 12 patients (42%) in the control group and 14 of 62 (23%) in the micafungin-plus-fluconazole groups had a suspected fungal infection during treatment which resulted in empirical treatment with amphotericin B. The combination of micafungin and fluconazole was found to be safe in this high-risk patient population. The MTD of micafungin was not reached even at doses up to 200 mg/day for 4 weeks. The pharmacokinetic profile of micafungin in adult cancer patients with blood or marrow transplants is consistent with the profile in healthy volunteers, and the area under the curve is proportional to dose.
Systemic fungal infections contribute to the morbidity and mortality of immunocompromised patients. The two most common invasive fungal infections, candidiasis and aspergillosis, are difficult to diagnose in immunocompromised individuals, and treatment of established infections is not always successful. Fluconazole has been shown to have significant activity against chronic disseminated candidiasis in patients with leukemia, and prophylactic administration of fluconazole to bone marrow transplant recipients reduces the incidence of systemic fungal infections. However, in patients at high risk for disseminated Candida infections, suppression of the more common Candida pathogens may permit some less pathogenic, but intrinsically fluconazole-resistant, Candida species to emerge as systemic pathogens (1, 6, 15).
Micafungin (FK463; Fujisawa Healthcare, Inc., Deerfield, Ill.) is an intravenous antifungal agent of the echinocandin class. A semisynthetic lipopeptide, micafungin possesses potent in vitro and in vivo activities against a broad spectrum of Candida and Aspergillus species, including activities against azole-resistant Candida spp. (5, 7, 8, 10, 12). Micafungin acts by inhibiting the production of 1,3-β-d-glucan, a key component in fungal cell wall synthesis (4). Safety and pharmacokinetic profiles of micafungin have been established for healthy volunteers following single- and repeated-dose administration (J. Azuma, I. Yamamoto, M. Ogura, T. Mukai, H. Suematsu, H. Kageyama, K. Nakahara, Y. Yoshida, and T. Takaya, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-146, 1998).
The objectives of this study included determining the maximum tolerated dose (MTD) of micafungin and the pharmacokinetic profile of micafungin, with concomitant fluconazole administration, in adult cancer patients undergoing bone marrow or peripheral stem cell transplantation.
MATERIALS AND METHODS
Study design.
This randomized, double-blind, sequential-group, dose escalation, tolerance study was conducted at five centers in the United States between June 1998 and May 1999. The protocol was approved by the institutional review board at each study site, and patients gave written informed consent prior to enrollment. Adult patients were randomized in groups in a ratio of 4:1 at each dose level to receive fluconazole (400 mg/day) and either micafungin (eight subjects at each dose level) or normal saline (control; two subjects at each dose level) for prophylactic antifungal therapy after bone marrow or stem cell transplantation. Micafungin was administered at dosages of 12.5, 25, 50, 75, 100, 150, and 200 mg/day. The first 10 patients were entered into the study at the lowest micafungin dose level. Escalation to the next micafungin dose level occurred only after enrollment was completed at the previous dose level and after at least six patients randomized to receive micafungin plus fluconazole had completed 7 days of therapy without meeting the criteria for unacceptable toxicity.
Patient selection.
Male and female patients, 18 to 55 years of age, who underwent a bone marrow or peripheral stem cell transplant were eligible for the study. Patients were excluded if they had abnormal liver test parameters (aspartate aminotransferase [AST], alanine aminotransferase [ALT], total bilirubin, or alkaline phosphatase greater than 2.5 times the upper limit of normal [ULN]), serum creatinine greater than 2.0 mg/day, clinical or other evidence of a deep or disseminated fungal infection, a requirement for systemic antifungal agents other than fluconazole, or a history of anaphylaxis attributed to azole compounds or echinocandins. Pregnant and nursing women were also excluded.
Procedures.
Patients underwent physical examination, clinical assessments of fungal infection, and blood collection for determination of baseline clinical laboratory profiles. Patients were evaluated for fungal infections once a week during treatment, within 48 h after the last treatment, and at 1 and 4 weeks posttreatment. Blood was collected for hematology and serum chemistry profile on days 3, 5, and 7 and then twice weekly for the duration of treatment and at 1 and 4 weeks posttreatment. The absolute neutrophil count (ANC) was measured daily during therapy. Vital signs were measured before and after treatment for the first 5 days of treatment and thereafter as clinically indicated. Treatment-emergent adverse events with onset occurring after the first administration of micafungin or saline were recorded through 72 h after the last dose.
Safety monitoring.
The medical monitor (D.B.) in collaboration with the investigators reviewed the safety data (adverse events and laboratory parameters) in order to ensure that the maximum tolerated dose criteria had not been fulfilled before escalation to the next micafungin dosage cohort.
Pharmacokinetic sampling.
Blood samples (2 ml) for plasma separation were collected for determination of pharmacokinetic parameters on days 1 (micafungin only) and 7 (micafungin and fluconazole) of study drug dosing at 0 (predose), 0.5, 1, 1.5, 2, 4, 6, 8, 10, 18, and 24 h after the start of study drug administration. The 24-h postdose blood sample was drawn prior to the start of the next day's dosing. Predose blood samples were also collected on days 3 and 5. Blood samples were obtained from a port site separate from that used to administer the study drug.
Assay methodology.
Plasma micafungin concentrations were assayed using a high-performance liquid chromatography (HPLC) method with a fluorescence detection system. A validated HPLC method for quantitation of micafungin and two metabolites (M1 and M2) in plasma (data on file) was transferred and validated by MDS Harris Laboratories, Inc. In brief, plasma samples were separated from whole blood, acidified with phosphoric acid, precipitated with acetonitrile, and centrifuged prior to dilution with buffer and injection into an HPLC system. Separation of micafungin, M1, and M2 was achieved with a TosoHaas TSK-GEL ODS80TM column. The analytes were quantified by fluorescence. Data were collected and integrated on a VG Multichrom data system for VAX/VMS. The lower limit of quantitation for micafungin was 0.05 μg/ml. The inter- and intra-assay coefficient of variation values were, respectively, ≤6.8 and 3.2% for micafungin, 5.3 and 2.7% for M1, and 6.1 and 3.2% for M2. Finally, all individual sample concentration data reported for micafungin and metabolites M1 and M2 were within the statistical performance of the assay.
A validated HPLC method for the quantitation of fluconazole was employed by MDS Harris Laboratories, Inc. In brief, octadecyl silane solid-phase extraction columns were used to extract fluconazole from plasma samples. Once eluted from the extraction column, samples were injected into a reverse-phase HPLC analytical system. The analyte was quantified with UV light. Data were collected and integrated on a VG Multichrom data system for VAX/VMS.
Treatment.
Study drug treatment was initiated between 48 h prior to transplant and 24 h posttransplant. Micafungin and saline were administered as 100-ml intravenous infusions over 1 h in a blind manner. Fluconazole was administered either orally (when clinically feasible) or intravenously. Patients were treated until neutrophil recovery (ANC, ≥500 cells/mm3) to a maximum of 4 weeks. At the investigator's discretion, study drugs could be continued for up to 5 days after neutrophil recovery.
Therapy was discontinued if unacceptable toxicity developed or if the patient developed an invasive fungal infection, which was considered to be empirically established if the patient was neutropenic (ANC, <500 cells/mm3), had a persistent or recurrent fever (>100°F or >38°C) for which there was no known etiology, and failed to respond to at least 96 h of adequate broad-spectrum antibacterial therapy.
Criteria for evaluation.
The primary analysis data set included all patients who received at least one dose of the study drug. Safety parameters included treatment-emergent adverse events, laboratory measurements, and changes in vital signs. Dose-limiting toxicity was reached if three separate patients at the same dose level developed the same Southwest Oncology Group (SWOG) grade 3 or greater toxicity, and that toxicity was considered at least probably related to the study drug. Hematologic abnormalities related to the chemotherapy regimen were excluded. Because the trial was primarily designed to be a safety and pharmacokinetics study, no primary efficacy endpoint was established. Efficacy parameters evaluated included incidence of treatment-emergent fungal infections during treatment and posttreatment and a requirement for empirical antifungal therapy.
Statistical methods.
At each dose level, the incidence rates of treatment-emergent adverse events, serious adverse events, and those events related to the study drug (micafungin plus fluconazole or saline plus fluconazole) were summarized. The incidence rates were estimated for systemic fungal infections at the end of therapy, the proportion of patients with systemic fungal infections during the posttreatment period, and the proportion of patients requiring additional systemic antifungal therapy during the posttreatment period.
The pharmacokinetic profiles for micafungin and fluconazole were computed from the drug concentration-time data by noncompartmental methods (2) with a reduced data set in which outlier concentrations were removed. The peak drug concentration in serum (Cmax) and the time of peak drug concentration (Tmax) were obtained directly from the observed data. The terminal elimination rate constant (kel) was obtained from a log linear regression of the plasma drug concentration-time data in the terminal postdistribution phase. The elimination half-life (t1/2) was calculated with the formula 0.693/kel. The area under the plasma drug concentration-time curve from 0 to 24 h (AUC0-24) and the area under the moment curve from 0 to 24 h (AUMC0-24) were calculated by the log-linear trapezoidal rule. The AUC from 0 h to infinity (AUC0-∞) was obtained from the equation AUC0-24 + Ct/kel, where Ct was the last measurable concentration. The total body clearance (CL) for micafungin on days 1 and 7 was obtained from the dose divided by the AUC0-∞ and from the dose divided by the AUC0-24, respectively (presumed steady state, where 24 h was the dosing interval). The volume of distribution (V) for micafungin was calculated as CL/kel. The steady-state volume of distribution (Vss) was calculated from the product of CL and the mean residence time. The mean residence time was obtained with the equation (AUMC0-∞/AUC0-∞) × T/2, where AUMC0-∞ was extrapolated with the equation [(Ct × t)/kel + Ct/kel2], where T was the infusion time. The apparent clearance (CL/F, where F is the bioavailability) for fluconazole on day 7 was obtained from the dose divided by the AUC0-24 (presumed steady state, where 24 is the dosing interval in hours). The apparent V for fluconazole was calculated as (CL/F)/kel.
Prior to the analysis, drug concentration data and case report forms were reviewed for outliers or documentation about errors in the timing of blood samples or drug infusion and for the contamination of blood samples. Based on this review, mistiming was found and selected drug concentration values were excluded for five patients. The second step was to apply a method described by Tukey (13). Tukey defined “outer fences” as values that are three times the interquartile range above the upper (75th percentile) quartile and three times the interquartile range below the lowest (25th percentile) quartile. This procedure was applied to the set of data for each time point for the patients in each dose group.
RESULTS
Patients.
A total of 79 patients were enrolled in the study, and 74 patients received at least one dose of study drug; 12 patients in the control group and 62 patients in the micafungin-fluconazole groups (8 patients at 12.5 mg/day, 9 patients at 25 mg/day, 9 patients at 50 mg/day, 9 patients at 75 mg/day, 9 patients at 100 mg/day, 10 patients at 150 mg/day, and 8 patients at 200 mg/day). Patient characteristics were comparable across dose levels and treatment groups (Table 1).
TABLE 1.
Patient demographics and baseline characteristics
| Demographic or baseline characteristic | Value for:
|
|
|---|---|---|
| Control patients (n = 12) | Micafungin-treated patients (n = 62) | |
| Gender | ||
| No. of females (%) | 7 (58.3) | 42 (67.7) |
| Race | ||
| No. of Caucasians (%) | 12 (100.0) | 50 (80.6) |
| Age (yr) | ||
| Mean ± SD | 43.5 ± 11.63 | 41.9 ± 11.32 |
| Range | 20-56 | 19-65 |
| Mean wt (kg) ± SD | 72.2 ± 13.95 | 82.6 ± 19.35 |
| Underlying disease (no. [%]) | ||
| Hematologic malignancy | 7 (58.3) | 35 (56.5) |
| Solid tumor | 4 (33.3) | 24 (38.7) |
| Other | 1 (8.3) | 3 (4.8) |
| Type of transplantation (no. [%]) | ||
| Allogeneic | 6 (50.0) | 26 (41.9) |
| Autologous | 6 (50.0) | 36 (58.1) |
| Type of cells (no. [%]) | ||
| Bone marrow cells | 3 (25.0) | 8 (12.9) |
| Peripheral stem cells | 8 (66.7) | 50 (80.6) |
| Cord blood cells | 1 (8.3) | 4 (6.5) |
Study drug administration.
The mean duration of study drug administration was 11.2 ± 3.35 days (range, 4 to 18 days) in the control group and 10.7 ± 4.31 days (range, 1 to 27 days) in the micafungin-fluconazole groups.
Safety.
Four patients in the micafungin treatment groups developed a toxicity of grade 3 or greater that was regarded by the investigator as possibly or probably related to study drug (Table 2). Of these four patients, three received either 150 or 200 mg of micafungin. The criteria for the MTD were not fulfilled.
TABLE 2.
Summary of SWOG grade 3 or 4 toxicities at least possibly related to the study drug
| Age (yr) | Sexa | Underlying disease | Type of transplant/cells | Micafungin dose (mg) | Event | Day of onset | Duration of dosing (days) |
|---|---|---|---|---|---|---|---|
| 49 | F | Breast carcinoma | Autologous/peripheral stem cells | 50 | Atrial fibrillation | 7 | 6 |
| 37 | F | Breast carcinoma | Autologous/peripheral stem cells | 150 | Hypokalemia | 3 | 10 |
| 28 | F | CMLb | Allogeneic/cord blood cells | 200 | Pancreatitis | 30 | 27 |
| 54 | F | Breast carcinoma | Autologous/peripheral stem cells | 200 | Maculopapular rash | 8 | 10 |
F, female.
CML, chronic myelogenous leukemia.
All patients experienced one or more adverse events during the study. There were no clinically significant differences in the incidences of adverse events between the control group and the micafungin-fluconazole-treated groups, nor was there any evidence of an increased incidence of adverse events in patients who received higher doses of micafungin. The most common events considered at least possibly related to micafungin were headache (6.8%), arthralgia (6.8%), hypophosphatemia (4.1%), insomnia (4.1%), maculopapular rash (4.1%), and rash (4.1%). There were no reports of infusion-related reactions.
No patients died during treatment. Five patients died during the posttreatment period, namely, one (8.3%) in the control group and four (6.5%) in the micafungin-fluconazole groups. None of the deaths were considered related to either study drug. Adverse events leading to the discontinuation of the study drug occurred in two micafungin-treated patients. One patient was discontinued for an episode of atrial fibrillation (50 mg/day), and one was discontinued for kidney failure (12.5 mg/day).
Mean serum creatinine, AST, ALT, and total bilirubin levels at baseline and at the end of therapy were comparable across treatment groups (Table 3). One patient each in the control group, the group receiving 25 mg of micafungin-fluconazole per day, and the group receiving 150 mg of micafungin-fluconazole per day experienced an increase in ALT of ≥2.5 times the ULN at the end of therapy. One patient each in the 50- and 75-mg/day micafungin-fluconazole groups experienced an increase in total bilirubin of ≥2.5 times the ULN at the end of therapy.
TABLE 3.
Mean laboratory values at baseline and end of therapy by treatment group
| Lab parameter | Visita | Mean value (SD) for indicated group
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 12)b | 12.5 mg (n = 8) | 25 mg (n = 9) | 50 mg (n = 9) | 75 mg (n = 9) | 100 mg (n = 9) | 150 mg (n = 10) | 200 mg (n = 8) | ||
| Creatinine (mg/dl) | Baseline | 0.69 (0.21) | 0.78 (0.51) | 0.68 (0.14) | 0.63 (0.17) | 0.76 (0.35) | 0.66 (0.17) | 0.75 (0.15) | 0.73 (0.44) |
| EOT | 0.68 (0.21) | 0.89 (0.73) | 0.70 (0.25) | 0.79 (0.39) | 0.72 (0.16) | 0.64 (0.13) | 0.74 (0.20) | 0.89 (0.29) | |
| AST (U/liter) | Baseline | 35.9 (21.4) | 28.0 (13.2) | 27.6 (20.4) | 46.3 (39.1) | 33.9 (33.1) | 43.6 (18.8) | 37.8 (15.7) | 33.0 (24.5) |
| EOT | 15.3 (5.2) | 19.1 (11.0) | 14.9 (5.8) | 18.6 (8.9) | 15.9 (7.2) | 19.4 (7.9) | 28.7 (14.9) | 21.0 (10.2) | |
| ALT (U/liter) | Baseline | 44.1 (27.4) | 31.0 (19.9) | 26.3 (7.2) | 63.1 (52.4) | 46.4 (34.7) | 38.8 (20.8) | 53.4 (28.6) | 44.6 (39.5) |
| EOT | 23.3 (12.7) | 23.9 (11.1) | 24.1 (25.2) | 35.8 (26.2) | 17.6 (8.0) | 30.3 (34.5) | 38.9 (31.8) | 32.4 (28.1) | |
| Total bilirubin (mg/dl) | Baseline | 0.63 (0.22) | 0.60 (0.29) | 0.71 (0.33) | 0.86 (0.87) | 0.93 0.60 | 0.73 (0.28) | 0.84 (0.72) | 0.68 (0.42) |
| EOT | 0.70 (0.26) | 0.64 (0.52) | 0.80 (0.38) | 1.63 (1.75) | 1.02 (0.61) | 0.89 (0.59) | 0.92 (0.33) | 1.16 (0.53) | |
EOT, end of therapy.
n, no. of subjects.
MTD assessment.
No dose-limiting toxicities were observed and the maximum tolerated dose for micafungin, when used in combination with fluconazole, was not achieved in this study.
Pharmacokinetics.
Prior to analysis, a review of the case report forms disclosed five samples that were seriously mistimed (e.g., the trough sample was drawn after the start of the next dose). The Tukey procedure identified 37 additional outliers. A total of 42 values were excluded from the analysis, which represented 3.2% of the total 1,307 samples obtained. The pharmacokinetic profiles of micafungin obtained on days 1 and 7 for patients who were treated with 12.5, 25, 50, 75, 100, 150, and 200 mg/day were not appreciably different (Table 4). The AUC0-∞ on day 1 was compared to the AUC0-24 on day 7 by using a paired t test, and they showed no statistically significant difference. This result indicates that the accumulation of micafungin concentrations from days 1 to 7 follows linear pharmacokinetics.
TABLE 4.
Mean pharmacokinetic parameters for plasma micafungin concentrations at days 1 and 7 of dosing by dose group
| Daily dose (mg) | No. of patients | Study day | Cmax (μg/ml) | AUC0-24 (h · μg/ml) | AUC0-∞ (h · μg/ml) | t1/2 (h) | CL (liters/h) | Vss (liters) |
|---|---|---|---|---|---|---|---|---|
| 12.5 | 8 | 1 | 0.9 | 9.0 | 11.6 | 11.3 | 1.09 | 17.7 |
| 8 | 7 | 1.1 | 11.9 | 16.7 | 11.5 | 1.11 | 16.4 | |
| 25 | 9 | 1 | 1.6 | 16.6 | 24.2 | 14.6 | 1.14 | 23.1 |
| 8 | 7 | 4.1 | 23.8 | 34.9 | 12.4 | 1.05 | 16.4 | |
| 50 | 9 | 1 | 3.6 | 33.9 | 44.6 | 12.5 | 1.20 | 21.7 |
| 9 | 7 | 4.4 | 44.3 | 64.0 | 12.2 | 1.06 | 18.0 | |
| 75 | 9 | 1 | 5.4 | 47.0 | 64.3 | 12.7 | 1.26 | 21.8 |
| 8 | 7 | 8.3 | 63.0 | 91.1 | 13.4 | 1.47 | 23.7 | |
| 100 | 9 | 1 | 7.1 | 59.9 | 81.1 | 13.0 | 1.25 | 23.0 |
| 8 | 7 | 22.0 | 101.6 | 126.2 | 12.0 | 1.08 | 17.3 | |
| 150 | 10 | 1 | 11.7 | 103.6 | 144.6 | 13.0 | 1.12 | 21.0 |
| 8 | 7 | 17.6 | 166.7 | 230.3 | 12.9 | 0.98 | 16.7 | |
| 200 | 8 | 1 | 13.1 | 118.1 | 164.3 | 14.3 | 1.28 | 25.6 |
| 8 | 7 | 22.6 | 210.6 | 438.0 | 20.1 | 0.96 | 23.4 |
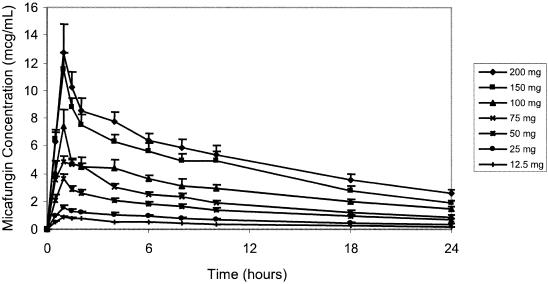
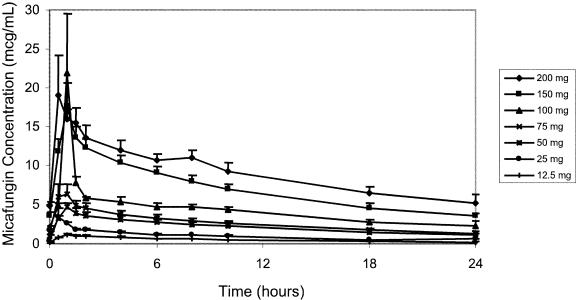
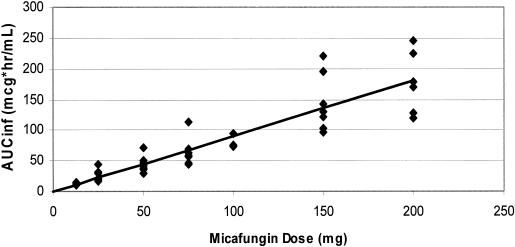
Following a 1-h infusion, biexponential decay was evident (Fig. 1 and 2). The mean terminal elimination half-life was approximately 13 h and remained consistent following repeated, and increasing, doses of micafungin. On day 1, both mean Cmaxs and AUC0-∞ values were approximately proportional to dose (Fig. 3). On day 7, mean Cmax and AUC0-24 estimates were slightly more varied, as was demonstrated for day 1, but still proportional to dose. There was some intersubject variability in day 7 Cmaxs, which influenced the means for the individual dose groups; however, mean Cmaxs on day 7 were proportional to dose. The mean CL values were slightly lower on day 7 than on day 1, but the ranges overlapped, indicating that a change in disposition was unlikely. Similar ranges of mean V values for days 1 and 7 further supported the lack of any change in the pharmacokinetics over the 7 days of dosing.
FIG. 1.
Day 1 plasma micafungin concentrations.
FIG. 2.
Day 7 plasma micafungin concentrations.
FIG. 3.
Proportionality of day 1 AUC00-∞ (AUCinf) to micafungin dose in adult bone marrow and stem cell transplant patients. For the regression line, r2 was equal to 0.83.
Day 7 fluconazole kinetic profiles were obtained for 16 patients who also received a range of micafungin doses from 12.5 to 200 mg. While there was a relatively wide range in the day 7 fluconazole AUC0-24 (range, 191.69 to 469.48 μg · h/ml), there was no discernible trend for different fluconazole AUC0-24s with increasing dose of micafungin. The range of plasma Cmaxs from approximately 11 to 30 μg/ml was obtained at approximately 1 to 8 h after dosing; plasma drug levels then declined in a monoexponential manner. The terminal t1/2 ranged from 11.0 to 41.5 h. Apparent clearance was in the range of 0.15 to 0.53 ml/min/kg of body weight, and the apparent volume of distribution was in the range of 0.33 to 0.79 liters/kg across the micafungin dose groups.
Efficacy.
Five out of 12 (41.7%) patients in the control group had a suspected fungal infection that required the initiation of empirical antifungal therapy at the end of treatment, compared with 14 of 62 (22.6%) patients in the micafungin-fluconazole groups. The numbers of patients who developed a suspected fungal infection that required empirical therapy by micafungin dose group were 2 of 8 (12.5 mg), 3 of 9 (25 mg), 2 of 9 (50 mg), 2 of 9 (75 mg), 2 of 9 (100 mg), 1 of 10 (150 mg), and 2 of 8 (200 mg). One micafungin-fluconazole-treated patient (12.5 mg/day) was discontinued due to a suspected fungal infection during treatment and was diagnosed with a probable fungal infection (histoplasmosis) based on a bone marrow biopsy on day 20 (posttreatment). The infection was not confirmed by antigen testing, and a subsequent bone marrow biopsy was negative. Two patients, both in the 75-mg/day group, developed proven fungal infections. One of these developed pulmonary infiltrates suggestive of a fungal infection and was initiated on empirical treatment with amphotericin B and itraconazole after 6 days of study drug therapy. On day 18, a culture from a lung tissue biopsy showed Cunninghamella bertholetia (in vitro studies indicate that micafungin is not active against Cunninghamella species (9). The second patient had evidence of intestinal candidiasis on autopsy, which was not confirmed by microscopic evaluation. Of the micafungin-fluconazole-treated patients who completed therapy with no indication of a fungal infection, none developed an infection during the 4-week posttreatment period.
DISCUSSION
Systemic fungal infections are a major concern in immunocompromised patients undergoing bone marrow transplantation. The two most common invasive fungal infections, candidiasis and aspergillosis, are difficult to diagnose, and treatment of established infections in immunocompromised patients is not always successful (1, 6). Amphotericin B is not effective in all patients and is associated with significant side effects. Fluconazole has been successfully used for the prevention of candidiasis in bone marrow transplant patients, but a number of resistant species have been identified (15).
The present study was designed to determine the MTD of micafungin in immunocompromised cancer patients. Since micafungin efficacy data for cancer patients were limited at the time the study was designed, it was considered prudent to administer micafungin in combination with a standard antifungal agent, fluconazole.
Based on the results of this study, micafungin, in combination with fluconazole, appears to be safe in adult cancer patients undergoing autologous or allogeneic bone marrow or peripheral stem cell transplantation. Patients were treated with up to 200 mg of micafungin per day, and the criteria for the MTD were not fulfilled, suggesting that the MTD is higher than 200 mg/day in this patient population. A subsequent MTD study of adult cancer patients administered micafungin at doses up to 8 mg/kg/day without identifying the MTD (R. Powles, B. Sirohi, R. Chopra, N. Russel, and H. G. Prentice, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 5217, 2001).
The pharmacokinetic profile of micafungin in combination with fluconazole in adult cancer patients was consistent with that of healthy adult males when micafungin was administered alone (J. Azuma, I. Yamamoto, M. Ogura, T. Mukai, H. Suematsu, H. Kageyama, K. Nakahara, Y. Yoshida, and T. Takaya, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-146, 1998). Importantly, the exposure (AUC) was proportional to dose. This observation extends beyond the 200-mg/day dose tested in this study. In another study of adult cancer patients, the AUC was proportional to dose at doses up to 8 mg/kg/day (R. Powles et al., Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother.). Additional analyses of these data indicate that gender and race have no effect on the kinetics of micafungin in adult cancer patients.
The kinetics of fluconazole at 400 mg/day in this study when administered concomitantly with a wide range of micafungin doses were comparable to published data suggesting no interaction between the two drugs (3). A subsequent drug interaction study (unpublished data) evaluated the pharmacokinetics of micafungin at 150 mg/day and fluconazole at 200 mg/day in healthy volunteers. Thirty subjects received fluconazole orally on day 1, followed by a 1-week wash-out (neither study drug administered). Micafungin was then administered for 15 consecutive days (days 8 to 22), followed by a single dose of fluconazole on day 22. Fluconazole pharmacokinetic parameters were not affected by micafungin at steady state (the mean ratio [90% confidence intervals around the ratio of the means] for days 22 to 1 for Cmax was 98.8% [94.5 to 103.4%] and for AUC0-72 was 102.3% [98.8 to 105.9%]). Similarly, micafungin pharmacokinetic parameters were not affected by single-dose fluconazole (mean ratio [90% confidence interval around the ratio of means] for days 22 to 1 for Cmax was 101.7% [99.7 to 103.7%] and for AUC0-72 was 101.1% [100.1 to 102.1%]).
While not designed to assess efficacy, this study suggested that prophylactic administration of micafungin, with or without fluconazole, may be useful in preventing opportunistic fungal infections in adult patients undergoing allogeneic or autologous bone marrow or peripheral cell transplantation. Subsequently, a large (882 subjects), randomized, multicenter trial that compared micafungin at 50 mg/day to fluconazole at 200 mg/day for prophylaxis in patients undergoing a hematopoietic stem cell transplant was conducted. Micafungin was significantly better in overall treatment success than fluconazole (80.0% success for micafungin compared to 73.5% success for fluconazole [P = 0.03]). Importantly, both drugs prevented the occurrence of invasive Candida infections (0.4% with micafungin versus 0.2% with fluconazole), and there was a nearly significant trend in the prevention of invasive aspergillosis (1 of 425 cases in the micafungin group versus 7 of 457 cases in the fluconazole group [P = 0.071]) (14).
The results of this study suggest that the combination of micafungin and fluconazole is safe and effective for the prevention of fungal infections in immunocompromised patients. Further, this study characterized the dose-proportional pharmacokinetics of micafungin in adult cancer patients at doses up to 200 mg/day. Additional clinical studies with micafungin in this high-risk patient population are warranted.
Acknowledgments
This study was supported by a grant from Fujisawa Healthcare, Inc., Deerfield, Ill. John W. Hiemenz has a consultancy agreement with Fujisawa Healthcare, Inc.
REFERENCES
- 1.De La Rosa, G. R., R. E. Champlin, and D. P. Kontoyiannis. 2002. Risk factors for the development of invasive fungal infections in allogeneic blood and marrow transplant recipients. Transplant. Infect. Dis. 4:3-9. [DOI] [PubMed] [Google Scholar]
- 2.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, 2nd ed., p. 409-416. Marcel Dekker, Inc., New York, N.Y.
- 3.Grant, S. M., and S. P. Clissold. 1990. Fluconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial and systemic mycoses. Drugs 39:877-916. [DOI] [PubMed] [Google Scholar]
- 4.Hatano, K., Y. Morishita, T. Nakai, and F. Ikeda. 2002. Antifungal mechanism of FK463 against Candida albicans and Aspergillus fumigatus. J. Antibiot. 55:219-222. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda, F., Y. Wakai, S. Matsumoto, K. Maki, E. Watabe, S. Tawara, T. Goto, Y. Watanabe, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob. Agents Chemother. 44:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marr, K. A., and R. A. Bowden. 1999. Fungal infections in patients undergoing blood and marrow transplantation. Transplant. Infect. Dis. 1:237-246. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto, S., Y. Wakai, Y. Nakai, K. Hatano, T. Ushitani, F. Ikeda, S. Tawara, T. Goto, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of pulmonary aspergillosis. Antimicrob. Agents Chemother. 44:619-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikamo, H., Y. Sato, and T. Tamaya. 2000. In vitro antifungal activity of FK463, a new water soluble echinocandin-like lipopeptide. J. Antimicrob. Chemother. 46:485-487. [DOI] [PubMed] [Google Scholar]
- 9.Nakai, T., J. Uno, K. Otomo, F. Ikeda, S. Tawara, T. Goto, K. Nishimura, and M. Miyaji. 2002. In vitro activity of FK463, a novel lipopeptide antifungal agent, against a variety of clinically important molds. Chemotherapy 48:78-81. [DOI] [PubMed] [Google Scholar]
- 10.Petraitis, V., R. Petraitiene, A. H. Groll, K. Roussillon, M. Hemmings, C. A. Lyman, T. Sein, J. Bacher, I. Bekersky, and T. J. Walsh. 2002. Comparative antifungal activities and plasma pharmacokinetics of micafungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 46:1857-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pettengell, K., J. Mynhardt, T. Kluyts, W. Lau, D. Facklam, D. Buell, and the FK463 South African Study Group. 2004. Successful treatment of oesophageal candidiasis by micafungin: a novel systemic antifungal agent. Aliment. Pharmacol. Ther. 20:475-481. [DOI] [PubMed] [Google Scholar]
- 12.Tawara, S., F. Ikeda, K. Maki, Y. Morishita, K. Otomo, N. Teratani, T. Goto, M. Tomishima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, K. Sakane, H. Tanaka, F. Matsumoto, and S. Kuwahara. 2000. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob. Agents Chemother. 44:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tukey, J. W. 1977. Exploratory data analysis, p. 43-45. Addison-Wesley, Reading, Mass.
- 14.van Burik, J.-A., V. Ratanatharathorn, D. E. Stepan, C. B. Miller, J. H. Lipton, D. H. Vesole, N. Bunin, D. A. Wall, J. W. Hiemenz, Y. Satoi, J. M. Lee, and T. J. Walsh for the National Institute of Allergy and Infectious Diseases Mycoses Study Group. 2004. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin. Infect. Dis. 39:1407-1416. [DOI] [PubMed] [Google Scholar]
- 15.Wolff, S., J. Fay, D. Stevens, R. Herzig, B. Pohlman, B. Bolwell, J. Lynch, S. Ericson, C. O. Freytes, F. LeMaistre, R. Collins, L. Pineiro, J. Greer, R. Stein, S. A. Goodman, and S. Dummer. 2000. Fluconazole versus low-dose amphotericin B for the prevention of fungal infections in patients undergoing bone marrow transplantation: a study of the North American Marrow Transplant Group. Bone Marrow Transplant. 25:853-859. [DOI] [PubMed] [Google Scholar]