Abstract
Penicillin-binding proteins (PBPs) of slightly different molecular masses (94, 62 or 68, 42 or 50, 25, and 22 kDa) were identified in one human and two porcine Brachyspira pilosicoli strains. Identification of PBPs of B. pilosicoli provides a basis for characterization of the genes encoding these proteins among pathogenic intestinal spirochetes of humans and animals.
Brachyspira pilosicoli is a spirochete that colonizes the hindgut of a broad range of vertebrate hosts, including humans (5). In all hosts where it is found, B. pilosicoli is associated with colonic spirochetosis (CS), a polymicrobial disease characterized by epithelial attachment of Brachyspira spirochetes alone or together with certain Helicobacter species and accompanied by damage and inflammation of the gut wall (5, 7). B. pilosicoli is widespread in stool specimens obtained from humans in developing countries, whereas in Western societies, it is found primarily among homosexual men and human immunodeficiency virus-infected patients, some with diarrhea, abdominal pain, and rectal bleeding (16). The systemic spread of B. pilosicoli has been documented by culture of blood specimens obtained from critically ill human patients, some with diarrheal disease suggestive of CS (13, 22).
A variable response to parenteral administration of β-lactam antibiotics has been recorded for patients with the enteric (3, 14) and systemic (13, 15) forms of CS. More recently, an assessment of the in vitro antimicrobial susceptibility of a large collection of human and porcine B. pilosicoli isolates revealed variable sensitivities to β-lactam antibiotics (2). The MIC of the aminobenzylpenicillin amoxicillin was between 0.25 and 128 μg/ml, whereas those of the cephalosporin ceftriaxone and the carbapenem meropenem were between 0.125 and 2 μg/ml and 0.125 and 0.5 μg/ml, respectively.
Penicillin-binding proteins (PBPs) are a group of enzymes anchored to the inner membrane of bacteria that catalyze the terminal stage of cell wall peptidoglycan synthesis (20). The PBPs of Escherichia coli have been classified as either high-molecular-weight (HMW) PBP-1a, PBP-1b, PBP-1c, PBP-2, and PBP-3, which exhibit transpeptidase and transglycosylase activities, or low-molecular-weight PBP-4, PBP-5/6, and PBP-7, which display endopeptidase and carboxypeptidase activities (10, 20). With the exception of the transglycosylases, these enzymes are inhibited by covalent binding of β-lactam antibiotics, leading to the lysis of susceptible bacteria (10). Therefore, β-lactam antibiotics are substrate analogs that bind irreversibly to the active site of the PBPs (8, 10, 20).
PBPs have been identified among certain pathogenic spirochetes, including Treponema pallidum, the syphilis spirochete, Borrelia burgdorferi, the Lyme disease spirochete, and Leptospira interrogans, the cause of leptospirosis (1, 18, 19). By contrast, relatively little is known about the function of membrane proteins of Brachyspira intestinal spirochetes, and with the exception of one report in which PBPs were used as a tool to verify the purity of outer membrane preparations from B. pilosicoli (23), the PBPs of intestinal spirochetes have not been characterized. The purpose of the present study was to examine B. pilosicoli for the presence of PBPs and comparison with known PBPs of E. coli.
(A preliminary report of these findings was presented at the 84th Annual Meeting of the Conference of Research Workers in Animal Diseases, St. Louis, Mo., 9 to 11 November 2003 [R. P. Dassanayake, G. Sarath, and G. E. Duhamel, abstr. 55P].)
B. pilosicoli strain SP16 (ATCC 49776) (6, 21), originally isolated from a diarrheal specimen obtained from a human immunodeficiency virus-positive individual (12), was propagated in prereduced anaerobically sterilized trypticase soy broth, as previously described (4). The reference porcine B. pilosicoli strain P43/6/78T (ATCC 51139) (6, 21) and the field strain UNL-8 (5) were included in some experiments. E. coli K-12 (Gene Choice, Frederick, Md.) was grown at 37°C in Luria-Bertani medium.
Bacterial membranes were prepared as previously described (24). Briefly, bacteria grown to the exponential phase were harvested by centrifugation, and the cell pellet was washed with phosphate-buffered saline (pH 7.2). After the addition of lysis buffer (10 mM Tris-HCl, 1 mM EDTA, and 1 mM PMSF [pH 7.2]) and sonication (six pulses of 30 s at 50% duty cycle) of the mixture, the cellular debris were removed by centrifugation and the supernatant was centrifuged at 100,000 × g for 60 min at 4°C. The pellet containing the membrane fraction was washed with phosphate-buffered saline, and after determination of the protein concentration (Pierce, Rockford, Ill.), the membrane portion was adjusted to 10 mg/ml.
Ampicillin sodium salt (Sigma Chemical Co., St. Louis, Mo.) and digoxigenin-NHS ester (Roche Applied Science, Indianapolis, Ind.) were conjugated, and digoxigenin-ampicillin conjugates (DIG-AMP) were purified by gel filtration, as previously described (11, 24). Detection of PBPs with DIG-AMP was accomplished as previously described (20, 24). Briefly, membrane preparations from either B. pilosicoli or E. coli (100 μg) were incubated with 2.5 μg of DIG-AMP (15 mM) for 10 min at 30°C. After the reaction was stopped by the addition of 5 μg of unlabeled ampicillin and 5 μl of 20% (wt/vol) Sarkosyl (Sigma), the Sarkosyl-soluble material was mixed with an equal volume of sample loading buffer, boiled for 5 min, separated by electrophoresis onto 4% polyacrylamide stacking and 12% polyacrylamide separating sodium dodecyl sulfate-polyacrylamide gel, and either stained with Coomassie brilliant blue R250 (Bio-Rad) or transferred onto nylon membranes (Midwest Scientific, St. Louis, Mo.). After being washed with Tris-buffered saline (TBS; pH 7.5) and blocked with 2% (wt/vol) skim milk, the membranes were incubated with anti-digoxigenin sheep immunoglobulin G Fab fragments conjugated to alkaline phosphatase (Roche) in blocking solution for 30 min at room temperature. After being washed with TBS containing 0.1% (vol/vol) Tween 20, the membranes were equilibrated with TBS containing 50 mM MgCl2 (pH 9.5) before incubation with chemiluminescent CSPD (disodium 3-(4-methoxyspiro{1,2-dioxetane-3,2′-[5′-chloro]tricyclo[3.3.1.13,7]decan}-4-yl)phenyl phosphate) substrate (Roche) for 1 min at room temperature. The DIG-AMP reactive bands were resolved by exposure of the membrane to X-OMAT-AR films (Eastman Kodak Co., Rochester, N.Y.) for up to 10 min at room temperature. In some experiments, nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (Bio-Rad Laboratories, Inc., Hercules, Calif.) was used as substrate.
Labeling of intact B. pilosicoli and E. coli cells with DIG-AMP was accomplished by harvesting bacteria grown to exponential growth phase and incubation with DIG-AMP (60 μg) for 30 min at 30°C. After incubation, whole-cell lysates or membrane fractions were prepared as described above.
Initial studies with either intact cells or membrane preparations from E. coli confirmed previous findings (Fig. 1; 24). When membrane preparations of the human B. pilosicoli were incubated with DIG-AMP, two prominent bands with molecular masses of approximately 94 and 62 kDa and less prominent bands of approximately 42 and 22 to 25 kDa were found (Fig. 1). Although several additional bands are seen when membrane preparations from B. burgdorferi reacted with DIG-AMP are exposed for longer period, up to 30 min (18), additional bands were not seen with B. pilosicoli exposed for up to 5 h (data not shown).
FIG. 1.
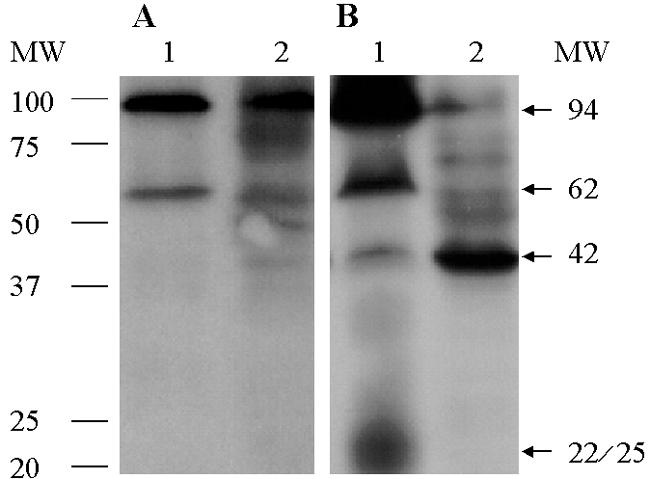
Results of chemiluminescence assays using whole-cell lysates (A) and membrane preparations (B) of human B. pilosicoli strain SP16 (lanes 1) and E. coli (lanes 2) reacted with ampicillin conjugated to digoxigenin followed by immunoblot and chemiluminescence assays. Two prominent PBPs with molecular masses of approximately 94 and 62 kDa and a minor 42-kDa reactive band present in B. pilosicoli migrate at a position similar to the high-molecular-weight PBP-1 and PBP-3 and the low-molecular-weight PBP-5/6 of E. coli. A less-defined band, of approximately 22 to 25 kDa, that is present in the membrane preparation of B. pilosicoli (panel B) but not in the intact cells (panel A), might represent a breakdown product of high-molecular-weight PBPs. MW, molecular weight in thousands.
Both porcine B. pilosicoli strains had identical PBPs that were slightly different from the human strain. Consistent with the chemiluminescence assay, the nitroblue tetrazolium-5-bromo-4-chloro-3-indolyl phosphate substrate revealed a prominent 94-kDa band in each strain; however, all three strains had a doublet of approximately 22 and 25 kDa. Instead of the 62- and 42-kDa bands seen in the human strain, the two porcine strains had bands of slightly higher molecular masses, of 68 and 50 kDa, respectively.
The low-molecular-mass (between 15 and 20 kDa) DIG-AMP-reactive bands of E. coli are interpreted to represent degradation products of HMW PBPs (9). Conversely, bands with molecular masses of 21 kDa and of 13, 21, and 22 kDa, identified in Helicobacter pylori (11) and B. burgdorferi (18), respectively, have been assigned to PBPs. The low-molecular-mass 22- and 25-kDa bands seen for B. pilosicoli might represent breakdown products of HMW PBPs rather than a low-molecular-weight PBP. The absence of the 42-kDa band in intact B. pilosicoli cells (Fig. 1A, lane 1) might be attributable to a HMW PBP that is of low abundance, has a low affinity for ampicillin, or both.
The therapeutic responses of patients with B. pilosicoli infections and the in vitro sensitivities of B. pilosicoli to β-lactam antibiotics are consistent with the identification of PBPs in this spirochete (2, 3, 13-15). The demonstration of PBPs in intact B. pilosicoli cells suggests that the cell membrane is permeable to β-lactam antibiotics. Conversely, the resistance of B. pilosicoli to amoxicillin and the restoration of susceptibility by the addition of clavulanic acid in a previous study (2) suggest that β-lactamase activity, rather than the ability of the drug to penetrate the cell membrane, is the predominant resistance mechanism in this spirochete. Consistent with these results were the low MICs of B. pilosicoli to the β-lactam antibiotics, with enhanced resistance to inactivation by β-lactamase, ceftriaxone, and meropenem (2).
Because PBPs have endopeptidase and carboxypeptidase activities, some of the PBPs identified in the present study might be related to endopeptidases and to a carboxypeptidase identified in membrane preparations of B. pilosicoli in previous studies (4, 17). The identification of PBPs of B. pilosicoli provides a basis for the characterization of the genes encoding these proteins among pathogenic intestinal spirochetes of humans and animals.
Acknowledgments
This work was supported in part by funds provided by the USDA Cooperative State Research, Education, and Extension Service (CSREES), National Research Initiative, Competitive Grants Program, Project NEB 14-114, USDA CSREES Multi-State Research Project NC-1007, and USDA CSREES Animal Health Project NEB 14-118 to G.E.D., and by the BRIN Program of the National Center for Research Resources, Project 1 P20 RR 16469, to G.S.
Footnotes
Published as Paper No. 14743, Agriculture Research Division, Institute for Agriculture and Natural Resources, University of Nebraska—Lincoln, Lincoln, Nebr.
REFERENCES
- 1.Brenot, A., D. J. Trott, I. Saint Girons, and R. Zuerner. 2001. Penicillin-binding proteins in Leptospira interrogans. Antimicrob. Agents Chemother. 45:870-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooke, C. J., D. J. Hampson, and T. V. Riley. 2003. In vitro antimicrobial susceptibility of Brachyspira pilosicoli isolates from humans. Antimicrob. Agents Chemother. 47:2354-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotton, D. W. K., N. Kirkham, and D. A. Hicks. 1984. Rectal spirochetosis. Br. J. Vener. Dis. 60:106-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dassanayake, R. P., N. E. Caceres, G. Sarath, and G. E. Duhamel. 2004. Biochemical properties of membrane-associated proteases of Brachyspira pilosicoli isolated from humans with intestinal disorders. J. Med. Microbiol. 53:319-323. [DOI] [PubMed] [Google Scholar]
- 5.Duhamel, G. E. 2001. Comparative pathology and pathogenesis of naturally acquired and experimentally induced colonic spirochetosis. Anim. Health Res. Rev. 2:3-17. [PubMed] [Google Scholar]
- 6.Duhamel, G. E., N. Muniappa, M. R. Mathiesen, J. L. Johnson, J. Toth, R. O. Elder, and A. R. Doster. 1995. Certain canine weakly β-hemolytic intestinal spirochetes are phenotypically and genotypically related to spirochetes associated with human and porcine intestinal spirochetosis. J. Clin. Microbiol. 33:2211-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duhamel, G. E., C. J. Stryker, G. Lu, V. J. Wong, and R. P. Tarara. 2003. Colonic spirochetosis of colony-raised rhesus macaques associated with Brachyspira and Helicobacter. Anaerobe 9:45-55. [DOI] [PubMed] [Google Scholar]
- 8.Eberhardt, C., L. Kuerschner, and D. S. Weiss. 2003. Probing the catalytic activity of a cell division-specific transpeptidase in vitro with β-lactams. J. Bacteriol. 185:3726-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgopapadakou, N. H., and F. Y. Liu. 1980. Penicillin-binding proteins in bacteria. Antimicrob. Agents Chemother. 18:148-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghuysen, J. M. 1991. Serine β-lactamases and penicillin-binding proteins. Annu. Rev. Microbiol. 45:37-67. [DOI] [PubMed] [Google Scholar]
- 11.Harris, A. G., S. L. Hazell, and A. G. Netting. 2000. Use of digoxigenin-labeled ampicillin in the identification of penicillin-binding proteins in Helicobacter pylori. J. Antimicrob. Chemother. 45:591-598. [DOI] [PubMed] [Google Scholar]
- 12.Jones, M. J., J. M. Miller, and W. L. George. 1986. Microbiological and biochemical characterization of spirochetes isolated from the feces of homosexual males. J. Clin. Microbiol. 24:1071-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanavaki, S., E. Mantadakis, N. Thomakos, A. Pefanis, P. Matsiota-Bernard, S. Karabela, and G. Samonis. 2002. Brachyspira (Serpulina) pilosicoli spirochetemia in an immunocompromised patient. Infection 30:175-177. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan, L. R., and A. Takeuchi. 1979. Purulent rectal discharge associated with a nontreponemal spirochete. JAMA 241:52-53. [PubMed] [Google Scholar]
- 15.Lambert, T., and G. Goursot. 1982. Diarrhée aiguë avec hémocultures et coprocultures positives à Tréponema. Med. Mal. Infect. 12:276-278. [Google Scholar]
- 16.Mikosza, A. S. J., and D. J. Hampson. 2001. Human intestinal spirochetosis: Brachyspira aalborgi and/or Brachyspira pilosicoli? Anim. Health Res. Rev. 2:101-110. [PubMed] [Google Scholar]
- 17.Muniappa, N., and G. E. Duhamel. 1997. Outer membrane-associated serine protease of intestinal spirochetes. FEMS Microbiol. Lett. 154:159-164. [DOI] [PubMed] [Google Scholar]
- 18.Norgard, M. V., S. I. Baker, and J. D. Radolf. 1995. Chemiluminescent analysis of Borrelia burgdorferi penicillin-binding proteins using ampicillin conjugated to digoxigenin. Microb. Pathog. 19:257-272. [DOI] [PubMed] [Google Scholar]
- 19.Radolf, J. D., C. Moomaw, C. A. Slaughter, and M. V. Norgard. 1989. Penicillin-binding proteins and peptidoglycan of Treponema pallidum subsp. pallidum. Infect. Immun. 57:1248-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spratt, B. G. 1977. Properties of the penicillin-binding proteins of Escherichia coli K12. Eur. J. Biochem. 72:341-352. [DOI] [PubMed] [Google Scholar]
- 21.Trott, D. J., T. B. Stanton, N. S. Jensen, G. E. Duhamel, J. L. Johnson, and D. J. Hampson. 1996. Serpulina pilosicoli sp. nov., the agent of porcine intestinal spirochetosis. Int. J. Syst. Bacteriol. 46:206-215. [DOI] [PubMed] [Google Scholar]
- 22.Trott, D. J., N. S. Jensen, I. S. Girons, S. L. Oxberry, T. B. Stanton, D. Lindquist, and D. J. Hampson. 1997. Identification and characterization of Serpulina pilosicoli isolates recovered from the blood of critically ill patients. J. Clin. Microbiol. 35:482-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trott, D. J., D. P. Alt, R. L. Zuerner, M. J. Wannemuehler, and T. B. Stanton. 2001. The search for Brachyspira outer membrane proteins that interact with the host. Anim. Health Res. Rev. 2:19-30. [PubMed] [Google Scholar]
- 24.Weigel, M. L., J. T. Belisle, J. D. Radolf, and M. V. Norgard. 1994. Digoxigenin-ampicillin conjugate for detection of penicillin-binding proteins by chemiluminescence. Antimicrob. Agents Chemother. 38:330-336. [DOI] [PMC free article] [PubMed] [Google Scholar]


