Abstract
Background
Studies have demonstrated low hepatitis B virus (HBV) vaccine series completion among persons with human immunodeficiency virus (HIV).
Methods
We conducted a retrospective record review of persons entering HIV care at 2 clinics in Houston, Texas, between 2010 and 2018. Kaplan-Meier curves summarized time to receipt of HBV vaccines for those eligible for vaccination. We estimated the proportions of patients who had received 1, 2, or 3 HBV vaccine doses at 12 and 24 months after entry to care. A Prentice Williams and Peterson total time model was used to evaluate associations between patient characteristics and time to vaccination.
Results
Of the 5357 patients who entered care, 2718 were eligible for HBV vaccination. After 2 years of follow-up, 51.2% of those eligible had received 1 HBV vaccine, 43.2% had received 2, and 28.4% received 3 vaccines. With adjustment for significant cofactors, patients whose CD4 cell count was ≥200/μL (adjusted hazard ratio [aHR], 1.43 [95% confidence interval (CI), 1.29–1.59]) and transgender patients (1.49 [1.08–2.04]) received any given vaccine dose sooner than those with CD4 cell counts <200/μL or cisgender patients, respectively. Compared with non-Hispanic whites, Hispanic patients were vaccinated sooner (aHR, 1.28 [95% CI, 1.07–1.53]). Those with an active substance use history had a significantly longer time to vaccination than those with no substance use history (aHR, 0.73 [95% CI, .62–.85]).
Conclusions
Strategies are needed to increase HBV vaccine completion rates in our study population, particularly among those with CD4 cell counts <200/μL or with a substance use disorder.
Keywords: hepatitis, hepatitis B, human immunodeficiency virus, immunization, vaccination
Approximately 37 million people worldwide are living with human immunodeficiency virus (HIV) infection, and 5%–20% of them also have chronic hepatitis B virus (HBV) infection [1, 2]. Ten percent of the 1.2 million people in the United States with HIV infection are coinfected with HBV [3, 4]. HIV increases the risk of chronic HBV infection and HBV replication, as well as the risk of fibrosis, cirrhosis, and hepatocellular carcinoma [5, 6]. HBV infection leads to increased rates of end-stage liver disease and death in persons with HIV (PWH) [7–9].
HBV infection is vaccine preventable. The US Centers for Disease Control and Prevention, Infectious Diseases Society of America, HIV Medicine Association, and the US National Institutes of Health recommend testing all PWH for HBV infection, vaccinating those who are uninfected and not immune to HBV, and checking postvaccination levels for antibody to hepatitis B surface antigen (HBsAg) (anti-HBs) to ensure seroprotection [10, 11]. The World Health Organization also recommends HBV vaccination for PWH and postvaccination antibody level monitoring [12]. Despite these recommendations, prior studies have demonstrated that HBV vaccination rates—in particular, completion of a 3-dose vaccine series—are suboptimal among PWH [13, 14].
Because there is increasing interest and use of antiretroviral therapy (ART) regimens that do not have activity against HBV, such as long-acting injectable cabotegravir/rilpivirine, knowing HBV infection status in PWH and vaccinating them against HBV if they are not immune is particularly relevant. Furthermore, various ART changes are made over a person's lifetime.
Most HBV vaccines require 3 doses (Recombivax HB, Engerix-B, Twinrix, and PreHevbrio), but the Centers for Disease Control and Prevention guidelines recommend up to Twinrix 4 doses on an accelerated schedule or 4 Engerix-B doses in immunocompromised patients and those receiving hemodialysis. The Heplisav-B vaccine, however, necessitates only 2 doses separated by 1 month, a significant decrease from the approximately 6-month schedule of 3–4 dose regimens [11, 15]. In PWH, protective anti-HBs levels after receipt of 3 standard doses of HBV vaccine range from 18% to 71% [16]. This is in contrast to the 90%–95% of healthy adults who develop seroprotective antibody levels after completion of the standard HBV vaccine series. The primary objective of the current study was to determine the proportions of PWH who had not previously received a HBV vaccine and received 1, 2, and 3 HBV-containing vaccines 12 and 24 months after entering care at the Thomas Street Health Center (TSHC) and Harris Health Northwest (NW HIV) Clinics in Houston, Texas. We also sought to evaluate patient demographic and clinical variables associated with time to HBV vaccine receipt.
METHODS
Study Design and Population
The Baylor College of Medicine Institutional Review Board approved the study. We abstracted data by retrospective record review. The study included PWH ≥13 years of age who entered care as new patients at TSHC and NW HIV Clinic in Houston, Texas, with ≥ 1 clinic visits between 1 January 2010 and 31 December 2018. The TSHC and NW HIV Clinics are funded by the Ryan White HIV/AIDS Program. These clinics administered the Engerix-B (GlaxoSmithKline Biologicals), Recombivax HB (Merck & Co), and Twinrix (GlaxoSmithKline Biologicals) HBV vaccines during the study period. In these clinics, the cost of the HBV vaccination series is generally covered, even if patients lack private health insurance; therefore, the cost of HBV vaccines is not a barrier to vaccination.
It is the standard practice in the clinics to check anti-HBs, HBsAg, and hepatitis core antibody (anti-HBc) with screening laboratory tests before the entry-to-care visit. The number of patients eligible to receive an HBV-containing vaccine at entry to care was determined. We defined eligibility for HBV vaccine receipt as no history of chronic HBV infection or prior HBV vaccination and no evidence of immunity to hepatitis B (negative anti-HBs, anti-HBc, and HBsAg results). We evaluated the dates of HBV vaccine receipt in patients who were eligible for vaccination, in addition to clinical and sociodemographic factors associated with higher risk of acquiring HBV infection or having complications of infection. Risk factors for HBV infection in this study included chronic liver disease (including history of chronic hepatitis C virus infection, identification as men who have sex with men [MSM], and history of injection drug use). We identified chronic hepatitis C virus infection and chronic liver disease by means of International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes. Data used in this study were deidentified and studied in aggregate; no factors necessitating patient consent were used.
Statistical Analyses
We analyzed data using Stata 16 software (StataCorp) with the support of the Baylor College of Medicine Institute for Clinic and Translational Research. Patient characteristics were summarized using descriptive statistics. Survival analysis was used to look at the time from entry to care to first, second, and third vaccination. Patients were censored either at their last follow-up or at 24 months, whichever came first.
Among patients eligible for HBV vaccine, Kaplan-Meier curves were used to summarize the time to receiving 1, 2, and 3 HBV vaccines. The cumulative incidence (95% confidence interval [CI])—which is equivalent to the proportion of patients who received 1, 2, and 3 HBV vaccines doses at 12 and 24 months from entry to care—was estimated. The Prentice Williams and Peterson total time (PWP-TT) model was used to evaluate associations between patient characteristics and time to vaccination. This model is essentially a stratified Cox proportional hazards model for multiple events, where the vaccine that the patient is eligible for during the time frame (first, second, or third) is used as the stratum [17]. Robust standard errors were also used. Unadjusted associations with vaccination were assessed using univariate PWP-TT regressions, and factors found significant were considered for inclusion in the multivariable PWP-TT model. To avoid collinearity, predictor variables found to be correlated with each other (r > |0.5|) were not included in the same model. A more parsimonious model was obtained by backward elimination. Statistical significance was defined as P < .05.
RESULTS
Patient Characteristics
Of 5357 patients who entered care between 2010 and 2018, 2718 patients were eligible for hepatitis B vaccination. Of the 2639 patients who were excluded, 2361 (89.5%) had evidence of prior hepatitis B immunity with a positive anti-HBs result due either to prior HBV infection or vaccination with positive anti-HBs; 236 (8.9%) were found to have chronic hepatitis B infection based on ICD codes or laboratory results (assessment based on anti-HBs, anti-HBc, anti-HBsAg, and HBV DNA); and 138 (5.2%) had history of prior hepatitis B vaccination. Eligible patients had a mean age of 40.1 years, and 33% were female at birth (Table 1).
Table 1.
Characteristics of the Study Cohort Eligible for Hepatitis B Virus Vaccinationa
| Patient Characteristics | Patients, No. (n = 2718) | Patients, % | |
|---|---|---|---|
| Sex at birth | Male | 1821 | 67.0 |
| Female | 897 | 33.0 | |
| Race/ethnicity | White | 287 | 10.6 |
| Black | 1443 | 53.1 | |
| Hispanic | 956 | 35.2 | |
| Other | 32 | 1.2 | |
| Previously sought care elsewhere | 908 | 33.4 | |
| Transgender | 27 | 1.0 | |
| History of IDU | 138 | 5.1 | |
| History of substance use disorderb | None | 1606 | 69.9 |
| Prior history | 383 | 16.7 | |
| Ongoing | 309 | 13.4 | |
| MSM | 816 | 30.0 | |
| Absolute CD4 cell count, cells/μLc | <200 | 1483 | 55.7 |
| ≥200 | 1179 | 44.3 | |
| History of hepatitis C | 74 | 2.7 | |
| Discontinued care before any vaccination | At 6-mo mark | 244 | 9.0 |
| At 12-mo mark | 390 | 14.3 | |
Abbreviations: IDU, injection drug use; MSM, men who have sex with men.
aEligibility as determined by seronegativity for hepatitis B surface antigen (HBsAg), antibody to HBsAg, and hepatitis core antibody.
bHistory of substance use disorder among 2298 patients for whom this was recorded.
cAbsolute CD4 cell count was evaluated among 2662 patients for whom CD4 count was available.
Approximately half of the eligible population, 1443 patients, identified as black (53.1%), 956 as Hispanic (35.2%), 287 as white (10.6%), and 32 identified as a race/ethnicity that did not align with the aforementioned 3 categories (1.2%). About one-third of the eligible patients (33.4%) had previously sought care at a different HIV clinic before entry to care in our clinics. Various clinical characteristics were also surveyed in study patients, including identification as transgender (1%), history of injection drug use (5.1%), substance use disorder history (13.4% current and 16.7% historically), and MSM (30%). The median absolute CD4 cell count was 246/μL, and 1179 of 2662 patients (44.3%) presented with an absolute CD4 cell count <200/μL. Of the patients eligible for hepatitis B vaccination, 74 (2.7%) had chronic hepatitis C.
The median number of visits per patient was 6 over 24 months of follow-up. A significant proportion of patients discontinued care (ie, did not have subsequent follow-up visits at or after the 6- or 12-month mark) in our clinics before hepatitis B vaccination could be administered. Two hundred forty-four patients (9%) discontinued care <6 months after their entry to care, and this number increased to 390 patients (14.3%) discontinuing care <12 months after their initial visit. Patients were censored in the survival analysis at their last follow-up visit.
Time to Vaccination
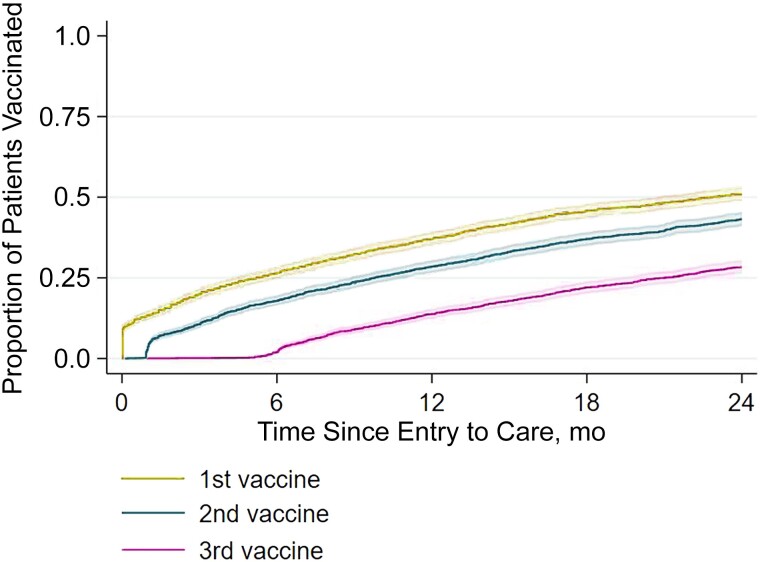
Time to completion of each dose in the 3-dose series is depicted in Figure 1. The cumulative incidences of having received the first, second, and third doses 1 year after entry to care were 37.4% (95% CI, 35.5%–39.3%), 28.5% (26.8%–30.3%), and 13.8% (12.5%–15.3%), respectively. Two years after entry to care, these cumulative incidences increased to 51.2% (95% CI, 49.2%–53.3%), 43.2% (41.1%–45.2%), and 28.4% (26.5%–30.3%), respectively.
Figure 1.
Proportions of patients eligible for hepatitis B virus (HBV) vaccination who had received their first, second, or third HBV vaccine up to 24 months after entry to care.
Univariate PWP-TT was used to assess the unadjusted association of each variable with time to hepatitis B vaccination. In univariate analysis, being Hispanic compared with non-Hispanic white race/ethnicity (hazard ratio [HR], 1.27 [95% CI, 1.09–1.49), being transgender (1.66 [1.28–2.15]), MSM status (1.20 [1.09–1.31]), active substance use disorder compared with no substance use history (0.73 [.63–.85]), and absolute CD4 cell count ≥200/μL (1.36 [1.24–1.50]) had significant associations with vaccination (Table 2). With adjustment for significant factors in a multivariable PWP-TT analysis, Hispanic patients received any given dose sooner than white patients (adjusted HR [aHR], 1.28 [95% CI, 1.07–1.53]), and transgender patients were vaccinated sooner than cisgender patients (1.49 [1.08–2.04]). Meanwhile, patients with ongoing substance use disorder were vaccinated later than those without such disorder (aHR, 0.7 [95% CI, .62–.85]). Finally, patients with an absolute CD4 cell count ≥200/μL were vaccinated sooner than those with a count <200/μL (aHR, 1.43 [95% CI, 1.29–1.59]) (Table 3).
Table 2.
Unadjusted Prentice Williams and Peterson Total Time Model Associations for Time to Vaccination
| Patient Characteristics | Unadjusted PWP-TT Associations for Time to Vaccination | P Value | ||
|---|---|---|---|---|
| HR | 95% CI | |||
| Age | Overall | … | … | .27a |
| <30 y (reference) | 1.00 | … | … | |
| 30–50 y | 0.97 | .88–1.07 | .56 | |
| >50 y | 1.06 | .93–1.21 | .35 | |
| Sex at birth | Male (reference) | 1.00 | … | … |
| Female | 0.94 | .86–1.03 | .20 | |
| Race/ethnicity | Overall | … | … | <.001a |
| White (reference) | 1.00 | … | … | |
| Black | 0.95 | .81–1.12 | .57 | |
| Hispanic | 1.27 | 1.09–1.49 | .003 | |
| Other | 0.83 | .52–1.33 | .44 | |
| Previously sought care elsewhere | … | 0.89 | .81–.98 | .02 |
| Transgender | … | 1.66 | 1.28–2.15 | <.001 |
| History of IDU | … | 0.90 | .75–1.09 | .29 |
| History of substance use disorder | Overall | … | … | <.001a |
| None (reference) | 1.00 | … | … | |
| Prior history | 0.96 | .85–1.08 | .52 | |
| Ongoing | 0.73 | .63–.85 | <.001 | |
| MSM | … | 1.20 | 1.09–1.31 | <.001 |
| Absolute CD4 cell count | <200/μL (reference) | 1.00 | … | … |
| ≥200/μL | 1.36 | 1.24–1.50 | <.001 | |
| History of hepatitis C | … | 0.97 | .78–1.22 | .81 |
| Years patient entered care | 2010–2015 (reference) | 1.00 | … | … |
| 2015–2018 | 1.09 | 1.00–1.18 | .06 | |
Abbreviations: CI, confidence interval; HR, hazard ratio; IDU, injection drug use; MSM, men who have sex with men; PWP-TT, Prentice Williams and Peterson total time.
aOverall P values are reported for variables are characterized by >2 categories.
Table 3.
Adjusted Prentice Williams and Peterson Total Time Model Associations for Time to Vaccination
| Characteristic | Adjusted PWP-TT Model for Time to Vaccination | P Value | ||
|---|---|---|---|---|
| HR | 95% CI | |||
| Race/ethnicity | Overall | … | … | <.001a |
| White (reference) | 1.00 | … | … | |
| Black | 0.95 | .79–1.13 | .54 | |
| Hispanic | 1.28 | 1.07–1.53 | .007 | |
| Other | 0.67 | .37–1.22 | .19 | |
| Transgender | 1.49 | 1.08–2.04 | .01 | |
| History of substance use disorder | Overall | … | … | <.001a |
| None (reference) | 1.00 | … | … | |
| Prior history | 0.96 | .84–1.09 | .53 | |
| Ongoing | 0.73 | .62–.85 | <.001 | |
| Absolute CD4 cell count | <200/μL (reference) | 1.00 | … | … |
| ≥200/μL | 1.43 | 1.29–1.59 | <.001 | |
Hazard ratio for each variable presented with a 95% CI and P value.
Abbreviation: CI, confidence interval; HR, hazard ratio; PWP-TT, Prentice Williams and Peterson total time.
aOverall P values (generated via Wald test) are reported for variables characterized by >2 categories.
DISCUSSION
Among our study population at 2 Ryan White–funded clinics in Houston, we noted low rates of HBV vaccine series completion 2 years after entry to care. We observed that at the 2-year follow-up mark, 51.2% of our patient population had received ≥1 vaccine, 43.2% had received ≥2, and 28.4% had received 3 vaccines. Previously conducted studies of HBV vaccination among PWH have also found low rates of vaccine completion, with 1 study noting a 32.4% single-dose vaccination rate and a 17% series completion rate [13]. Another study that used data from the Medical Monitoring Project, a surveillance system designed to produce nationally representative, annual cross-sectional estimates of behavior and clinical characteristics of PWH in the United States, found that 44% of 18 089 patients evaluated in 2009 were eligible to receive hepatitis B vaccination. By the end of the surveillance period, 9.6% of candidates were vaccinated, 7.5% had no documented vaccination but had documented infection or immunity, and 82.9% remained eligible for hepatitis B vaccination [14].
Yet another study (n = 717 eligible vaccination candidates) found that at 1 year follow-up, up to 49.7% of eligible patients had completed the series. Documentation regarding why patients did not complete their vaccine series indicated that in 85% of cases providers did not offer vaccination owing to concerns of diminished immunogenicity in patients with reduced CD4 cell counts [18]. However, current guidelines indicate that HBV vaccination should be used in PWH to reduce disease incidence in this particularly susceptible population, regardless of CD4 cell count. The Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV [10] note that vaccination should not be deferred in people with lower CD4 cell counts, as such deferment may lead to missed opportunities for vaccination.
Although 51.2% of PWH entering care at both studied Houston clinics eventually received ≥1 dose of hepatitis B vaccine, fewer than a third of at-risk people had completed the HBV vaccine series 2 years after entering HIV care. The increased vaccination rate among transgender patients was an unexpected finding. Prior studies have noted some that transgender PWH experience relatively poorer health outcomes than cisgender PWH, such as reduced HIV viral suppression [19, 20]. The increased vaccination rate among transgender PWH in our study suggests that, among other potential reasons, acceptability of the vaccine was prevalent among this population, providers were more likely to order vaccinations, or barriers to vaccine uptake were relatively low. Meanwhile, the low vaccination rate among ongoing patients with active substance use disorders could reflect a variety of causative factors, including socioeconomic barriers, poor follow-up, and biases against this population [21]. Vaccination rates among those with substance use disorder have historically lagged behind that of the general population in multiple diseases [21, 22]. Finally, a significantly higher hazard for Hispanic compared with white PWH seems to contradict prior findings that HBV vaccine coverage is diminished in Hispanic Americans, suggesting that HIV coinfection or practices in our clinics may modulate previously studied disparities in vaccination [23].
Limitations of our study include its retrospective nature and reliance on the electronic medical record, which may have led to incomplete data capture. Our study was limited to 2 Ryan White–funded county clinics in Houston, Texas, and our results may not be generalizable to all clinic populations. We excluded patients with a history of HBV vaccination or a history of HBV infection or active HBV infection. Some patients with prior HBV infection may have been anti-HBc positive without a positive anti-HBs result and would have been candidates for HBV vaccination. A potential confounder that we did not evaluate in this study is adherence to clinic visits. A patient who misses clinic visits is also missing opportunities for HBV vaccination. Another variable that we did not assess is provider effect. It may be that some providers were more likely to order and promote HBV vaccinations. However, a strength of our study is that we did control for the effect of loss to follow-up in our analyses by censoring patients at the time of their last follow-up visit. Other strengths of our study include a large sample size, a 9-year study period, and multivariate analysis of factors associated with time to vaccination.
Our finding that fewer than a third of our patients who were at risk of developing HBV infection completed the HBV vaccination series has major public health implications. Moreover, our findings are consistent with the findings of other studies evaluating HBV series completion rates in PWH [13, 14, 18]. Not only are many PWH not completing the HBV vaccine series, but prior studies also show that PWH achieve seroprotection at lower rates than people without HIV infection when completing the vaccine series [16]. Inadequate HBV series completion and lower rates of HBV vaccine immunogenicity among PWH mean that a significant number of PWH are vulnerable to developing HBV infection. HBV is a vaccine-preventable infection, and chronic HBV infection may require lifelong treatment. ART without activity against HBV is increasingly being used, which highlights the importance of vaccine protection. Owing to similar modes of transmission, both HBV and HIV are commonly found in the same patient, making it even more crucial to vaccinate PWH [3, 4].
HIV coinfection with HBV has been reported to hasten the progression of HBV infection, characterized by increased HBV chronicity, viral replication, and reactivation, eventually leading to an acceleration of liver cirrhosis, a higher risk of hepatocellular carcinoma, and a higher risk of death due to liver disease [24, 25]. Coinfection has also been associated with an increased all-cause mortality rate in a meta-analysis study of a cohort including >12 000 patients [26]. It may be beneficial for providers and clinics to evaluate their overall HBV vaccine completion rates and evaluate system-wide strategies to improve these rates in their practices. HBV vaccine series completion has also been shown to increase dramatically when vaccination is performed in combination with existing HIV treatment/support initiatives. In a study of primarily MSM, 80% of high-risk individuals who sought HIV testing and received HBV vaccinations completed the series, with a median time to completion close to the recommended 6-month period [27]. The use of dedicated vaccination clinics at HIV care centers, patient text messaging reminder systems, vaccination record cards, and patient education have also been shown to improve series completion in PWH by up to 75.5% [28].
In our clinics, a concerted effort to improve the poor completion rates in general and particularly among persons with active substance use disorders and low CD4 cell counts may require additional strategies, including provider education of HBV immunization guidelines, the recommendation to not delay vaccination based on CD4 cell counts, electronic medical record notification alerts to patients and providers, and the lowering of barriers to clinic access and immunization visits, given the higher risk of infection and complications among these patients.
Finally, our study highlights the timeliness of evaluating alternative HBV vaccination series that require fewer doses to circumvent the problem of diminished 3-dose series completion rates. One such strategy warranting further consideration is the 5′-cytosine-phosphate-guanine-3′ (CpG)-adjuvanted recombinant hepatitis B vaccine that requires 2 vaccination doses ≥1 month apart in immunocompetent adults. The CpG-adjuvanted vaccine has been shown to induce seroconversion in a greater proportion of PWH, compared with those receiving conventional alum-based vaccines [29]. The Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV [10] recommend the CpG-adjuvanted recombinant HBV vaccine series for PWH as a 2-dose series with doses 1 month apart at a CIII recommendation level (weak recommendation based on expert opinion).
The AIDS Clinical Trials Group 5379 clinical trial of 3 doses of the CpG-adjuvanted recombinant hepatitis B vaccine at 0, 1, and 6 months among 68 PWH recently reported that, after 3 doses in the vaccine-naive group, 100% of participants achieved seroprotective anti-HBs titers, and 98.5% achieved seroprotective titers after 2 doses when titers were checked 6 months before administration of the third dose [30]. While higher anti-HBs titers are substantially lower after 2 doses than after 3 doses, if patients complete 2 doses and do not complete the third, they are more likely to have protection against HBV infection than with the current alum-adjuvanted HBV vaccine strategies, with either standard or double-dose vaccines [30, 31].
Although CpG-adjuvanted vaccines cost more than alum-adjuvanted vaccines, studies of cost-effectiveness find that the benefits of CpG-adjuvanted vaccines outweigh costs when factoring for the associated greater and earlier seroprotection rates [32]. In our clinic population, where 43.2% of patient had completed 2 doses of HBV vaccine 2 years after entry to care, as opposed to 28.4% completing the 3-dose series, this may be an important tool. However, as only 43.2% of patients in our clinics completed 2 doses of HBV vaccines, a combination of health care system–wide strategies to increase vaccine series completion rates and alternative vaccination strategies resulting in greater immunogenicity with fewer vaccine doses is most likely to benefit our patient population.
Acknowledgments
Data availability. Data used in this study are not publicly available.
Contributor Information
Daanish Sheikh, Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas, USA.
Kristen A Staggers, Institute for Clinical and Translational Research, Baylor College of Medicine, Houston, Texas, USA.
Jennifer Carey, Thomas Street Health Center, Harris Health System, Houston, Texas, USA.
Wendy A Keitel, Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas, USA; Department of Medicine, Section of Infectious Diseases, Baylor College of Medicine, Houston, Texas, USA.
Robert L Atmar, Department of Medicine, Section of Infectious Diseases, Baylor College of Medicine, Houston, Texas, USA.
Hana M El Sahly, Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas, USA; Department of Medicine, Section of Infectious Diseases, Baylor College of Medicine, Houston, Texas, USA.
Jennifer A Whitaker, Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas, USA; Department of Medicine, Section of Infectious Diseases, Baylor College of Medicine, Houston, Texas, USA.
References
- 1. World Health Organization . Global hepatitis report 2017. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 2. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021 . Accountability for the global health sector strategies 2016–2021: actions for impact. Geneva, Switzerland: World Health Organization, 2021. [Google Scholar]
- 3. Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis 2003; 188:571–7. [DOI] [PubMed] [Google Scholar]
- 4. Spradling PR, Richardson JT, Buchacz K, Moorman AC, Brooks JT; HIV Outpatient Study (HOPS) Investigators . Prevalence of chronic hepatitis B virus infection among patients in the HIV outpatient study, 1996-2007. J Viral Hepat 2010; 17:879–86. [DOI] [PubMed] [Google Scholar]
- 5. Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology 2009; 49(5 suppl):S138–45. [DOI] [PubMed] [Google Scholar]
- 6. Sterling RK, Wahed AS, King WC, et al. Spectrum of liver disease in hepatitis B virus (HBV) patients co-infected with human immunodeficiency virus (HIV): results of the HBV-HIV cohort study. Am J Gastroenterol 2019; 114:746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh KP, Crane M, Audsley J, Avihingsanon A, Sasadeusz J, Lewin SR. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS 2017; 31:2035–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klein MB, Althoff KN, Jing Y, et al. Risk of end-stage liver disease in HIV-viral hepatitis coinfected persons in North America from the early to modern antiretroviral therapy eras. Clin Infect Dis 2016; 63:1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chun HM, Mesner O, Thio CL, et al. HIV outcomes in hepatitis B virus coinfected individuals on HAART. J Acquir Immune Defic Syndr 2014; 66:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV . Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. National Institutes of Health, Centers for Disease Control and Prevention, and the HIV Medicine Association of the Infectious Disease Society of America. Available at: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection. Accessed 7 December 2022.
- 11. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep 2018; 67:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization . Hepatitis B vaccines: WHO position paper, July 2017—recommendations. Vaccine 2019; 37:223–5. [DOI] [PubMed] [Google Scholar]
- 13. Tedaldi EM, Baker RK, Moorman AC, et al. Hepatitis A and B vaccination practices for ambulatory patients infected with HIV. Clin Infect Dis 2004; 38:1478–84. [DOI] [PubMed] [Google Scholar]
- 14. Weiser J, Perez A, Bradley H, King H, Shouse RL. Low prevalence of hepatitis B vaccination among patients receiving medical care for HIV infection in the United States, 2009 to 2012. Ann Intern Med 2018; 168:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weng MK, Doshani M, Khan MA, et al. Universal hepatitis B vaccination in adults aged 19–59 years: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whitaker JA, Rouphael NG, Edupuganti S, Lai L, Mulligan MJ. Strategies to increase responsiveness to hepatitis B vaccination in adults with HIV-1. Lancet Infect Dis 2012; 12:966–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amorim LD, Cai J. Modelling recurrent events: a tutorial for analysis in epidemiology. Int J Epidemiol 2015; 44:324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bailey CL, Smith V, Sands M. Hepatitis B vaccine: a seven-year study of adherence to the immunization guidelines and efficacy in HIV-1-positive adults. Int J Infect Dis 2008; 12:e77–83. [DOI] [PubMed] [Google Scholar]
- 19. Lemons A, Beer L, Finlayson T, McCree DH, Lentine D, Shouse RL. Characteristics of HIV-positive transgender men receiving medical care: United States, 2009–2014. Am J Public Health 2018; 108:128–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mizuno Y, Frazier EL, Huang P, Skarbinski J. Characteristics of transgender women living with HIV receiving medical care in the United States. LGBT Health 2015; 2:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quaglio G, Lugoboni F, Mezzelani P, Des Jarlais DC, Lechi A. Hepatitis vaccination among drug users. Vaccine 2006; 24:2702–9. [DOI] [PubMed] [Google Scholar]
- 22. Iversen J, Wand H, Kemp R, et al. Uptake of COVID-19 vaccination among people who inject drugs. Harm Reduct J 2022; 19:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhuiyan AR, Kabir N, Mitra AK, Ogungbe O, Payton M. Disparities in hepatitis B vaccine coverage by race/ethnicity: the National Health and Nutrition Examination Survey (NHANES) 2015–2016. Diseases 2020; 8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization . Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. [PubMed]
- 25. Cheng Z, Lin P, Cheng N. HBV/HIV coinfection: impact on the development and clinical treatment of liver diseases. Front Med (Lausanne) 2021; 8:713981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis 2009; 48:1763–71. [DOI] [PubMed] [Google Scholar]
- 27. Savage RB, Hussey MJ, Hurie MB. A successful approach to immunizing men who have sex with men against hepatitis B. Public Health Nurs 2000; 17:202–6. [DOI] [PubMed] [Google Scholar]
- 28. Rock C, de Barra E, Sadlier C, et al. Impact of a new vaccine clinic on hepatitis B vaccine completion and immunological response rates in an HIV-positive cohort. J Infect Public Health 2013; 6:173–8. [DOI] [PubMed] [Google Scholar]
- 29. Reilly-Evans B, Dudzik B, Costlow DJ, et al. Observational study evaluating the seroprotection of HepB-alum vaccine and HepB-CpG vaccine in people with HIV. Open Forum Infect Dis 2023; 10:ofad267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marks KM, Kang M, Umbleja T, et al. Immunogenicity and safety of hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant in HBV vaccine-naïve people with human immunodeficiency virus. Clin Infect Dis 2023; 77:414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tian Y, Hua W, Wu Y, et al. Immune response to hepatitis B virus vaccine among people living with HIV: a meta-analysis. Front Immunol 2021; 12:745541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hirst A, Hyer RN, Janssen RS. Comparative cost-effectiveness of a 2-dose versus 3-dose vaccine for hepatitis B prevention in selected adult populations. Vaccine 2021; 39:4733–41. [DOI] [PubMed] [Google Scholar]