Abstract
Background and hypothesis
Neuroimaging-based machine learning (ML) algorithms have the potential to aid the clinical diagnosis of schizophrenia. However, literature on the effect of prevalent comorbidities such as substance use disorder (SUD) and antisocial personality (ASPD) on these models’ performance has remained unexplored. We investigated whether the presence of SUD or ASPD affects the performance of neuroimaging-based ML models trained to discern patients with schizophrenia (SCH) from controls.
Study design
We trained an ML model on structural MRI data from public datasets to distinguish between SCH and controls (SCH = 347, controls = 341). We then investigated the model’s performance in two independent samples of individuals undergoing forensic psychiatric examination: sample 1 was used for sensitivity analysis to discern ASPD (N = 52) from SCH (N = 66), and sample 2 was used for specificity analysis to discern ASPD (N = 26) from controls (N = 25). Both samples included individuals with SUD.
Study results
In sample 1, 94.4% of SCH with comorbid ASPD and SUD were classified as SCH, followed by patients with SCH + SUD (78.8% classified as SCH) and patients with SCH (60.0% classified as SCH). The model failed to discern SCH without comorbidities from ASPD + SUD (AUC = 0.562, 95%CI = 0.400–0.723). In sample 2, the model’s specificity to predict controls was 84.0%. In both samples, about half of the ASPD + SUD were misclassified as SCH. Data-driven functional characterization revealed associations between the classification as SCH and cognition-related brain regions.
Conclusion
Altogether, ASPD and SUD appear to have effects on ML prediction performance, which potentially results from converging cognition-related brain abnormalities between SCH, ASPD, and SUD.
Keywords: schizophrenia, machine learning, MRI, comorbidity, antisocial personality, substance use
Introduction
Neuroimaging is often performed in patients with serious psychiatric symptoms to rule out somatic conditions that could cause psychiatric manifestations,1 but it is not currently clinically used for psychiatric differential diagnostics. Previous work has shown that neuroimaging-based machine learning (ML) algorithms have the potential to distinguish controls from patients with schizophrenia (SCH) with up to 80% balanced accuracy (BAC).2 However, the prediction performance of an ML model is affected by multiple factors, one being sample heterogeneity, such as patient comorbidities.3 Previous ML studies were mainly conducted in patient samples without comorbidities and were compared to control groups without psychiatric disorders, thereby decreasing the generalizability of the findings to real-world clinical samples. A prior study reported varying ML model prediction results (BAC ranged from 63% to 73%) among patients with first-episode psychosis depending on comorbidities and disorder courses.4
Approximately 50% of patients with SCH have psychiatric comorbidities,5–7 and in forensic psychiatric populations, comorbidities such as antisocial personality disorder (ASPD) and substance use disorder (SUD) are even more prevalent. According to an Epidemiological Catchment study, 84% of ASPD patients suffer from an SUD.8 Also, in a nationally representative sample of psychotic homicide offenders, 52% of patients with SCH had ASPD, 74% had SUD as a comorbidity, and all offenders diagnosed with some form of personality disorder had SUD.9 In addition, approximately 50% of patients with SCH in general psychiatric populations have SUD, and 20% have ASPD comorbidity.5,10 Thus, ASPD and SUD have a high prevalence in forensic psychiatric populations,9 which implies that “true” healthy controls in these populations are rare.
Addressing the issue of comorbidities in neuroimaging-based ML studies is important since previous research has shown overlapping brain abnormalities in SCH, ASPD, and SUD.11–18 Specifically, MRI studies on SCH have shown volumetric reductions in total brain volume and cortical gray matter, as well as in frontal and temporal lobes and the thalamus. In addition, increased volumes in basal ganglia and cerebral ventricle were reported.11–13 Similar brain abnormalities have been reported for ASPD, as previous studies have found reductions in total brain volume, thalamus, and frontal and temporal lobes.14 Also, for SUD patients, volumetric gray matter alterations in the thalamus, insula, putamen, and anterior cingulate cortex, as well as white matter alterations in thalamic radiations and internal capsule, have been reported.15,16 Moreover, individuals with ASPD and SUD have shown reduced volumes in the prefrontal cortex,18 posterior cingulate cortex, and insula.17
Since SCH, ASPD, and SUD appear to share brain abnormalities, ML models trained to classify SCH from controls may fail to correctly classify nonpsychotic individuals with ASPD + SUD. However, no previous ML study has investigated this possibility. Also, to the best of our knowledge, it has remained elusive whether ASPD and SUD comorbidities affect the predictions of neuroimaging-based ML models in SCH. Here, using individuals undergoing forensic psychiatric evaluation, we investigated whether SUD or ASPD affects the performance of neuroimaging-based ML models trained to discern SCH from controls.
Methods
Analytical Strategy and Samples
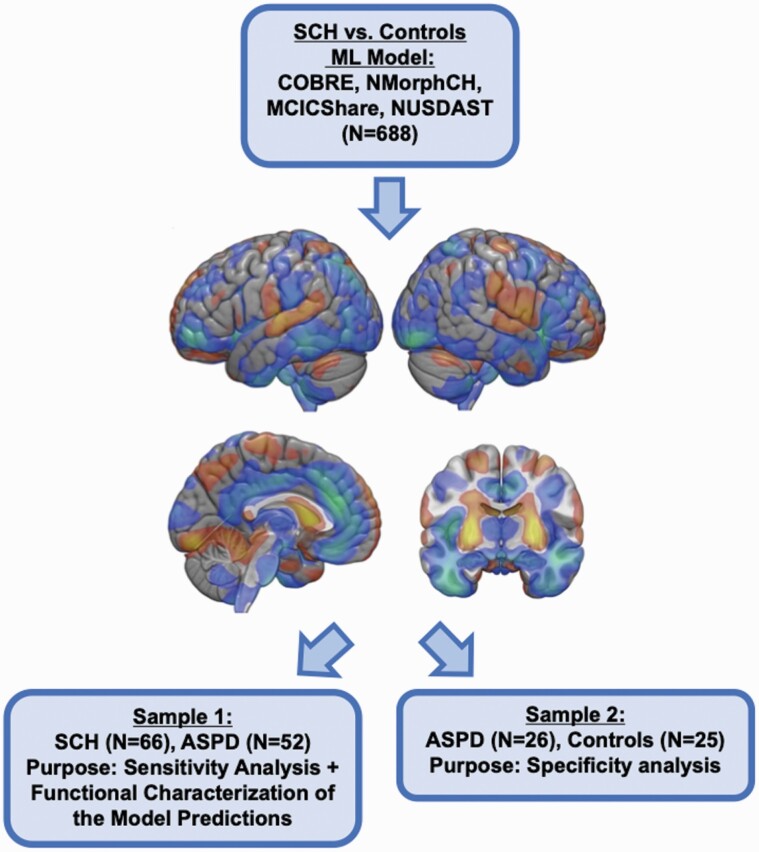
The workflow describing the analyses of the present study is described in Figure 1. First, we utilized four publicly available data repositories (available at http://schizconnect.org) for training our neuroimaging-based ML model to discern SCH from controls: Centre for Biomedical Research Excellence (COBRE), the Neuromorphometry by Computer Algorithm Chicago (NMorphCH), MIND Clinical Imaging Consortium (MCICShare) and Northwestern University Schizophrenia Data and Software Tool (NUSDAST). These samples have been widely used in previous schizophrenia-related neuroimaging studies.19–21 The samples were imaged using four different MRI scanners with field strengths ranging from 1.5T to 3T with varying voxel size. The COBRE sample excluded individuals with comorbid SUD, but we had no information about the presence of comorbid SUD in the other three samples. Also, we had no information about whether any sample included individuals with comorbid ASPD. More detailed information is described in the Supplement.
Fig. 1.
Flowchart describing the analyses of the study. Training the schizophrenia vs. controls machine learning model using discovery samples. The SCH vs. Controls ML model is visualized as an SVM weight map (thresholded at 0.0005 for visualization purposes). Warm colors indicate gray matter volume increases, and cold colors decrease in the VBM data. The trained ML model was then applied, without any in-between-retraining, to samples 1 and 2.
Next, we utilized two samples (Samples 1 and 2) of individuals undergoing a forensic psychiatric examination to evaluate the effect of ASPD and SUD on the ML model’s prediction. Both samples were drawn from Niuvanniemi Forensic Psychiatric Hospital, which granted permission to conduct the present study with permission from the local ethical committee. In Finland, full forensic psychiatric examinations are ordered by the court of law to evaluate the level of criminal responsibility (a detailed description is provided in the Supplement).22 In the present study, we used two samples of individuals. Due to very few females in forensic evaluations during the data gathering period, we focused on males.
Sample 1 (N = 118) comprised individuals who underwent a forensic psychiatric evaluation at Niuvanniemi forensic hospital between 2014 and June 2021. The purpose of this sample was to evaluate the predictive performance of the two ML models on discerning SCH (ICD-10: any F20 and any F25) from ASPD (ICD-10: F60.2). The effect of comorbid substance use disorders (ICD-10: F10-F19) was also examined. We also conducted data-driven functional characterization to investigate whether the ML model’s predictions were associated with known behavioral abnormalities in SCH and ASPD (details below). Data were obtained retrospectively from hospital files. The exclusion criteria for sample 1 were traumatic brain injury and neurological disorders. Imaging quality was visually inspected and assessed using the automatic quality control provided by the analysis software (details below). Since we utilized real-world clinical data, the whole brain MRI scans were obtained with different 1.5T scanners (Philips Achieva, Siemens Avanto, GE Signa HDxt, or GE Signa Voyager) and variable parameters. MRI slice thickness varied from 1 to 2 mm.
Sample 2 (n = 51) has been described in a previous study.17 This sample was used for specificity analyses to evaluate whether our ML model can discern ASPD with a comorbid substance use disorder (SUD, ICD-10: F10-F19) from controls (N = 25). Data from ASPD + SUD were obtained retrospectively from hospital files, and controls gave informed consent. All participants were Finnish males. Inclusion criteria for controls (students, hospital staff, and skilled workers) were the absence of any current or past mental disorder or substance misuse based on unstructured interviews. The intelligence quotient (IQ) of controls was not available. Inclusion criteria for the offender group in sample 2 were the absence of current or past psychosis or schizotypal personality disorder.
Processing of the Structural MRI
We analyzed the structural MRI (sMRI) data using voxel-based morphometry (VBM) provided by the CAT12 toolbox version 1743 (Structural Brain Mapping Group, Jena University Hospital, Jena, Germany http://dbm.neuro.uni-jena.de/cat12/) on SPM12 in MATLAB r2017b (mathworks.com). T1-weighted images were processed using the standard processing pipeline implemented in the toolbox, including denoising, skull stripping, and spatial normalization to the Montreal Neurological Institute (MNI-152) space using the DARTEL algorithm. Preprocessed images were then segmented into gray matter (GM), white matter, and cerebrospinal fluid. We conducted quality control (QC) by utilizing the automatic QC provided by the CAT12 toolbox and visually inspecting the processed images.
Machine Learning Pipeline
SCH and controls from the model discovery samples were matched for age, sex, and scanner field strength using nearest-neighbor matching before training our ML model (resulting in 347 SCH and 341 controls). The ML model, using VBM maps in 4mm MNI-space as features, was trained using R version 4.0.3 (https://cran.r-project.org) accompanied by “e1071”23 and caret24 wrapped in the “mlr”25 package. We trained our ML model using repeated nested cross-validation (10 outer folds with 10 repetitions, 5 inner folds with 5 repetitions) to prevent information leakage between training and testing and to provide unbiased predictive generalizability. In the inner cross-validation loop, we used the following preprocessing steps: correction for age, sex, and scanner field strength using linear regression; principal component analysis for dimensionality reduction (80% variance retained); and scaling and mean centering. The preprocessed features were trained using a linear support vector machine (SVM), which was chosen over other ML algorithms due to its frequent usage in previous psychiatric ML studies.26 We tuned a range of SVM hyperparameters (0.0039, 0.0156, 0.0625, 0.25, 1, 4, 16, 64, and 256) using balanced accuracy (BAC; i.e., an average of sensitivity and specificity) as the optimization criterion for the winning model. We then applied the winning models to the respective out-of-training test folds. Finally, without any in-between retraining, we applied the ML model to Samples 1 and 2. Across the three samples, we plotted our results as schizophrenia classification probability provided by the “svm” function. Specifically, this function provides a probability estimation by fitting a logistic distribution to the SVM decision values using maximum likelihood estimation. The resulting probabilities can range from 0% (i.e., the model has 100%”certainty” that an individual is a control) to 100% (i.e., the model has 100%”certainty” that the individual is SCH). Individuals with classification probability > 50% were classified as SCH.
Statistical Analyses
We used R accompanied by “ggplot2”27 package for statistical analyses and visualizations of the present study. We compared the demographic characteristics using t-test, χ2-test, and Fisher’s exact test as specified in the Results section. To assess the association between the ML model’s prediction and clinical variables in the discovery samples, we conducted random-effect meta-analyses (using “metafor”28 package) on the four available variables: positive symptoms, negative symptoms, education, and time from diagnosis. In individuals with ASPD + SUD, we tested whether being classified as SCH was associated with poor functional outcome, which was defined as no further education or vocational training after compulsory nine-year education and never in full-time employment. In SCH, in addition to functional outcome, we tested the association between time since the first psychiatric hospitalization with ML model’s probability of being classified as schizophrenia. Finally, we tested the effect of scanner and image quality on the ML model’s predictions due to the usage of multiple scanners.
Univariate Analyses to Characterize ML Model’s Relation to Behavioral Abnormalities
We also aimed to investigate whether the brain abnormalities related to the ML model’s predictions were associated with known behavioral abnormalities. First, we identified the brain abnormality distinguishing those classified as SCH from those as controls. For this purpose, we conducted univariate analyses using FSL version 6.0.1. The model used total intracranial volume (TIV), age, substance abuse, and image quality as covariates. We conducted a voxel-wise test to assess for statistical significance using the FSL’s29 randomize tool30 using 5000 repetitions, applying threshold-free cluster enhancement (TFCE),31 and a family-wise error (FWE) correction to account for multiple voxel-wise comparisons. Statistically significant clusters were considered at P value < .05 (FWE-corrected).
Next, we binarized the statistically significant clusters that were transformed into Talairach space. We then imported these clusters into Mango v4.1. with “Behavioral Analysis” plugin v3.1., which is based on BrainMap’s database (http://brainmap.org) of previously published neuroimaging studies with coordinate-based results (4.118 papers on 30th of October 2022).32 The plugin automatically compares the clusters with functional metadata of the BrainMap database’s 60 behavioral domains that are classified as “Action,” “Cognition,” “Emotion,” “Interoception,” and “Perception “. The analytical process is described in detail elsewhere.33 Briefly, for each of the 60 behavioral domains, the fraction of coordinates falling within the clusters was computed and compared with the fraction expected if coordinates for the behavior were not clustered. We used the recommended thresholding of Z-score > 3.0 (corresponding Bonferroni corrected P-value < .05) to designate a statistically significant association with a given behavioral domain.
Results
Sociodemographic and Clinical Characteristics of Samples 1 and 2
In sample 1, there was no difference between SCH and ASPD patients in age, sex, IQ, duration of SUD, number of attempted homicides, and global anatomical volumes (Table 1). There were differences in comorbidities in sample 1: 27.3% of SCH patients had ASPD, and there were more SUD in ASPD patients compared to SCH patients (X2 = 11.6 P < 0.001). There were more committed homicides in ASPD patients compared to SCH patients in sample 1 (Fisher, P = 0.002). In sample 2, there was no difference between age, sex, and mean global white matter volume. The mean global gray matter volume was larger in control men compared to ASPD patients in sample 2 (t(46) = 2.8, P = 0.007). Mean global cerebrospinal fluid volume was larger in ASPD compared to control men in sample 2 (t(43) = −3.8, P < 0.001).
Table 1.
Sociodemographic and Clinical Characteristics of Samples 1 and 2.
| Sample 1 | Sample 2 | |||||
|---|---|---|---|---|---|---|
| SCZ (N = 66) | ASPD (N = 52) | Test Statistic/P value | ASPD (N = 26) | Controls (N = 25) | Test Statistic/P value | |
| Age mean (SD) | 32.0 (9.8) | 32.8 (12.2) | t = 0.36, P = .722 | 32.5 (8.4) | 34.6 (10.7) | t = 0.78, P = .442 |
| Sex (male) | 66 (100%) | 52 (100%) | FET, P = 1.000 | 26 (100%) | 25 (100%) | FET, P = 1.000 |
| IQ, mean (SD) | 87.8 (13.4)* | 87.7 (16.0)* | F = 0.006, P = .941 | 91.5 (9.0) | NA | |
| Time since first hospitalization in years, mean (SD) | 9.8 (8.1) | NA | NA | NA | ||
| ASPD (%) | 18 (27.3 %) | 52 (100%) | X2= 16.5, P < .001 | 26 (100%) | 0 (0%) | FET, P < .001 |
| Substance use disorder, (%) | 51 (77.3%) | 52 (100%) | Χ2 = 11.6, P < .001 | 26 (100%) | 0 (0%) | FET, P < .001 |
| Duration of substance use disorder in years, mean (SD) | 17.6 (10.4)* | 19.9 (11.9)* | t = 1.05, P = .300 | 19.0 (9.2) | NA | |
| Number of comitted homicides, median (range) | 0 (0–3) | 1 (0–3)* | FET, P = .002 | 0.5 (0–3) | NA | |
| Number of attempted homicides, median (range) | 0 (0–9)* | 0 (0–2)* | FET, P = .321 | 0 (0–1) | NA | |
| PCL-R, mean (SD) | NA | NA | 29.9 (5.2) | NA | ||
| Mean global grey matter volume, ml (SD) | 673.71 (60.8) | 687.44 (79.6) | t = 1.030, P = .306 | 633.01 (51.9) | 679.68 (65.0) | t = 2.8, P = .007 |
| Mean global white matter volume, ml (SD) | 568.71 (52.5) | 568.00 (67.7) | t = −0.063, P = .950 | 610.18 (58.1) | 630.68 (54.6) | t = 1.3, P = .200 |
*Missing values.
FET, fisher exact test; NA, Not available.
The Performance of the Model in the Discovery Samples
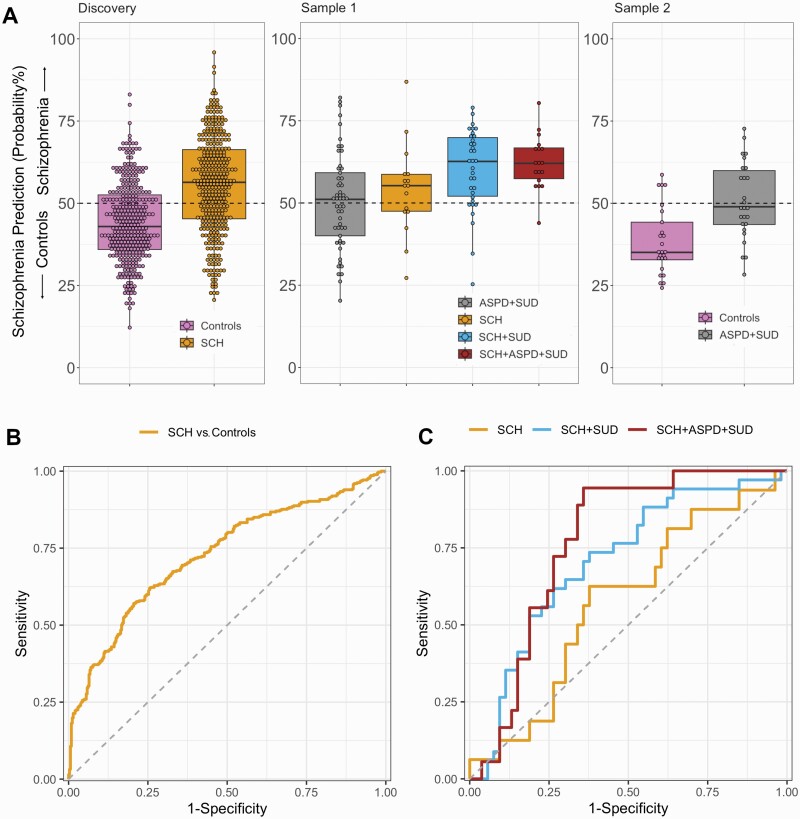
Out-of-training classification of SCH from controls in the model discovery samples resulted in a balanced accuracy (BAC) of 67.0% (sensitivity = 65.7%, specificity = 68.3%), and AUC = 0.727 (95%CI = 0.689–0.765, Figure 2b). Prediction performances across the individual discovery samples are described in the Supplement.
Fig. 2.
(A) Schizophrenia prediction probability with and without comorbidities in discovery sample, sample 1 and sample 2. Individuals with probability above 50% were labeled as schizophrenia. (B) ROC-curve for the classification of schizophrenia vs. controls in the discovery samples (out-of-training classification results). (C) ROC-curves for discerning between schizophrenia with and without comorbidity vs. ASPD + SUD in Sample 1. Abbreviations: SCH, schizophrenia; SUD, substance use disorder; ASPD, antisocial personality disorder.
Across the four discovery samples (Supplementary Figures 1–3), we did not find associations between the probability of being classified as schizophrenia with positive symptoms (R = 0.045, 95%CI = −0.061–0.152, P-value = .404), negative symptoms (R = 0.069, 95%CI = −0.038–0.176, P-value = .204), or time from diagnosis (R = −0.167, 95%CI = −0.613–0.280, P-value = .464). However, the probability of being predicted as schizophrenia was associated with low education (R = −0.198, 95%CI = −0.378–0.018, P-value = .031, Supplementary Figure 4).
The Performance of the ML Model in Samples 1 and 2
When the ML model was applied to sample 1 (Figure 2, Table 2), we found that the SCH vs. controls ML model classified 51.9% of ASPD + SUD patients as SCH, 60.0% of SCH patients without comorbidities as SCH, 78.8% of SCH + SUD patients as SCH, followed by 94.4% of SCH + SUD + ASPD as SCH. The individuals with SCH + ASPD + SUD were significantly more likely classified as SCH by the model compared to SCH without comorbidities (Fisher’s test, P-value = .03). When the ML model was applied to sample 2, we found that the model classified 16% of controls as SCH and 46.2% of ASPD + SUD as SCH. Misclassification of ASPD + SUD as SCH was similar between Samples 1 and 2 (X2= 0.23, P-value = .63).
We then investigated the model’s performance in discerning SCH from ASPD + SUD in sample 1 (Figure 2c). We found that AUC-values for discerning from ASPD + SUD in sample 1 were as follows: AUC = 0.562 (95%CI = 0.400–0.723) for SCH, AUC = 0.704 (95%CI = 0.590–0.818) for SCH + SUD, and AUC = 0.770 (95%CI = 0.660–0.880) for SCH + SUD + ASPD.
In sample 1, misclassification of schizophrenia did not relate to functional outcome (Χ2=0.166, P-value = .68) or duration since the first psychiatric hospitalization (t(19.6) = −0.603, P-value = .554). We found that those ASPD + SUD classified as SCH had a lower functional outcome than those classified as controls (Χ2 = 6.522, P-value = .01).
The scanner effect was addressed during the training of the model in discovery samples, and we did not observe a statistically significant scanner (F(3,62) = 1.243, P-value = .302) or image quality effect (F(1,64) = 0.146, P-value = .704) for the ML model’s predictions in SCH. Likewise, in ASPD + SUD, there was no statistically significant scanner (F(2,49) = 1.961, P-value = .152) or image quality effect (F(1,50) = 1.036, P-value = .314) for ML model’s predictions.
ML Model’s Predictions’ Association with Behavioral Domains
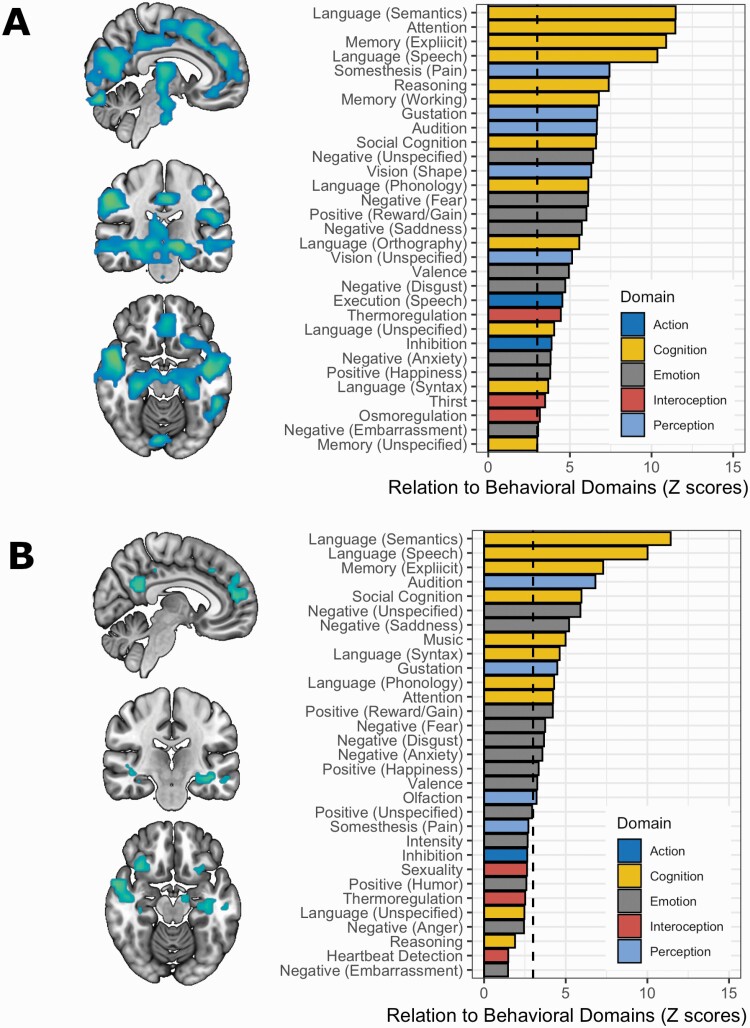
Figure 3 shows the gray matter deficits associated with classification to SCH. As shown in Figure 3a, correct classification (vs. incorrect) in SCH was associated with decreased gray matter volume in the prefrontal cortex, thalamus, posterior cingulate cortex, and hippocampus. Similar findings (Figure 3b), albeit more nuanced, were observed in those ASPD misclassified as SCH (vs. classified as controls). A detailed description of these abnormalities is provided in Supplementary Tables 1-2. In SCH, volumetric abnormalities in those correctly (vs. incorrectly) classified as schizophrenia related to 31 behavioral domains that were mainly related to cognition (39%) and emotion processing (29%). Similarly, in ASPD, abnormalities associated with classification as SCH related to 19 behavioral domains mainly related to cognition (42%) and emotion processing (42%).
Fig. 3.
(A) Gray matter deficits (TFCE-corrected P value < .05) in patients correctly classified as schizophrenia (N = 52) compared to patients incorrectly classified as controls (N = 14). Correct classification was mostly related to behavioral domains related to cognition (39%) and emotion processing (29%). The dashed line presents Z > 3, Bonferroni-Corrected P-value < .05. (B) Gray matter deficits (TFCE-corrected P value < .05)) in ASPD classified as schizophrenia (N = 27) vs. classified as controls (N = 25). Misclassification of ASPD as SCH was primarily related to behavioral domains related to cognition (42%) and emotion processing (42%).
Discussion
To the best of our knowledge, this is the first study to explore the effect of SUD and ASPD on the neuroimaging-based ML prediction of SCH. Our findings indicate that ML models trained to predict SCH have different performances depending on the test sample’s comorbidities. Consequently, comorbidities may hamper the clinical use of neuroimaging-based ML classification in heterogenous samples if the comorbidities’ effect remains uncontrolled.
Although many previous ML studies have shown high prediction performances in discerning SCH from controls,2 these studies were largely conducted in samples where the effect of potential comorbidities was not addressed. In our study, the prediction of SCH with comorbidities was substantially better compared to solely SCH implicating that our model trained to discern SCH from controls is using a neurobiological signature that overlaps with abnormalities observed in SUD and ASPD. Specifically, the accumulation of both ASPD and SUD comorbidities in SCH (SCH + SUD + ASPD) resulted in 94.4% being classified as SCH, while patients without such comorbidities (SCH only) were classified 60.0% as SCH. Furthermore, the model failed to discern SCH without comorbidities from ASPD + SUD. Altogether, these results suggest that SUD and ASPD comorbidities affect the model performance, thereby potentially contributing to variance in prediction performance estimates among previous ML studies.3
Previous longitudinal studies examining individuals at risk for psychosis have also found the varying performance of ML models. For example, a recent longitudinal study with a nine-year follow-up showed that the SCH vs. controls model’s performance increased in SCH but not in controls.34 A study investigating at-risk patients found that the performance of the SCH vs. controls ML model improved as the clinical stages of psychosis went further from ultra-high risk for psychosis to chronic schizophrenia.35 While these findings might partially stem from progressive brain alterations related to SCH, our findings suggest that some of these variations may also be due to the accumulation of comorbidities with converging brain abnormalities. For example, a large prospective longitudinal study found that schizophrenia was significantly associated with the risk of subsequent substance abuse, and the risk was highest one year after the diagnosis of schizophrenia.36
To the best of our knowledge, neuroimaging-based ML studies utilizing a transdiagnostic approach are scarce. One previous ML study on separate patient groups with SCH, Alzheimer’s disease, and behavioral-variant frontotemporal dementia (bvFTD) found neuroanatomical overlaps between the multivariate patterns discerning these disorders from controls. Specifically, the ML model trained to discern SCH from controls classified 85.5% of bvFTD and 70.5% of Alzheimer’s disease patients as SCH.37 Also, a multivariate pattern that was trained to distinguish bvFTD from controls labeled 41% of SCH as bvFTD.37 Thus, bvFTD appears to share a common frontal degeneration type with SCH. Intriguingly, however, recent research mainly agrees that patients with depression are rarely labeled as SCH by ML models trained to predict SCH from controls.34,37
In our study, the ML model’s classification as SCH related to volumetric reductions in the prefrontal cortex, thalamus, posterior cingulate cortex, and hippocampus in ASPD and SCH. These volumetric abnormalities were primarily associated with cognition, which aligns with the previous epidemiological research demonstrating such deficiencies in SCH.38,39 Interestingly, a previous neuroimaging-based ML study found that the most consistent predictor of being classified as SCH was poor verbal cognition.34 Furthermore, the association between poor cognition and predictive performance in ML classification has been reported in other previous studies.40,41 In the present study, poor education was the only significant predictor of being labeled as SCH by the ML model. Furthermore, the misclassification of ASPD as SCH was associated with low functional outcome (meaning no education after compulsory nine-year education and never adhered to working life), which potentially results from the above-mentioned deficits in brain regions central to cognitive processing. Specifically, deficits in cognitive processing can hamper adhering to working life or education.42,43 Note, however, that in addition to cognitive impairments, low functional outcome could also result from factors such as medication43 and substance abuse.44 Overall, our findings imply that neuroimaging-based ML models trained to distinguish SCH from controls adopt a pattern that weights cognitive abnormalities, potentially leading to false positives in other diagnostic groups with cognitive impairments.
Further research is required to determine whether it is possible to build a diagnostic ML model to distinguish only between SCH and controls without producing false positives for other disorders. Previous meta-analysis has shown that the ML model can reach high sensitivity and specificity, but the prediction performance varies between studies.2,3 Based on our results, ML models trained in samples without comorbidities can learn a general mental disorder pattern rather than a disorder-specific neuroanatomical signature. It is also remarkable that the ML model’s predictions did not associate with positive or negative symptoms of schizophrenia. Therefore, the potential transdiagnostic nature of these models should be considered when conducting large studies to assess ML models’ generalizability since sample heterogeneity increases as the sample size increases.3 Also, future studies should investigate whether disorder-specificity could be reached by utilizing multimodal data (e.g., by combining sMRI with fMRI, psychological tests, and blood tests). In developing more specific predictive ML models, one should consider other possible sources of heterogeneity in addition to comorbidities such as illness severity, illness course, medications, and genetic factors.3 In summary, our study suggests that future research should pivot from the traditional SCH vs. healthy controls approach when developing neuroimaging-based ML classifiers. A more comprehensive approach considering a range of psychiatric disorders and their comorbidities vs. SCH with and without comorbidities might better discern shared and disorder-specific neurobiological signatures, potentially offering enhanced specificity in ML-based schizophrenia diagnosis.
Our study has limitations. First, while our discovery samples were large and from multiple sites, our testing samples were small and from one site. Thus, future studies from other forensic psychiatric samples are needed to replicate our results. Second, clinical populations with other comorbidities, such as ADHD or affective disorders, were lacking in our samples. Third, our sample contains forensic patients with violent criminal behavior who may differ from nonviolent patients with the same disease burden (e.g., a tendency for violence, impulsiveness, and nonadherence). Fourth, the effects of medication on the low functional outcome could not be assessed due to the lack of data on medication history. Finally, we used real-world data with multiple scanners, potentially affecting our model’s performance by inducing substantial scanner-related heterogeneity. However, the scanner effect was taken into account during the model’s training, and we found no effect of a scanner or image quality on the ML model performance in our testing samples.
In conclusion, ASPD and SUD appear to affect ML prediction performance, which potentially results from converging cognition-related brain abnormalities between SCH, ASPD, and SUD. Consequently, more efforts to characterize and control the contributions of comorbidities on neuroimaging-based ML predictions are needed in future studies. This is particularly important in forensic contexts where precise diagnosis and prediction are also crucial for society.
Supplementary Material
Supplementary material is available at https://academic.oup.com/schizophreniabulletin/.
Table 2.
Classification as Schizophrenia or Control Across the Study Populations using the Machine Learning Model Trained to Discern Schizophrenia from Controls Trained in the Discovery Sample (Out-Of-Training Predictions are Provided for the Discovery Sample).
| Population Name | Predicted as Schizophrenia % (N) | Predicted as Controls % (N) |
|---|---|---|
| SCH (N = 347, Discovery sample) | 65.7% (228) | 34.3% (119) |
| Controls (N = 341, Discovery sample) | 31.7% (108) | 68.3% (233) |
| ASPD + SUD (N = 52, Sample 1) | 51.9% (27) | 48.1% (25) |
| SCH (N = 15, Sample 1) | 60.0% (9) | 40.0% (6) |
| SCH + SUD (N = 33, Sample 1) | 78.8% (26) | 21.2% (7) |
| SCH + SUD + ASPD (N = 18, Sample 1) | 94.4% (17) | 5.6% (1) |
| Controls (N = 25, Sample 2) | 16.0% (4) | 84.0% (21) |
| ASPD + SUD (N = 26, Sample 2) | 46.2% (12) | 53.8% (14) |
Note: SCH, individuals with schizophrenia; ASPD + SUD, individuals with antisocial personality disorder with substance use disorder; SCH + SUD, individuals with schizophrenia and substance use disorder; SCH + SUD + ASPD, individuals with schizophrenia, substance use disorder and antisocial personality disorder.
Acknowledgements
Data sharing and collection for the NMorphCH project was funded by the National Institute of Mental Health Grant R01 MH056584. For COBRE, Data was downloaded from the COllaborative Informatics and Neuroimaging Suite Data Exchange tool (COINS; https://coins.trendscenter.org/) and data collection was performed at the Mind Research Network, and funded by a Center of Biomedical Research Excellence (COBRE) grant 5P20RR021938/P20GM103472 from the NIH to Dr Vince Calhoun. For the MCICShare, the imaging data and demographic information was collected and shared by [University of Iowa, University of Minnesota, University of New Mexico, Massachusetts General Hospital] the Mind Research Network supported by the Department of Energy under Award Number DE-FG02-08ER64581. The NUSDAST project was funded by the National Institute of Mental Health (NIMH): 1R01 MH084803.
Contributor Information
Matias Taipale, Department of Forensic Psychiatry, Niuvanniemi Hospital, University of Eastern Finland, Kuopio, Finland.
Jari Tiihonen, Department of Forensic Psychiatry, Niuvanniemi Hospital, University of Eastern Finland, Kuopio, Finland; Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; Center for Psychiatry Research, Stockholm City Council, Stockholm, Sweden.
Juuso Korhonen, Department of Computer Science, Aalto University, Espoo, Finland.
David Popovic, Max Planck Institute of Psychiatry, Munich, Germany; International Max Planck Research School for Translational Psychiatry (IMPRS-TP), Munich, Germany; Department of Psychiatry and Psychotherapy, Ludwig-Maximilian-University, Munich, Germany.
Olli Vaurio, Department of Forensic Psychiatry, Niuvanniemi Hospital, University of Eastern Finland, Kuopio, Finland.
Markku Lähteenvuo, Department of Forensic Psychiatry, Niuvanniemi Hospital, University of Eastern Finland, Kuopio, Finland.
Johannes Lieslehto, Department of Forensic Psychiatry, Niuvanniemi Hospital, University of Eastern Finland, Kuopio, Finland; Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden.
Declaration of interestJT have participated in research projects funded by grants from Janssen-Cilag and Eli Lilly to their employing institution. JT has been a consultant and/or advisor to and/or has received honoraria from: Eli Lilly, Evidera, Janssen-Cilag, Lundbeck, Orion, Otsuka, Mediuutiset, Sidera, and Sunovion. ML is a board member of Genomi Solutions ltd. and Nursie Health ltd., has received honoraria from Sunovion ltd., Orion Pharma ltd., Lunbdbeck ltd., Otsuka Pharma ltd., and Janssen-Cilag.
FundingThis work was supported by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. DP was supported by the “Else-Kröner-Fresenius-Stiftung” through the Clinician Scientist Program “EKFS-Translational Psychiatry.”
Data Availability
The COBRE, the NMoprhCH, the NUSDAST, and the MCIC are publicly available datasets for research purposes (http://schizconnect.org). The datasets for Samples 1 and 2 in this study are not publicly available due to participant privacy and security concerns. Researchers can apply for access for these data from from pertinent registry holders.
Code AvailabilityThe codes and machine learning model to reproduce the present study’s findings are available from the corresponding author upon request.
References
- 1. Treadway MT, Buckholtz JW.. On the use and misuse of genomic and neuroimaging science in forensic psychiatry: current roles and future directions. Child Adolesc Psychiatr Clin N Am. 2011;20(3):533–546. doi: 10.1016/J.CHC.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kambeitz J, Kambeitz-Ilankovic L, Leucht S, et al. Detecting neuroimaging biomarkers for schizophrenia: a meta-analysis of multivariate pattern recognition studies. Neuropsychopharmacology. 2015;40(7):1742–1751. doi: 10.1038/NPP.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schnack HG, Kahn RS.. Detecting neuroimaging biomarkers for psychiatric disorders: sample size matters. Front Psychiatry. 2016;7. doi: 10.3389/FPSYT.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zanetti MV, Schaufelberger, Doshi MS, J et al. Neuroanatomical pattern classification in a population-based sample of first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:116–125. doi: 10.1016/J.PNPBP.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 5. Tsai J, Rosenheck RA.. Psychiatric comorbidity among adults with schizophrenia: a latent class analysis. Psychiatry Res. 2013;210(1):16–20. doi: 10.1016/J.PSYCHRES.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bermanzohn PC, Porto L, Arlow PB, Pollack S, Stronger R, Siris SG.. Hierarchical diagnosis in chronic schizophrenia: a clinical study of co-occurring syndromes. Schizophr Bull. 2000;26(3):517–525. doi: 10.1093/OXFORDJOURNALS.SCHBUL.A033472. [DOI] [PubMed] [Google Scholar]
- 7. Green AI, Canuso CM, Brenner MJ, Wojcik JD.. Detection and management of comorbidity in patients with schizophrenia. Psychiatr Clin North Am. 2003;26(1):115–139. doi: 10.1016/S0193-953X(02)00014-X. [DOI] [PubMed] [Google Scholar]
- 8. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area (ECA) study. JAMA. 1990;264(19):2511–2518. doi: 10.1001/JAMA.1990.03450190043026. [DOI] [PubMed] [Google Scholar]
- 9. Putkonen A, Kotilainen I, Joyal CC, Tiihonen J.. Comorbid personality disorders and substance use disorders of mentally ill homicide offenders: a structured clinical study on dual and triple diagnoses. Schizophr Bull. 2004;30(1):59–72. doi: 10.1093/OXFORDJOURNALS.SCHBUL.A007068. [DOI] [PubMed] [Google Scholar]
- 10. Mcmillan KA, Enns MW, Cox BJ, Sareen J.. Comorbidity of axis I and II mental disorders with schizophrenia and psychotic disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Can J Psychiatry. 2009;54(7):477–486. [DOI] [PubMed] [Google Scholar]
- 11. Shenton ME, Dickey CC, Frumin M, McCarley RW.. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1-2):1–52. doi: 10.1016/S0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET.. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157(1):16–25. doi: 10.1176/AJP.157.1.16. [DOI] [PubMed] [Google Scholar]
- 13. Brugger SP, Howes OD.. Heterogeneity and homogeneity of regional brain structure in schizophrenia: a meta-analysis. JAMA psychiatry. 2017;74(11):1104–1111. doi: 10.1001/JAMAPSYCHIATRY.2017.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johanson M, Vaurio O, Tiihonen J, Lähteenvuo M.. A systematic literature review of neuroimaging of psychopathic traits. Front Psychiatry. 2020;10:1–20. doi: 10.3389/fpsyt.2019.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pando-Naude V, Toxto S, Fernandez-Lozano S, Parsons CE, Alcauter S, Garza-Villarreal EA.. Gray and white matter morphology in substance use disorders: a neuroimaging systematic review and meta-analysis. Transl Psychiatry. 2021;11(1):29. doi: 10.1038/S41398-020-01128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Long Y, Pan N, Ji S, et al. Distinct brain structural abnormalities in attention-deficit/hyperactivity disorder and substance use disorders: a comparative meta-analysis. Transl Psychiatry. 2022;12(1). doi: 10.1038/S41398-022-02130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tiihonen J, Rossi R, Laakso MP, et al. Brain anatomy of persistent violent offenders: more rather than less. Psychiatry Res Neuroimaging. 2008;163(3):201–212. doi: 10.1016/J.PSCYCHRESNS.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 18. Laakso MP, Gunning-Dixon F, Vaurio O, Repo-Tiihonen E, Soininen H, Tiihonen J.. Prefrontal volumes in habitually violent subjects with antisocial personality disorder and type 2 alcoholism. Psychiatry Res - Neuroimaging. 2002;114(2):95–102. doi: 10.1016/S0925-4927(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 19. Cabral C, Kambeitz-Ilankovic L, Kambeitz J, et al. Classifying schizophrenia using multimodal multivariate pattern recognition analysis: evaluating the impact of individual clinical profiles on the neurodiagnostic performance. Schizophr Bull. 2016;42(Suppl 1):S110–S117. doi: 10.1093/SCHBUL/SBW053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oh J, Oh BL, Lee KU, Chae JH, Yun K.. Identifying schizophrenia using structural MRI with a deep learning algorithm. Front Psychiatry. 2020;11:1. doi: 10.3389/fpsyt.2020.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Czepielewski LS, Wang L, Gama CS, Barch DM.. The relationship of intellectual functioning and cognitive performance to brain structure in schizophrenia. Schizophr Bull. 2017;43(2):355. doi: 10.1093/SCHBUL/SBW090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seppänen A, Joelsson P, Ahlgren-Rimpiläinen A, Repo-Tiihonen E.. Forensic psychiatry in Finland: an overview of past, present and future. Int J Ment Health Syst. 2020;14(1):29. doi: 10.1186/S13033-020-00362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meyer D, Dimitriadou E, Hornik K, et al. Package “e1071.” R J.2019.
- 24. Kuhn M. caret: Classification and Regression Training. R Package Version 6.0-90. https://CRAN.R-project.org/package=caret. Published online 2021.
- 25. Bischl B, Lang M, Kotthoff L, et al. mlr: machine learning in R. J Mach Learn Res. 2016;17(1):5938–5942. [Google Scholar]
- 26. Dwyer DB, Falkai P, Koutsouleris N.. Machine learning approaches for clinical psychology and psychiatry. Annu Rev Clin Psychol. 2018;14:91–118. doi: 10.1146/annurev-clinpsy-032816-045037. [DOI] [PubMed] [Google Scholar]
- 27. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2009;(Journal Article). [Google Scholar]
- 28. Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J Stat Softw. 2010;36(3):1–48. doi: 10.18637/JSS.V036.I03. [DOI] [Google Scholar]
- 29. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL.. Neuroimage. 2012;62(2):782–790. doi: 10.1016/J.NEUROIMAGE.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 30. Nichols TE, Holmes AP.. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15(1):1–25. doi: 10.1002/HBM.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith SM, Nichols TE.. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/J.NEUROIMAGE.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 32. Kochunov P, Lancaster J, Thompson P, et al. An optimized individual target brain in the talairach coordinate system 1. Published online 2002. doi: 10.1006/nimg.2002.1084 [DOI] [PubMed]
- 33. Lancaster JL, Laird AR, Eickhoff SB, Martinez MJ, Mickle Fox P, Fox PT.. Automated regional behavioral analysis for human brain images. Front Neuroinform. 2012;6. doi: 10.3389/FNINF.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lieslehto J, Jääskeläinen E, Kiviniemi V, et al. The progression of disorder-specific brain pattern expression in schizophrenia over 9 years. NPJ Schizophr. 2021;7(1):1–11. doi: 10.1038/s41537-021-00157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu Y, Nakatani H, Yassin W, et al. Application of a machine learning algorithm for structural brain images in chronic schizophrenia to earlier clinical stages of psychosis and autism spectrum disorder: a multiprotocol imaging dataset study. Schizophr Bull. 2022;48(3):563–574. doi: 10.1093/SCHBUL/SBAC030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petersen SM, Toftdahl NG, Nordentoft M, Hjorthøj C.. Schizophrenia is associated with increased risk of subsequent substance abuse diagnosis: a nation-wide population-based register study. Addiction. 2019;114(12):2217–2226. doi: 10.1111/ADD.14746. [DOI] [PubMed] [Google Scholar]
- 37. Koutsouleris N, Pantelis C, Velakoulis D, et al. Exploring links between psychosis and frontotemporal dementia using multimodal machine learning: dementia praecox revisited. JAMA Psychiatry. 2022;79(9):907–919. doi: 10.1001/JAMAPSYCHIATRY.2022.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fatouros-Bergman H, Cervenka S, Flyckt L, Edman G, Farde L.. Meta-analysis of cognitive performance in drug-naïve patients with schizophrenia. Schizophr Res. 2014;158(1-3):156–162. doi: 10.1016/J.SCHRES.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 39. Weinreb S, Li F, Kurtz MM.. A meta-analysis of social cognitive deficits in schizophrenia: does world region matter? Schizophr Res. 2022;243:206–213. doi: 10.1016/J.SCHRES.2022.04.002. [DOI] [PubMed] [Google Scholar]
- 40. Ambrosen KS, Skjerbæk MW, Foldager J, et al. A machine-learning framework for robust and reliable prediction of short- and long-term treatment response in initially antipsychotic-naïve schizophrenia patients based on multimodal neuropsychiatric data. Transl Psychiatry. 2020;10(1). doi: 10.1038/S41398-020-00962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Viviano JD, Buchanan RW, Calarco N, et al. ; Social Processes Initiative in Neurobiology of the Schizophrenia(s) Group. Resting-state connectivity biomarkers of cognitive performance and social function in individuals with schizophrenia spectrum disorder and healthy control subjects. Biol Psychiatry. 2018;84(9):665–674. doi: 10.1016/J.BIOPSYCH.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Green MF, Kern RS, Heaton RK.. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72(1):41–51. doi: 10.1016/J.SCHRES.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 43. Sharma T, Antonova L.. Cognitive function in schizophrenia. Deficits, functional consequences, and future treatment. Psychiatr Clin North Am. 2003;26(1):25–40. doi: 10.1016/S0193-953X(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 44. Jackson CT, Fein D, Essock SM, Mueser KT.. The effects of cognitive impairment and substance abuse on psychiatric hospitalizations. Community Ment Health J. 2001;37(4):303–312. doi: 10.1023/A:1017593423538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The COBRE, the NMoprhCH, the NUSDAST, and the MCIC are publicly available datasets for research purposes (http://schizconnect.org). The datasets for Samples 1 and 2 in this study are not publicly available due to participant privacy and security concerns. Researchers can apply for access for these data from from pertinent registry holders.
Code AvailabilityThe codes and machine learning model to reproduce the present study’s findings are available from the corresponding author upon request.