Abstract
Amphotericin B (AmB) is a ligand of toll-like receptor 2 (TLR2). Here, we demonstrate the participation of TLR1 in AmB-induced cell activation that led to the secretion of tumor necrosis factor alpha, interleukin 6 (IL-6), and IL-8. Hence, TLR2-TLR1 coactivation serves as the underlying mechanism for the proinflammatory toxicities associated with AmB.
The proinflammatory response to amphotericin B (AmB) is mediated by a toll-like receptor (TLR)-dependent mechanism. Recently, Sau et al. reported that primary murine macrophages and human cell lines expressing TLR2, CD14, and MyD88 responded to AmB stimulation with nuclear factor κB-dependent activity and cytokine secretion, whereas cells deficient in these proteins did not respond (12). Here, we expand these observations by demonstrating the critical participation of TLR1 in AmB-induced cell activation.
AmB-induced cellular activation is TLR2 dependent.
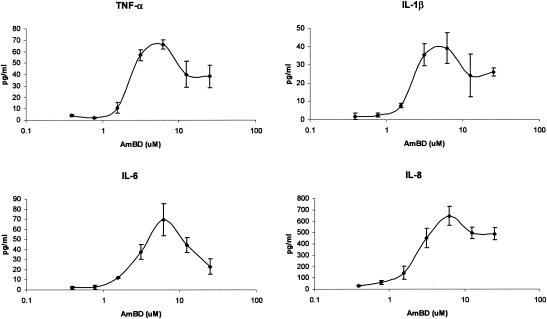
In a series of experiments, we confirmed the TLR2-dependent nature of AmB-induced cell activation. The THP1 cell line (ATCC no. TIB-202; cell density, 106/ml), which expresses abundant TLR2 mRNA (as assessed by reverse transcription-PCR), secreted interleukin 1β (IL-1β), IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) (assessed by Fluorokine multianalyte profiling; R&D Systems, Minneapolis, Minn.) during an 18-h incubation (at 37°C, 5% CO2) with AmB deoxycholate (Fungizone Intravenous; Bristol Myers Squibb, Princeton, N.J.) (Fig. 1); IL-2, IL-4, IL-10, IL-12(p70), granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor were not secreted. At concentrations that approximate the systemic exposure during AmB infusion at doses of 0.4 to 0.6 mg/kg of body weight, IL-1β was 10- to 43-fold higher, IL-6 was >580-fold higher, IL-8 was 7- to 21-fold higher, and TNF-α was 4- to 22-fold higher than levels in unstimulated cells (P < 0.05 for all comparisons).
FIG. 1.
Dose-dependent secretion of cytokines by THP1 monocytic cells during exposure to amphotericin B. Amphotericin B deoxycholate induced human monocytic cells to secrete, in a concentration-dependent manner, interleukin (IL)-1β, IL-6, IL-8, and tumor-necrosis factor alpha. IL-8 was secreted at the highest absolute concentration, while lower but significant (compared to results with unstimulated cells) levels of IL-1β, IL-6, and TNF-α were observed. The highest level of secretion was observed at a stimulation dose of 6.25 μM.
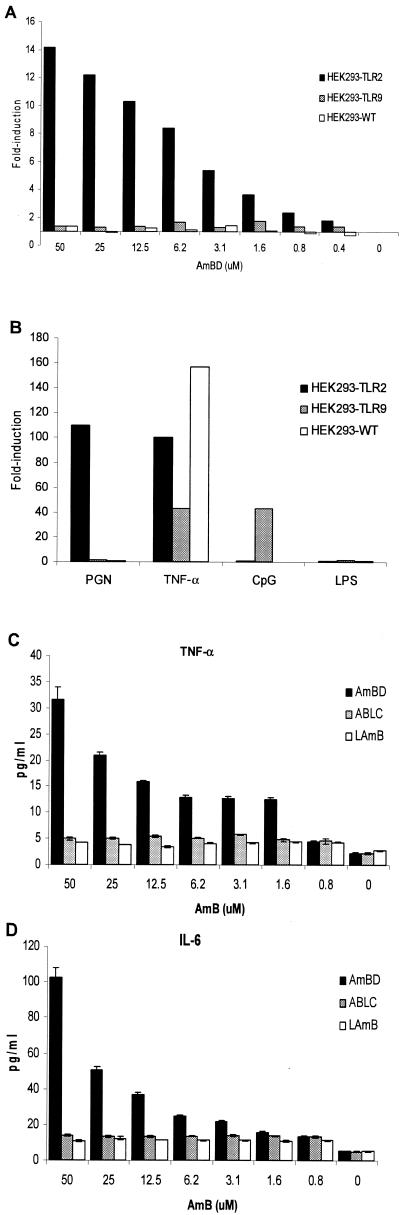
In contrast, the human embryonic kidney (HEK) 293 wild-type (wt) cell line (HEK293-wt) (ATCC no. CRL-1573; cell density, 106/ml), which is deficient in TLR2 mRNA expression (by reverse transcription-PCR), failed to respond to AmB during an 18-h incubation (37°C, 5% CO2) (Fig. 2A). Likewise, HEK293-wt did not respond to the TLR2 ligand peptidoglycan (PGN) (10 μg/ml; Sigma) (Fig. 2B). However, when HEK293-wt was manipulated to express TLR2 (HEK293-TLR2), the cells acquired responsiveness to AmB, as indicated by the dose-dependent nuclear factor κB activity and IL-6, IL-8, and TNF-α secretion (Fig. 2); IL-2, IL-4, IL-10, IL-12(p70), gamma interferon, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor were not secreted above baseline levels. HEK293-TLR2 also acquired responsiveness to PGN but remained nonresponsive to the TLR4 ligand lipopolysaccharide (LPS) (Sigma) and the TLR9 ligand CpG (Fig. 2B).
FIG. 2.
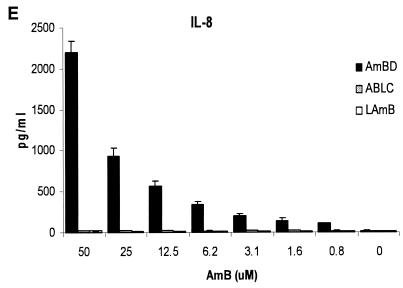
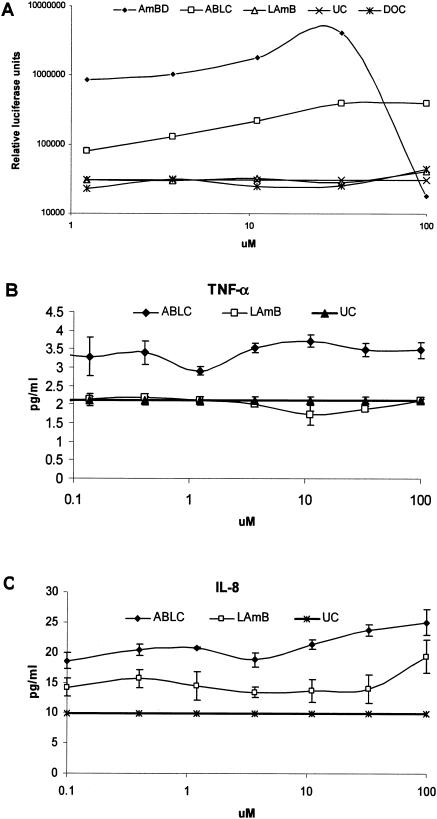
Cellular activation of various cell lines by amphotericin B formulations and other stimuli. A, Amphotericin B deoxycholate (AmBD) induced cellular activation, as indicated by a concentration-dependent increase in nuclear factor κB activity in HEK293-TLR2 cells but not in wild type (HEK293-wt) and TLR9-transfected HEK293 (HEK293-TLR9) cells. B, HEK293-wt responds to stimulation with TNF-α but not to PGN (a TLR2 ligand), unmethylated CpG (a TLR9 ligand), and LPS (a TLR4 ligand). Upon transfection with TLR2, HEK293-TLR2 acquired responsiveness to PGN but not to LPS and CpG. On the other hand, HEK293-TLR9 acquired responsiveness to CpG but not to PGN and LPS. C-E, AmBD induces a dose-dependent secretion of TNF-α, IL-6, and IL-8 in HEK293-TLR2 cells. HEK293-TLR2 cells did not secrete or secreted markedly lower levels of TNF-α (C), IL-6 (D), and IL-8 (E) during stimulation with the lipid formulations of AmB: ABLC and LAmB.
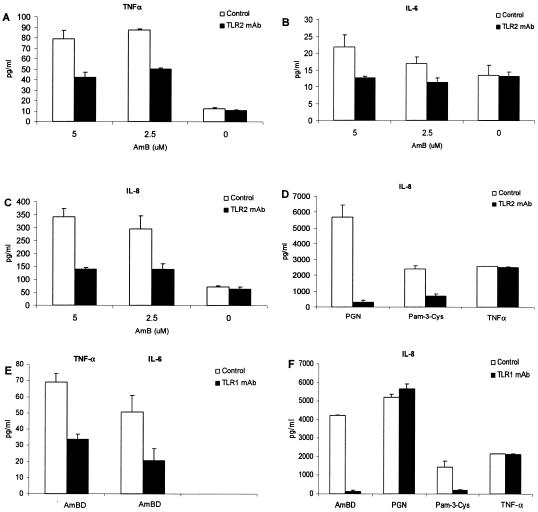
Moreover, preincubation of THP1 with murine anti-human TLR2 monoclonal antibody (MAb) (eBioscience) significantly reduced IL-6, IL-8 and TNF-α secretion in response to AmB (Fig. 3A to C). As expected, anti-TLR2 MAb also reduced cytokine secretion in response to PGN and tripalmitoyl cysteinyl lipopeptide (Pam-3-Cys) but not to TNF-α (10 ng/ml; R&D). Taken together, these series of studies, which utilized a modified “lack → gain → loss of function” experimental design, confirm the TLR2-dependent nature of AmB-induced cellular activation.
FIG. 3.
Neutralization-inhibition experiments using murine anti-human TLR1 and TLR2 monoclonal antibodies demonstrate the critical role that the TLR2-TLR1 complex plays in cellular activation by AmB. AmB-induced secretion of TNF-α (A), IL-6 (B), and IL-8 (C) by THP1 cells is inhibited by preincubation with anti-TLR2 monoclonal antibody, compared to results with cells preincubated with an MAb isotype control. Likewise, cytokine secretion in response to the TLR2 ligands PGN and Pam-3-Cys was inhibited by anti-TLR2 MAb (D). Preincubation of THP1 cells with murine anti-human TLR1 MAb inhibited TNF-α and IL-6 (E) and IL-8 (F) secretion in response to AmB. Anti-TLR1 MAb also inhibited IL-8 secretion in response to the TLR2-TLR1 ligand Pam-3-Cys but not to PGN and TNF-α (F).
AmB-induced cellular activation is TLR1 dependent.
Since TLR2-mediated signaling is facilitated by other TLRs (7, 11, 13), we determined whether TLR1 participates in AmB-induced cellular activation. Preincubation of THP1 with murine anti-human TLR1 MAb (eBioscience) reduced IL-6, IL-8, and TNF-α secretion in response to AmB (Fig. 3E to F). Anti-TLR1 MAb also inhibited IL-8 secretion in response to the TLR2-TLR1 ligand Pam-3-Cys (10-ng/ml; Calbiochem) but not the TLR-independent TNF-α (Fig. 3F).
Notably, the inhibition of IL-6, IL-8, and TNF-α secretion during preincubation with anti-TLR1 MAb was observed even without specific inhibition of the TLR2 molecule. Indeed, IL-8 inhibition with anti-TLR1 MAb was comparably greater than that with anti-TLR2 MAb (P = 0.098), and the addition of anti-TLR1 MAb significantly increased the degree of IL-8 inhibition by anti-TLR2 MAb (P = 0.0201). All these data suggest that TLR1 participation is essential in TLR2-mediated AmB-induced cell activation (12).
AmB-induced cellular activation is not TLR9 dependent.
In contrast, TLR9 does not participate in AmB-induced cellular activation. HEK293-wt manipulated to express TLR9 (HEK293-TLR9) (cell density, 106/ml) remained nonresponsive to AmB and PGN, while it gained responsiveness to CpG 2006S (2 μM; Integrated DNA Technologies, Coralville, Iowa) (Fig. 2A and B).
Our collective observations and those by Sau et al. (12) suggest that AmB is recognized as a pathogen-associated molecular pattern by TLR2 and that the ensuing TLR2-AmB interaction results in the costimulation of TLR1, the cooperation of TLR2-TLR1 signaling pathways, the activation of nuclear factor κB, and the secretion of proinflammatory cytokines and chemokines. Interestingly, Sau et al. suggested a possible role of TLR4, since macrophages from TLR4 mutant mice were less responsive to high-dose AmB than macrophages expressing normal TLR4 (12). However, during stimulation with conventional doses of AmB, TLR4 mutant macrophages were as responsive as the cells with normal TLR4. Moreover, TLR4 transfection of HEK293 did not render responsiveness to AmB. These observations suggest that TLR4 is not the critical pattern recognition receptor that mediates AmB-induced cellular activation. Indeed, Sau et al. suggested that TLR4 may play a role in only some systems (12). Instead, TLR2 and TLR1 appear to be the dominant receptors for AmB-induced cell activation. Our multiple attempts to assess the role of other TLRs, such as TLR6, which comediates the TLR2-mediated response to PGN, were limited by the lack of reliable anti-human TLR6 MAb.
The TLR2-TLR1 coactivation could serve as the molecular basis for the infusion-related fever and rigors that occur in up to 70% of patients receiving intravenous AmB (1, 3, 10). Currently, the use of lipid-based AmB has reduced the systemic inflammatory toxicity associated with AmB (14). Sau et al. demonstrated that lipid-based AmB did not induce nuclear factor κB activation or significant cytokine secretion (12). Here, we report that cellular activation was still induced by lipid-based AmB (particularly AmB lipid complex [ABLC]), albeit at significantly much lower levels.
Lipid-based AmB induced varying degrees of cellular activation.
Nuclear factor κB activation in HEK293-TLR2 cells was significantly lower during exposure to ABLC (The Liposome Company, Princeton, N.J.) and liposomal AmB (LAmB) (AmBisome; Fujisawa Healthcare, Deerfield, Ill.) than with AmBD, although the degree of activation was also significantly higher than that in unstimulated cells. Importantly, there was a contrast in cellular activation by the lipid-based formulations—nuclear factor κB activation (Fig. 4A) (P ≤ 0.05) and TNF-α (Fig. 4C) (P ≤ 0.05) and IL-8 (Fig. 4B) (P ≤ 0.05) secretion were significantly higher in ABLC-stimulated than in LAmB-stimulated HEK293-TLR2 cells. However, the levels of TNF-α and IL-8 were very low, and these were abrogated by anti-TLR2 or -TLR1 MAb (data not shown).
FIG. 4.
Differential degree of cell activation in response to amphotericin B deoxycholate (AmBD), amphotericin B lipid complex (ABLC), and amphotericin B liposome (LAmB). The degrees of nuclear factor κB activation in HEK293-TLR2 cells in response to the two lipid formulations are significantly lower than that with AmBD but are significantly higher than that in unstimulated cells (UC, panel A). ABLC induced a significantly higher degree of nuclear factor κB activation (A) and TNF-α (B) and IL-8 (C) secretion in HEK293-TLR2 cells than did LAmB. Deoxycholate (DOC) did not induce nuclear factor κB activation in HEK293-TLR2 cells (A).
The intercalation of AmB into lipid carriers could have provided hindrance that limited TLR2-AmB interaction. Lipid complexation stabilizes AmB in a self-associated state so that it is unavailable for interaction with cell membranes (4, 6, 8). Nonetheless, ABLC was still able to induce cell activation, at a level that is significantly higher than that with LAmB. The physicochemical characteristics of lipid carriers and the process by which AmB is incorporated into lipid vehicles could account for these contrasting biologic properties (5), which could be correlated clinically to the higher incidence of infusion-related reactions with ABLC than with LAmB (2, 9).
In conclusion, AmB promotes inflammation through a TLR2-TLR1-dependent mechanism. This ability of AmB to activate TLRs may relate to the fact that it is a fermentation product of Streptomyces nodosus (15). The role of TLRs in the pathogenesis of adverse inflammatory toxicities to other drug therapies deserves study.
Acknowledgments
There was no external source of funding for this project. Raymund R. Razonable is a Mayo Foundation Scholar (Mayo Clinic College of Medicine, Rochester, Minn.) and was a visiting scientist at the Lilly Research Laboratories (Indianapolis, Ind.) during the conducting of this research.
REFERENCES
- 1.Arning, M., K. O. Kliche, A. H. Heer-Sonderhoff, and A. Wehmeier. 1995. Infusion-related toxicity of three different amphotericin B formulations and its relation to cytokine plasma levels. Mycoses 38:459-465. [DOI] [PubMed] [Google Scholar]
- 2.Blau, I. W., and A. A. Fauser. 2000. Review of comparative studies between conventional and liposomal amphotericin B (Ambisome) in neutropenic patients with fever of unknown origin and patients with systemic mycosis. Mycoses 43:325-332. [DOI] [PubMed] [Google Scholar]
- 3.Cleary, J. D., S. W. Chapman, and R. L. Nolan. 1992. Pharmacologic modulation of interleukin-1 expression by amphotericin B-stimulated human mononuclear cells. Antimicrob. Agents Chemother. 36:977-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legrand, P., A. Vertut-Doi, and J. Bolard. 1996. Comparative internalization and recycling of different amphotericin B formulations by a macrophage-like cell line. J. Antimicrob. Chemother. 37:519-533. [DOI] [PubMed] [Google Scholar]
- 5.Mehta, J. 1997. Do variations in molecular structure affect the clinical efficacy and safety of lipid-based amphotericin B preparations? Leukoc. Res. 21:183-188. [DOI] [PubMed] [Google Scholar]
- 6.Mehta, R., G. Lopez-Berestein, R. Hopfer, K. Mills, and R. L. Juliano. 1984. Liposomal amphotericin B is toxic to fungal cells but not to mammalian cells. Biochim. Biophys. Acta 770:230-234. [DOI] [PubMed] [Google Scholar]
- 7.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perkins, W. R., S. R. Minchey, L. T. Boni, C. E. Swenson, M. C. Popescu, R. F. Pasternack, and A. S. Janoff. 1992. Amphotericin B-phospholipid interactions responsible for reduced mammalian cell toxicity. Biochim. Biophys. Acta 1107:271-282. [DOI] [PubMed] [Google Scholar]
- 9.Prentice, H. G., I. M. Hann, R. Herbrecht, M. Aoun, S. Kvaloy, D. Catovsky, C. R. Pinkerton, S. A. Schey, F. Jacobs, A. Oakhill, R. F. Stevens, P. J. Darbyshire, and B. E. Gibson. 1997. A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients. Br. J. Haematol. 98:711-718. [DOI] [PubMed] [Google Scholar]
- 10.Rogers, P. D., J. K. Jenkins, S. W. Chapman, K. Ndebele, B. A. Chapman, and J. D. Cleary. 1998. Amphotericin B activation of human genes encoding for cytokines. J. Infect. Dis. 178:1726-1733. [DOI] [PubMed] [Google Scholar]
- 11.Sandor, F., E. Latz, F. Re, L. Mandell, G. Repik, D. T. Golenbock, T. Espevik, E. A. Kurt-Jones, and R. W. Finberg. 2003. Importance of extra- and intracellular domains of TLR1 and TLR2 in NFkappa B signaling. J. Cell Biol. 162:1099-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sau, K., S. S. Mambula, E. Latz, P. Henneke, D. T. Golenbock, and S. M. Levitz. 2003. The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism. J. Biol. Chem. 278:37561-37568. [DOI] [PubMed] [Google Scholar]
- 13.Takeda, K., O. Takeuchi, and S. Akira. 2002. Recognition of lipopeptides by Toll-like receptors. J. Endotoxin Res. 8:459-463. [DOI] [PubMed] [Google Scholar]
- 14.Tiphine, M., V. Letscher-Bru, and R. Herbrecht. 1999. Amphotericin B and its new formulations: pharmacologic characteristics, clinical efficacy, and tolerability. Transpl. Infect. Dis. 1:273-283. [DOI] [PubMed] [Google Scholar]
- 15.Trejo, W. H., and R. E. Bennett. 1963. Streptomyces nodosus sp. n., the amphotericin-producing organism. J. Bacteriol. 85:436-439. [DOI] [PMC free article] [PubMed] [Google Scholar]