Abstract
Background
To investigate the impact of the M184V/I mutation on virologic response to dolutegravir plus lamivudine (DTG + 3TC) in suppressed-switch populations, a meta-analysis was performed using virologic outcomes from people with human immunodeficiency virus type 1 (PWH) with and without M184V/I before DTG + 3TC switch in real-world studies identified via systematic literature review. Sensitivity analyses were performed using data from PWH with M184V/I in interventional studies identified via targeted literature review.
Methods
Single-arm meta-analyses using common- and random-effects models were used to estimate proportions of PWH with virologic failure (VF) among real-world populations with and without M184V/I and interventional study participants with M184V/I at 24, 48, and 96 weeks.
Results
Literature reviews identified 5 real-world studies from 3907 publications and 51 abstracts meeting inclusion criteria and 5 interventional studies from 1789 publications and 3 abstracts. All time points had low VF incidence in PWH with M184V/I (real-world: 1.43%–3.81%; interventional: 0.00%) and without (real-world: 0.73%–2.37%). Meta-analysis–estimated proportions (95% confidence interval) with VF were low at weeks 24, 48, and 96, respectively, for PWH with M184V/I (real-world: 0.01 [.00–.04], 0.03 [.01–.06], and 0.04 [.01–.07]; interventional: 0.00 [.00–.02], 0.00 [.00–.01], and 0.00 [.00–.03]) and without (real-world: 0.00 [.00–.02], 0.02 [.01–.04], and 0.02 [.00–.05]). One real-world study (n = 712) reported treatment-emergent M184V at VF in 1 of 652 (0.15%) PWH without prior M184V/I.
Conclusions
Results suggest that prior M184V/I has minimal impact on virologic suppression after switching to DTG + 3TC and provide reassurance when considering switching regimens in virologically suppressed PWH with incomplete treatment history or limited treatment options.
Keywords: dolutegravir/lamivudine, HIV drug resistance, integrase inhibitor, M184V/I, real-world evidence
Meta-analysis results from real-world evidence suggest that prior M184V/I has minimal impact on virologic suppression after switching to dolutegravir plus lamivudine, providing reassurance when considering switching regimens in virologically suppressed people with HIV-1 with incomplete treatment history or limited options.
The human immunodeficiency virus type 1 (HIV-1) reverse transcriptase enzyme is prone to spontaneous transcription errors, generating drug-resistant variants [1]. People with HIV-1 (PWH) can acquire drug resistance–associated mutations (RAMs) through accumulated exposure to antiretrovirals with suboptimal virologic suppression [2]. Immediate treatment modification is advised when a RAM selective for an antiretroviral is detected due to increased virologic failure (VF) risk [3]. Additionally, historically selected RAMs are considered to be archived in the genome of long-term surviving CD4+ cells and can remain undetectable but could reemerge under a recycled, nonsuppressive antiretroviral therapy regimen [4]. However, current or historical resistance results are not always available when switching treatment in clinical practice. If RAMs are unintentionally missed due to lack of genotype or cumulative resistance history, clinicians may inadvertently prescribe regimens with higher VF risk.
The RAM M184V/I is commonly selected in PWH with ≥1 VF on lamivudine (3TC)– or emtricitabine (FTC)–containing regimens [4] and confers high-level phenotypic resistance to 3TC and FTC [1]. However, M184V decreases viral fitness versus wild-type virus in vitro [1], and some data suggest that 3TC monotherapy retains some antiviral activity in PWH with M184V/I [5, 6]. The M184V mutation may also delay appearance of integrase strand transfer inhibitor (INSTI) RAMs [7]. Whether benefits outweigh risks of using 3TC-inclusive regimens in PWH with M184V/I is debated, and too few studies have been performed to establish consensus on how to approach treatment modification in this population.
The 2-drug regimen dolutegravir (DTG)/3TC demonstrated durable efficacy for maintaining virologic suppression in phase 3 clinical trials [8–10]. These studies excluded PWH with known or suspected RAMs at screening; however, in post hoc analyses, presence of M184V/I detected via next-generation sequencing in proviral DNA at baseline (SALSA, n = 5; TANGO, n = 4) did not impact virologic efficacy of DTG + 3TC up to week 48 (SALSA) or week 144 (TANGO) [9, 11]. Similarly, absence of historical resistance results or availability of prior genotype did not impact virologic efficacy through week 48 (pooled SALSA/TANGO, n = 294) [12]. However, DTG + 3TC is only indicated for PWH with no known RAMs selective for its components [10]. Real-world evidence (RWE) can help inform whether switching to DTG + 3TC is safe in clinical practice when full treatment history or previous genotype are unavailable and M184V/I can be inadvertently missed.
We conducted a systematic literature review and meta-analysis of published studies to estimate VF rates in PWH receiving DTG + 3TC in suppressed-switch settings, with and without historical M184V/I. A sensitivity analysis was then performed using interventional study data identified via targeted literature review.
METHODS
Systematic Literature Review: RWE Studies
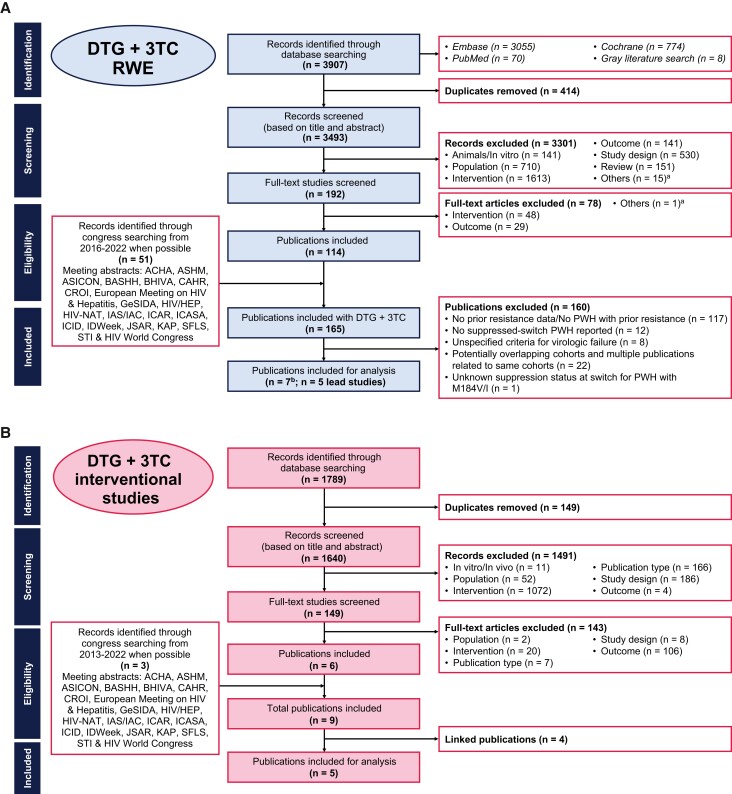
A systematic literature review was conducted to identify real-world studies reporting outcomes for PWH receiving DTG + 3TC (Figure 1A). Embase, Ovid Medline, Medline In-Process, and the Cochrane library were searched from 1 January 2013 to 1 November 2022, and relevant conference archives were searched from 2016 to 2021 (search criteria in Supplementary Table 1). Controlled clinical trials, case studies, studies with <10 PWH, reviews, editorials, and preclinical studies were excluded. At least 2 reviewers independently assessed full-text articles and abstracts meeting selection criteria for full-text review, with discrepancies resolved by consensus.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowcharts for RWE studies (A) and interventional studies (B). aOthers indicates records that were not classified into key categories. bTwo lead publications did not report all information reported within the analyses and were each manually supplemented with data from an additional publication from the same cohort. Abbreviations: 3TC, lamivudine; ACHA, Asian Conference on Hepatitis and AIDS; ASHM, Australasian HIV & AIDS Conference; ASICON, National Conference of AIDS Society of India; BASHH, British Association for Sexual Health and HIV; BHIVA, British HIV Association; CAHR, Canadian Conference on HIV/AIDS Research; CROI, Conference on Retroviruses and Opportunistic Infections; DTG, dolutegravir; GeSIDA, Grupo de Estudio del SIDA-SEIMC; HIV/HEP, HIV & Hepatitis in the Americas; HIV-NAT, The HIV Netherlands Australia Thailand Research Collaboration; IAS/IAC, International AIDS Society/International AIDS Conference; ICAR, International Conference on Antiviral Research; ICASA, International Conference on AIDS and STIs in Africa; ICID, International Congress on Infectious Diseases; JSAR, Japanese Society for AIDS Research; KAP, Kenya Association of Physicians; PWH, people with human immunodeficiency virus type 1; RWE, real-world evidence; SFLS, Société Française de Lutte contre le Sida; STI, sexually transmitted infection.
Studies were screened for adult suppressed-switch populations reporting historical M184V/I before DTG + 3TC initiation, regardless of whether PWH with M184V/I were intentionally included via study exclusion criteria, and assessed for overlapping cohorts. For cohorts represented by multiple publications, the publication with the largest or most up-to-date information was considered the “lead” study and used in the analysis. The full study protocol is available at https://www.viiv-studyregister.com/en/study/?id=219155.
Targeted Literature Review: Interventional Studies
Virologic data from interventional studies were used in sensitivity analyses to assess virologic response to DTG + 3TC in PWH with M184V/I under controlled settings. A targeted literature review was conducted to identify interventional studies (ie, those in which participants received assigned treatment) assessing impact of historical M184V/I on DTG + 3TC efficacy in suppressed-switch populations (Figure 1B; search criteria in Supplementary Table 2).
Feasibility Assessment
A feasibility study was performed to determine whether identified studies were clinically and methodologically comparable enough to combine in the meta-analysis. Primary outcomes of interest were proportions of participants with study-defined VF (see Table 1 for VF definitions by study) and virologic suppression (Snapshot algorithm; HIV-1 RNA <50 copies/mL). These outcomes had to be reported consistently and at comparable time points for inclusion. Proportions were derived in cases where studies reported total number of participants and number with the outcome of interest.
Table 1.
Characteristics of Studies That Met All Systematic or Targeted Literature Review Criteria and Were Included in the Meta-analysis
| VF Outcomes, no./No. (%) | ||||||
|---|---|---|---|---|---|---|
| Lead Study (Cohort) | M184V/I Identification Method | PWH With Pre-Switch M184V/I, no./No. (%) | VF Time Point, Week | With M184V/I | Without M184V/I | VF Definition |
| RWE studies | ||||||
| Hocqueloux 2021 (Dat’AIDS) [13] | RNA and proviral DNA genotypes (pooling both) | 105/695 (15.11) | 24 | 1/105 (0.95) | 0/590 | 2 consecutive confirmed VL >50 copies/mL or 1 VL >200 copies/mL |
| 48 | 2/105 (1.90) | 1/590 (0.17) | ||||
| 96 | 2/105 (1.90) | 3/590 (0.51) | ||||
| Santoro 2022 (LAMRES) [14, 15] | RNA and proviral DNA genotypes | 60/712 (8.43) | 24 | 2/60 (3.33) | 10/652 (1.53) | 2 consecutive confirmed VL >50 copies/mL or 1 VL ≥200 copies/mL |
| 48 | 3/60 (5.00) | 18/652 (2.76) | ||||
| 96 | 4/60 (6.67) | 28/652 (4.29) | ||||
| Borghetti 2021 (ODOACRE) [16, 17] | Historical genotypes; does not specify RNA or proviral DNA | 48/669 (7.17)a | 24 | 0/45 | 2/406 (0.49) | 2 consecutive VL ≥50 copies/mL or 1 VL ≥200 copies/mL |
| 48 | 1/45 (2.22) | 5/406 (1.23) | ||||
| 96 | 2/45 (4.44) | 8/406 (1.97) | ||||
| Galizzi 2020 (NR) [18] | Either RNA or proviral DNA genotypes at baseline (before switch) | 47/174 (27.01)b | 24 | … | … | 2 consecutive confirmed VL >50 copies/mL or 1 VL >50 copies/mL followed by ART modification or 1 VL >1000 copies/mL |
| 48 | 2/47 (4.26) | 7/127 (5.51) | ||||
| 96 | … | … | ||||
| Hidalgo-Tenorio 2019 (DOLAMA) [19] | Baseline RNA genotype | 4/178 (2.25) | 24 | … | … | 2 consecutive VL >50 copies/mL |
| 48 | 1/4 (25.00)c | 4/147 (2.72)c | ||||
| 96 | … | … | ||||
| RWE study total VF outcomes | 24 | 3/210 (1.43) | 12/1648 (0.73) | |||
| 48 | 9/261 (3.45) | 35/1922 (1.82) | ||||
| 96 | 8/210 (3.81) | 39/1648 (2.37) | ||||
| Lead Study (Cohort) | M184V/I Identification Method | PWH With Pre-Switch M184V/I, no./No. (%) | VF Time Point, Week | VF Outcomes, no./No. (%) | VF Definition |
|---|---|---|---|---|---|
| Interventional studies | |||||
| DOLULAM [20] | RNA and proviral DNA genotypes | 17/27 (62.96) | 24 | 0/17 | VL >50 copies/mL |
| 48 | 0/17 | ||||
| 96 | 0/17 | ||||
| TANGO [10] | Proviral DNA genotype | 4/322 (1.24) | 24 | 0/4d | VL ≥50 copies/mL followed by consecutive VL ≥200 copies/mL |
| 48 | 0/4d | ||||
| 96 | … | ||||
| ART PRO [21] | Historical RNA genotype | 21/41 (51.22)e | 24 | 0/21f | VL ≥50 copies/mL |
| 48 | 0/21 | ||||
| 96 | 0/21 | ||||
| SALSA [11] | Proviral DNA genotype | 5/192 (2.60) | 24 | … | VL ≥40 copies/mL |
| 48 | 0/5 | ||||
| 96 | … | ||||
| SOLAR 3D [22] | Historical genotypes; does not specify RNA or proviral DNA | 50/100 (50.00) | 24 | … | VL ≥50 copies/mL followed by consecutive VL >200 copies/mL |
| 48 | 0/50 | ||||
| 96 | … | ||||
| Interventional study total VF outcomes | 24 | 0/42 | |||
| 48 | 0/97 | ||||
| 96 | 0/38 | ||||
Abbreviations: ART, antiretroviral therapy; NR, not reported; PWH, people with human immunodeficiency virus type 1; RWE, real-world evidence; VF, virologic failure; VL, viral load.
aCohort reference reporting the proportion with VF for individuals with M184V/I was used for analysis (n = 45 individuals with M184V/I) [17].
bAssumption: n = 60 PWH with M184V/I were reported out of N = 220 total PWH with available pre-switch genotype resistance data across 2 groups but not reported for dolutegravir plus lamivudine (DTG + 3TC) specifically. Table no. with M184V/I was calculated according to the proportion of PWH in the DTG + 3TC (n = 174) vs other group (n = 46).
cN = 151 total PWH remained on study at week 48.
dAssumption: Week 24 was not reported, but reports described no VF to week 48.
eOf the 20 PWH without known M184V/I at baseline, next-generation sequencing identified n = 7, n = 3, and n = 1 with M184I at 1%, 5%, and 20% thresholds, respectively.
fRefers to the number of PWH with historical 3TC resistance (M184V/I and/or K65R/E/N); 3 PWH with historical 3TC resistance discontinued before week 24 but had VL <50 copies/mL at time of discontinuation (2 protocol violations and 1 adverse event–related discontinuation).
Meta-analysis
Meta-analyses were unadjusted, single-arm pooling of data acquired via literature reviews. The primary objective was to produce point estimates and 95% confidence intervals (CIs) for proportions of participants in RWE studies with M184V/I and study-defined VF at 24, 48, and 96 weeks. Within-cohort sensitivity analyses were performed at each time point for participants without M184V/I. Sensitivity analyses were performed using interventional study data to produce point estimates and 95% CIs for proportions of participants with M184V/I and study-defined VF at 24, 48, and 96 weeks.
For both RWE and interventional study data sets, base analyses were performed at each time point pooling data only from studies sharing similar VF definitions. To maximize sample size and confirm robustness of results, sensitivity analyses were performed at each time point pooling data from all studies regardless of VF definition.
Proportions of PWH experiencing VF were estimated from base and sensitivity analyses using common- and random-effects models. Proportions from RWE and interventional study data sets were transformed using arcsine transformations. Meta-analyses were conducted using inverse-variance methods. Additional sensitivity analyses were performed using logit transformations and Freeman-Tukey double-arcsine transformations. Logit-transformed data were fit to general linear mixed models; Freeman-Tukey double-arcsine transformations followed inverse-variance methods.
Statistical Analysis
Meta-analyses were conducted by 2 analysts independently using R (version 4.0.3 or greater; R Foundation, Vienna, Austria) meta [23] and metafor [24] packages. Study heterogeneity was assessed to quantify inconsistency according to the equation I2 = ([Q – df] / Q) × 100, where Q is the χ2 statistic and df is degrees of freedom. Threshold I2 values were used to indicate that heterogeneity might not be important (0%–40%), may be moderate (30%–60%), may be substantial (50%–90%), or is considerable (75%–100%). The DerSimonian-Laird residual heterogeneity estimator was used to determine τ2 values.
Patient Consent Statement
This study does not include factors necessitating patient consent or ethical approval.
RESULTS
RWE Study Characteristics
Of 3907 publications and 51 conference abstracts identified via systematic literature review, 165 were considered for screening. Characteristics of the 5 real-world cohorts that met all search criteria and were included in the meta-analysis are summarized in Table 1 and indicated by lead cohort publication. Of these cohorts, 2 used similar criteria to define VF [13, 14]; 3 reported VF outcomes at all time points [13, 14, 16].
RWE VF Outcomes
Overall, the 5 RWE cohorts reported low incidence of VF events at weeks 24, 48, and 96 in PWH with prior M184V/I and without M184V/I (Table 1). Key findings related to VF in RWE cohorts are summarized in Table 2. One cohort observed that presence of M184V/I had no impact on virologic suppression with DTG + 3TC [19], and another found that presence of M184V/I did not predict VF [17] but did predict shorter time to VF (vs absence [16]); 3 cohorts found that evidence of M184V/I did not affect probability of VF for ≥1 year [13, 14, 18].
Table 2.
Summary of Resistance Selection in Participants From Real-World Evidence Studies at Virologic Failure According to Study-Defined Virologic Failure Criteria
| Study (Cohort) | GRT Availability at Time of VF | INSTI and NRTI RAMs at VF | Key Findings |
|---|---|---|---|
| RWE studies | |||
| Hocqueloux 2021 (Dat’AIDS) [13] | 4/9 total VFs (0 with known pre-switch M184V/I) | No M184V/I present in participants at VF | Archived M184V/I did not affect the probability of VF after median follow-up of 1.2 y (log-rank test, P = .81), even when stratified by time since last M184V/I detection (log-rank test, P = .94) |
| Santoro 2022 (LAMRES) [14, 15] | 4/32 total VFs (0 with known pre-switch M184V) | No INSTI resistance was reported at VF; n = 1 report of NRTI RAM (M184V) at VFa |
|
| Borghetti 2021 (ODOACRE) [16, 17] | 11/12 VFs (2 with known pre-switch M184V/I)b | No participants experiencing VF developed RAMs after failure |
|
| Galizzi 2020 (NR) [18] | 8/17 total VFs (2 with known pre-switch M184V/I) | No development of INSTI resistance was reported |
|
| Hidalgo-Tenorio 2019 (DOLAMA) [19] | 2/5 total VFs (1 with known pre-switch M184V)c | No development of INSTI resistance was reported | Presence of M184V did not affect virologic response to DTG + 3TC |
| Interventional studies | |||
| DOLULAM [20] | 0 VFs | No participants met VF criteria | No participants met VF criteria despite potential risk factors: 15/27 (56%) had highest pre-ART VL ≥100 000 copies/mL and 17/27 (63%) had nadir CD4+ cell count <200 cells/μL |
| TANGO [10] | 0 VFs | No participants met VF criteria | All 4 participants with baseline M184V/I maintained virologic suppression at all on-treatment study visits |
| ART PRO [21] | 0 VFs | No participants met VF criteria | 6/12 participants who experienced transient viral rebound had historical 3TC resistance; all resuppressed with no change in treatment |
| SALSA [11] | 1/1 VF (0 with known pre-switch M184V)d | NR | 5/5 (100%) participants with M184V at baseline maintained VL <40 copies/mL; of these, 4/5 (80%) had VL <40 copies/mL and qualitative target not detected |
| SOLAR 3D [22] | 0 VFs | No RAMs observed through week 48 | Similar proportions of participants with vs without historical M184V/I, respectively, had VL <20 copies/mL and target not detected at weeks 24 (80% vs 78%) and 48 (84% vs 80%) |
Abbreviations: 3TC, lamivudine; aHR, adjusted hazard ratio; ART, antiretroviral therapy; CI, confidence interval; DTG, dolutegravir; GRT, genotypic resistance test; INSTI, integrase strand transfer inhibitor; NR, not reported; NRTI, nucleoside reverse transcriptase inhibitor; RAM, resistance-associated mutation; VF, virologic failure; VL, viral load.
aIndividual was virologically suppressed for 1 month before switch, had previous VF due to low adherence, and had VF on the regimen switched to after the VF under DTG + 3TC.
bAssumption: presence of M184V/I was listed for 2 of 12 participants with VF; whether these indicate pre-switch or time of VF results was not explicit [17].
cWhether the participant with known pre-switch M184V is 1 of the 2 participants with GRT available at time of VF was not explicit.
dParticipant had VL ≥50 copies/mL and did not meet confirmed virologic withdrawal criteria.
No INSTI RAMs were reported in PWH experiencing VF in 4 cohorts [14, 17–19]; the remaining cohort did not report presence/absence of INSTI RAMs in PWH at VF [13]. Among all cohorts, only 1 PWH in 1 cohort (n = 712) developed M184V at VF, among 652 PWH with no previously known M184V/I (1/652 [0.15%]) [14].
RWE VF Estimates
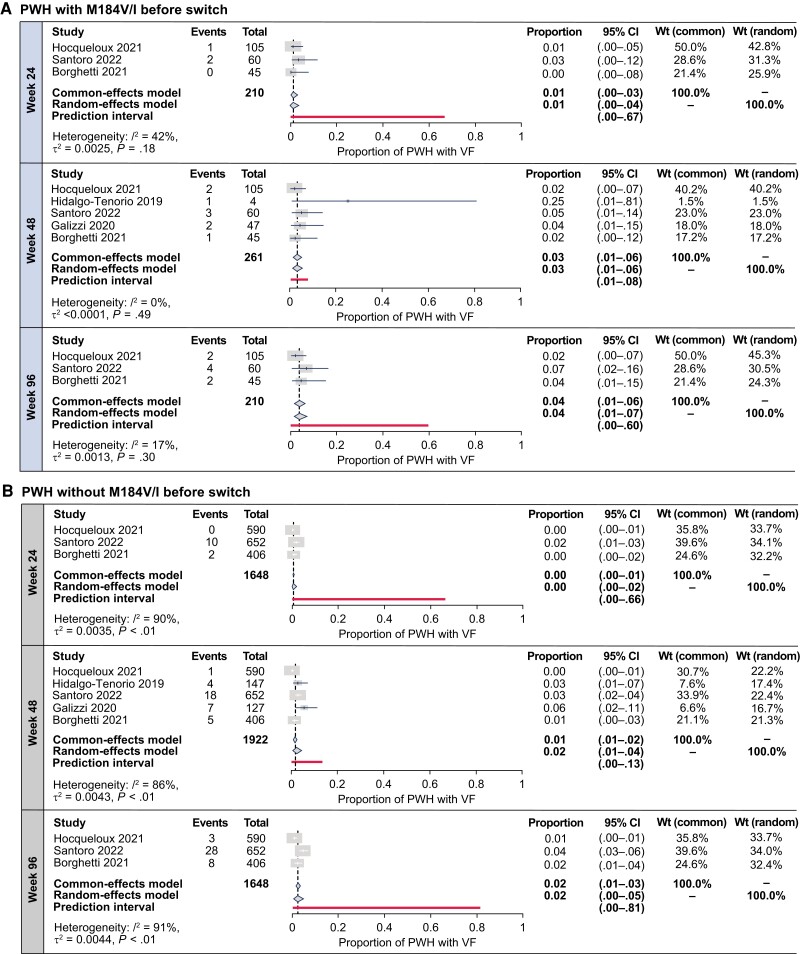
Base analyses at weeks 24, 48, and 96 included only the 2 RWE cohorts that shared similar VF definitions and M184V/I identification methods [13, 14]. Sensitivity analyses were performed to maximize sample sizes and pooled all RWE cohorts with all results reported at each time point, regardless of VF definition or M184V/I identification method. Sensitivity analyses were also performed at each time point using data from within-cohort participants without M184V/I. Random-effects and common-effects model meta-analysis–estimated proportions with VF using arcsine-transformed proportions were low in PWH with M184V/I in sensitivity analyses inclusive of all VF definitions at weeks 24, 48, and 96 (Figure 2A) and in within-cohort PWH without historical M184V/I (Figure 2B).
Figure 2.
Meta-analysis estimates of virologic failure (VF) incidence after switch to dolutegravir plus lamivudine in people with human immunodeficiency virus type 1 with (A) and without (B) historical M184V/I from systematic literature review–identified real-world evidence studies, inclusive of all VF definitions. Abbreviations: CI, confidence interval; PWH, people with human immunodeficiency virus type 1; VF, virologic failure; Wt, weight.
Base and sensitivity analysis estimates were similar at each time point (Figure 2, Supplementary Figure 1), indicating that all cohorts regardless of VF definition could be pooled to increase sample sizes without affecting estimates.
Though estimated proportions for VF in PWH without M184V/I were numerically lower than those with M184V/I at all time points, these populations had overlapping estimate CIs. Estimated proportions with VF marginally increased over time regardless of M184V/I presence. Base and sensitivity analyses for estimates in PWH with historical M184V/I generally had low proportions of variance due to heterogeneity (I2; Figure 2A, Supplementary Figure 1A). Estimates in PWH without M184V/I had substantial-to-considerable I2 values at all time points (Figure 2B, Supplementary Figure 1B).
Meta-analysis results using logit- and Freeman-Tukey double-arcsine–transformed proportions were mostly consistent with those using arcsine-transformed proportions (Supplementary Figures 2 and 3, respectively).
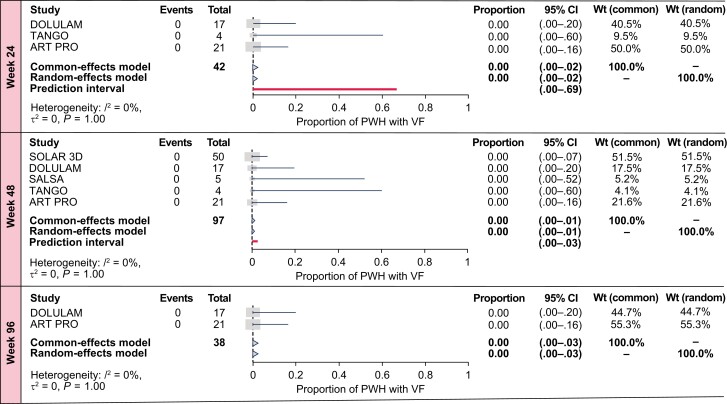
Sensitivity Analysis in Interventional Studies
The targeted literature review identified 5 relevant interventional studies from 1789 publications and 3 conference abstracts, summarized in Table 1. Two interventional studies reported VF outcomes at all time points [20, 21]; 3 shared similar VF definitions [10, 21, 22].
Total number of VF outcomes in PWH with M184V/I was 0 at all time points. No treatment-emergent RAMs were reported at VF. Sample sizes were smaller than RWE studies, with multiple studies listing historical nucleoside reverse transcriptase inhibitor (NRTI) resistance as an exclusion criterion [10, 11]. Estimated proportions with VF in PWH with historical M184V/I at weeks 24, 48, and 96 were consistently low in interventional studies at each time point in base and sensitivity analyses. Sensitivity analyses including all studies regardless of VF definition increased sample sizes versus base analyses, without impacting VF estimates (Figure 3, Supplementary Figure 4). In both common- and random-effects model–estimated proportions of PWH with M184V/I with VF, zero total VF events were observed at all time points (Figure 3).
Figure 3.
Meta-analysis estimates of virologic failure (VF) incidence after switch to dolutegravir plus lamivudine in people with human immunodeficiency virus type 1 with historical M184V/I from targeted literature review–identified interventional studies, inclusive of all VF definitions. Abbreviations: CI, confidence interval; PWH, people with human immunodeficiency virus type 1; VF, virologic failure; Wt, weight.
Sensitivity analyses performed using logit- and Freeman-Tukey double-arcsine–transformed proportions were consistent with those using arcsine-transformed proportions (Supplementary Tables 3 and 4, respectively).
DISCUSSION
Using a systematic literature review and meta-analysis approach with real-world antiretroviral switch studies, we have estimated that low proportions of PWH with historical M184V/I meet VF criteria through 96 weeks after switching to DTG + 3TC, with similarly low rates observed among PWH without previously documented M184V/I. This was consistent with the sensitivity analysis performed using data from interventional studies in PWH with historical M184V/I. Meta-analysis–estimated proportions were consistently low across different proportion transformations.
Despite M184V/I conferring high-level resistance against 3TC/FTC [1], its impact on subsequent virologic responses to treatment has been debated. A randomized study in PWH with M184V failing 3TC-inclusive regimens reported significantly higher median (interquartile range) viral load (VL) in a treatment interruption group versus a 3TC monotherapy group (1.22 [0.8–1.48] vs 0.61 [0.4–0.87] log10 copies/mL, respectively; P = .001). At week 48 (or discontinuation), M184V was undetectable in 28 of 29 (97%) treatment interruption participants and persistent in all 29 3TC monotherapy participants, but HIV-1 replication capacity was significantly higher with treatment interruption versus 3TC monotherapy at weeks 24 and 48 [25], supporting decreased viral fitness with M184V versus wild-type virus observed in vitro [1]. M184V can also gradually clear from the viral reservoir in PWH with long-term suppression, even under active 3TC/FTC pressure [26].
Using 3TC/FTC in PWH with M184V/I failing their current regimen may not be detrimental to virologic suppression. A post hoc analysis of PWH with M184V/I failing first-line treatment in the DAWNING study showed no evidence of decreased virologic suppression when 3TC or FTC was used in a DTG-based 3-drug regimen after VF (vs M184V/I absence and vs switching to regimens without 3TC/FTC) [27]. The Nucleosides and Darunavir/Dolutegravir in Africa (NADIA) trial in PWH failing nonnucleoside reverse transcriptase inhibitor and tenofovir disoproxil fumarate (TDF)–based first-line regimens found higher odds of virologic suppression through 96 weeks with presence versus absence of M184V/I when 3TC + TDF or zidovudine was used in a 3-drug regimen [28], consistent with improved viral susceptibility to TDF and zidovudine observed with M184V/I presence [1, 4]. Furthermore, switching to bictegravir/FTC/tenofovir alafenamide demonstrated durable efficacy in PWH with pre-switch M184V/I in a suppressed-switch setting (last on-treatment suppression rate, 98%), with no treatment-emergent RAMs at VF [29]. Other studies have also reported low VF incidence in individuals with M184V/I and prior VF receiving 3TC as part of different 3-drug regimens [30, 31].
Using 3TC/FTC as a component in a 2-drug regimen may still provide protection in PWH with historical M184V/I. In the randomized switch study MOBIDIP, in which 82% of participants were virologically suppressed at baseline, boosted protease inhibitor (bPI) + 3TC demonstrated superiority over bPI monotherapy at week 48 (VF treatment difference, 21.8% [95% CI, 13.9%–29.7%]) despite near-universal M184V presence at first-line VF (96%) [32]. A retrospective study using the Antiviral Response Cohort Analysis database of virologically suppressed PWH switching to 2-drug regimens of 3TC + bPI or INSTI reported low VF incidence in PWH with M184V (5.1 [95% CI, 2.2%–9.9%] per 100 person-years of follow-up [PYFU]) and without M184V (3.1 [95% CI, 1.8%–4.8%] per 100 PYFU) and determined that historical M184V was not a significant predictor of VF with the 3TC-inclusive 2-drug regimens used (adjusted hazard ratio, 1.11 [95% CI, .38–3.23]) [33]. Collectively, these results support that 3TC/FTC-containing regimens can remain effective in PWH with historical M184V/I.
The low meta-analysis estimates of VF and lack of emergent INSTI resistance through 96 weeks in PWH with M184V/I suggest not only an effect of decreased viral fitness but also that DTG + 3TC is unlikely to function as DTG monotherapy in this population. Tissue cultures exposed to DTG monotherapy selected INSTI RAMs when infected with wild-type virus but not with virus containing M184V or M184I variants [7], suggesting an antagonism of either M184V and K65R in the variant toward selecting DTG resistance. A meta-analysis reported that PWH on DTG monotherapy developed INSTI RAMs at VF and had higher estimated proportions with VF versus PWH receiving DTG dual therapy [34]. The randomized MONotherapy of TiviCAY (MONCAY) study evaluating switch to DTG monotherapy reported 7 VFs among 78 PWH on DTG monotherapy through week 48 (vs 0/80 maintaining DTG/abacavir/3TC), at weeks 24 (n = 2), 29 (n = 1), 36 (n = 2), and 48 (n = 2). Of these VFs, new INSTI RAMs were reported for 2 (29%) PWH at weeks 36 and 48 (n = 1 each) [35]. Based on the high risk of VF and emergent on-treatment resistance, DTG monotherapy is not recommended.
Pooling studies regardless of VF definition did not affect predicted VF estimates in RWE or interventional studies. Most VF definitions were consistent in the interventional sensitivity analysis but less consistent in RWE sensitivity analyses. In 4 of 5 RWE studies, VF criteria included a single elevated VL ranging from ≥200 to >1000 copies/mL [13, 14, 16, 18]. Without details on which individuals met which criteria, VF may be underreported in studies with less stringent definitions. Despite this, with only 5 studies each meeting RWE and interventional study search criteria, the increase in sample size due to including all studies regardless of VF definition is likely a net benefit versus calculating estimates from fewer studies sharing similar VF definitions.
In PWH with M184V/I, analysis estimates of VF from RWE studies remained low but increased over time from 24 to 96 weeks, whereas sensitivity analysis estimates from interventional studies remained low (zero) through 96 weeks. Rates of VF beyond week 96 could remain low, as DTG + 3TC demonstrated durable efficacy in the TANGO study through week 196 with only 1 case of confirmed VF at year 4 [36]; however, TANGO excluded PWH with known historical NRTI resistance. Additionally, confounding factors beyond the presence of historical M184V/I could impact the likelihood of PWH meeting VF criteria in real-world settings versus controlled interventional settings, such as adherence or baseline characteristics.
One strength of our study is the meta-analysis approach employed. Random-effects model estimates are generalizable, whereas common-effects (or fixed-effects) models assume that only the included cohorts are the population of interest [24]. Arcsine transformation is recommended for meta-analysis single proportion estimates [37]; though sensitivity analysis estimates using other transformations were mostly consistent with arcsine transformation results, logit proportion estimates can have uninformatively wide CIs if zero events are reported [38], as in interventional study estimates at all time points, and Freeman-Tukey proportion estimates can be misleading if data sets have a large range of population sizes [37], as in 48-week RWE estimates for PWH with historical M184V/I. Arcsine transformation is not affected by these limitations [37] and had the most reasonable proportion estimates of all transformations. The similarity between base and sensitivity analyses results confirmed the robustness of our approach of grouping studies regardless of VF definition.
There are some limitations to these analyses. We were limited to the data reported. Although no studies reported any INSTI resistance, genotypic data at VF were not always available (Table 2); therefore, occurrence of RAMs selective for 3TC or DTG at failure could not be fully ascertained. The number of PWH with pre-switch M184V/I and reported virologic outcomes in RWE studies represented a modest percentage of total RWE PWH at each time point (weeks 24 and 96, 11% [210/1858]; week 48, 12% [261/2183]), which limits the power to detect differences in VF between PWH with and without pre-switch M184V/I. Though reported VF rates were low across PWH with and without M184V/I and estimated CIs overlapped between these populations, larger sample sizes could determine whether the numerically higher proportions of PWH with versus without M184V/I meeting VF criteria would eventually reach statistical significance. Time of detection of M184V/I before DTG + 3TC switch and time with suppressed viremia were noted as criteria that impact VF in the LAMRES cohort [14, 15] but not the Dat’AIDS cohort [13]; however, these comparisons may not have been sufficiently powered with the low VF incidence in each cohort. Our analysis is not suitable to draw conclusions based on factors such as time to VF and time to M184V/I identification, as they were not consistently reported and were not filtering criteria for the systematic literature review. It may be difficult to derive meaningful estimates if the number of studies was narrowed to only those including these or other specific criteria. Some estimates had high I2 values (up to 96%), indicating that a high proportion of variability would remain in the true effect estimate if sampling error was removed [39], but this statistic is expected to be biased toward overestimating or underestimating the actual true effect variance in meta-analyses with fewer (especially <10) included studies and should be interpreted with this caveat in mind [40]. Finally, because the M184I variant can develop as a hypermutated provirus, often with replication-incompetent genomes [4, 41], using proviral DNA detection may overestimate the prevalence of functional M184V/I. Indeed, real-world studies specifying reports of M184V [14, 19] versus M184V/I [13, 16, 18] had lower overall proportions of participants with historical variants. Pooling studies that reported historical RNA and archived proviral DNA detection may misrepresent the number of PWH with functional M184V/I.
In summary, pre-switch M184V/I prevalence was variable in PWH in RWE studies. Incidence of VF among PWH who switched to DTG + 3TC was low and minimally impacted by preexisting historical M184V/I through 96 weeks, and no treatment-emergent INSTI RAMs were reported, consistent with results from interventional studies. This meta-analysis suggests that M184V/I may have no impact on the effectiveness of DTG + 3TC as a switch strategy when considering treatment changes in PWH with an incomplete history, where historical M184V/I may be inadvertently missed, and where other options are limited or contraindicated. More outcomes data are needed for future analyses to resolve whether duration of virologic suppression pre-switch, M184V/I timing, or other factors impact DTG + 3TC effectiveness in PWH with historical M184V/I.
Supplementary Material
Contributor Information
Madhusudan Kabra, ViiV Healthcare, Brentford, United Kingdom.
Tristan J Barber, Ian Charleson Day Centre, Royal Free London National Health Service Foundation Trust, London, United Kingdom; Institute for Global Health, University College London, London, United Kingdom.
Clotilde Allavena, Department of Infectious and Tropical Diseases, Centre Hospitalier Universitaire Hôtel-Dieu, Nantes, France.
Anne-Geneviève Marcelin, Sorbonne Université, Inserm, Institut Pierre Louis d’Epidémiologie et de Santé Publique, Assistance Publique–Hôpitaux de Paris, Laboratoire de Virologie, Hôpital Pitié-Salpêtrière, Paris, France.
Simona Di Giambenedetto, Laboratory and Infectious Diseases Sciences, Fondazione Policlinico Universitario Agostino Gemelli Istituto di Ricovero e Cura a Carattere Scientifico and Department of Safety and Bioethics, Università Cattolica del Sacro Cuore, Rome, Italy.
Juan Pasquau, Unit of Infectious Diseases, Virgen de las Nieves University Hospital, Granada, Spain.
Nicola Gianotti, Infectious Diseases Unit, Istituto di Ricovero e Cura a Carattere Scientifico Ospedale San Raffaele, Milan, Italy.
Josep M Llibre, Infectious Diseases Division and Fight Infections Foundation, Hospital Universitari Germans Trias i Pujol, Barcelona, Spain.
David Rial-Crestelo, HIV Unit, Hospital Universitario 12 de Octubre, Madrid, Spain; Centro de Investigación Biomédica en Red de Enfermedades Infecciosas, Madrid, Spain.
Rosa De Miguel-Buckley, Centro de Investigación Biomédica en Red de Enfermedades Infecciosas, Madrid, Spain; Infectious Diseases Unit, Hospital Universitario La Paz, Madrid, Spain.
Gary Blick, Health Care Advocates International, Stratford, Connecticut, USA.
Matthew Turner, HEOR Ltd, Cardiff, United Kingdom.
Cale Harrison, HEOR Ltd, Cardiff, United Kingdom.
Tammy Wynne, HEOR Ltd, Cardiff, United Kingdom.
Gustavo Verdier, ViiV Healthcare, Montréal, Quebec, Canada.
Chris M Parry, ViiV Healthcare, Brentford, United Kingdom.
Bryn Jones, ViiV Healthcare, Brentford, United Kingdom.
Chinyere Okoli, ViiV Healthcare, Brentford, United Kingdom.
Cynthia Donovan, ViiV Healthcare, Durham, North Carolina, USA.
Julie Priest, ViiV Healthcare, Durham, North Carolina, USA.
Emilio Letang, ViiV Healthcare, Madrid, Spain.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Editorial assistance was provided under the direction of the authors by Lindsay Walton, PhD, and Jennifer Rossi, MA, ELS, MedThink SciCom, and funded by ViiV Healthcare.
Author contributions. M. K., B. J., J. Pr., and E. L. contributed to the conception of the study. M. K., J. M. L., G. V., C. M. P., B. J., C. O., J. Pr., and E. L. contributed to the design of the study. M. K., M. T., C. H., T. W., J. Pr., and E. L. contributed to the acquisition of data. M. T., C. H., and T. W. contributed to the analysis of data. M. K., C. D., J. Pr., and E. L. contributed to drafting the manuscript. M. K., T. J. B., C. A., A.-G. M., S. D. G., J. Pa., N. G., J. M. L., D. R.-C., R. D. M.-B., G. B., G. V., C. M. P., B. J., C. O., C. D., J. Pr., and E. L. contributed to critically revising the manuscript for important intellectual content. All authors contributed to the interpretation of data and approved the manuscript for publication.
Data availability. Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Financial support. This work was supported by ViiV Healthcare.
References
- 1. Diallo K, Götte M, Wainberg MA. Molecular impact of the M184V mutation in human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother 2003; 47:3377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . HIV drug resistance report 2021. Available at: https://www.who.int/publications/i/item/9789240038608. Accessed 12 October 2022.
- 3. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society–USA panel. JAMA 2020; 324:1651–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geretti AM, Blanco JL, Marcelin AG, et al. HIV DNA sequencing to detect archived antiretroviral drug resistance. Infect Dis Ther 2022; 11:1793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schuurman R, Nijhuis M, van Leeuwen R, et al. Rapid changes in human immunodeficiency virus type 1 RNA load and appearance of drug-resistant virus populations in persons treated with lamivudine (3TC). J Infect Dis 1995; 171:1411–9. [DOI] [PubMed] [Google Scholar]
- 6. Eron JJ, Benoit SL, Jemsek J, et al. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. N Engl J Med 1995; 333:1662–9. [DOI] [PubMed] [Google Scholar]
- 7. Oliveira M, Ibanescu RI, Pham HT, Brenner B, Mesplède T, Wainberg MA. The M184I/V and K65R nucleoside resistance mutations in HIV-1 prevent the emergence of resistance mutations against dolutegravir. AIDS 2016; 30:2267–73. [DOI] [PubMed] [Google Scholar]
- 8. Llibre JM, Brites C, Cheng C-Y, et al. Efficacy and safety of switching to the 2-drug regimen dolutegravir/lamivudine versus continuing a 3- or 4-drug regimen for maintaining virologic suppression in adults living with HIV-1: week 48 results from the phase 3, noninferiority SALSA randomized trial. Clin Infect Dis 2023; 76:720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Osiyemi O, De Wit S, Ajana F, et al. Efficacy and safety of switching to dolutegravir/lamivudine versus continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: results through week 144 from the phase 3, noninferiority TANGO randomized trial. Clin Infect Dis 2022; 75:975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, noninferiority TANGO study. Clin Infect Dis 2020; 71:1920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Underwood M, Osiyemi O, Rubio R, et al. Archived resistance and response to <40 c/mL and TND–DTG/3TC FDC at week 48 in SALSA [Poster 481]. Presented at: Conference on Retroviruses and Opportunistic Infections, Virtual, 12–16 February 2022.
- 12. Scholten S, Cahn P, Portilla J, et al. Dolutegravir/lamivudine efficacy outcomes in people living with HIV with or without resistance results: 48-week pooled analysis [Poster P019]. Presented at: BHIVA Spring Conference 2022, Manchester, UK, 20–22 April 2022.
- 13. Hocqueloux L, Allavena C, Sécher C, et al. Archived mutation M184V does not increase virologic failure during maintenance therapy with dolutegravir + lamivudine in the French DAT'AIDS cohort [Slides OS1/2]. Presented at: 18th European AIDS Conference, Virtual and London, UK, 27–30 October 2021.
- 14. Santoro MM, Armenia D, Teyssou E, et al. Virological efficacy of switch to DTG plus 3TC in a retrospective observational cohort of suppressed HIV-1 patients with or without past M184V: the LAMRES study. J Glob Antimicrob Resist 2022; 31:52–62. [DOI] [PubMed] [Google Scholar]
- 15. Santoro MM, Armenia D, Teyssou E, et al. Impact of M184V on the virological efficacy of switch to 3TC/DTG in real life [Poster 429]. Presented at: Conference on Retroviruses and Opportunistic Infections, Virtual, 6–11 March 2021.
- 16. Borghetti A, Giacomelli A, Borghi V, et al. Nucleoside reverse-transcriptase inhibitor resistance mutations predict virological failure in human immunodeficiency virus–positive patients during lamivudine plus dolutegravir maintenance therapy in clinical practice. Open Forum Infect Dis 2021; 8:ofab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baldin G, Ciccullo A, Rusconi S, et al. Long-term data on the efficacy and tolerability of lamivudine plus dolutegravir as a switch strategy in a multi-centre cohort of HIV-1-infected, virologically suppressed patients. Int J Antimicrob Agents 2019; 54:728–34. [DOI] [PubMed] [Google Scholar]
- 18. Galizzi N, Poli A, Galli L, et al. Retrospective study on the outcome of two-drug regimens based on dolutegravir plus one reverse transcriptase inhibitor in virologically-suppressed HIV-infected patients. Int J Antimicrob Agents 2020; 55:105893. [DOI] [PubMed] [Google Scholar]
- 19. Hidalgo-Tenorio C, Cortés LL, Gutiérrez A, et al. DOLAMA study: effectiveness, safety and pharmacoeconomic analysis of dual therapy with dolutegravir and lamivudine in virologically suppressed HIV-1 patients. Medicine (Baltimore) 2019; 98:e16813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reynes J, Meftah N, Tuaillon E, Charpentier C, Montes B. Dual regimen with dolutegravir and lamivudine maintains virologic suppression even in heavily treatment experienced HIV-infected patients: 96 weeks results from maintenance DOLULAM study [Poster MOPEB0322]. Presented at: 9th IAS Conference on HIV Science, Paris, France, 23–26 July 2017.
- 21. Rial-Crestelo D, de Miguel R, Montejano R, et al. Long-term efficacy of dolutegravir plus lamivudine for maintenance of HIV viral suppression in adults with and without historical resistance to lamivudine: week 96 results of ART-PRO pilot study. J Antimicrob Chemother 2021; 76:738–42. [DOI] [PubMed] [Google Scholar]
- 22. Blick G, Cerreta E, Mancini G, Cosenza A. SOLAR 3D: a prospective study switching to DTG/3TC from 3- or 4-drug ART for maintenance of viral suppression with historic M184V/I mutation and prior virological failures: 48 week primary endpoint results [Poster PE2/65]. Presented at: 18th European AIDS Conference, Virtual and London, UK, 27–30 October 2021.
- 23. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019; 22:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36:1–48. [Google Scholar]
- 25. Castagna A, Danise A, Menzo S, et al. Lamivudine monotherapy in HIV-1-infected patients harbouring a lamivudine-resistant virus: a randomized pilot study (E-184V study). AIDS 2006; 20:795–803. [DOI] [PubMed] [Google Scholar]
- 26. Palich R, Teyssou E, Sayon S, et al. Kinetics of archived M184V mutation in treatment-experienced virally suppressed HIV-infected patients. J Infect Dis 2022; 225:502–9. [DOI] [PubMed] [Google Scholar]
- 27. Brown D, Kaplan R, Losso M, et al. Efficacy of second-line dolutegravir plus 2 nucleoside reverse transcriptase inhibitors by baseline nucleoside reverse transcriptase inhibitor resistance and nucleoside reverse transcriptase inhibitor use in the DAWNING study. Antivir Ther 2022; 27:13596535221077487. [Google Scholar]
- 28. Paton NI, Musaazi J, Kityo C, et al. Efficacy and safety of dolutegravir or darunavir in combination with lamivudine plus either zidovudine or tenofovir for second-line treatment of HIV infection (NADIA): week 96 results from a prospective, multicentre, open-label, factorial, randomised, non-inferiority trial. Lancet HIV 2022; 9:e381–93. [DOI] [PubMed] [Google Scholar]
- 29. Sax PE, Andreatta K, Molina J-M, et al. High efficacy of switching to bictegravir/emtricitabine/tenofovir alafenamide in people with suppressed HIV and preexisting M184V/I. AIDS 2022; 36:1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jary A, Marcelin A-G, Charpentier C, et al. M184V/I does not impact the efficacy of abacavir/lamivudine/dolutegravir use as switch therapy in virologically suppressed patients. J Antimicrob Chemother 2020; 75:1290–3. [DOI] [PubMed] [Google Scholar]
- 31. Olearo F, Nguyen H, Bonnet F, et al. Impact of the M184V/I mutation on the efficacy of abacavir/lamivudine/dolutegravir therapy in HIV treatment-experienced patients. Open Forum Infect Dis 2019; 6:ofz330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ciaffi L, Koulla-Shiro S, Sawadogo AB, et al. Boosted protease inhibitor monotherapy versus boosted protease inhibitor plus lamivudine dual therapy as second-line maintenance treatment for HIV-1-infected patients in sub-Saharan Africa (ANRS12 286/MOBIDIP): a multicentre, randomised, parallel, open-label, superiority trial. Lancet HIV 2017; 4:e384–92. [DOI] [PubMed] [Google Scholar]
- 33. Gagliardini R, Ciccullo A, Borghetti A, et al. Impact of the M184V resistance mutation on virological efficacy and durability of lamivudine-based dual antiretroviral regimens as maintenance therapy in individuals with suppressed HIV-1 RNA: a cohort study. Open Forum Infect Dis 2018; 5:ofy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wandeler G, Buzzi M, Anderegg N, et al. Virologic failure and HIV drug resistance on simplified, dolutegravir-based maintenance therapy: systematic review and meta-analysis. F1000Res 2018; 7:1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hocqueloux L, Raffi F, Prazuck T, et al. Dolutegravir monotherapy versus dolutegravir/abacavir/lamivudine for virologically suppressed people living with chronic human immunodeficiency virus infection: the randomized noninferiority MONotherapy of TiviCAY trial. Clin Infect Dis 2019; 69:1498–505. [DOI] [PubMed] [Google Scholar]
- 36. De Wit S, Bonnet F, Osiyemi O, et al. Durable efficacy of switching from a 3-/4-drug tenofovir alafenamide (TAF)-based regimen to the 2-drug regimen dolutegravir/lamivudine (DTG/3TC) in the TANGO study through week 196 [Slides MO41]. Presented at: HIV Drug Therapy Glasgow 2022, Virtual and Glasgow, UK, 23–26 October 2022.
- 37. Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rücker G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods 2019; 10:476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health 2013; 67:974–8. [DOI] [PubMed] [Google Scholar]
- 39. Borenstein M, Higgins JPT, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods 2017; 8:5–18. [DOI] [PubMed] [Google Scholar]
- 40. von Hippel PT. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med Res Methodol 2015; 15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Y, Etemad B, Dele-Oni R, et al. Drug resistance mutations in HIV provirus are associated with defective proviral genomes with hypermutation. AIDS 2021; 35:1015–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.