Abstract
Background
Social cognitive impairment is a recognized feature of psychotic disorders. However, potential age-related differences in social cognitive impairment have rarely been studied.
Study Design
Data came from 905 individuals with a psychotic disorder, 966 unaffected siblings, and 544 never-psychotic controls aged 18–55 who participated in the Genetic Risk and Outcome of Psychosis (GROUP) study. Multilevel linear models were fitted to study group main effects and the interaction between group and age on emotion perception and processing (EPP; degraded facial affect recognition) and theory of mind (ToM; hinting task) performance. Age-related differences in the association between socio-demographic and clinical factors, and EPP and ToM were also explored.
Study Results
Across groups, EPP performance was associated with age (β = −0.02, z = −7.60, 95% CI: −0.02, −0.01, P < .001), with older participants performing worse than younger ones. A significant group-by-age interaction on ToM (X2(2) = 13.15, P = .001) indicated that older patients performed better than younger ones, while no age-related difference in performance was apparent among siblings and controls. In patients, the association between negative symptoms and ToM was stronger for younger than older patients (z = 2.16, P = .03).
Conclusions
The findings point to different age-related performance patterns on tests of 2 key social cognitive domains. ToM performance was better in older individuals, although this effect was only observed for patients. EPP was less accurate in older compared with younger individuals. These findings have implications with respect to when social cognitive training should be offered to patients.
Keywords: aging, schizophrenia, siblings, psychosis, social cognition, functional impairment
Introduction
Individuals with schizophrenia and related psychotic disorders often experience impairments in social cognition.1,2 Social cognition may impact functional outcomes more strongly than nonsocial cognition and psychotic symptoms.3 While social cognition, referring to the psychological processes that enable people to understand social contexts, spans multiple domains (eg, mentalization or theory of mind [ToM]), emotion perception and processing (EPP), and social perception, the vast amount of existing psychosis research has focused on ToM and EPP. However, even within these domains, there are knowledge gaps.
One concerns the age-related development of ToM and EPP impairment. Some studies suggest that impairments in EPP and ToM are already present prior to the first psychotic episode,4,5 and that social cognitive performance in individuals with a psychotic disorder is relatively stable over time6–9 and across illness phases.2,10,11 However, the effect of age on social cognitive performance remains largely unexplored and it is unclear whether potential age-related differences in patients with a psychotic disorder are comparable to those of never-psychotic individuals.12 One study showed comparable ToM impairment in young individuals in the early phase and older individuals in more chronic phase of the illness.10 However, the study did not include a control group, which limits conclusions relating to “typical” age-related functioning.
Furthermore, most, but not all,13 studies suggest that the social cognitive performance of unaffected siblings of patients lies in between never-psychotic individuals and those diagnosed with a psychotic disorder (for a review see14). As siblings share genetic and/or environmental factors that might be related to an increased risk for psychosis but are not exposed to the effects that are related to having a psychotic disorder (eg, medication, stigma), investigations of social cognition in siblings can help to understand the impact of risk vs. disorder-related mechanisms. Similar social cognitive performance in siblings and controls while patients diverge as they age, would suggest age-related processes specific to the disorder. Similar social cognitive performance in siblings and patients, would suggest familial rather than disorder-related mechanisms.
It remains unknown whether illness-related factors, such as symptom severity, illness duration, and nonsocial cognitive impairment relate differentially to social cognition throughout adulthood,15 or whether possible age-related cognitive processes in psychotic disorders differ by sex. While previous psychosis studies found only small sex differences (eg,16,17), or no sex difference,18 the potential interaction between age and sex on social cognitive performance in adults with psychotic disorders has not yet been studied. Finally, social cognition has been related to functional outcomes, but this relationship may vary with age. Identifying age-related patterns can inform interventions that aim to improve or retain patients' independent functioning, social connections, and a better quality of life as they age.
We investigated these knowledge gaps in a large sample of patients with a non-affective psychotic disorder, unaffected siblings, and controls from the Genetic Risk and Outcome of Psychosis (GROUP) study. The cross-sectional data on ToM and EPP comprised 2415 individuals aged 18 to 55. Specifically, we examined (1) whether possible group differences in EPP and ToM varied by age, (2) age-related differences in the cross-sectional associations between EPP, ToM and socio-demographic, and illness-related factors, and (3) explored associations between age-related patterns of EPP, ToM at baseline, and everyday functioning of the patient group 3 years later. Based on previous cross-sectional and longitudinal work, we expected to find relatively similar ToM and EPP scores by age.
Methods
Study Population
Data on 2415 study participants, including 905 individuals with a non-affective psychotic disorder, 966 unaffected siblings, and 544 never-psychotic controls aged 18–55 were collected in 35 mental health care institutes across the Netherlands and Belgium. The control group was selected through a random system of mailings to addresses in the catchment area and were excluded if they had a (history of a) psychotic disorder, of a first-degree family member with a lifetime psychotic disorder. The GROUP study, a multi-site longitudinal observational study was carried out between April 2004 and December 2013, with the overall goal to identify vulnerability and protective factors that influence the onset and course of psychotic disorders.19 Here we report on the baseline and 3-year follow up assessment. The study was approved by the Medical Ethics Committee of the University Medical Center Utrecht and subsequently by local review boards of each participating institute.
Procedure
Nonsocial and social cognitive tests were administered first to make sure participants were focused and fully concentrated. These tests were followed by the Positive and Negative Syndrome Scale interview and social functioning scale.19
Materials
Demographic information (ie, sex, age, ethnicity, and study site) and information on illness duration were captured by means of a general demographic questionnaire at baseline assessment.
Assessments of Social Cognitive Functioning
All participants completed at least one social cognition measure in the domain of (1) EPP (ie, the ability to infer emotional information from facial expressions), and/or (2) ToM (ie, the ability to comprehend the mental states of others) at baseline assessment:
(1) Emotion Perception and Processing (EPP).
The computerized degraded facial affect recognition (DFAR) task, presented participants with 64 photographs of 4 individuals (2 males/2 females) expressing happy, angry, fearful, and neutral emotions (16 images for each).20 To increase difficulty and enhance the contribution of perceptual expectancies and interpretation, photographs of the faces were passed through a filter resulting in a reduced visual contrast by 30%. The total scores were calculated by the percentage of total correct answers per domain, and across domains (DFAR).
(2) ToM.
The hinting task21 measures the ability to infer real intentions behind indirect speech. Ten short passages read aloud by the interviewer, present an interaction between 2 characters that included dropping a hint. Participants had to indicate what the hint meant. If the first response was inaccurate, a second hint was given, allowing participants to earn partial credit. An example question from the task is: Rebecca’s birthday is approaching. She says to her Dad, “I love animals, specially dogs.” Question 1: What does Rebecca really mean when she says this? Answer: She wants her dad to get her a dog for her birthday. If an incorrect answer given to question 1: Rebecca goes on to say, “Will the pet shop be open on my birthday, Dad?” Question 2: What does Rebecca want her dad to do? Answer: She wants her dad to get her a dog for her birthday. Total scores range from 0 to 20. The Hinting Task has adequate test–retest reliability, small practice effects, and limited potential for floor effects, although ceiling effects have been noted.22,23
Face Recognition
A measure of the ability to match unfamiliar faces, the short version of the Benton Facial Recognition Test24 was used at baseline to assess whether potential deficits in facial affect recognition are accounted for by general facial-recognition ability.
Nonsocial Cognitive Functioning
An abbreviated Wechsler Adult Intelligence Scale III (WAIS-III),25 comprising the information, arithmetic, block design, and digit symbol coding sub-tests, was used to measure performance in the domains of verbal knowledge, working memory, visuospatial processing, and processing speed, respectively. Here we used the IQ estimate at baseline.
Symptom Severity
For patients, clinical psychotic symptoms at baseline were assessed with the Positive And Negative Syndrome Scale,26 consisting of 3 subscales: Positive syndrome scale (item P1–P7), a negative syndrome scale (items N1–N7) and general psychopathology scale (item G1–G16).
Social Functioning
The social functioning scale27 measures broad social functioning in people diagnosed with a psychotic disorder. . Here, we focused specifically on the interpersonal behavior and prosocial activities subscales (interpersonal behavior (eg, number of friends), prosocial activities (eg, sports, visiting friends)). The social functioning scale was assessed at the 3-year follow-up.
Data Analyses
Data release 8.0 were used for the analyses. Group differences in sample characteristics were examined using χ2, t- and Mann–Whitney-U-tests. Raw scores on the social cognitive tests were z transformed against the average scores of the entire study sample (ie, combining patients, siblings, and controls). Thus, β values throughout represent standardized effect sizes, with values 0.2, 0.5, and 0.8 indicating small, medium, and large effect sizes, respectively. These z scores were used in all statistical analyses. To account for multiple comparisons, a P-value of (.05/ 3 (number of groups) ≤) .017 was considered statistically significant.
(1) To examine whether group differences in EPP and ToM varied by age, multilevel linear models (MLM) were fitted to account for the hierarchical structure of the data (ie, with random intercepts for family and study site). We examined age-by-group interactions, group, and age as predictors (main effects), if interactions were nonsignificant. Statistically significant age-by-EPP or ToM interactions were followed up with simple effects analyses. Nonsignificant age-by-group interactions were removed from the models before interpretation of any main effects. Sex was entered as a covariate in all models that investigated between-group effects. Separate models including IQ as additional covariate (for both EPP and ToM analyses) and face recognition ability scores (for EPP analyses) were also conducted.
(2) To explore whether the association between symptom severity (PANSS positive, negative, and general), illness duration, nonsocial cognitive impairment (IQ), and sociodemographic characteristics (sex, ethnicity [white/non-white]), in the patient group, differed by age, the predictors were added as interaction (with age) and main effects to the MLM’s separately. Statistically significant age-by-factor of interest interactions were followed up with simple effects analyses. Nonsignificant interactions were removed from the models before interpretation of any main effects.
(3) To explore whether the association between social cognition and social functioning outcome varied by age similar MLM models were run, this time with the social functioning score at 3-year follow-up as dependent variable, and social cognition at baseline, age, and their interaction as predictors. In sensitivity analyses, we separately explored age-related patterns in social functioning. In this MLM model, age-by-group interactions on social functioning were run without adding the social cognition scores as predictors to the model.
For visualization purposes and ease of interpretation, figures in the main text are presented based on age categorizations (very early adulthood: 18–25; early adulthood: 26–30; mid-adulthood: 31–40; later-adulthood: 41–55). The distribution of age categorizations for each group can be found in supplementary table 1.
Results
Sample characteristics are displayed in Table 1.
Table 1.
Displays the Sociodemographic and Clinical Characteristics of the GROUP Study Sample
| PatientsP (n = 905) |
SiblingsS (n = 966) |
ControlsC (n = 544) |
Statistics** | |
|---|---|---|---|---|
| Female (n, [%]) | 198 (21.88) | 526 (54.45) | 301 (55.33) | P<S,C |
| Age (mean, [sd]) | 27.74 (7.47) | 28.61 (7.77) | 31.26 (10.21) | P<S<C |
| IQ (mean, [sd]) | 94.74 (16.26) | 102.88 (15.60) | 110.11 (15.14) | P<S<C |
| White (n, [%]) | 673 (78.81) | 780 (83.60) | 492 (92.83) | P<S<C |
| Antipsychotics yes (n, %) | 874 (99.5) | |||
| Benton face recognition (mean, [sd]) | 22.75 (2.32) | 23.22 (2.15) | 23.19 (2.02) | P<S,C |
| Social Functioning (mean, [sd])* | 112.43 (9.42) | 122.20 (6.70) | 123.94 (5.63) | P<S<C |
| Interpersonal Score | 124.47 (19.53) | 138.48 (12.69) | 140.74 (10.38) | P<S<C |
| Prosocial activities | 113.1 (13.88) | 120.95 (11.58) | 123.39 (10.12) | P<S<C |
| DFAR (mean, [sd]) | 68.30 (10.75) | 72.47 (9.27) | 73.35 (9.18) | P<S,C |
| DFAR neutral | 77.92 (17.29) | 81.71 (14.81) | 82.41 (14.42) | P<S,C |
| DFAR happy | 86.85 (12.69) | 88.11 (10.63) | 87.45 (11.02) | P<S |
| DFAR fearful | 48.27 (20.24) | 53.04 (19.20) | 54.78 (18.38) | P<S,C |
| DFAR angry | 63.95 (21.02) | 70.34 (19.27) | 72.05 (18.48) | P<S,C |
| Hinting Task (mean, [sd]) | 17.54 (2.78) | 18.87 (1.62) | 19.13 (1.26) | P<S<C |
| PANSS positive (mean, [sd]) | 1.81 (.76) | |||
| PANSS negative (mean, [sd]) | 2.03 (.87) | |||
| PANSS general (mean, [sd]) | 1.75 (.52) | |||
| Age of first psychosis (mean, [sd]) | 23.31 (6.95) | |||
| Illness duration in yrs. (mean, [sd]) | 4.62 (4.50) |
Note: *measured at 3-year follow-up, ** differences significant at .05. DFAR, degraded facial affect recognition; IQ, intelligence quotient; PANSS, Positive and Negative Syndrome Scale; GROUP, Genetic Risk and Outcome of Psychosis.
Group Differences in Social Cognitive Performance
EPP.
DFAR performance (X2(2) = 91.45, P < .001) and performance in subcategories neutral (X2(2) = 25.49, P < .001), angry (X2(2) = 42.62, P < .001), fearful (X2(2) = 49.33, P < .001), and happy (X2(2) = 11.01, P < .01) differed significantly between patients, siblings, and controls. Patients showed medium-sized deficits in the recognition of most emotions in comparison to controls and siblings (DFAR: z = −8.29 and −8.11, P < .001; neutral z = −4.51 and −3.95, P < .001; angry: z = −6.03 and −4.91, P < .001; fearful: z = −6.23 and −5.95, P < .001, respectively), except for the recognition of happy faces, where patients only differed from siblings (z = −3.29, P < .01), but not controls (z = −1.19, P = .23). Siblings performed marginally worse than controls on the total DFAR (z = −2.55, P = .011). Specifically, they were less accurate in recognizing fearful emotions (z = −2.35, P < .01), but performed similar to controls for neutral (z = −1.19, P = .32), angry (z = −2.07, P = .04), and happy emotions (z = 1.7, P = .08).
ToM.
The groups differed significantly on the Hinting Task (X2(2) = 240.85, P < .001). Patients performed worse than controls (z = −13.16, P < .001) and siblings (z = −13.32, P < .001). Siblings performed in between patients (z = −14.64, P < .001) and controls (z = −2.41, P = .016).
See supplementary table 2 for an EPP and ToM score distribution by group.
(1) Age-related patterns for EPP and ToM skills differ by group.
EPP.
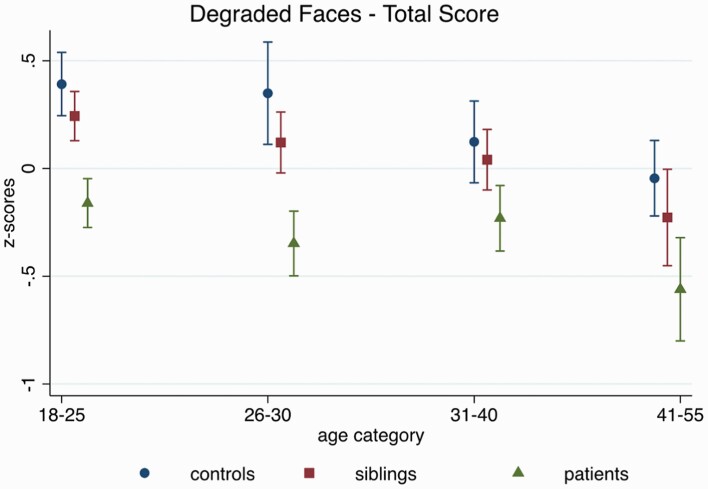
The group-by-age interactions for DFAR, neutral, happy, and angry were not significant (all P >.16), suggesting that age differences in EPP followed a similar pattern in all groups (see figure 1). Across groups, age was associated with the DFAR (β = −0.02, z = −7.60, 95% CI: −0.02, −0.01, P < .001), and with the recognition of happy (β = −0.01, z = −4.05, 95% CI: −0.02, −0.005, P < .001), and angry faces (β = −0.006, z = −2.60, 95% CI: −0.01, −0.002, P < .01), such that older participants performed worse than younger ones. The age effects for DFAR and happy affect were comparable after controlling for IQ (DFAR: β = −0.02, z = −6.92, 95% CI: −0.02, −0.01, P < .001; happy: β = −0.009, z = −3.38, 95% CI: −0.01, −0.004, P = .001), and marginally reduced for the recognition of angry faces (β = −0.005, z = −2.10, 95% CI: −0.01, −0.0004, P = .04).
Fig. 1.
Age-related performance on the degraded facial affect recognition (DFAR) task across groups, adjusting for sex, family ID (in case multiple individuals from one family participated in the study), and study site.
No age effect was observed for the recognition of neutral faces (β = −0.003, z = −1.16, 95% CI: −0.01, 0.002, P = 0.25). For fearful faces a group-by-age interaction was observed (X2(2) = 7.63, P = .02), which remained after controlling for IQ (X2(2) = 8.58, P = .01); age effects were significant in all groups, although effects were stronger in siblings (β = −0.03, z = −7.25, 95% CI: −0.04, −0.02, P < .001) than controls (β = −0.02, z = −3.83, 95% CI: −0.04, −0.001, P <.001) and patients (β = −0.02, z = −3.87, 95% CI: −0.03, −0.01, P < .001).
No age-related differences in face recognition ability, ie, Benton Facial Recognition Test scores were observed (β = −0.003, z = −1.44, 95% CI: −0.009, 0.001, P = .15), and all observed DFAR effects were comparable after controlling for Benton scores. Supplementary figure 1 shows the individual graphs by emotion.
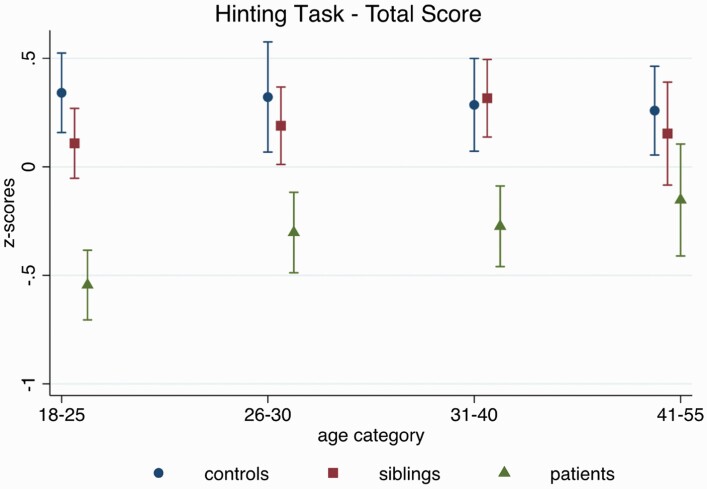
ToM.
There was a significant group-by-age interaction on Hinting Task performance (X2(2) = 13.15, P = .001), where older patients performed better than younger ones (β = 0.02, z = 4.13, 95% CI: 0.01, 0.03, P < .001), while no such age-related pattern emerged for controls (see figure 2; β = −0.003, z = −0.89, 95% CI: −0.01, 0.004, P = .38) or siblings (β = 0.01, z = 1.78, 95% CI: −0.001, 0.01, P = .08). These findings were not accounted for by IQ differences (group-by-age interaction after controlling for IQ: X2(2) = 10.35, P = .006).
Fig. 2.
Age-related performance on the Hinting Task across groups, adjusting for sex, family ID (in case multiple individuals from one family participated in the study), and study site.
(2) The effect of age on the association between illness factors and EPP and ToM performance in patients.
We conducted exploratory analyses to investigate whether the effect of predictors of social cognitive performance differed by age. Sociodemographic and clinical correlates (r) of performance on DFAR and Hinting Task in the patient group are displayed in supplementary figure 2.
Patient Characteristics
There were no age-by-sex interactions for DFAR (β = −0.007, z = −0.66, 95% CI: −0.03, 0.01, P = .51), and Hinting Task performance (β = −0.003, z = −0.28, 95% CI: −0.03, 0.02, P = .78). Removing the interaction from the models, females outperformed males on the DFAR task (DFAR: β = 0.24, z = 2.63, 95% CI: 0.06, 0.41, P = .009).
Similarly, no age-by-ethnicity interactions were found (DFAR: β = 0.02, z = 1.21, 95% CI: −0.001, 0.04, P = .23; Hinting Task: β = 0.01, z = 0.97, 95% CI: −0.01, 0.04, P = .33). Non-white participants did not differ from white participants on the DFAR (β = −0.02, z = −0.27, 95% CI: −0.20, 0.14, P = .79), but had lower scores on the Hinting Task (β = −0.32, z = −3.14, 95% CI: −0.52, −0.26, P = .002).
Finally, there were no age-by-illness duration interactions (DFAR: β = 0.0005, z = −0.64, 95% CI: −0.002, 0.001, P = .53; Hinting: β = 0.0007, z = 0.76, 95% CI: −0.001, 0.002, P = .44). No main effect of illness duration was detected for DFAR or Hinting Task performance (DFAR: β = 0.005, z = 0.55, 95% CI: −0.01, 0.02, P = 0.58; Hinting: β = −0.006, z = −0.63, 95% CI: −0.03, .01, P = 0.53).
Symptom Severity
We observed a marginal age-by-negative symptom interaction for the Hinting Task, suggesting a slightly stronger association between negative symptoms and ToM performance in younger participants (β = 0.01, z = 2.16, 95% CI: 0.001, 0.03, P = .031). There was no interaction for the DFAR (β = 0.004, z = 0.68, 95% CI: −0.007, 0.02, P = .50). Regardless of age, higher negative symptom severity was associated with poorer DFAR performance (DFAR: β = −0.18, z = −4.24, 95% CI: −0.26, −0.10, P = .009).
There were no significant age-by-positive symptom interactions on social cognitive performance (DFAR: β = 0.006, z = 0.88, 95% CI: −0.007, 0.02, P = .38; Hinting: β = 0.005, z = 0.62, 95% CI: −0.01, 0.02, P = .54).
Participants with higher positive symptoms performed worse on DFAR and Hinting task (DFAR: β = −0.13, z = −2.72, 95% CI: −0.23, −0.04, P = .007; Hinting: β = −0.23, z = −4.14, 95% CI: −0.35, −0.12, P < .001).
No age-by-general symptom interactions were observed (DFAR: β = 0.003, z = 0.35, 95% CI: −0.01, 0.02, P = .72; Hinting: β = 0.009, z = 0.93, 95% CI: −0.01, 0.03, P = .35), but general symptoms had a negative association with performance of both social cognitive tests (DFAR: β = −0.21, z = −2.95, 95% CI: −0.35, −0.07, P = .003; Hinting: β = −0.46, z = −5.60, 95% CI: −0.61, −0.30, P < .001).
Nonsocial Cognition
There were no age-by-IQ interactions for DFAR (β < 0.0001, z = −0.05, 95% CI: −0.0006, 0.0005, P = .96) or Hinting Task (β = −0.0002, z = −0.91, 95% CI: −0.0009, 0.0003, P = .36). IQ predicted DFAR (β = 0.01, z = 5.97, 95% CI: 0.009, 0.02, P < .0001) and Hinting Task performance (β = 0.02, z = 10.95, 95% CI: 0.02, 0.03, P < .0001), regardless of age.
(3) EPP and ToM as a predictor of social functioning across ages.
We explored the association between DFAR and Hinting Task performance, age and 2 indices of social functioning 3 years later (see supplementary table 3 for a partial correlation matrix). There was no interaction between DFAR and age at baseline on interpersonal functioning or prosocial activities at follow-up (both P = .15). Regardless of age, DFAR performance was significantly associated with interpersonal functioning (β = 1.91, z = 2.51, 95% CI: 0.42, 3.40, P < .01), but not with prosocial activities (P = .30). There was no interaction between Hinting Task performance and age at baseline on interpersonal functioning or prosocial activities at follow-up (both P = .22). Regardless of age, Hinting Task performance was marginally associated with interpersonal functioning (β = 1.59, z = 2.35, 95% CI: 0.26, 2.91, P = .02), but not with prosocial activities (P = .76).
Sensitivity Analyses: Age-Related Patterns in Social Functioning Among the Patient Group
MLMs exploring age-related differences in social functioning revealed an age effect for prosocial activities. In this model, participation in prosocial activities was significantly lower among older patients (β = −0.23, z = −2.96, 95% CI: −0.39, −0.08, P = .003). No age-related differences were observed for interpersonal functioning (β = −0.19, z = −0.12, 95% CI: −0.41, 0.03, P = .09).
Discussion
This large cross-sectional study provides the first comprehensive analysis of age-related differences in EPP and ToM impairment in individuals with a non-affective psychotic disorder, unaffected siblings, and never-psychotic controls. Group differences in EPP were unrelated to age. Across groups, EPP was worse in older than younger individuals. In contrast, in patients, ToM was better in older than in younger individuals, while ToM was not significantly related to age in never-psychotic controls or siblings. In patients, associations between EPP and ToM and most sociodemographic and clinical factors of interest did not vary with age. Interestingly, however, the association between negative symptoms and ToM appeared somewhat stronger for younger than for older patients. Finally, both ToM and EPP were positively associated with interpersonal functioning, but not with prosocial activities across all ages. Findings from this study add to the literature in several important ways.
First, our results suggest that EPP impairment in patients diagnosed with a non-affective psychotic disorder is stable across ages. Regardless of group, EPP was worse in older individuals, with group differences between patients, unaffected siblings, and never-psychotic controls that were of similar magnitude across the adult lifespan. The observed age-related worsening of EPP is in congruence with studies of normative aging that show declining EPP from mid-adulthood,28 and previous work in samples with schizophrenia.17 Several, non-exclusive, theories have been proposed to explain this decline. From an evolutionary perspective, it might be more important for younger people to accurately recognize others’ emotions to enable learning from others, to promote partner selection, or to avoid risk in social situations.29 This “survival” theory is also supported by neuroimaging research, which showed that viewing negative emotional expressions is associated with less amygdala activation in older than younger adults.30,31 Other work suggests that age-related changes in EPP might be due to changes in the visual scan paths of faces; changes that may be related to normative age-related changes in executive functioning.32 We also cannot rule out that task characteristics contributed to the age-related differences in EPP, as suggested previously,33 for example, bias may arise due to participants being presented with mostly young faces in the DFAR. However, studies that test whether own-age faces are recognized more accurately than other-age faces are limited in number and report inconsistent results.34
Second, our results suggest that differences in ToM between patients, unaffected siblings, and never-psychotic controls are smaller in individuals in their early to mid-50’s, in line with previous work.35 Age-related improvements on the Hinting Task in patients with schizophrenia, but not controls have previously been reported.17 There are various potential explanations. First, the smaller difference may suggest that patients “regain some function” during the later phase of the illness when they might have adjusted to some of the negative consequences, such as negative symptoms. The weaker association between Hinting Task performance and negative symptoms in older individuals supports this idea. In addition, initial ceiling effects in controls and siblings may have prevented us from noticing any further age-related improvement. Ceiling effects on the Hinting Task have been reported previously.36 Studies making use of different ToM tasks could shed light on this. However, possibly patients may be catching up with controls in ToM performance as they age because of gained experience and/or practice with social situations. Alternatively, around the age of 50 controls and siblings (to a lesser degree) may start to show decline that already occurred earlier in patients. Supportive of this hypothesis are studies that show age-related declines in ToM in healthy individuals that are associated with declines in other cognitive functions, such as attention and working memory.28,37 It is also possible that the larger deficits in our younger patient group are due to their earlier onset of the disorder. An early illness onset may disrupt the development of ToM and/or may be related to a distinct neural pathology where disease mechanisms interact with neural development.38 As recently suggested by Armando, Hutsebaut, Debbané39 the arrested development in the specialization of social cognition during adolescence and early adulthood may account for residual social functioning impairment in psychosis. Thus, individuals with an earlier illness onset may follow different cognitive and social functioning trajectories over time. While meta-analyses suggest that age of onset might not relate to the magnitude of ToM difficulties, whereas illness duration does,40 longitudinal studies including large social cognitive test batteries are needed to formally investigate this. If our findings were corroborated by such studies, this would suggest that young adulthood may offer a very good window of opportunity for social cognitive interventions that can promote the development of social cognitive skills41 and that may positively impact on patients' social functioning further down the line.3,42 Vice versa, a potential beneficial downstream effect on social functioning and the additional early application of interventions that address social skills and deficits may foster ToM across the life span.
Finally, associations between EPP and ToM and most sociodemographic and clinical factors of interest did not vary with age and ToM and EPP significantly predicted interpersonal functioning, but not prosocial activities across all ages. These results tentatively suggest that interventions that aim to improve or retain patients' interpersonal functioning are useful across the entire adult lifespan.
Our results need to be interpreted considering several limitations. First, we cannot rule out age-associated effects in EPP or ToM in early life or late adulthood since our youngest and oldest participants were 18 and 55, respectively. For instance, in nonsocial cognition and functional outcome psychosis studies, a second period of decline beyond the age of 65 has been suggested.43,44 Future research is needed to further explore whether cognitive processes show different patterns in individuals with a psychotic disorder from healthy controls after the age of 55.
Second, individuals with better EPP/ToM may have been more likely to participate in this study, particularly in certain age groups. For example, the older age group, which was substantially smaller may have presented individuals with overall better functioning in various domains. In a similar vein, it is possible that young patients with more severe EPP/ToM impairments may have been overrepresented in the study because they were more likely to be pushed to participate by family members or staff, in comparison to older patients.
Third, our findings regarding the 2 social cognitive domains require replication using larger test batteries that cover each domain in greater detail and that tap into other domains as well. Future studies should consider the reliability of tests in older age groups. For example, DFAR faces are all young to mid-adulthood and it may be that participants are particularly skilled in recognizing emotions of similar-aged peers. Relatedly, and regarding social cognition, ceiling effects, cultural differences, and cultural sensitivity of the stimulus and test materials should be considered.
Fourth, it is important to note that some individuals in the control and sibling groups had diagnoses that might have impacted on their social-cognitive functioning. As such the current findings represent a conservative estimate of differences between the included participant groups.
Finally, any age-related differences in EPP and ToM need to be investigated longitudinally; the cross-sectional nature of our study cannot speak to lifetime trajectories of ToM and EPP within-person and may be subject to cohort effects.
To conclude, our findings suggest that, at least without intervention, EPP and ToM impairments are present in individuals with a non-affective psychosis across adulthood. Our results show that EPP and ToM impairment is associated with poorer interpersonal functioning both in younger and older patient groups but suggest that younger individuals might benefit particularly from support with ToM skills, whereas older individuals might be more likely to benefit from support with EPP skills. Longitudinal research is needed to gain much-needed insights into age-related trajectories of social cognition.
Supplementary Material
Acknowledgements
We are grateful for the generosity of time and effort by the patients, their families and healthy subjects. Furthermore, we would like to thank all research personnel involved in the GROUP project, in particular: Joyce van Baaren, Erwin Veermans, Ger Driessen, Truda Driesen, Erna van’t Hag.
Contributor Information
Eva Velthorst, Department of Research, Mental Health Organization “GGZ Noord-Holland-Noord,” Heerhugowaard, The Netherlands.
Adam Socrates, Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Seaver Center for Autism Research and Treatment, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Behrooz Z Alizadeh, Rob Giel Research Center, University of Groningen, University Medical Center Groningen, University Center for Psychiatry, Groningen, The Netherlands; Department of Epidemiology, University Medical Center Groningen, Groningen, The Netherlands.
Therese van Amelsvoort, Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, Maastricht University, Maastricht, The Netherlands.
Agna A Bartels-Velthuis, Rob Giel Research Center, University of Groningen, University Medical Center Groningen, University Center for Psychiatry, Groningen, The Netherlands.
Richard Bruggeman, Rob Giel Research Center, University of Groningen, University Medical Center Groningen, University Center for Psychiatry, Groningen, The Netherlands; Department of Clinical and Developmental Neuropsychology, University of Groningen, Groningen, The Netherlands.
Wiepke Cahn, University Medical Center Utrecht, Department of Psychiatry, Brain Centre Rudolf Magnus, Utrecht University, Utrecht, The Netherlands; Altrecht, General Mental Health Care, Utrecht, The Netherlands.
Lieuwe de Haan, Seaver Center for Autism Research and Treatment, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Arkin, Institute for Mental Health, Amsterdam, The Netherlands.
Frederike Schirmbeck, Seaver Center for Autism Research and Treatment, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Arkin, Institute for Mental Health, Amsterdam, The Netherlands.
Claudia J P Simons, Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, Maastricht University, Maastricht, The Netherlands; GGzE Institute for Mental Health Care, Eindhoven, The Netherlands.
Jim van Os, University Medical Center Utrecht, Department of Psychiatry, Brain Centre Rudolf Magnus, Utrecht University, Utrecht, The Netherlands; Department of Psychosis Studies, Institute of Psychiatry, King’s College London, King’s Health Partners, London, UK.
Anne-Kathrin Fett, Department of Psychology, City, University of London, London, UK; Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Funding
The infrastructure for the GROUP study was funded through the Geestkracht programme of the Dutch Health Research Council (Zon-Mw, grant number 10-000-1001), and matching funds from participating pharmaceutical companies (Lundbeck, AstraZeneca, Eli Lilly, Janssen Cilag) and universities and mental health care organizations (Amsterdam: Academic Psychiatric Centre of the Academic Medical Center and the mental health institutions: GGZ Ingeest, Arkin, Dijk en Duin, GGZ Rivierduinen, Erasmus Medical Centre, GGZ Noord Holland Noord. Groningen: University Medical Center Groningen and the mental health institutions: Lentis, GGZ Friesland, GGZ Drenthe, Dimence, Mediant, GGNet Warnsveld, Yulius Dordrecht, and Parnassia psycho-medical center The Hague. Maastricht: Maastricht University Medical Centre and the mental health institutions: GGzE, GGZ Breburg, GGZ Oost-Brabant, Vincent van Gogh voor Geestelijke Gezondheid, Mondriaan, Virenze riagg, Zuyderland GGZ, MET ggz, Universitair Centrum Sint-Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint-Truiden, PZ Sancta Maria Sint-Truiden, GGZ Overpelt, OPZ Rekem. Utrecht: University Medical Center Utrecht and the mental health institutions Altrecht, GGZ Centraal, and Delta). EV is supported by the Seaver Foundation.
References
- 1. Vaskinn A, Horan WP.. Social cognition and schizophrenia: unresolved issues and new challenges in a maturing field of research. Schizophr Bull. 2020;46(3):464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Green MF, Bearden CE, Cannon TD, et al. Social cognition in schizophrenia, Part 1: performance across phase of illness. Schizophr Bull. 2012;38(4):854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L.. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35(3):573–588. [DOI] [PubMed] [Google Scholar]
- 4. Lee TY, Hong SB, Shin NY, Kwon JS.. Social cognitive functioning in prodromal psychosis: a meta-analysis. Schizophr Res. 2015;164(1–3):28–34. [DOI] [PubMed] [Google Scholar]
- 5. van Donkersgoed RJ, Wunderink L, Nieboer R, Aleman A, Pijnenborg GH.. Social cognition in individuals at ultra-high risk for psychosis: a meta-analysis. PLoS One. 2015;10(10):e0141075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horan WP, Green MF, DeGroot M, et al. Social cognition in schizophrenia, Part 2: 12-month stability and prediction of functional outcome in first-episode patients. Schizophr Bull. 2012;38(4):865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCleery A, Lee J, Fiske AP, et al. Longitudinal stability of social cognition in schizophrenia: a 5-year follow-up of social perception and emotion processing. Schizophr Res. 2016;176(2–3):467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lysaker PH, Olesek KL, Warman DM, et al. Metacognition in schizophrenia: correlates and stability of deficits in theory of mind and self-reflectivity. Psychiatry Res. 2011;190(1):18–22. [DOI] [PubMed] [Google Scholar]
- 9. Hamm JA, Renard SB, Fogley RL, et al. Metacognition and social cognition in schizophrenia: stability and relationship to concurrent and prospective symptom assessments. J Clin Psychol. 2012;68(12):1303–1312. [DOI] [PubMed] [Google Scholar]
- 10. Vohs JL, Lysaker PH, Francis MM, et al. Metacognition, social cognition, and symptoms in patients with first episode and prolonged psychoses. Schizophr Res. 2014;153(1–3):54–59. [DOI] [PubMed] [Google Scholar]
- 11. Sprong M, Schothorst P, Vos E, Hox J, van Engeland H.. Theory of mind in schizophrenia: meta-analysis. Br J Psychiatry. 2007;191:5–13. [DOI] [PubMed] [Google Scholar]
- 12. Moran JM, Jolly E, Mitchell JP.. Social-cognitive deficits in normal aging. J Neurosci. 2012;32(16):5553–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mucci A, Galderisi S, Green MF, et al. ; Italian Network for Research on Psychoses. Familial aggregation of MATRICS Consensus Cognitive Battery scores in a large sample of outpatients with schizophrenia and their unaffected relatives. Psychol Med. 2018;48(8):1359–1366. [DOI] [PubMed] [Google Scholar]
- 14. Lavoie MA, Plana I, Bedard Lacroix J, Godmaire-Duhaime F, Jackson PL, Achim AM.. Social cognition in first-degree relatives of people with schizophrenia: a meta-analysis. Psychiatry Res. 2013;209(2):129–135. [DOI] [PubMed] [Google Scholar]
- 15. Balogh N, Egerhazi A, Berecz R, Csukly G.. Investigating the state-like and trait-like characters of social cognition in schizophrenia: a short term follow-up study. Schizophr Res. 2014;159(2-3):499–505. [DOI] [PubMed] [Google Scholar]
- 16. Ferrer-Quintero M, Green MF, Horan WP, Penn DL, Kern RS, Lee J.. The effect of sex on social cognition and functioning in schizophrenia. npj Schizophr. 2021;7(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pinkham AE, Kelsven S, Kouros C, Harvey PD, Penn DL.. The effect of age, race, and sex on social cognitive performance in individuals with schizophrenia. J Nerv Ment Dis. 2017;205(5):346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Navarra-Ventura G, Vicent-Gil M, Serra-Blasco M, et al. Group and sex differences in social cognition in bipolar disorder, schizophrenia/schizoaffective disorder and healthy people. Compr Psychiatry. 2021;109:152258. [DOI] [PubMed] [Google Scholar]
- 19. Korver N, Quee PJ, Boos HB, Simons CJ, de Haan L, investigators G.. Genetic Risk and Outcome of Psychosis (GROUP), a multi-site longitudinal cohort study focused on gene-environment interaction: objectives, sample characteristics, recruitment and assessment methods. Int J Methods Psychiatr Res. 2012;21(3):205–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van’t Wout M, Aleman A, Kessels RP, Larøi F, Kahn RS.. Emotional processing in a non-clinical psychosis-prone sample. Schizophr Res. 2004;68(2–3):271–281. [DOI] [PubMed] [Google Scholar]
- 21. Corcoran R, Mercer G, Frith CD.. Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophr Res. 1995;17(1):5–13. [DOI] [PubMed] [Google Scholar]
- 22. Ludwig KA, Pinkham AE, Harvey PD, Kelsven S, Penn DL.. Social cognition psychometric evaluation (SCOPE) in people with early psychosis: a preliminary study. Schizophr Res. 2017;190:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fett A-KJ, Maat A, Investigators G.. Social cognitive impairments and psychotic symptoms: what is the nature of their association? Schizophr Bull. 2013;39(1):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benton AL, Van Allen MW (1968). Impairment in facial recognition in patients with cerebral disease. Cortex, 4(4), 344-IN1. [PubMed] [Google Scholar]
- 25. Wechsler D. WAIS-III: Administration and Scoring Manual: The Psychological Corporation. TX: San Antonio. 1997. [Google Scholar]
- 26. Kay SR, Fiszbein A, Opler LA.. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 27. Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S.. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry. 1990;157:853–859. [DOI] [PubMed] [Google Scholar]
- 28. Kemp J, Després O, Sellal F, Dufour A.. Theory of mind in normal ageing and neurodegenerative pathologies. Ageing Res Rev. 2012;11(2):199–219. [DOI] [PubMed] [Google Scholar]
- 29. Frith CD, Frith U.. Mechanisms of social cognition. Annu Rev Psychol. 2012;63(1):287–313. [DOI] [PubMed] [Google Scholar]
- 30. Gunning-Dixon FM, Gur RC, Perkins AC, et al. Age-related differences in brain activation during emotional face processing. Neurobiol Aging. 2003;24(2):285–295. [DOI] [PubMed] [Google Scholar]
- 31. Fischer H, Nyberg L, Bäckman L.. Age-related differences in brain regions supporting successful encoding of emotional faces. Cortex. 2010;46(4):490–497. [DOI] [PubMed] [Google Scholar]
- 32. Circelli KS, Clark US, Cronin-Golomb A.. Visual scanning patterns and executive function in relation to facial emotion recognition in aging. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2013;20(2):148–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sze JA, Goodkind MS, Gyurak A, Levenson RW.. Aging and emotion recognition: not just a losing matter. Psychol Aging. 2012;27(4):940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Short LA, Mondloch CJ.. Aging faces and aging perceivers: young and older adults are less sensitive to deviations from normality in older than in young adult faces. Perception. 2013;42(8):795–812. [DOI] [PubMed] [Google Scholar]
- 35. Smeets-Janssen MMJ, Meesters PD, Comijs HC, et al. Theory of Mind differences in older patients with early-onset and late-onset paranoid schizophrenia. Int J Geriatr Psychiatry. 2013;28(11):1141–1146. [DOI] [PubMed] [Google Scholar]
- 36. Klein HS, Springfield CR, Bass E, et al. Measuring mentalizing: a comparison of scoring methods for the hinting task. Int J Methods Psychiatr Res. 2020;29(2):e1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johansson Nolaker E, Murray K, Happé F, Charlton RA.. Cognitive and affective associations with an ecologically valid test of theory of mind across the lifespan. Neuropsychology. 2018;32(6):754–763. [DOI] [PubMed] [Google Scholar]
- 38. Burke L, Androutsos C, Jogia J, Byrne P, Frangou S.. The Maudsley Early Onset Schizophrenia Study: the effect of age of onset and illness duration on fronto-parietal gray matter. Eur Psychiatry. 2008;23(4):233–236. [DOI] [PubMed] [Google Scholar]
- 39. Armando M, Hutsebaut J, Debbané M.. A mentalization-informed staging approach to clinical high risk for psychosis. Front Psychiatry. 2019;10:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bora E, Yucel M, Pantelis C.. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109(1–3):1–9. [DOI] [PubMed] [Google Scholar]
- 41. Yamada Y, Inagawa T, Sueyoshi K, et al. Social cognition deficits as a target of early intervention for psychoses: a systematic review. Front Psychiatry. 2019;10:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Halverson FT, Orleans-Pobee M, Merritt C, Sheeran P, Fett AK, Penn DL.. Pathways to functional outcomes in schizophrenia spectrum disorders: meta-analysis of social cognitive and neurocognitive predictors. Neurosci Biobehav Rev. 2019;105:212–219. [DOI] [PubMed] [Google Scholar]
- 43. Friedman JI, Harvey PD, Coleman T, et al. Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: a comparison with Alzheimer’s disease and normal aging. Am J Psychiatry. 2001;158(9):1441–1448. [DOI] [PubMed] [Google Scholar]
- 44. Moran JM. Lifespan development: the effects of typical aging on theory of mind. Behav Brain Res. 2013;237:32–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.