Abstract
The pharmacokinetics of tigecycline was evaluated in 46 healthy young and elderly men and women. Except for the volumes of distribution at steady state (approximately 350 liters in women versus 500 liters in men), there were no significant differences in tigecycline pharmacokinetic parameters. Based on pharmacokinetics, no dosage adjustment is warranted based on age or sex.
Tigecycline is a novel intravenously administered glycylcycline antibiotic exhibiting an expanded spectrum of in vitro and in vivo activity against gram-positive, gram-negative, atypical, anaerobic, and other difficult-to-treat pathogens (1-8, 10, 13, 18, 20-23, 25, 26). Clinical studies suggest that tigecycline is generally well tolerated and easy to use with a twice-daily dose regimen (17, 21). The clinical dosing regimen presently being evaluated is 100 mg followed by 50 mg every 12 h (17).
The primary objective of this open-label study was to determine if subject age or sex affects the pharmacokinetic profile of a single 100-mg intravenous dose of tigecycline, and the secondary objective was to compare the levels of observed open-label safety and tolerability of tigecycline among the age and sex groups.
Forty-six healthy men and women from the following three age categories were enrolled: young (18 to 50 years, inclusive), young-elderly (65 to 75 years, inclusive), and elderly (>75 years). Subjects were in good health on the basis of medical histories, physical examinations, electrocardiograms, and laboratory evaluations. The Institutional Review Board of The Methodist Hospital in Philadelphia, Pennsylvania approved the study, and all subjects gave written informed consent before enrollment. The demographic profile of each age-sex group is presented in Table 1.
TABLE 1.
Subject demographics
| Parameter | Patient characteristic by age (in yrs) and sex
|
|||||
|---|---|---|---|---|---|---|
| 18-50
|
65-75
|
>75
|
||||
| Women (n = 9) | Men (n = 9) | Women (n = 7) | Men (n = 8) | Women (n = 5) | Men (n = 8) | |
| Age (years) (mean ± SD) | 39.9 ± 6.6 | 32.3 ± 7.1 | 67.9 ± 2.0 | 68.4 ± 3.3 | 78.0 ± 3.7 | 77.5 ± 1.3 |
| Weight (kg) (mean ± SD) | 65.0 ± 9.7 | 81.0 ± 10.5 | 61.5 ± 9.1 | 85.8 ± 13.5 | 68.9 ± 9.5 | 74.4 ± 11.6 |
| Creatinine clearance (ml/min/1.73 m2) (mean ± SD) | 89 ± 18 | 100 ± 14 | 70 ± 22 | 79 ± 25 | 59 ± 6 | 61 ± 15 |
| Ethnic origin n (%) | ||||||
| Black | 5 (56) | 6 (67) | 1 (14) | 0 | 2 (40) | 0 |
| Hispanic | 0 | 2 (22) | 0 | 0 | 0 | 0 |
| White | 4 (44) | 1 (11) | 6 (86) | 8 (100) | 3 (60) | 8 (100) |
Tigecycline (100 mg) was administered as a single intravenous dose infused over 60 min. Serial blood samples for the determination of tigecycline concentrations in serum were collected over 120 h after the start of tigecycline infusion, and complete urine output was collected over 48 h after the start of the tigecycline infusion. Tigecycline concentrations in serum and urine were quantified by using validated analytical methods that are similar to those described previously (17).
Pharmacokinetic parameters based on serum data for tigecycline were estimated with standard noncompartmental methods (11) using a validated SAS (version 8.02) program. Pharmacokinetic parameters were compared among the age-sex groups by using a two-factor analysis of variance with factors for age, sex, and age-by-sex interaction.
Based on the intersubject variability observed in previous studies (17), it was estimated that having at least 20 subjects per sex and at least 17 in the younger cohort compared with the pooled elderly cohorts would provide a statistical power of at least 80% to detect a 30% difference for both the maximum concentrations of the drug in serum and the areas under the concentration-time curve (AUCs) between sexes or age groups.
Safety was evaluated from spontaneously reported signs and symptoms and from the results of physical examinations, including weight and height, vital sign measurements, 12-lead electrocardiograms, clinical laboratory evaluations (hematology and blood chemistry), and routine urinalyses. Adverse events were recorded throughout the study.
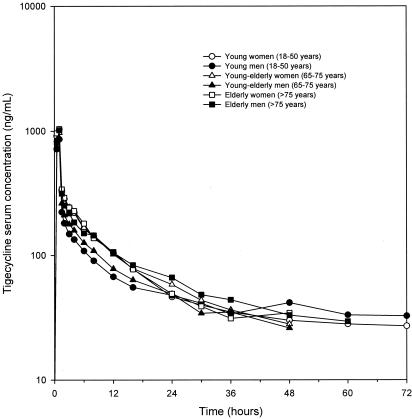
For the first 12 h after administration, tigecycline concentrations in serum were typically higher in women than in men for all age groups (Fig. 1). With the exception of the volumes of distribution at steady state (Vss), there were no other statistically significant differences in pharmacokinetic parameters among the age and sex groups (Table 2). Tigecycline Vss were large and approximated 350 liters (or 5.8 liters/kg of body weight) for women and 500 liters (or 6.2 liters/kg) for men. The difference in non-weight-normalized Vss between men and women attained statistical significance (P = 0.001), but the weight-normalized Vss did not differ significantly (P = 0.40), indicating that the observed differences were primarily caused by differences in body size between the men and women in this study.
FIG. 1.
Mean tigecycline concentrations in serum of subjects by age and sex group.
TABLE 2.
Pharmacokinetic parameters for tigecycline in young and elderly men and women
| Patients by age (yrs) and sex or P valuea | Pharmacokinetic parameter (mean ± SD)b or P value
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cmax (ng/ml) | t1/2 (h) | AUC (ng · h/ml) | CL (liters/h) | CL (liters/h/kg) | Vss (liters) | Vss (liters/kg) | Clr (liters/h) | Ae,0-48h (% dose) | |
| 18-50 | |||||||||
| Women | 1,033 ± 158 | 17.1 ± 8.4 | 5,112 ± 1,312 | 20.6 ± 4.8 | 0.31 ± 0.06 | 355 ± 95 | 5.6 ± 2.1 | 2.6 ± 1.0 | 9.5 ± 3.7 |
| Men | 861 ± 154 | 22.3 ± 15.3 | 4,218 ± 2,033 | 28.5 ± 11.8 | 0.34 ± 0.11 | 554 ± 158 | 7.1 ± 2.6 | 2.9 ± 1.1 | 8.3 ± 1.7 |
| 65-75 | |||||||||
| Women | 993 ± 269 | 16.5 ± 4.1 | 5,120 ± 1,156 | 20.4 ± 4.7 | 0.34 ± 0.10 | 367 ± 96 | 6.1 ± 2.1 | 2.2 ± 0.7 | 9.2 ± 3.6 |
| Men | 900 ± 174 | 19.5 ± 3.1 | 4,317 ± 689 | 23.8 ± 4.3 | 0.28 ± 0.03 | 499 ± 78 | 5.9 ± 1.2 | 2.7 ± 1.2 | 8.8 ± 2.0 |
| >75 | |||||||||
| Women | 1,088 ± 147 | 21.2 ± 12.5 | 5,273 ± 1,105 | 19.6 ± 3.6 | 0.29 ± 0.05 | 377 ± 123 | 5.6 ± 1.9 | 2.2 ± 0.3 | 9.6 ± 0.8 |
| Men | 1,017 ± 112 | 19.0 ± 5.0 | 5,472 ± 901 | 18.7 ± 3.0 | 0.26 ± 0.06 | 401 ± 58 | 5.5 ± 1.2 | 2.6 ± 0.6 | 11.3 ± 3.0 |
| Variable P value | |||||||||
| Age | 0.15 | 0.80 | 0.18 | 0.18 | 0.19 | 0.38 | 0.67 | 0.57 | 0.24 |
| Sex | 0.05 | 0.42 | 0.14 | 0.14 | 0.31 | 0.001 | 0.40 | 0.17 | 0.82 |
| Age by sex | 0.61 | 0.79 | 0.35 | 0.35 | 0.41 | 0.16 | 0.51 | 0.99 | 0.69 |
P values for indicated variables were determined by a two-way analysis of variance of log-transformed data.
Cmax, maximum concentration of drug in serum; t1/2, half-life; CL, clearance; CLR, renal clearance; Ae,0-48h, amount of tigecycline excreted in urine over 48h.
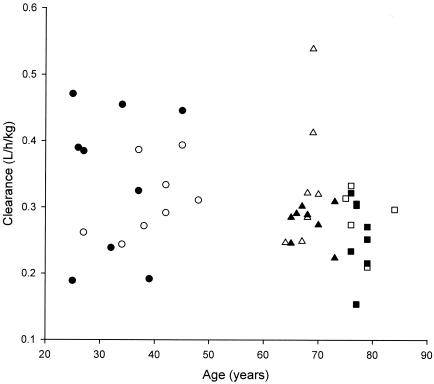
Tigecycline weight-normalized clearance was approximately 10% higher in young men than in young women (Table 2 and Fig. 2). The greatest between-sex difference for this parameter was the clearance in young-elderly women being 22% higher than that in young-elderly men; weight-normalized clearance among elderly women was 11% higher than that of their elderly male counterparts. None of these differences in weight-normalized clearance achieved statistical significance (Table 2).
FIG. 2.
Weight-normalized tigecycline clearances for individual subjects by age and sex group. ○, young women (18 to 50 years); •, young men (18 to 50 years); ▵, young-elderly women (65 to 75 years); ▴, young-elderly men (65 to 75 years); □, elderly women (>75 years); ▪, elderly men (>75 years).
Mean tigecycline AUCs ranged from 4,218 ng · h/ml for the young men to 5,472 ng · h/ml for the elderly men. Mean AUC values for each of the three female age groups were approximately 5.1 to 5.3 μg · h/ml, compared with 4.2 to 5.5 μg · h/ml for men. In addition, tigecycline AUC values were approximately 21% higher for young women than for young men, whereas AUC values were only 4% higher for elderly women than for elderly men, which is consistent with the differences in clearances observed between groups.
One subject, a 46-year-old woman, withdrew from the study because of a rash that developed during the tigecycline infusion. This subject's vital sign data showed no evidence of a systemic allergic response, and the rash resolved in 12 h after the administration of diphenhydramine.
No serious adverse effects occurred during this study. Overall, tigecycline was reasonably well tolerated. Nine (36%) men and 16 (76%) women experienced one or more treatment-emergent adverse events (TEAEs). Most TEAEs were mild; two subjects reported nausea of moderate severity. The most common TEAE was nausea, which was more common in women (48%) than in men (24%). Only 1 (8%) of 13 subjects over 75 years of age reported nausea, compared with 4 of 15 (27%) subjects in the young-elderly group and 11 of 18 (61%) in the young age group. No clinically important changes in laboratory values or vital signs were observed during this study.
The aging process is known to affect the disposition of drugs by altering both body composition and organ function and, thus, may influence the pharmacokinetics of some drugs (9, 12, 14, 15, 19); these effects have been reviewed recently with respect to age (16) and sex (24). Overall, the results of this study demonstrate that the pharmacokinetic parameters of tigecycline do not differ significantly between sexes of the same age group or across age groups. Therefore, based upon pharmacokinetics, no dosage adjustment is necessary based on a patient's age or sex.
Acknowledgments
This clinical study was supported by Wyeth Research, Collegeville, Pa.
We thank Scott A. Saunders for professional medical writing services in preparation of the manuscript.
REFERENCES
- 1.Betriu, C., E. Culebras, I. Rodriguez-Avial, M. Gomez, B. A. Sanchez, and J. J. Picazo. 2004. In vitro activities of tigecycline against erythromycin-resistant Streptococcus pyogenes and Streptococcus agalactiae: mechanisms of macrolide and tetracycline resistance. Antimicrob. Agents Chemother. 48:323-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betriu, C., I. Rodriguez-Avial, B. A. Sanchez, M. Gomez, J. Alvarez, J. J. Picazo, and the Spanish Group of Tigecycline. 2002. In vitro activities of tigecycline (GAR-936) against recently isolated clinical bacteria in Spain. Antimicrob. Agents Chemother. 46:892-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biedenbach, D. J., M. L. Beach, and R. N. Jones. 2001. In vitro antimicrobial activity of GAR-936 tested against antibiotic-resistant gram-positive blood stream infection isolates and strains producing extended-spectrum beta-lactamases. Diagn. Microbiol. Infect. Dis. 40:173-177. [DOI] [PubMed] [Google Scholar]
- 4.Cazzola, M., F. Blasi, S. Centanni, C. F. Donner, and L. Allegra. 2001. Advances in the research and development of chemotherapeutic agents for respiratory tract bacterial infections. Pulm. Pharmacol. Ther. 14:367-381. [DOI] [PubMed] [Google Scholar]
- 5.Chopra, I. 2002. New developments in tetracycline antibiotics: glycylcyclines and tetracycline efflux pump inhibitors. Drug Resist. Updates 5:119-125. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande, L. M., A. C. Gales, and R. N. Jones. 2001. GAR-936 (9-t-butylglycylamido-minocycline) susceptibility test development for streptococci, Haemophilus influenzae and Neisseria gonorrhoeae: preliminary guidelines and interpretive criteria. Int. J. Antimicrob. Agents 18:29-35. [DOI] [PubMed] [Google Scholar]
- 7.Edelstein, P. H., W. J. Weiss, and M. A. Edelstein. 2003. Activities of tigecycline (GAR-936) against Legionella pneumophila in vitro and in guinea pigs with L. pneumophila pneumonia. Antimicrob. Agents Chemother. 47:533-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edlund, C., and C. E. Nord. 2000. In-vitro susceptibility of anaerobic bacteria to GAR-936, a new glycylcycline. Clin. Microbiol. Infect. 6:159-163. [DOI] [PubMed] [Google Scholar]
- 9.Hockings, N., A. A. Ajayi, and J. L. Reid. 1986. Age and the pharmacokinetics of angiotensin converting enzyme inhibitors enalapril and enalaprilat. Br. J. Clin. Pharmacol. 21:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones, R. N. 1999. Disk diffusion susceptibility test development for the new glycylcycline, GAR-936. Diagn. Microbiol. Infect. Dis. 35:249-252. [DOI] [PubMed] [Google Scholar]
- 11.Jusko, W. J. 1992. Guidelines for collection and analysis of pharmacokinetic data, p. 2-1-2-43. In W. E. Evans, J. J. Schentag, and W. J. Jusko (ed.), Applied pharmacokinetics: principles of therapeutic drug monitoring, 3rd ed. Applied Therapeutics, Vancouver, Wash.
- 12.LaCreta, F. P., G. D. Kollia, G. Duncan, D. Behr, and D. M. Grasela. 2000. Age and gender effects on the pharmacokinetics of gatifloxacin. Pharmacotherapy 20:67S-75S. [DOI] [PubMed] [Google Scholar]
- 13.Lefort, A., M. Lafaurie, L. Massias, Y. Petegnief, A. Saleh-Mghir, C. Muller-Serieys, D. Le Guludec, and B. Fantin. 2003. Activity and diffusion of tigecycline (GAR-936) in experimental enterococcal endocarditis. Antimicrob. Agents Chemother. 47:216-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyers, B. R., and P. Wilkinson. 1989. Clinical pharmacokinetics of antibacterial drugs in the elderly. Implications for selection and dosage. Clin. Pharmacokinet. 17:385-395. [DOI] [PubMed] [Google Scholar]
- 15.Muck, W., H. P. Breuel, and J. Kuhlmann. 1996. The influence of age on the pharmacokinetics of nimodipine. Int. J. Clin. Pharmacol. Ther. 34:293-298. [PubMed] [Google Scholar]
- 16.Muhlberg, W., and D. Platt. 1999. Age-dependent changes of the kidneys: pharmacological implications. Gerontology 45:243-253. [DOI] [PubMed] [Google Scholar]
- 17.Muralidharan, G., M. Micalizzi, J. Speth, D. Raible, and S. Troy. 2005. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob. Agents Chemother. 49:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nannini, E. C., S. R. Pai, K. V. Singh, and B. E. Murray. 2003. Activity of tigecycline (GAR-936), a novel glycylcycline, against enterococci in the mouse peritonitis model. Antimicrob. Agents Chemother. 47:529-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norrby, S. R., and B. Ljungberg. 1989. Pharmacokinetics of fluorinated 4-quinolones in the aged. Rev. Infect. Dis. 11:S1102-S1106. [DOI] [PubMed] [Google Scholar]
- 20.Patel, R., M. S. Rouse, K. E. Piper, and J. M. Steckelberg. 2000. In vitro activity of GAR-936 against vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus and penicillin-resistant Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 38:177-179. [DOI] [PubMed] [Google Scholar]
- 21.Postier, R. G., S. L. Green, S. R. Klein, E. J. Ellis-Grosse, and E. Loh. 2004. Results of a multicenter, randomized, open-label efficacy and safety study of two doses of tigecycline for complicated skin and skin-structure infections in hospitalized patients. Clin. Ther. 26:704-714. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen, B. A., Y. Gluzman, and F. P. Tally. 1994. Inhibition of protein synthesis occurring on tetracycline-resistant, TetM-protected ribosomes by a novel class of tetracyclines, the glycylcyclines. Antimicrob. Agents Chemother. 38:1658-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnappinger, D., and W. Hillen. 1996. Tetracyclines: antibiotic action, uptake, and resistance mechanisms. Arch. Microbiol. 165:359-369. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz, J. B. 2003. The influence of sex on pharmacokinetics. Clin. Pharmacokinet. 42:107-121. [DOI] [PubMed] [Google Scholar]
- 25.Someya, Y., A. Yamaguchi, and T. Sawai. 1995. A novel glycylcycline, 9-(N,N-dimethylglycylamido)-6-demethyl-6-deoxytetracycline, is neither transported nor recognized by the transposon Tn10-encoded metal-tetracycline/H+ antiporter. Antimicrob. Agents Chemother. 39:247-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Ogtrop, M. L., D. Andes, T. J. Stamstad, B. Conklin, W. J. Weiss, W. A. Craig, and O. Vesga. 2000. In vivo pharmacodynamic activities of two glycylcyclines (GAR-936 and WAY 152,288) against various gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 44:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]