Abstract
Flocculosin, a glycolipid isolated from the yeast-like fungus Pseudozyma flocculosa, was investigated for in vitro antifungal activity. The compound displayed antifungal properties against several pathogenic yeasts. Synergistic activity was observed between flocculosin and amphotericin B, and no significant cytotoxicity was demonstrated when tested against human cell lines.
Although progress has been made in antifungal therapy, amphotericin B (AMB) and triazoles are still the most commonly used drugs for this purpose (9). However, their use is limited because of toxicity and resistance (4, 5). Recently, the Food and Drug Administration approved caspofungin, a new promising agent with excellent antifungal activity and low toxicity. However, it has a low oral bioavailability and is only available in an intravenous formulation (1). Thus, there is a need for the isolation (or synthesis) of new drugs with different modes of action and low toxicity (3, 7).
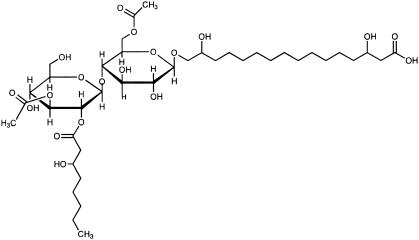
Flocculosin (Fig. 1) is a novel low-molecular-weight glycolipid isolated from the yeast-like fungus Pseudozyma flocculosa (Traquair, Shaw and Jarvis) Boekhout and Traquair. This molecule is known to be one of the active components of Sporodex, a biological control agent of powdery mildew fungi (2, 6). Screening against plant fungal pathogens has already confirmed the broad spectrum of flocculosin (2). In an attempt to determine if flocculosin has potential against fungi commonly associated with human mycoses, the objective of this study was to evaluate its antifungal activity under different conditions and against yeast strains with various characteristics.
FIG. 1.
Chemical structure of flocculosin.
Flocculosin was extracted from liquid culture of P. flocculosa as described previously (2), and a stock solution was prepared in methanol. Antifungal activity was determined against a panel of pathogenic yeasts, including Candida albicans, Candida glabatra, Candida lusitaniae, Saccharomyces cerevisiae, and Trichosporon asahii, or reference strains, including C. albicans (ATCC 90028, ATCC 18804, and ATCC 66027), C. krusei (ATCC 6258), C. parapsilosis (ATCC 22019), and Cryptococcus neoformans (ATCC 90112). All strains were obtained from the Laboratoire de Microbiologie de l'Hôtel-Dieu de Québec du Centre Hospitalier Universitaire de Québec, Québec, Canada.
MICs were determined by the NCCLS broth microdilution M27-A2 reference method (8). Modifications were made to test the influence of pH (from 3 to 9) and temperature (25 and 37°C). Drug interactions were assessed by the same method, adapted in a checkerboard fashion. Interactions of flocculosin with AMB (Bristol-Myers Squibb Co., St-Laurent, Québec, Canada) and fluconazole (FLC) (Pfizer, Montréal, Québec, Canada) were evaluated. The MIC was defined as the lowest concentration at which growth inhibition was 100% for flocculosin and AMB and 80% for FLC. The interactions were determined by the fractional inhibitory concentration (FIC) index which is defined as follows:
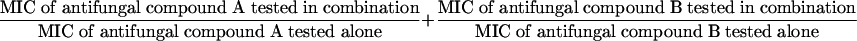 |
The interaction was defined as synergistic if the FIC index was ≤0.5, indifferent if >0.5 but ≤4, and antagonistic if >4. Flocculosin activity was found to be greater under acidic conditions. All strains were inhibited at 50 μg or less of flocculosin per ml at pH 5.0. However, low antifungal activity was observed at pH 7.0. The MICs were 500 μg/ml for C. lusitaniae, C. neoformans, T. asahii, and the four C. albicans isolates and above 500 μg/ml for all other yeasts tested. Temperature (25 or 37°C) had little effect on the activity of flocculosin (Table 1).
TABLE 1.
Influence of temperature and pH on activity of flocculosin against C. albicans ATCC 18804
| pH | MIC (μg/ml) at tempa:
|
|
|---|---|---|
| 25°C | 37°C | |
| 3.0 | 10 | 20 |
| 4.0 | 10 | 20 |
| 5.0 | 17 | 30 |
| 6.0 | 40 | 50 |
| 7.0 | >200b | >200b |
| 8.0 | >200b | >200b |
| 9.0 | >200b | >200b |
Values are averages of triplicate measurements.
No complete growth inhibition was detected at concentrations of 200 μg/ml.
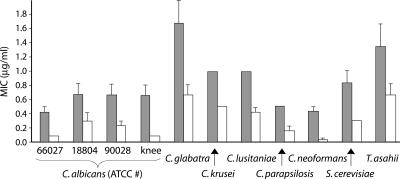
At pH 7.0, interaction between flocculosin and AMB was observed with as little as 0.005 μg of flocculosin per ml, and AMB MICs were lowered for all strains tested when 0.5 μg or more per ml was added. The most relevant results were the effect of the addition of 5 μg of flocculosin per ml to AMB (Fig. 2). The FIC index was between 0.1 and 0.5 for all the strains tested, indicating synergistic interactions. Of particular importance, the addition of flocculosin significantly reduced AMB MICs for C. glabatra and C. lusitaniae, two species considered resistant to AMB. This result is all the more surprising considering that flocculosin had very low activity at pH 7.0 and this concentration is much lower than the flocculosin MIC under optimal acidic conditions. This would indicate that the interaction between the two compounds is not only the sum of two distinct actions on the membrane but also a cooperative effect leading to cell death. It is possible that this small amount of flocculosin is sufficient to disturb the cell membrane surface and facilitate AMB binding to ergosterol. No interaction was observed between flocculosin and FLC.
FIG. 2.
Influence of the addition of 5 μg of flocculosin per ml on the activity of amphotericin B against yeasts of medical importance at pH 7.0. Shaded columns are MICs for AMB used alone, and white columns represent MICs for AMB after the addition of 5 μg of flocculosin per ml. Vertical bars show the standard error of the mean based on three independent measurements.
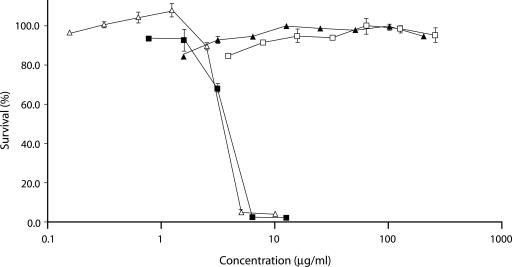
AMB, FLC, flocculosin, and a mixture of 5 μg of flocculosin per ml with AMB were evaluated for their cytotoxicities against six different human cancer cell lines: T24 (ATCC, HTB-4, urinary bladder), Rupp2 (kidney), Lovo (ATCC, CCL-229, colon), HepG2 (ATCC, HB-8065, liver), HACAT (skin), and CHODOFF (kidney). These cell lines were obtained from René C. Gaudreault at the Centre de Recherche de l'Hôpital Saint-François D'Assise, Québec, Canada. The sulforhodamine B colorimetric method was used in microtiter plates to evaluate cytotoxicity (10). Flocculosin did not affect the growth of the six cancer cell lines tested at concentrations up to 250 μg/ml. As a positive control, AMB was toxic at all concentrations greater than 1.5 μg/ml, with a 50% inhibitory concentration of around 4.0 μg/ml. FLC did not show any toxicity at concentrations up to 200 μg/ml. The addition of 5 μg of flocculosin per ml to AMB did not significantly modify AMB toxicity curves. The cytotoxic effect of each drug on the Rupp2 cell line is shown in Fig. 3 as an example. The fact that flocculosin activity is synergistic with AMB but does not increase its toxicity is another positive outcome. This supports the potential use of these molecules together to reduce toxicity by lowering the AMB dosage while increase efficacy over AMB alone.
FIG. 3.
Cytotoxicities of different antifungal compounds on the Rupp2 (human kidney) cell line. Antifungal compounds are AMB (▪), FLC (▴), flocculosin (□), and a mixture of AMB and 5 μg of flocculosin per ml (▵). Vertical bars show the standard error of the mean based on four independent measurements.
The hemolytic activity of flocculosin and AMB was tested against defibrinated sheep erythrocytes (Quélab, Montréal, Québec, Canada). A similar volume of erythrocytes (108 cells per ml) and twofold drug solutions in phosphate-buffered saline were mixed together and incubated at 37°C for 1 h. Phosphate-buffered saline was used as a nonhemolytic, negative control blank, and distilled water was used as a hemolytic, 100% lysis control. After incubation, cells were centrifuged at 800 × g for 10 min to remove nonhemolyzed erythrocytes. A550 was determined, and the percentage of hemolysis was calculated as follows: [(ODexp − ODblank)/(OD100% − ODblank)] × 100. ODexp is the optical density of the experimental sample. After absorbance measurements, cells were resuspended by vortexing and a sample was observed on a Olympus BX-40 optical microscope at ×1,000 for membrane integrity (11, 13). Flocculosin did not cause hemolysis of sheep erythrocytes even at the highest dose tested (500 μg/ml). On the other hand, AMB caused hemolysis of sheep erythrocytes at a concentration of 8 μg/ml. Microscopic observations showed the presence of echinocytes at a high flocculosin concentration (>250 μg/ml), indicating a slight interaction between flocculosin and erythrocyte membranes. This phenomenon is often associated with a high concentration of amphipathic molecule and is reversible (12).
This is the first report of antifungal activity against agents of mycoses in humans with flocculosin, a novel glycolipid isolated from P. flocculosa. Flocculosin demonstrated promising activity against all strains tested under acidic conditions and significantly lowered the AMB MIC under standard conditions. This synergistic activity could increase the efficacy of AMB while lowering its toxicity and side effects. Since flocculosin was only tested in its native form, combinatorial chemistry may provide means to retrieve its activity at pH 7.0 and reduce its MIC.
Acknowledgments
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Biocontrol Network, and the Canada Research Chairs program to R. R. Bélanger.
We thank Sébastien Bolduc for technical assistance with cytotoxicity assays.
REFERENCES
- 1.Boucher, H. W., A. H. Groll, C. C. Chiou, and T. J. Walsh. 2004. Newer systemic antifungal agents. Drugs 64:1997-2020. [DOI] [PubMed] [Google Scholar]
- 2.Cheng, Y., D. J. McNally, C. Labbé, N. Voyer, F. Belzile, and R. R. Bélanger. 2003. Insertional mutagenesis of a fungal biocontrol agent led to discovery of a rare cellobiose lipid with antifungal activity. Appl. Environ. Microbiol. 69:2595-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Pauw, B. E. 2000. New antifungal agents and preparation. Int. J. Antimicrob. Agents 16:147-150. [DOI] [PubMed] [Google Scholar]
- 4.Deray, G. 2002. Amphotericin B nephrotoxicity. J. Antimicrob. Chemother. 49(Suppl. 1):37-41. [DOI] [PubMed] [Google Scholar]
- 5.Georgopapadakou, N. K., and T. J. Walsh. 1996. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob. Agents Chemother. 40:279-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajlaoui, M. R., J. A. Traquair, W. R. Jarvis, and R. R. Bélanger. 1994. Antifungal activity of extracellular metabolites produced by Sporothrix flocculosa. Biocontrol Sci. Technol. 4:229-237. [Google Scholar]
- 7.Masia Canuto, M., and F. Gutierrez Rodero. 2002. Antifungal drug resistance to azoles and polyenes. Lancet Infect. Dis. 2:550-563. [DOI] [PubMed] [Google Scholar]
- 8.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 2nd ed. NCCLS document M27-A2. NCCLS, Wayne, Pa.
- 9.Sheehan, D. J., C. A. Hitchcock, and C. M. Sibley. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skehan, P., R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney, and M. R. Boyd. 1990. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 82:1107-1112. [DOI] [PubMed] [Google Scholar]
- 11.Sorensen, K. N., K.-H. Kim, and J. Y. Takemoto. 1996. In vitro antifungal and fungicidal activities and erythrocyte toxicities of cyclic lipodepsinonapeptides produced by Pseudomonas syringae pv. syringae. Antimicrob. Agents Chemother. 40:2710-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yawata, Y. 2003. Cell membrane: the red blood cell as a model, p. 251-260. Wiley-VCH Verlag GmbH, Weinheim, Germany.
- 13.Zarif, L., J. R. Graybill, D. Perlin, L. Najvar, R. Bocanegra, and R. J. Mannino. 2000. Antifungal activity of amphotericin B cochleates against Candida albicans infection in a mouse model. Antimicrob. Agents Chemother. 44:1463-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]