Abstract
We have reported previously that prolonged caspofungin (CAS) dosing enhances survival in a murine model of central nervous system aspergillosis. In this study we determined by quantitative PCR (qPCR) and CFU enumeration whether CAS could reduce fungal burdens, prior to the deaths of untreated animals, and also assessed progressive infection in untreated mice. Mice were infected intracranially and treated for 4 days with CAS (1, 5, or 10 mg/kg of body weight/day) or amphotericin B (AMB) (3 mg/kg/day) starting 1 day postinfection. Fungal burdens in brains and kidneys of untreated controls were determined on days 1, 3, and 5 to assess progressive infection; burdens in treated animals were determined on day 5. qPCR showed higher burdens than CFU enumeration in all comparisons. In untreated animals, qPCR showed transiently increased burdens in brains, while CFU enumeration showed a decrease. qPCR showed increased burdens in kidneys, but CFU enumeration did not. Neither method indicated drug efficacy in the brain. Both methods showed AMB efficacy in the kidneys, and qPCR demonstrated CAS efficacy at all doses. Spearman correlations of qPCR and CFU determination results showed a significant correlation for most untreated groups; results correlated well for kidneys (P ≤ 0.03) but not for brains in treated mice. Regression analyses of qPCR and CFU groups indicated different slopes for progressive infection in untreated animals but the same slopes for CAS dose-response efficacy. qPCR appeared to better reflect the progression of untreated infection. The lack of demonstration of efficacy in the brain suggests that longer dosing is necessary to cause burden reduction. These results also suggest that, when there is drug efficacy in a therapeutic study, either method appears to be useful for determining Aspergillus fumigatus burdens.
Caspofungin (CAS) has proven effective against aspergillosis in animal models of invasive disease (1-3, 14, 20, 22), prolonging survival as well as reducing fungal burdens in kidneys and lungs after dosing. Because central nervous system (CNS) aspergillosis is a frequent and often dire manifestation of disease, we have examined the efficacy of CAS in our murine model of this disease. In an initial study, we found that 10 days of CAS therapy was effective against CNS infection in mice, prolonging survival and reducing fungal burden as assessed by CFU (G. Singh, J. Imai, K. V. Clemons, and D. A. Stevens, Abstr. XV Congr. Int. Soc. Hum. Anim. Mycol., abstr. 144, 2003). However, reduction of fungal burden by CFU did not appear to be dose responsive and could not be compared directly to that from untreated animals because of deaths in the control group, nor did CAS effect cure in both the brains and kidneys. Although prolongation of survival is one standard endpoint of efficacy, it is important to assess the capacity of an antifungal agent to lower tissue burdens and to effect cure.
Although enumerating CFU in tissue homogenates is the customary approach in quantifying fungal burdens, some investigators question whether numbers of CFU are an accurate measure of infectious burden with a mycelial organism, such as Aspergillus fumigatus (3). When organs are homogenized, multiple fungal cells and interwoven hyphae may not be disarticulated (thus, multiple viable cells may yield only 1 CFU), or cells may be disrupted when the hyphae are broken (thus, a viable cell may yield no CFU). The concern then is that conventional homogenization techniques fail to effectively disperse the viable cells composing hyphal extensions, with CFU generated by large hyphal masses grossly underrepresenting the viable burden of a filamentous fungus in tissue and subsequently causing any drug efficacy to be misinterpreted or missed entirely (3).
At issue is the assessment of drugs that merely inhibit hyphal growth and the potential that drug efficacy and dose responsiveness are not apparent if reduction in number of CFU is used as the gauge, the thought being that apically inhibited organisms will generate the same number of CFU as untreated organisms. Our laboratory and others have used CFU of experimental Aspergillus-infected tissue as a correlate of progressive infection and therapeutic failure, e.g., various doses of a partially effective antimicrobial produce a continuum of residual CFU in a dose-responsive configuration (5-7, 9, 12, 13, 16, 21). We have noted that CFU in these settings may not show a linear correlation, but rather a blunted one, with drug efficacy (at the upper end of the CFU range, CFU quantified may be lower than expected).
Several techniques have been used in an attempt to quantify fungal burdens of mycelial organisms more accurately, including a quantitative PCR (qPCR) (TaqMan) assay and chitin assay (3, 17). The qPCR-based assay for A. fumigatus is a real-time technique that measures DNA by employing a fluorescent reporter and quencher complexed to a probe that is complementary to a sequence from the 18S rRNA gene of A. fumigatus. A standard curve relates fluorescence to conidial equivalents (CE) (i.e., one nucleus), a unit derived from tissues spiked with known numbers of conidia, and CE is used to quantify fungal burdens in tissues from the resulting fluorescent signal (3).
In the present study we sought to determine whether CAS reduced the burden of A. fumigatus in the brains and kidneys of infected mice at a time when few untreated control animals had succumbed to infection in order to directly compare the fungal burdens in the tissues and determine whether CAS caused any reduction in burden. Because the utility of CFU enumeration has been called into question, and the qPCR method has been put forward as a better assay, we used both methods on each tissue sample homogenate to assay fungal burden and thus determine CAS efficacy. Furthermore, we analyzed the relationships between CFU determination and the qPCR assay to assess how fungal burdens determined by the two techniques correlate in progressive infection, as well as in terms of their utility in determining the early efficacy of CAS against CNS aspergillosis.
MATERIALS AND METHODS
Preparation of the inoculum for infection.
A. fumigatus 10AF was maintained as a stock isolate at −80°C (8). The isolate was thawed and grown on potato dextrose agar (Difco Laboratories, Sparks, Md.) plates for 72 h at 35°C to allow sufficient production of conidia. Conidia and mycelial fragments were washed and harvested in sterile saline with 0.05% Tween 80 (vol/vol), and the conidial suspension was stored at 4°C and used within 2 weeks (7, 8, 12). Viable conidium counts were determined by preparing serial dilutions of the stock suspension, plating duplicates on Sabouraud dextrose agar (Difco) plates with chloramphenicol (50 mg/liter), and enumerating CFU after 48 h of growth at 35°C.
Animals.
Six-week-old male CD-1 mice (Charles River Laboratories) were housed under conventional conditions, with five mice per cage, and provided irradiated food and acidified water ad libitum. Manipulations of animals were performed in accordance with the standards set by the Institutional Animal Care and Use Committee of the California Institute for Medical Research. On the day of infection, mice averaged 29 g in weight. All mice were randomly assigned to groups of 10 to 12 animals prior to infection.
Infection model.
Each animal was rendered neutropenic by an intraperitoneal injection of 200 mg of cyclophosphamide (Cytoxan; Mead Johnson, Princeton, N.J.) per kg of average body weight 2 days prior to infection (day 0) and again on day 3 after infection (5). This regimen has been demonstrated to maintain leukocyte counts well below the normal range of 6 × 103 to 15 × 103 leukocytes/mm3 (5, 6). On day 0, mice were anesthetized by inhalation of methoxyflurane fumes, and each animal was inoculated intracerebrally with 5.75 × 106 conidia of A. fumigatus (total volume, 50 μl) to initiate cerebral aspergillosis as previously described (5, 6). All mice survived the procedure and had resumed normal activity within approximately 5 min.
Drugs and therapy regimens.
Once-daily therapy was initiated 24 h after infection and given for 4 consecutive days. Groups of 10 mice received 1, 5, or 10 mg of CAS (Cancidas; Merck, Rahway, N.J.)/kg, 10 mice received amphotericin B (AMB) (Novaplus; Bristol-Myers Squibb, Princeton, N.J.) at 3 mg/kg, and 32 untreated mice served as controls. Untreated control mice were preassigned to groups for euthanasia on day 1 (n = 10), 3 (n = 10), or 5 (n = 12) postinfection. Drug regimens were based on the average weight of all animals on day 0. AMB was reconstituted and diluted in sterile 5% dextrose water (vol/vol) according to the manufacturer's instructions and stored at −20°C until used. Fresh CAS solutions were prepared daily and diluted in sterile saline. Both drugs were administered intraperitoneally in a volume of 0.2 ml.
Homogenization of organs.
Using CO2 asphyxiation, survivors in the untreated control groups were euthanatized on day 1, 3, or 5, as previously indicated. Surviving animals treated with CAS or AMB were euthanatized on day 5 postinfection. The brain and kidneys were aseptically removed and placed in sterile preweighed Whirl-Pak bags (Nasco) (3). After organ weights were determined, 8 volumes of sterile saline containing a known number of copies of a plasmid carrying a nonfungal, nonmurine gene, which served as a control for DNA extraction efficiency, were added to the organs for each gram of tissue. The organs were then homogenized by rolling a large bottle over the bags with applied pressure for approximately 10 passes, dispersing the tissues evenly (3).
Determination of fungal burdens.
Tissue homogenate samples for CFU determinations were removed from the bags, and serial dilutions were prepared in sterile saline with penicillin and streptomycin. Both the undiluted homogenates and the dilutions were plated as duplicates on Sabouraud dextrose agar plates with 50 mg of chloramphenicol per liter. CFU were enumerated after incubation at 35°C for 48 h. The fungal burden in the tissue is given as the log10 CFU per organ or per gram of tissue. The remainder of each homogenate sample in the Whirl-Pak bags was frozen at −80°C.
Subsequent qPCR TaqMan analysis was performed by Cell and Molecular Technologies, Inc. (Phillipsburg, N.J.), with the primers and hybridization probe derived from A. fumigatus 18S rRNA gene using the methodology described by Bowman et al. (3). Five-point standard curves were generated by spiking naïve brain or kidney tissues with known numbers of conidia from A. fumigatus 10AF. DNA extraction efficiency from each sample was determined by the quantitative amplification of a nonmurine, nonfungal DNA sequence in a control plasmid, which was added to all samples prior to homogenization. The plasmid contains a 3-kb fragment of DNA with the coding region for the protein PKG from Eimeria tenella (J. Bowman, C. M. Douglas, J. W. Anderson, and P. Liberator, unpublished data). The quantification of fungal DNA in each sample was normalized on the basis of the percent recovery of DNA predicted from the plasmid control. Fungal burden was reported as number of CE per gram of tissue. Because conidia are uninucleate, CE equates to numbers of nuclei in the mycelial form of the organism. Each tissue sample extract was run in triplicate, and the mean CE for each sample was used in subsequent data analyses.
Statistical analyses.
Residual fungal burdens in tissues, which were converted to numbers of log10 CFU for CFU data or log10 CE for qPCR data prior to analysis, were compared using the nonparametric Mann-Whitney U test. Statistical correlations between CFU and qPCR data were assessed for each group by determining nonparametric Spearman correlations for burdens in individual animals and performing a Spearman correlation test for each group. Analysis of covariance (ANCOVA) was used to assess the equivalency of slopes of the linear regression lines of whole CFU and qPCR groups for progressive infection, from day 1 to 3 and 5, and for comparing dose escalation efficacy of CAS. P values were considered significant at the 0.05 level. All data were analyzed using Graphpad Prism software (version 3.02 for Windows; Graphpad Software, San Diego, Calif.).
RESULTS
In evaluating fungal burdens in tissues from a murine model of CNS aspergillosis for progressive infection in untreated mice and in mice treated with CAS or AMB, three separate parameters were analyzed: (i) comparative fungal burdens from CFU and qPCR data, as analyzed by the Mann-Whitney U test; (ii) direct comparisons of CFU and qPCR results for each individual mouse to determine nonparametric Spearman correlations for each group; and (iii) comparisons of the linear regression lines of whole CFU and qPCR groups using ANCOVA. The efficacy study was performed with escalating doses of CAS and a single dose of AMB previously shown to be effective in this model (6), and drug efficacy was evaluated at a single time point following infection and therapy.
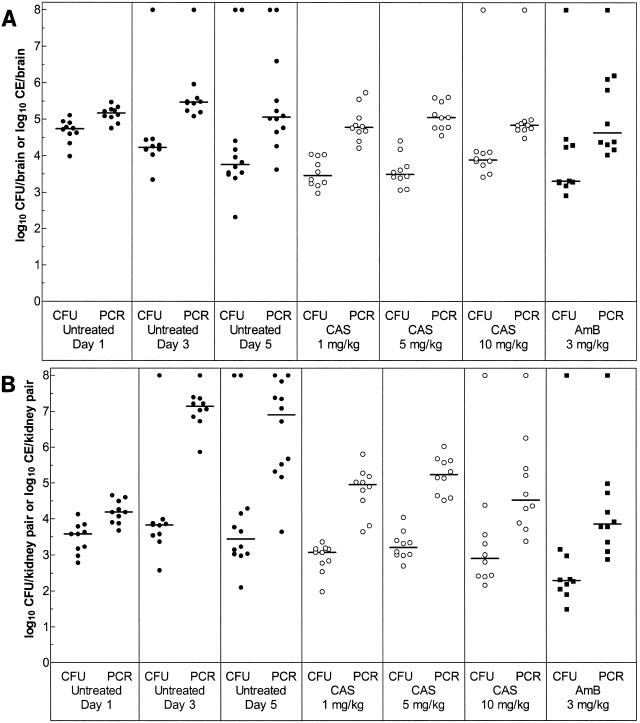
A. fumigatus recovered from each surviving animal and quantified using either CFU determinations or qPCR analyses is presented in scattergrams for burdens in brains (Fig. 1A) and kidneys (Fig. 1B). For all comparisons, qPCR reflected a higher organism burden than did CFU. Animals that died before the conclusion of the study were assigned a higher arbitrary value of log10 8, which assures that death is evaluated as a worse outcome than survival with any amount of fungal burden (15).
FIG. 1.
Scattergrams of CFU or CE recovered from the brains (A) and kidneys (B) of surviving mice. Each data point corresponds to the number of log10 CFU/organ or log10 CE/organ for an individual mouse, with CFU and qPCR data placed adjacently for each group. A log10 value of 8 was assigned to mice that died before the conclusion of the study. The bars represent median group values. In the group consisting of untreated mice euthanatized on day 5, there were 12 animals, and all other groups included 10 animals.
Analyses of progressive infection.
Evaluating progressive infection by CFU determination demonstrated a steady decrease in median numbers of log10 CFU in the brains of untreated animals. Statistical analyses using the Mann-Whitney U test showed that the numbers of log10 CFU decreased significantly in untreated mice euthanatized on day 3 or 5 compared to day 1 (P = 0.02 for both). qPCR analyses revealed a different pattern for the progression of infection. The median number of log10 CE increased in the brain from day 1 to 3 in untreated mice and then decreased on day 5. Numbers of CE were significantly lower in the brains of mice sacrificed on day 1 than on day 3 (P = 0.02), whereas day 1 and day 5 were equivalent in terms of numbers of CE recovered.
In the kidneys, the recovery of CFU showed a small nonsignificant increase from day 1 to 3 but overall illustrated no differences in median number of log10 CFU among any of the untreated groups (P > 0.05). In contrast, by qPCR, the median number of log10 CE increased dramatically from day 1 to day 3 in the untreated controls and then decreased slightly on day 5; numbers of CE in untreated mice were significantly higher on day 3 or 5 than on day 1 (both P = 0.0001); numbers of CE were no different on days 3 and 5.
A Spearman correlation coefficient (rS) was determined for each untreated group by comparing qPCR and CFU data for each individual mouse. From these rS values, a Spearman correlation test was performed to determine whether there was a significant correlation between the two different techniques (i.e., whether they varied regularly). For progressive infection in most untreated groups, rS values were close to +1, and their respective P values were accordingly small (Table 1), indicating a significant positive correlation between CFU and qPCR data. Brains from untreated mice euthanatized on day 3 and kidneys from mice euthanatized on day 5 were the exceptions, with Spearman correlations of CFU and qPCR analyses indicating a nonsignificant correlation between the techniques.
TABLE 1.
Spearman correlation coefficients (rS) and corresponding P values to determine significant correlations between results of CFU (log10 CFU/organ) and qPCR (log10 CE/organ) assays for progressive infection
| Tissue | Group | rS | Pa |
|---|---|---|---|
| Brain | Untreated day 1 | 0.9030 | 0.0008 |
| Brain | Untreated day 3 | −0.4000 | 0.3 |
| Brain | Untreated day 5 | 0.8085 | 0.007 |
| Kidney | Untreated day 1 | 0.6565 | 0.04 |
| Kidney | Untreated day 3 | 0.8000 | 0.01 |
| Kidney | Untreated day 5 | 0.5030 | 0.1 |
Correlations were considered significant at the 0.05 level.
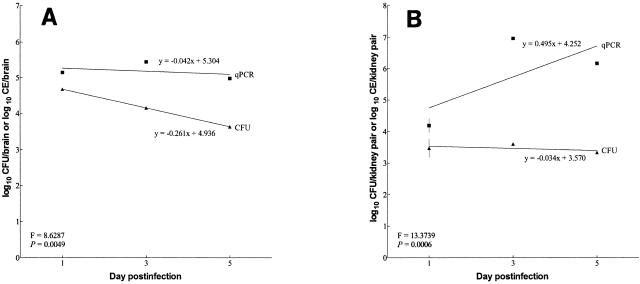
Linear regressions of CFU and qPCR data following progressive infection in untreated mice, on days 1 to 3 and 5, were computed for brains (Fig. 2A) and kidneys (Fig. 2B). Animals that died before the conclusion of the study were omitted from these analyses. ANCOVA was used to evaluate the equivalency of the linear regression lines of CFU and qPCR data and demonstrated that the slopes of the CFU and qPCR linear regression lines were statistically different for progressive infection in both brains and kidneys (P = 0.005 and 0.0006, respectively).
FIG. 2.
Linear regression lines of whole CFU and qPCR groups following progressive infection in the brains (A) and kidneys (B) of untreated mice. ANCOVA was used to assess the equivalency of CFU and qPCR slopes of the linear regression lines. Equations of slopes, F statistics, and P values are displayed for each organ. The predicted regression line is shown. Data points are shown as the mean log10 value (n = 9 or 10), and the error bars represent the 95% confidence interval. Animals that died prior to sampling were not included in the analysis.
Comparative analyses of drug efficacy.
For the drug efficacy study comparing CAS and AMB, Mann-Whitney U tests were used to compare fungal burdens among the treatment groups and with day 5 untreated control animals. In terms of CFU data, most treatment groups were equivalent to each other and to day 5 untreated controls (P > 0.05) in fungal burdens in the brain; the exception was that CAS at 1 mg/kg was superior to CAS at 10 mg/kg (P = 0.04). qPCR data analyses indicated that all treatment groups were equivalent to each other and to the day 5 untreated controls (P > 0.05). CAS showed no dose responsiveness.
For the kidneys, CFU data showed that all CAS regimens equally reduced fungal burdens and that these were not significantly different from burdens in day 5 untreated mice (P > 0.05). However, 1 mg of CAS/kg demonstrated a trend toward efficacy (P = 0.07). AMB at 3 mg/kg significantly reduced numbers of log10 CFU in comparison to no treatment and CAS at 5 mg/kg (P = 0.01 and 0.009, respectively) but was equivalent to CAS at 1 mg/kg or 10 mg/kg (P > 0.05). In contrast, qPCR results indicated that all regimens of CAS (P = 0.003 to 0.03, dependent on comparison) and AMB (P = 0.005) significantly lowered numbers of log10 CE relative to no treatment in the kidneys. AMB at 3 mg/kg was again superior to CAS at 5 mg/kg (P = 0.009) and equivalent to CAS at 1 mg/kg or 10 mg/kg (P > 0.05) based on the qPCR results.
For each treatment group, rS values and corresponding P values were determined to provide an indication of how well results obtained by use of CFU and qPCR methodologies correlated for drug efficacy (Table 2). A significant positive correlation was observed for burdens in the kidneys of mice given any treatment regimen, with rS values of >0.85 for mice receiving any dose of CAS. For burdens in the brain, results of CFU determinations and qPCR analyses did not correlate well for any treatment regimen, as all rS values were ≤0.50.
TABLE 2.
Spearman correlation coefficients (rS) and corresponding P values to determine significant correlations between results of CFU (log10 CFU/organ) and qPCR (log10 CE/organ) assays for drug efficacy
| Tissue | Group | rS | Pa |
|---|---|---|---|
| Brain | CAS, 1 mg/kg | 0.2242 | 0.5 |
| Brain | CAS, 5 mg/kg | 0.3818 | 0.3 |
| Brain | CAS, 10 mg/kg | 0.1667 | 0.7 |
| Brain | AMB, 3 mg/kg | 0.5000 | 0.2 |
| Kidney | CAS, 1 mg/kg | 0.9394 | 0.0002 |
| Kidney | CAS, 5 mg/kg | 0.8545 | 0.003 |
| Kidney | CAS, 10 mg/kg | 0.9500 | 0.0004 |
| Kidney | AMB, 3 mg/kg | 0.7500 | 0.03 |
Correlations were considered significant at the 0.05 level.
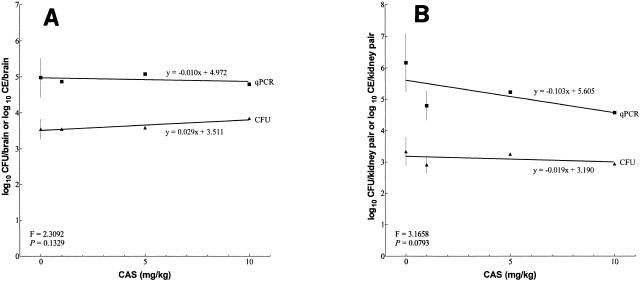
Using recovered fungal burdens from increasing doses of CAS, linear regression lines of CFU and qPCR groups were calculated for brains (Fig. 3A) and kidneys (Fig. 3B). ANCOVA indicated that the slopes of the CFU and qPCR linear regression lines were not statistically different for either the brains or the kidneys (P = 0.1 and 0.08, respectively). Although qPCR indicated a larger burden of A. fumigatus than did CFU, the profiles of CFU and qPCR groups are nearly identical for mice receiving therapy. Namely, the distribution of data points and the relative positions of the medians within the CFU treatment groups were similar to those of the qPCR treatment groups, so that the difference between the medians was reasonably constant from one treatment group to another. The largest differences were in the untreated controls, as discussed above.
FIG. 3.
Linear regression lines of whole CFU and qPCR groups following increasing doses of CAS and resultant fungal burdens in the brains (A) and kidneys (B) of untreated mice. Burdens from untreated mice sacrificed on day 5 were used for the 0-mg/kg dose of CAS. ANCOVA was used to assess the equivalency of CFU and qPCR slopes of the linear regression lines. Equations of slopes, F statistics, and P values are displayed for each organ. The predicted regression line is shown. Data points are shown as the mean log10 value (n = 9 or 10), and the error bars represent the 95% confidence interval. Animals that died prior to sampling were not included in the analysis.
DISCUSSION
An aim of the present study was to determine whether CAS showed efficacy in reduction of A. fumigatus burdens early in the course of CNS disease and to assess whether quantitative values derived from the CFU and qPCR determinations correlated, especially in a drug efficacy study. Each analysis was performed on the same tissue homogenate from each animal for the progression of CNS infection in untreated animals and for the efficacy of CAS or AMB against CNS aspergillosis evaluated at a single time point postinfection.
Convincing evidence is available that a qPCR-based assay (TaqMan) provides a more accurate reflection of Aspergillus burdens than does traditional CFU enumeration when assessing progressive infection in untreated animals (3). In evaluating the kidneys of animals infected systemically with A. fumigatus, the qPCR assay detected a nearly 10,000-fold increase in numbers of CE between days 1 and 4 postinfection, and the incidence of significant mortality corresponded with maximal burdens. In contrast, CFU determinations done on a separate cohort of animals detected only slight escalations in fungal burden (3). However, a direct comparison using the same homogenates for both methods was not done. Our results for evaluating progressive infection in untreated animals corroborate these data (3), as qPCR analyses showed a greater increase in fungal burden in the kidneys with time than did CFU determinations on the same samples. In addition, we extend those results by demonstrating that progressive infection also takes place in the brain. In the brain, qPCR showed a modest increase from day 1 to day 3, followed by a slight decrease by day 5, whereas CFU enumeration showed a steady decline from day 1 to day 5. In the kidneys, both methodologies showed an increase in fungal burden from day 1 to 5, although the CFU values were of considerably smaller magnitude in comparison to the qPCR values. Regression analyses of burdens recovered from untreated animals during progression of infection revealed significant differences between the two assays, with qPCR analyses having steeper slopes than CFU determinations for both the brains and kidneys. The greater dynamic range of the qPCR technique at the upper end of detection enhances the likelihood of differential statistical significance of fungal burdens.
The previous study examined single dosages of CAS and AMB and showed by qPCR that 1 mg of CAS/kg had efficacy in the kidneys (3). We also studied the 1-mg/kg dosage plus two additional higher dosages, as well as a higher dosage of AMB. Our evaluation of drug efficacy by comparative fungal burden from the brain and kidneys of treated and untreated animals was more complex. We obtained equivalent results by CFU enumeration and qPCR for the brain, but only the qPCR showed that CAS had efficacy in the kidneys; these analyses were based on statistical comparisons by the conservative Mann-Whitney U test. However, regression analyses of the CAS dose versus CFU or qPCR CE data indicated that the slopes were not significantly different; different y-intercepts and elevations were present. Thus, while the qPCR method resulted in higher numerical values for the fungal burden than did the CFU method, the respective ratios of median fungal burden in a treatment group to that in an untreated control group were the same. This suggested that the assays were equivalent for the evaluation of drug efficacy when burdens were determined at a single time point after a set duration of therapy. Furthermore, no dose responsiveness for CAS was found by either method.
Interestingly, AMB, an agent with a different mechanism of action, showed efficacy in the kidney by both the CFU enumeration and qPCR methods. These findings are similar to those reported in a recent publication (20) describing a PCR assay using fluorescent resonance energy transfer (FRET), where AMB efficacy against aspergillosis was demonstrated by both CFU determination and the PCR-FRET methods. However, we found the CFU method more sensitive than the qPCR, whereas CFU determination was less sensitive than the PCR-FRET assay (20).
The inability of qPCR to detect a significant change in fungal burden for increasing doses of CAS may be due to the mode of action of the drug. CAS blocks fungal cell wall synthesis by inhibiting the enzyme (1,3)β-glucan synthase (10). Accordingly, CAS is effective in damaging areas of active hyphal growth, namely the hyphal tips and branch points of Aspergillus hyphae. This mechanism of action causes CAS to inhibit hyphal tip extension and branching, without completely damaging nongrowing hyphae of the organism. Images of CAS-treated colonies of Aspergillus in vitro illustrate significantly reduced numbers of hyphal buds with abnormal morphologies (e.g., the formation of flattened and distended tips) (4). Several published studies have shown that CAS fails to kill Aspergillus in vitro (23), and investigations conducted by Petraitiene et al. (22) in a rabbit model of pulmonary aspergillosis demonstrated that CAS causes dose-dependent hyphal damage without reducing residual fungal burden (i.e., is fungistatic), as measured by CFU enumeration. In contrast, CAS demonstrated significant fungicidal activity and efficacy against Candida species (10, 18).
The septate hyphal structure, in which organelles—including ribosomes, mitochondria, and nuclei—move freely between compartments (11), makes it difficult to define what a single cell is in a hypha. If the entire length of a hypha is considered a single cell, then CFU plating that yields a single colony is an accurate reflection of fungal load. The septate compartment of the hyphal tip represents the site of new growth (i.e., cell wall extension) and usually contains six to eight nuclei, whereas older, nonelongating septate compartments are most often uninucleate (19). The multinucleate nature of the tips, as well as the frequency of branching hyphae may result in qPCR overestimating the burden of fungus in comparison with CFU when the fungi are not inhibited by an antifungal agent, whereas after CAS treatment and loss of hyphal tip growth the two methods give more equivalent results. In the qPCR study (3), therapy with CAS or AMB reduced the gap between CFU and qPCR data to an average log10 value of 1 in the kidneys, whereas the difference reached nearly 10,000-fold in untreated mice. We observed a similar trend for fungal burdens in the treatment study of both the kidney and brain. Thus, in addition to producing very similar profiles of drug effectiveness as defined by fungal burden for treated animals, the CFU and qPCR assays detected comparable values for fungal burden, in our evaluation of CAS efficacy.
Regardless of the mechanism of CAS activity or the definition of a cell for filamentous organisms, such as A. fumigatus, a study employing tissue plating and CFU enumeration has demonstrated the efficacy of CAS against CNS aspergillosis in a murine model of infection (Singh et al., Abstr. XV Congr. Int. Soc. Hum. Anim. Mycol.). Doses of CAS and AMB identical to those used in the present study significantly prolonged survival and equally reduced fungal burdens in the brains of immunosuppressed, A. fumigatus-infected mice. At a dosage of 1 mg/kg, CAS showed efficacy comparable to AMB at 3 mg/kg, which has been identified as an effective dose in a murine model of CNS aspergillosis (6).
Although CFU determinations did not detect a dose-dependent reduction of fungal load with CAS therapy in either the previous or the present study, neither did qPCR analyses in the present study. Increasing dosages of amphotericin B lipid complex (Abelcet), from 0.8 to 4 to 8 mg/kg, exhibited a dose-responsive reduction in numbers of CFU in the brains of A. fumigatus-infected mice (J. Imai, G. Singh, B. Fernandez, K. V. Clemons, and D. A. Stevens, Abstr. 103rd Gen. Mtg. Am. Soc. Microbiol., abstr. F-087, 2003); the difference in burden between the low and high doses was over 10-fold. Dose responsiveness with another amphotericin B lipid delivery system, liposomes, as well as with Abelcet, has also been shown in both brain and kidney, in other studies [K. V. Clemons, M. Espiritu, R. Parmar, and D. A. Stevens, Abstr. 14th Eur. Congr. Clin. Microbiol. Infect. Dis., Clin. Microbiol. Infect. 10(Suppl. 3):41-42, 2004]. Furthermore, we have shown posaconazole efficacy with significant reduction of CFU in other studies (13). Thus, CFU quantification is clearly capable of detecting statistically significant efficacy and dose responsiveness.
Neither the CFU or the qPCR method showed CAS to have significant efficacy in the brain, the target organ in this model, in the present study. This lack of efficacy may be due in part to the shortened treatment duration of 4 days in comparison with the 10-day regimens used in our previous study, where efficacy was shown. From our data on progression of infection in the untreated animals the organism appears to be growing more slowly in brain than in kidney. Thus, the full effect of CAS may require a longer duration of therapy to become fully apparent in the brain, since the inhibitory activity of CAS relies on growing organisms. However, qPCR did detect CAS efficacy in the kidneys that was not detected by the CFU method, indicating the utility of the increased sensitivity of the qPCR technology.
It has recently been shown that with some isolates of various Candida species, growth in the presence of CAS in vitro is paradoxical, i.e., greater, with altered morphology, at higher drug concentrations (24, 25). If this phenomenon also occurs with Aspergillus, it could also contribute to nonlinear dose responses in vivo with caspofungin and to the CFU versus qPCR discrepancies in CAS therapy studies (altered cellular morphology could correlate with differences in cellular disruption during tissue homogenization).
Although traditional CFU counting methodologies lack the sensitivity of qPCR, both techniques present inherent advantages and disadvantages. CFU enumeration is inexpensive and performed rapidly. However, the dynamic range of CFU determinations can be narrow, and conventional techniques used to homogenize tissues may not completely disperse the fungus, leading to inconsistent results. Discrepancies can be circumvented by employing standardized procedures for homogenizing organs, as with a mechanical homogenizer for fixed times. In preliminary studies, we found that CFU determinations done after 4 and 48 h of systemic infection by roller-bottle-homogenization of organs in Whirl-Pak bags, which was used for this study, gave results equivalent to mechanical homogenization for both brains and kidneys (data not shown).
In comparison to the CFU method, the qPCR-based assay has a larger dynamic range. However, it is expensive and potentially time-consuming and requires specialized equipment. Another drawback to the qPCR method is the lower limit of detection, which has been reported to be log10 3.3 CE/g of tissue in the standard assay, reduced to log10 1.7 in a modified assay (3). These values would equate to a lower limit of 50 to about 2,000 CE per gram of tissue. This lower limit may make the assay incapable of detecting fungal load in tissues bearing few residual organisms; thus, cure may be better detected by conventional CFU enumeration, which has a lower limit of about 5 CFU per entire organ. Because the primer and probes used for the qPCR assay detect nine other species of Aspergillus (3), potential contamination could also skew results just above the limit of detection.
Distinct conclusions can be derived from our study. First, we agreed with previous investigators that qPCR is a better method for monitoring the progression of infection with a mycelial organism such as A. fumigatus in untreated animals (3). Secondly, we feel that both CFU enumeration and qPCR are usable methodologies for determining drug efficacy at a single time point; both showed that CAS was not efficacious in reducing the burden of A. fumigatus from the brain in the present short-term study of reduction of fungal burden in our model of CNS aspergillosis. However, neither is a perfect technique, and each presents inherent shortcomings. It is important to take into account the caveat that the increased sensitivity and dynamic range of the qPCR assay detected significant CAS efficacy in the kidneys where the less robust CFU method did not. This could be especially important for detecting efficacy when a drug is only fungistatic in vivo. Regardless, the level and sensitivity of fungal burdens detected by the two techniques for therapeutic efficacy were quite comparable. The fact that the qPCR assay is not readily available to many laboratories and entails a sizeable expense is important because usable drug efficacy data, even with echinocandins like CAS, can be derived from conventional CFU determinations, and these types of data should not summarily be dismissed as unreflective of antifungal efficacy in reducing fungal burdens in the tissues.
Acknowledgments
These studies were funded in part by a grant from Merck and Co., Inc.
We thank Marife Espiritu and Rachana Parmar for their assistance in these studies.
REFERENCES
- 1.Abruzzo, G. K., A. M. Flattery, C. J. Gill, L. Kong, J. G. Smith, V. B. Pikounis, J. M. Balkovec, A. F. Bouffard, J. F. Dropinski, H. Rosen, H. Kropp, and K. Bartizal. 1997. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 41:2333-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abruzzo, G. K., C. J. Gill, A. M. Flattery, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, and H. Rosen. 2000. Efficacy of the echinocandin caspofungin against disseminated aspergillosis and candidiasis in cyclophosphamide-induced immunosuppressed mice. Antimicrob. Agents Chemother. 44:2310-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman, J. C., G. K. Abruzzo, J. W. Anderson, A. M. Flattery, C. J. Gill, V. B. Pikounis, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman, J. C., P. S. Hicks, M. B. Kurtz, H. Rosen, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2002. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob. Agents Chemother. 46:3001-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiller, T. M., J. Capilla Luque, R. A. Sobel, K. Farrokhshad, K. V. Clemons, and D. A. Stevens. 2002. Development of a murine model of cerebral aspergillosis. J. Infect. Dis. 186:574-577. [DOI] [PubMed] [Google Scholar]
- 6.Chiller, T. M., R. A. Sobel, J. Capilla Luque, K. V. Clemons, and D. A. Stevens. 2003. Efficacy of amphotericin B or itraconazole in a murine model of central nervous system Aspergillus infection. Antimicrob. Agents Chemother. 47:813-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemons, K. V., T. K. Miller, C. P. Selitrennikoff, and D. A. Stevens. 2002. fos-1, a putative histidine kinase as a virulence factor for systemic aspergillosis. Med. Mycol. 40:259-262. [DOI] [PubMed] [Google Scholar]
- 8.Denning, D. W., K. V. Clemons, and D. A. Stevens. 1992. Quantitative preservation of viability of Aspergillus fumigatus. J. Med. Vet. Mycol. 30:485-488. [DOI] [PubMed] [Google Scholar]
- 9.Denning, D. W., L. Hall, M. Jackson, and S. Hollis. 1995. Efficacy of D0870 compared with those of itraconazole and amphotericin B in two murine models of invasive aspergillosis. Antimicrob. Agents Chemother. 39:1809-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deresinski, S. C., and D. A. Stevens. 2003. Caspofungin. Clin. Infect. Dis. 36:1445-1457. [DOI] [PubMed] [Google Scholar]
- 11.Fischer, R. 1999. Nuclear movement in filamentous fungi. FEMS Microbiol. Rev. 23:39-68. [DOI] [PubMed] [Google Scholar]
- 12.Hanson, L. H., K. V. Clemons, D. W. Denning, and D. A. Stevens. 1995. Efficacy of oral saperconazole in systemic murine aspergillosis. J. Med. Vet. Mycol. 33:311-317. [DOI] [PubMed] [Google Scholar]
- 13.Imai, J., G. Singh, K. V. Clemons, and D. A. Stevens. 2004. Efficacy of posaconazole in a murine model of central nervous system aspergillosis. Antimicrob. Agents Chemother. 48:4063-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkpatrick, W. R., S. Perea, B. J. Coco, and T. F. Patterson. 2002. Efficacy of caspofungin alone and in combination with voriconazole in a guinea pig model of invasive aspergillosis. Antimicrob. Agents Chemother. 46:2564-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lachin, J. M. 1999. Worst-rank score analysis with informatively missing observations in clinical trials. Control. Clin. Trials 20:408-422. [DOI] [PubMed] [Google Scholar]
- 16.Leenders, A. C., S. de Marie, M. T. ten Kate, I. A. Bakker-Woudenberg, and H. A. Verbrugh. 1996. Liposomal amphotericin B (AmBisome) reduces dissemination of infection as compared with amphotericin B deoxycholate (Fungizone) in a rat model of pulmonary aspergillosis. J. Antimicrob. Chemother. 38:215-225. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann, P. F., and L. O. White. 1975. Chitin assay used to demonstrate renal localization and cortisone-enhanced growth of Aspergillus fumigatus mycelium in mice. Infect. Immun. 12:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mora-Duarte, J., R. Betts, C. Rotstein, A. L. Colombo, L. Thompson-Moya, J. Smietana, R. Lupinacci, C. Sable, N. Kartsonis, and J. Perfect. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020-2029. [DOI] [PubMed] [Google Scholar]
- 19.Muller, C., A. B. Spohr, and J. Nielsen. 2000. Role of substrate concentration in mitosis and hyphal extension of Aspergillus. Biotechnol. Bioeng. 67:390-397. [DOI] [PubMed] [Google Scholar]
- 20.O'Sullivan, C. E., M. Kasai, A. Francesconi, V. Petraitis, R. Petraitiene, A. M. Kelaher, A. A. Sarafandi, and T. J. Walsh. 2003. Development and validation of a quantitative real-time PCR assay using fluorescence resonance energy transfer technology for detection of Aspergillus fumigatus in experimental invasive pulmonary aspergillosis. J. Clin. Microbiol. 41:5676-5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson, T. F., D. George, P. Miniter, and V. T. Andriole. 1992. Saperconazole therapy in a rabbit model of invasive aspergillosis. Antimicrob. Agents Chemother. 36:2681-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petraitiene, R., V. Petraitis, A. H. Groll, T. Sein, R. L. Schaufele, A. Francesconi, J. Bacher, N. A. Avila, and T. J. Walsh. 2002. Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition, and relationship to galactomannan antigenemia. Antimicrob. Agents Chemother. 46:12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens, D. A. 2001. Challenges and new approaches to managing aspergillosis. J. Crit. Illn. 16:S43-S49. [Google Scholar]
- 24.Stevens, D. A., M. Espiritu, and R. Parmar. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 48:3407-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens, D. A., T. C. White, D. S. Perlin, and C. P. Selitrennikoff. 2005.. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn. Microbiol. Infect. Dis. 51:173-178. [DOI] [PubMed]