ABSTRACT
We describe a case of hepatoportal sclerosis (HPS) identified in an 81-year-old woman taking a traditional Chinese herbal supplementation, Cordyceps. The patient presented with splenomegaly and weight loss. After an extensive evaluation, liver biopsy confirmed loss of the small portal veins with characteristics of obstruction at the level of the small and large portal veins, suggestive of HPS. After a comprehensive history and exclusion of other etiological factors, patient's HPS was attributed to Cordyceps use. Ultimately, the patient's features of HPS improved with the cessation of Cordyceps.
KEYWORDS: cordyceps, mushroom poisoning, hepatoportal sclerosis, noncirrhotic portal hypertension
INTRODUCTION
Hepatoportal sclerosis (HPS) as a cause of noncirrhotic intrahepatic portal hypertension is a rare clinicopathologic condition of uncertain etiology.1 The exact precipitants of HPS remain unclear. To date, there are only limited studies that evaluate the role of Chinese herbal supplementation in the development of HPS. A more recent report described HPS in female patients who used Herbalife products and anorexigenic agents in the form of herbal infusions.2 Although several mechanisms of HPS have been proposed, the effect of drugs and toxins still remains unclear. The following case highlights a patient with noncirrhotic portal hypertension because of herbal supplement use.
CASE REPORT
An 81-year-old woman presented to outpatient clinic with left-sided abdominal pain and 12-pound unintentional weight loss over a span of 3 months. She reported drinking 1–2 alcoholic beverages a month with no history of chronic liver disease. Physical examination was notable for palpable liver edge at the level of the costal margin and splenomegaly. No stigmata of cirrhosis were evident. Laboratory test results revealed platelet count 76 ×103/μL (reference range 150–450 ×103/μL), albumin 4.1 g/dL (reference range 3.6–4.6 g/dL), total bilirubin 0.7 mg/dL (reference range 0.0–1.2 mg/dL), alkaline phosphatase 72 U/L (reference range 44–121 U/L), aspartate aminotransferase 28 U/L (reference range 0–40 U/L), and alanine transaminase 26 U/L (reference range 0–32 U/L). R factor (also known as the R ratio or R value) score for liver injury was 1.1, indicating likely cholestatic injury pattern.3 A complete blood count from 1 year earlier showed a platelet count of 144 ×103/μL. Infectious and autoimmune liver disease workup was negative. An abdominal ultrasound noted a normal liver size and echogenicity, hepatopetal flow in the portal vein, and an enlarged spleen measuring 21.5 cm. Transient elastography demonstrated liver stiffness of 8.9 kPa with a controlled attenuation parameter of 217 dB/m. Esophagogastroduodenoscopy noted grade 1, small esophageal varices without high-risk stigmata.
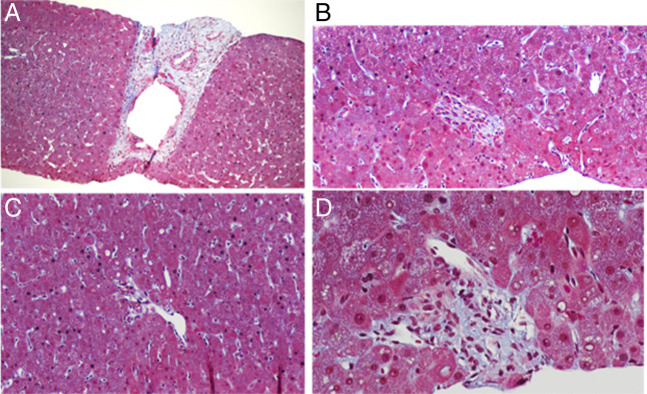
Given the findings of splenomegaly and thrombocytopenia, the patient was evaluated by a hematologist who performed a bone marrow biopsy, which was negative for lymphoma or other malignancy. A transjugular liver biopsy with portal pressure measurements was performed. The hepatic-venous portal pressure gradient was 2–4 mm Hg with normal free and wedged hepatic pressures, suggesting presinusoidal portal hypertension. Liver biopsy pathology (Figure 1) demonstrated loss of the small portal veins with features, suggestive of obstruction including several small portal areas with no visible portal vein profile or with a portal vein invaginated into adjacent parenchyma. One portal vein showed muscular hypertrophy of its walls. Otherwise, no significant fibrosis, cirrhosis, outflow obstruction, or nodular regenerative hyperplasia was identified. Therefore, the diagnosis of HPS was made. Further history revealed that the patient took a traditional Chinese herbal supplement, Cordyceps, as a daily tablet for 5 years for irritable bowel syndrome. Our patient was not taking any additional herbal supplements, teas, or prescription drugs or over-the-counter drugs that are associated with liver injury. By applying the Roussel Uclaf Causality Assessment Method, a widely used instrument to facilitate the causality attribution for suspected drug-induced liver injury, the patient was found to have a score of 4, indicating that drug-induced liver injury is possible.3 The patient discontinued Cordyceps approximately 6 months after the onset of abdominal pain symptoms. The importance of avoiding herbal supplements was discussed with the patient. Six months after discontinuing the Cordyceps, the patient reported improved abdominal pain and appropriate weight gain. Two years after discontinuation of Cordyceps, imaging showed a spleen size of 17.8 cm, decreased from 21.5 cm. Repeat laboratory studies showed improved platelet count to 106 ×103/μL (Table 1).
Figure 1.
(A) Portal vein with muscular hypertrophy of its wall (trichrome stain magnification 200×). (B) Portal tract with no portal vein profile (trichrome stain magnification 200×). (C) Portal tract with eccentric portal vein profile invaginated into liver parenchyma (trichrome stain magnification 200×). (D) Portal tract with eccentric portal vein profile invaginated into liver parenchyma.
Table 1.
Laboratory and imaging data before and after Cordyceps use
| During Cordyceps use | 2 yr after Cordyceps discontinuation | |
| Spleen size (cm) | 21.5 | 17.8 |
| Platelet count (×103/μL) | 76 | 106 |
| Albumin (g/dL) | 4.1 | 4.3 |
| Total bilirubin (mg/dL) | 0.7 | 0.7 |
| ALP (U/L) | 72 | 64 |
| AST (U/L) | 28 | 20 |
| ALT (U/L) | 26 | 13 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
DISCUSSION
HPS is an uncommon condition that causes noncirrhotic portal hypertension because of fibrous intimal thickening of the portal vein or its branches.4–6 HPS is characterized by presinusoidal intrahepatic portal hypertension associated with splenomegaly and/or complications of portal hypertension.6–8 The diagnosis is based on evidence of portal hypertension, in addition to clinical and histologic evidence of HPS.8 Liver biopsy is essential to confirm the diagnosis of HPS and often reveals obliterative or sclerotic changes.4,7 One series of 18 patients demonstrated that HPS is characterized by caudate and right hepatic lobe atrophy, preserved liver volume, and lack of the liver nodularity that may be associated with cirrhotic portal hypertension.5
There are various disorders associated with noncirrhotic portal hypertension including chronic or recurrent infections, genetic disorders, thrombophilia, immunological disorders, and exposure to drugs or toxins.1,2,9 These factors are believed to contribute to portal vascular endothelium aggregation; however, the precise pathophysiology is likely multifactorial and remains unknown.2 Our patient had no recent or history of infections, bleeding issues, or genetic/immunological disorders. Furthermore, the hematology workup for thrombophilia and malignancy was negative. To date, there are few studies of herbal causes of noncirrhotic portal hypertension. Previous studies have shown that many phytochemicals, which are found in traditional Chinese herbs, have the potential to injure the liver in an idiosyncratic manner.10
Interestingly, our patient was using Cordyceps, a rare entomopathogenic fungi, that has been a highly valued supplement in traditional Chinese medicine and has various biological activities.11,12 More than 350 Cordyceps species have been found worldwide; however, since 1964, only Cordyceps sinensis has been recorded officially as an herbal drug in Chinese medicine.13 Cordycepin, a bioactive component of Cordyceps, is believed to competitively inhibit the synthesis and metabolism of DNA and RNA as well as affect the activity of adenosine deaminase and the mechanistic target of rapamycin signaling pathway.14 Cordycepin has been reported in a variety of pharmacological actions including antioxidant, anti-inflammatory, antimicrobial, antiviral, antitumor, and hypoglycemic properties.12 Because of these properties, Cordyceps have been used for the treatment of liver disease in humans for thousands of years. In more recent years, Cordyceps sinensis has been reported to alleviate inflammation and fibrosis in mice/rats.15–17
Although Cordyceps have been used for centuries in China, there are few studies reviewing the adverse profile of this herbal supplement; in addition, the effects on the human liver have not been studied. The exact mechanism of action still remains unclear, and the risk of developing HPS is unknown. Although HPS is rare and Cordyceps-related research is limited, Cordyceps remain a backbone of traditional Chinese medicine for treatment of liver disease.
Treatment for HPS is primarily directed at managing the complications of portal hypertension; however, it is crucial to eliminate potential triggers.7 HPS may lead to hepatic synthetic dysfunction especially in the setting of diminished liver volume. The combination of portal hypertension and synthetic dysfunction may lead to liver transplantation in patients with advanced HPS; however, this is rare.18,19
This case demonstrates the importance of obtaining a detailed and careful review of herbal drugs and supplements in patients with noncirrhotic portal hypertension of unclear etiology. After excluding infectious, genetic, and autoimmune causes, a normal hepatic-venous portal pressure gradient without hepatic fibrosis was seen because of presinusoidal portal hypertension from HPS. A high index of suspicion is needed for herbal supplement-induced noncirrhotic portal hypertension to prompt timely withdrawal of the offending agent.
DISCLOSURES
Author contributions: B. Kaur: case report design, writing, and analysis. A. Vipani: case report analysis and writing. W. Ayoub: writing the manuscript and is the article guarantor. All authors contributed to interpretation, critical revision, and approval of the final version.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Bhupinder Kaur, Email: b.rosekaur@gmail.com.
Aarshi Vipani, Email: aarshi.vipani@cshs.org.
Hirsh Trivedi, Email: hirsh.trivedi@cshs.org.
Alexander Kuo, Email: alexander.kuo@cshs.org.
Ju Dong Yang, Email: judong.yang@cshs.org.
REFERENCES
- 1.Goel A, Ramakrishna B, Zachariah U, et al. What makes non-cirrhotic portal hypertension a common disease in India? Analysis for environmental factors. Indian J Med Res. 2019;149(4):468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rios FF, de Freitas LAR, Codes L, Santos Junior GO, Schinoni MI, Paraná R. Hepatoportal sclerosis related to the use of herbals and nutritional supplements. Causality or coincidence? Ann Hepatol. 2016;15(6):932–8. [DOI] [PubMed] [Google Scholar]
- 3.Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG clinical guideline: The diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950–66. [DOI] [PubMed] [Google Scholar]
- 4.Bioulac-Sage P, Bail BLE, Bernard PH, Balabaud C. Hepatoportal sclerosis. Semin Liver Dis. 1995;15(4):329–39. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan P, Fiel MI, Rosenkrantz AB, et al. Hepatoportal sclerosis: CT and MRI appearance with histopathologic correlation. AJR Am J Roentgenol. 2012;198(2):370–6. [DOI] [PubMed] [Google Scholar]
- 6.Mikkelsen WP, Edmondson HA, Peters RL, Redeker AG, Reynolds TB. Extra- and intrahepatic portal hypertension without cirrhosis (hepatoportal sclerosis). Ann Surg. 1965;162(4):602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-Caselles A, Pons-Miñano JA, Vargas-Acosta Á, et al. Hepatoportal sclerosis clinical different evolutionary stages: Presentation of 3 cases and literature review. Rev Esp Enferm Dig. 2012;104(2):94–7. [DOI] [PubMed] [Google Scholar]
- 8.Sarin SK, Kapoor D. Non-cirrhotic portal fibrosis: Current concepts and management. J Gastroenterol Hepatol. 2002;17(5):526–34. [DOI] [PubMed] [Google Scholar]
- 9.Schouten JNL, Garcia-Pagan JC, Valla DC, Janssen HLA. Idiopathic noncirrhotic portal hypertension. Hepatology. 2011;54(3):1071–81. [DOI] [PubMed] [Google Scholar]
- 10.Kaplowitz N. Herb-induced liver injury: A global concern. Chin J Integr Med. 2018;24(9):643–4. [DOI] [PubMed] [Google Scholar]
- 11.Yue K, Ye M, Zhou Z, Sun W, Lin X. The genus Cordyceps: A chemical and pharmacological review. J Pharm Pharmacol. 2013;65(4):474–93. [DOI] [PubMed] [Google Scholar]
- 12.Ashraf SA, Elkhalifa AEO, Siddiqui AJ, et al. Cordycepin for health and wellbeing: A potent bioactive metabolite of an entomopathogenic medicinal fungus Cordyceps with its nutraceutical and therapeutic potential. Molecules. 2020;25(12):2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin BQ, Li SP. Cordyceps as an herbal drug. In: Herbal Medicine: Biomolecular and Clinical Aspects. 2nd edn. CRC Press: Boca Raton, 2011, pp 73–105. [Google Scholar]
- 14.Wong YY, Moon A, Duffin R, et al. Cordycepin inhibits protein synthesis and cell adhesion through effects on signal transduction. J Biol Chem. 2010;285(4):2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Y, Huang K, Shen L, Tao YY, Liu CH. Cultured Mycelium Cordyceps sinensis alleviates CCl4-induced liver inflammation and fibrosis in mice by activating hepatic natural killer cells. Acta Pharmacol Sin. 2015;37(2):204–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu YK, Shen W. Inhibitive effect of cordyceps sinensis on experimental hepatic fibrosis and its possible mechanism. World J Gastroenterol. 2003;9(3):529–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng J, Li X, Feng Q, Chen L, Xu L, Hu Y. Anti-fibrotic effect of Cordyceps sinensis polysaccharide: Inhibiting HSC activation, TGF-β1/Smad signalling, MMPs and TIMPs. Exp Biol Med (Maywood). 2013;238(6):668–77. [DOI] [PubMed] [Google Scholar]
- 18.Fiel MI, Thung SN, Hytiroglou P, Emre S, Schiano TD. Liver failure and need for liver transplantation in patients with advanced hepatoportal sclerosis. Am J Surg Pathol. 2007;31(4):607–14. [DOI] [PubMed] [Google Scholar]
- 19.Ataide EC, Dos Santos IN, Martins DL, et al. Liver failure and the need for transplantation in 6 patients with hepatoportal sclerosis. Transplant Proc. 2013;45(5):1907–9. [DOI] [PubMed] [Google Scholar]