Abstract
Certain mutations in the C-terminal region of the Escherichia coli division inhibitor protein MinC cause loss of function of the division inhibitor by making MinC more sensitive to degradation by Lon protease, implying a possible role for the C-terminal region in regulating the stability and cellular concentration of MinC.
The gene products of the min gene cluster of Escherichia coli—MinC, MinD, and MinE—are required to ensure that the division septum is properly placed at the midpoint of the cell. In the absence of normal function of the min gene products, septation occurs at potential division sites located adjacent to the cell poles, leading to the formation of small, spherical minicells that lack chromosomal DNA. Suppression of septation at the polar sites is normally accomplished by the action of a septation inhibitor whose function requires the minC and minD gene products. MinC is believed to be the proximate cause of the inhibition of septation by the MinCD division inhibitor, with MinD acting as an activator of the septation-inhibitory activity of the MinC protein (5). The MinC division inhibitor can also be activated by the product of dicB (4), a gene that is normally not expressed and is thought to be part of a cryptic prophage in E. coli K-12 strains (1). The MinCD division inhibitor is given specificity for polar sites by the MinE topological specificity protein (3).
A number of minC mutants have been identified in which the ability to respond to MinD has been lost, as shown by the observation that overexpression of MinD no longer leads to division inhibition (6). Most of the mutations are located in the 3′ region of minC, resulting in amino acid changes or truncations in the region between amino acids 160 and 200 of the 232-amino-acid MinC protein. This led to the suggestion that this region of MinC participates in interaction with MinD (6).
In this communication we report that mutations in the carboxy-terminal region of minC lead to instability of the MinC protein as shown by a marked decrease in MinC concentration in cells that contain a functional Lon protease. Restoration of the normal MinC concentration by growth in Lon− cells was associated with a return of the division inhibition that is normally seen when minC is coexpressed with minD. Thus, determinants in the carboxy-terminal region of MinC impart protection against intracellular degradation, and the loss of function of the mutant MinC proteins reflects changes in their cellular concentrations rather than loss of specific sites that interact with the MinD activator protein.
Plasmids containing seven mutant minC alleles under control of Plac or the constitutive PaadA promoter were introduced into isogenic lon+ and lon mutant strains that contained a chromosomal deletion of the minCDE gene cluster. Five of the mutations were missense mutations (minC19, minC24, minC25, minC27, and minC28); two were nonsense mutations that led to formation of truncated MinC proteins (minC36 and minC37) (Fig. 1). Plasmids pMS706, pMS709, and pMS755 were prepared by replacing the 579-bp Bsu36I/PstI fragment of pDB201 (Plac-minC) (5) that originates within minC with the corresponding Bsu36I/PstI fragments from minC mutant plasmids (6) pJPB120m4 (minC24), pJPB120m12 (minC28), and pJPB120m11 (minC27). Plasmids pMS1 (minC36) and pMS2 (minC37) were prepared by digesting the pBluescript derivative pDB201 (minC+) with BstEII or PstI, respectively, and then treating with T4 DNA polymerase or Klenow polymerase and religating the blunt ends to give 3′ minC deletion mutations.
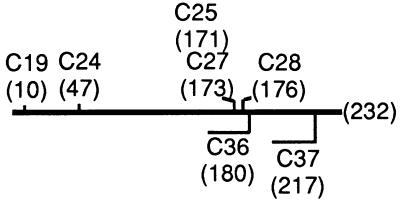
FIG. 1.
minC mutations. Missense minC alleles are shown above the line representing the 232-amino-acid minC gene. Alleles leading to premature termination are shown below the line. Sites of amino acid replacement or premature termination of translation are indicated by amino acid position in parentheses. The min36 and min37 gene products also contain 11 and 1 additional amino acids from the cloning vector at their C termini, respectively.
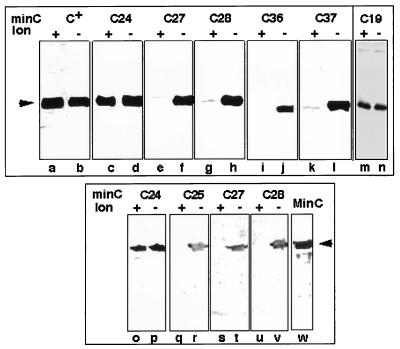
Mutations that led to changes in the C-terminal region of the protein (minC25, minC27, minC28, minC36, and minC37) were associated with a marked decrease in the MinC immunoreactive band in lon+ cells compared with lon mutant cells (Fig. 2). The results were the same both in the absence (Fig. 2, lanes a to n) and presence (Fig. 2, lanes o to v) of MinD. In contrast, in the two mutants in which the mutations were located in the amino-terminal region of the protein (minC19 and minC24), the cellular concentrations of MinC were similar in lon+ and lon mutant hosts.
FIG. 2.
MinC immunoblot analysis in lon+ and lon mutant cells. Immunoblot analysis (5) was performed on 0.05 A600 U of strain PB115 (lon+ ΔminCDE) (lanes a, c, e, g, i, and k), RC7 (lon ΔminCDE) (lanes b, d, f, h, j and l), PB114 (lon+ ΔminCDE) (lane m), MS114 (lon ΔminCDE) (lane n), PB114(λDB164) (lon+ ΔminCD/Plac-minD) (lanes o, q, s, and u), or MS114(λDB164) (lon ΔminCDE/Plac-minD) (lanes p, r, t, and v) containing the Plac-minC plasmids pDB201 (minC+) (lanes a and b), pMS706 (minC24) (lanes c and d), pMS755 (minC27) (lanes e and f), pMS709 (minC28) (lanes g and h), pMS1 (minC36) (lanes i and j), pMS2 (minC37) (lanes k and l), or pCL45 (Pmin-minC19) (lanes m and n) or containing the PaadA-minC plasmid pJPB120m4 (minC24) (lanes o and p), pJPB120m5 (minC25) (lanes q and r), pJPB120m11 (minC27) (lanes s and t), or pJPB120m12 (minC28) (lanes u and v). Lane w contained purified MinC (100 μg). Cells were grown for 5 h in the presence of 1 mM (lanes a to l) or 0.2 mM (lanes o to v) IPTG (isopropyl-β-d-thiogalactopyranoside). Arrowheads indicate the position of authentic MinC.
For quantitation, the cellular concentrations of the mutant proteins were estimated by comparing the intensity of the mutant MinC band with that of the MinC band in serial dilutions of a MinC+ extract from strain PB114/pDB201 (ΔminCDE/Plac-minC+) grown and immunoblotted under the same conditions. This showed a 93 to 97% reduction in MinC concentration in cells expressing the C-terminal MinC mutations in a lon+ background as compared with a lon mutant background.
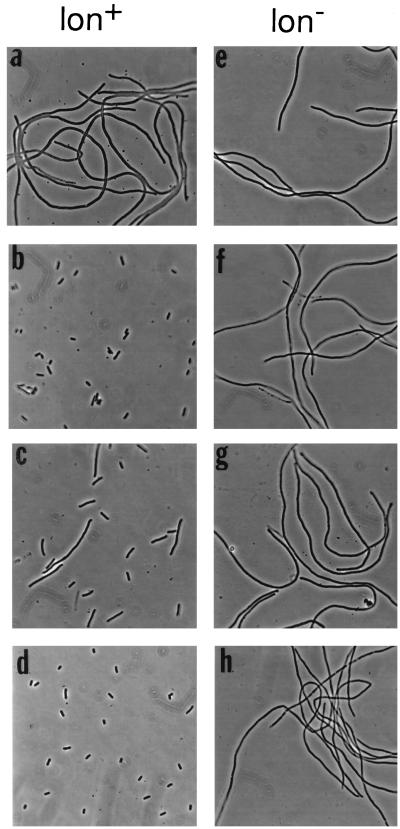
In the case of missense mutants in the carboxy-terminal region of MinC (minC25, minC27, and minC28), the restoration of MinC concentration to near normal levels in lon mutant cells was accompanied by restoration of function of the MinCD-mediated division inhibition system, as shown by the formation of nonseptate filaments when the mutant alleles were coexpressed with minD (Table 1 and Fig. 3f to h). In contrast, in minC mutants in which immunoblotting showed that MinC concentration was unaffected by the lon status of the host (minC19 and minC24), the phenotypes were also unaffected by the lon status of the host (Table 1 and Fig. 3).
TABLE 1.
Phenotypes of minC mutants in lon+ and lon mutant cells in the presence of MinDa
| Plasmid | Genotype | Phenotype
|
|
|---|---|---|---|
| lon+ host | lon mutant host | ||
| pJBP120m4 (6) | PaadA-minC24 | Sep− | Sep− |
| pJPBm5 (6) | PaadA-minC25 | Min− | Sep− |
| pJPBm11 (6) | PaadA-minC27 | Min− | Sep− |
| pJPBm12 (6) | PaadA-minC28 | Min− | Sep− |
| pDB201 (3) | Plac-minC+ | Sep− | Sep− |
| pMS706 | Plac-minC24 | Sep− | ND |
| pMS755 | Plac-minC27 | Min− | ND |
| pMS709 | Plac-minC28 | Min− | Sep− |
| pMS1 | Plac-minC36 | Min− | Min− |
| pMS2 | Plac-minC37 | Min− | Min− |
| pCL45 (6) | Pmin-minC19 | Min− | Min− |
The indicated minC plasmids were present in either a lon+ (PB115 [lon+ ΔminCDE]) or lon mutant (RC7 [lon ΔminCDE]) host. For the PaadA-minC plasmids, the host was lysogenic for λDB164 (Plac-minD) (3). For the Plac-minC plasmids, the host also contained pDB211 (PaadA-minD). Cells were grown overnight in the presence of 0.2 mM IPTG before examination by phase-contrast microscopy (2). In the absence of IPTG or in hosts containing the vector plasmids pBluescript or pGB2 the phenotypes were Min−, reflecting the ΔminCDE genotype of the host strains. ND, not done because stable transformants could not be obtained, even when transformants were grown in the presence of glucose. Min−, the culture contained large numbers of spherical minicells plus cells that were up to 6 times as long as normal cells, Sep−, essentially all cells were long filaments at least 10 times as long as normal cells.
FIG. 3.
Phenotypes of minC mutants in the presence of MinD in lon+ and lon mutant cells. Representative examples are shown; other results are summarized in Table 1. The lon+ ΔminCDE hosts were PB114 (λDB164 Plac-minD) (a to c) and RC3F−/pDB211 (PaadA-minD) (d). The lon ΔminCDE hosts were MS114 (λDB164) (e to g) and MS114/pDB211 (h). The strains contained minC plasmid pJPB120m4 (PaadA-minC24) (a and e), pJPB120m11 (PaadA-minC27) (b and f), pMS709 (Plac-minC28) (c and g), or pJPB120m5 (PaadA-minC25) (d and h). Cells were grown overnight in the presence of 0.2 mM IPTG (isopropyl-β-d-thiogalactopyranoside) prior to examination by phase-contrast microscopy (2).
We conclude that determinants in the carboxy-terminal region of MinC are required to maintain the protein in a state that is resistant to the lon protease since alteration of individual amino acids in the C-terminal domain or removal of the C-terminal 15 or 52 amino acids was associated with a marked reduction in cellular concentration of MinC in lon+ cells. The reduction of MinC concentration, in turn, appeared responsible for the previously observed loss of the ability to respond to MinD activation. Therefore, the loss of the ability of the mutant MinC proteins to support the MinCD-mediated division inhibition reaction cannot be used to support the view that this region is directly implicated in interactions with MinD. The results instead imply a role for the C-terminal region in regulating the stability and cellular concentration of MinC. It is not known whether this reflects a role in MinC folding or whether it indicates the presence of a site in the C-terminal region that is involved in other interactions that affect the susceptibility of MinC to intracellular turnover.
Acknowledgments
We thank J.-P. Bouché for generously providing the minC mutant plasmids.
We thank the National Institute of General Medical Sciences, Public Health Service, for supporting this work.
REFERENCES
- 1.Béjar S, Bouché F, Bouché J-P. Cell division inhibition gene dicB is regulated by a locus similar to lambdoid bacteriophage immunity loci. Mol Gen Genet. 1988;212:11–19. doi: 10.1007/BF00322439. [DOI] [PubMed] [Google Scholar]
- 2.de Boer P A J, Crossley R E, Rothfield L I. Isolation and properties of minB, a complex genetic locus involved in correct placement of the division site in Escherichia coli. J Bacteriol. 1988;170:2106–2112. doi: 10.1128/jb.170.5.2106-2112.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Boer P A J, Crossley R E, Rothfield L I. A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum in E. coli. Cell. 1989;56:641–649. doi: 10.1016/0092-8674(89)90586-2. [DOI] [PubMed] [Google Scholar]
- 4.de Boer P A J, Crossley R E, Rothfield L I. Central role for the Escherichia coli minC gene product in two different cell division-inhibition systems. Proc Natl Acad Sci USA. 1990;87:1129–1133. doi: 10.1073/pnas.87.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer P A J, Crossley R E, Rothfield L I. Roles of MinC and MinD in the site-specific septation block mediated by the MinCDE system of Escherichia coli. J Bacteriol. 1992;174:63–70. doi: 10.1128/jb.174.1.63-70.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulder E, Woldringh C L, Tétart F, Bouché J-P. New minC mutations suggest different interactions of the same region of division inhibitor MinC with proteins specific for minD and dicB coinhibition pathways. J Bacteriol. 1992;174:35–39. doi: 10.1128/jb.174.1.35-39.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]