Abstract
Background
Malnutrition is prevalent among patients with inflammatory bowel disease (IBD). Nutritional profiles among Asian patients with IBD have seldom been investigated. We assessed the prevalence of and risk factors for malnutrition, use of nutrition support, and sociopsychological status associated with malnutrition among patients with IBD in China.
Methods
Patients with ulcerative colitis and Crohn’s disease (CD) recruited from 43 tertiary referral hospitals were screened for malnutrition and nutrient deficiencies in this cross-sectional study. The use of nutrition support was recorded. The sociopsychological status was assessed by subjective questionnaires. Factors associated with malnutrition were analyzed, and multivariate regression was used to determine independent predictors for malnutrition.
Results
We recruited 1013 patients with a median age of 35.0 years, 58.5% of them had CD, and 61.4% of all patients were male. Overall, 49.5% (n = 501) of patients were diagnosed with malnutrition, including 57.0% of patients with CD, 38.8% of patients with ulcerative colitis, and 44.1% of patients with quiescent or mildly active disease. Nutrient deficiencies were prevalent despite the absence of malnutrition. Malnutrition was associated with adverse sociopsychological status, including decreased social support, higher perceived stress, and impaired quality of life. Moderate to severe disease activity and extensive disease were 2 independent risk factors for malnutrition. In total, 41.6% of patients received nutrition support, and patients with risk factors were more likely to receive nutrition support.
Conclusions
Malnutrition was highly prevalent and associated with adverse consequences in Chinese patients with IBD. Malnutrition screening and early initiation of nutrition support are essential components in IBD care.
Keywords: inflammatory bowel disease, malnutrition, nutrition support
Introduction
Malnutrition is prevalent among patients with inflammatory bowel disease (IBD). Malnutrition is often present already at IBD diagnosis and is associated with increased hospitalizations, worse disease prognosis, and higher mortality rates.1-3 The prevalence of malnutrition is high among patients with IBD, ranging from 10% to 70% across different studies, with most reports describing rates between 10% and 35%.1,4-7 Although malnutrition screening has been recommended for patients with IBD, it is not routine practice, which has led to an underestimation of malnutrition and delays in nutrition therapy, especially in Asia, where IBD emerged only in the past decade.8,9
Evidence for malnutrition is mainly drawn from Western IBD populations, for which overnutrition, with the obesity epidemic, became an emerging issue in recent years.10 Anthropologic characteristics for the population in Asia-Pacific regions differ from those in Latin America and Europe, including lower obesity prevalence and mean body mass index (BMI).11 To date, the prevalence of malnutrition, characteristics associated with malnutrition, and the use of nutrition support have been seldom investigated among IBD patients in Asia. Malnutrition has adverse sociopsychological impacts on patients with IBD, including anxiety and depression.12 The association between malnutrition and psychological well-being has rarely been assessed among IBD patients in Asia on a large scale. Therefore, the current study aimed to investigate the prevalence of malnutrition and the use of nutrition support among patients with IBD from tertiary referral hospitals in China. The sociopsychological status among these patients and risk factors for malnutrition were also investigated.
Methods
Study Design and Study Population
This was a cross-sectional, observational study conducted at 43 hospitals across China. Patients were consecutively recruited between July 2017 and December 2017 from participating hospitals regardless of hospitalization status. Inclusion criteria were (1) a confirmed diagnosis of either ulcerative colitis (UC) or Crohn’s disease (CD); (2) age between 18 and 75 years; (3) ability to complete all assessments, including subjective questionnaires; and 4) consent to participate in the study. Exclusion criteria were (1) a history of malignancy; (2) pregnancy; (3) diseases with growth retardation, such as Turner’s syndrome; and (4) inability to complete the assessment due to intellectual disabilities.
Data Collection
Baseline Characteristics and Malnutrition Assessment
Demographic and clinical features including sex, age, BMI, smoking status, type and classification of IBD, disease duration (time interval between IBD diagnosis and malnutrition screening), disease activity, current and past use of IBD medication, and biochemical and sociopsychological profiles were all recorded using standardized paper-based questionnaires. Malnutrition was evaluated in all patients. Use of nutrition support, including partial enteral nutrition with oral nutrition supplements, exclusive enteral nutrition through nasal gastric tube, and parenteral nutrition, within 2 weeks of the assessment was also recorded.
Patients were diagnosed with malnutrition if they fulfilled the following diagnostic criteria according to the European Society for Parenteral and Enteral Nutrition (ESPEN) consensus statement13:
BMI lower than 18.5 kg/m², or
-
unintentional weight loss (either more than 10% irrespective of time or 5% within 3 months), together with 1 of the following 2 criteria:
fat-free mass index lower than 15 and 17 kg/m² for female and male participants, respectively, or
reduced BMI (lower than 20 kg/m² for patients younger than 70 years of age or lower than 22 kg/m² for patients over 70 years of age).
A BMI of <18.5 kg/m² was considered underweight, a BMI of ≥25 kg/m² was considered overweight, and a BMI of >40 kg/m² was considered obese. In addition to this malnutrition evaluation, the malnutrition risk using the Nutritional Risk Screening (NRS) 2002 system was also assessed. Patients who scored more than 2 were defined as having an increased risk for malnutrition according to a previous validation study.14
Disease Activity and Biochemical Profiles
Disease activity was assessed using the Crohn’s Disease Activity Index (CDAI) for CD and the Truelove and Witts score for UC. For patients with CD, a CDAI score ≤220 was classified as quiescent or mildly active disease, a CDAI score between 221 and 450 was classified as moderately active disease, and a CDAI score >450 was classified as severely active disease.15 For UC patients, the occurrence of diarrhea ≤4 times/d, together with an erythrocyte sedimentation rate ≤30 mm/h, normal hemoglobin, and absence of fever and tachycardia, was classified as mild disease presentation, whereas diarrhea ≥6 times/d, together with erythrocyte sedimentation rate >30 mm/h, hemoglobin decreased by 25% or the patient requiring blood transfusion, and fever and tachycardia, was classified as severe disease presentation. Patients in between these parameters were classified as having moderately active disease.16 The disease extent was classified according to the Montreal classification; ileocolonic disease in CD and pancolitis in UC were classified as extensive gastrointestinal disease.
Biochemical profiles including serum albumin, hemoglobin, and calcium levels were measured at each participating hospital within 2 weeks of the malnutrition screening. Nutrient deficiencies including hypoalbuminemia, anemia, and hypocalcemia were defined using the reference ranges at each participating hospital.
Sociopsychological Assessment
For sociopsychological assessments, patients were asked to complete 4 questionnaires assessing the quality of life, perceived social support, anxiety and depression, and perceived stress. All questionnaires had been adapted into Chinese versions and have previously been confirmed in Chinese populations as valid and reliable.17-20 Quality of life was assessed using the Inflammatory Bowel Disease Questionnaire (IBDQ), a 32-item questionnaire well recognized as an assessment tool for quality of life in patients with IBD, which has previously been validated in a Chinese IBD population.18 Scores range from 32 to 224 with each item rated on a 7-point scale, and a higher score indicates better quality of life. Social support was assessed using the Multidimensional Scale of Perceived Social Support, a 12-item multidimensional scale encompassing social support from friends, family, and significant others. Total scores range from 12 to 84 with each item rated on a 4-point scale, and a lower score indicates lack of social support. The questionnaire was initially adapted into Chinese in 1996 and had been validated among various Chinese popualtions.19,21 Anxiety and depression were assessed using the Hospital Anxiety and Depression Scale, a 14-item questionnaire consisting of 2 equally numbered subscales for depression and anxiety, respectively. The questionnaire has previously been validated among Chinese patients with cardiovascular disease.17 Scores range from 0 to 21 for each subscale, with each item rated on a 4-point scale; a score of ≥8 on each subscale was validated as indicative of possible mood disorder.22 Stress was assessed using the 14-item perceived stress scale; scores range from 0 to 56 with each item rated on a 5-point scale, and a cutoff of 25 has been validated in Chinese urban residents as indicative of abnormally high perceived stress.20,23
Statistical Analysis
Continuous variables were presented as either the mean ± SD and compared using the independent sample Student’s t test or the median (interquartile range) and compared using the Mann-Whitney U test, depending on whether the variable was normally distributed or not. Categorical variables were presented as percentages and compared using the chi-square test. Relative risk was calculated using chi-square analysis. Fisher’s exact test was used when the sample size was under 5. In the risk factor analysis, all variables with P < .1 in the univariate analysis were stepwise entered into the logistic regression. Results from the multivariate analysis were displayed as odds ratio and 95% confidence intervals. A P value of <.05 was considered statistically significant. All analyses were conducted using SPSS software (version 22; IBM Corporation, Armonk, NY, USA).
Ethical Considerations
This study was approved by the Institutional Review Board and ethics committee of the primary participating hospital. All patients signed the consent form prior to participation.
Results
Study Population
A total of 1013 patients were recruited including 805 (79.5%) hospitalized patients and 96 (9.5%) ambulatory patients; the hospitalization status of 112 patients was not reported. Overall, 593 (58.5%) patients were diagnosed with CD (Table 1), and 622 (61.4%) patients were male, comprising the dominant group, especially among patients with CD. The median age was 35.0 (interquartile range, 27.0-48.0) years for all patients, and patients with CD were significantly younger than those with UC. The mean BMI was 19.43 ± 3.11 kg/m² for all patients, and patients with CD had lower BMI than patients with UC. In total, 437 (43.1%) patients were underweight, and only 42 (4.1%) patients were overweight, with 3 patients (1 with CD and 2 with UC) being obese. Patients with ileocolonic, nonstricturing, nonpenetrating CD and those with extensive UC dominated. The majority of patients with CD had disease remission or mild disease activity, whereas the majority of patients with UC had moderate to severe disease. The use of steroids was also more common in patients with UC than in those with CD.
Table 1.
Baseline characteristics of all patients who underwent nutritional status assessment
| Variable | CD (n = 593) | UC (n = 420) | P Value |
|---|---|---|---|
| Female | 196 (33.1) | 195 (46.4) | <.01 |
| Age, y 2 | 30.0 (24.0-41.0) | 45.5 (31.8-54.3) | <.01 |
| BMI, kg/m² | 18.73 ± 2.90 | 20.41 ± 3.12 | <.01 |
| <18.5 kg/m² | 310 (52.3) | 127 (30.2) | <.01 |
| 18.5-25 kg/m² | 265 (44.7) | 269 (64.1) | <.01 |
| ≥25 kg/m² | 18 (3.0) | 24 (5.7) | <.01 |
| Current smokers | 48 (8.1) | 45 (10.7) | <.01 |
| Disease duration, y 22 | 3.0 (1.0-7.0) | 2.9 (1.0-6.6) | <.01 |
| Disease location of CD | — | — | — |
| Distal ileum/limited cecal | 184 (31.1) | — | — |
| Colonic | 104 (17.5) | — | — |
| Ileocolonic | 305 (51.4) | — | — |
| Upper GI a | 40 (8.5) | — | — |
| Disease behavior of CD | — | — | — |
| Nonstricturing, nonpenetrating | 287 (48.4) | — | — |
| Stricturing | 190 (32.0) | — | — |
| Penetrating | 116 (19.6) | — | — |
| Perianal disease | 206 (34.7) | — | — |
| Disease extent of UC | — | — | — |
| Proctitis | — | 54 (12.9) | — |
| Left-sided colitis | — | 134 (31.9) | — |
| Extensive colitis | — | 232 (55.2) | — |
| Disease activity | — | — | <.01 |
| Quiescent/mild | 487 (82.2) | 145 (34.5) | — |
| Moderate | 82 (13.8) | 146 (34.8) | — |
| Severe | 24 (4.0) | 129 (30.7) | — |
| Current medication | — | — | — |
| Aminosalicylic acid | 135 (22.8) | 346 (82.4) | <.01 |
| Steroids | 76 (12.8) | 125 (29.8) | <.01 |
| Immunosuppressants | 186 (31.4) | 27 (6.4) | <.01 |
| Biologics | 208 (35.1) | 24 (5.7) | <.01 |
| Previous medication | — | — | — |
| Aminosalicylic acid | 296 (49.9) | 353 (84.0) | <.01 |
| Steroids | 152 (25.6) | 181 (43.1) | <.01 |
| Immunosuppressants | 189 (31.9) | 35 (8.3) | <.01 |
| Biologics | 173 (29.2) | 21 (5.0) | <.01 |
Values are n (%), median (interquartile range), or mean ± SD.
Abbreviations: BMI, body mass index; CD, Crohn’s disease; GI, gastrointestinal tract; UC, ulcerative colitis.
A total of 120 patients were not assessed regarding upper GI involvement.
Prevalence of Malnutrition
A total of 501 patients were diagnosed with malnutrition, yielding a prevalence of 49.5%. BMI was 17.11 ± 1.73 kg/m² for malnourished patients, and 437 (87.2%) patients with malnutrition were underweight. There was 1 out of 42 overweight patients that was also diagnosed with malnutrition. A total of 193 patients were diagnosed with malnutrition based on low BMI alone. Among patients with malnutrition, the proportion of underweight patients with CD was significantly higher than patients with UC (91.7% vs 77.9%, P < .01). The proportion of underweight patients did not differ between different sexes, by hospitalization status, or among different age categories. The prevalence of malnutrition was higher in CD than UC, higher in female patients than in male patients, and decreased with advanced age (Table 2). Among the 632 patients with quiescent or mildly active disease, 44.1% (n = 279 of 632) were malnourished, which increased to 50.7% (n = 247 of 487) in CD and decreased to 22.1% (n = 32 of 145) in UC. For patients with moderate to severe disease, the prevalence of malnutrition increased significantly, with 58.2% (n = 222 of 381; P < .05) in patients overall, 85.5% (n = 91 of 106; P < .05) in patients with CD, and 47.6% (n = 131 of 275; P < .05) in patients with UC.
Table 2.
Prevalence of malnutrition stratified by IBD subtype, age, and sex
| Patient Group | Malnutrition | RR (95% CI) | P Value |
|---|---|---|---|
| Overall | 501 (49.5) | — | — |
| IBD subtype | — | — | — |
| UC | 163 (38.8) | 1 | — |
| CD | 338 (57.0) | 2.090 (1.620-2.696) | <.01 |
| Age | — | — | — |
| 18 y ≤ age < 45 y | 384 (55.8) | 1 | — |
| 45 y ≤ age < 65 y | 102 (38.1) | 0.486 (0.364-0.649) | <.01 |
| 65 y ≤ age ≤ 75 y | 15 (26.3) | 0.283 (0.154-0.520) | <.01 |
| Sex | — | — | — |
| Male | 286 (46.0) | 1 | — |
| Female | 215 (55.0) | 1.435 (1.113-1.850) | <.01 |
Values are n (%), unless otherwise indicated.
Abbreviations: CD, Crohn’s disease; CI, confidence interval; IBD, inflammatory bowel disease; RR, relative risk; UC, ulcerative colitis.
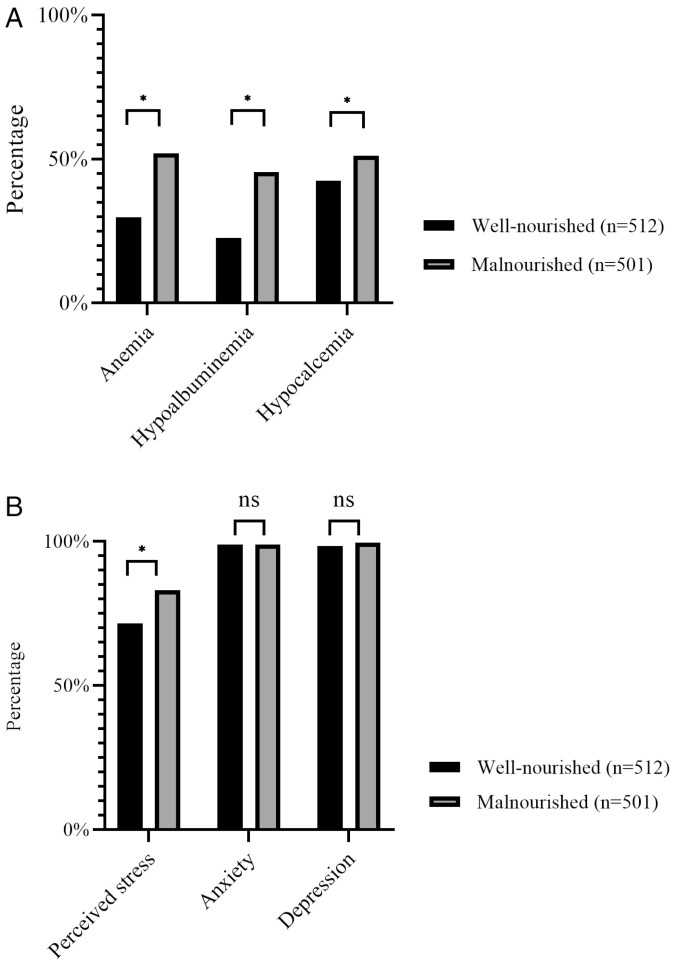
Biochemical and Sociopsychological Profiles
Biochemical and sociopsychological profiles were recorded and compared between patients with and without malnutrition (Figure 1A, B). Although anemia, hypoalbuminemia, and hypocalcemia were more frequent in malnourished patients (52%, 45.5%, and 51.1%, respectively), these nutrient deficiencies also existed among well-nourished patients, with 29.8%, 22.7%, and 42.5% of patients being affected by anemia, hypoalbuminemia, and hypocalcemia, respectively. A total of 741 (73.1%) patients returned valid questionnaires on sociopsychological assessments, including 375 (73.2%) well-nourished patients and 366 (73.1%) malnourished patients. The average IBDQ score among all patients was 150.0 ± 42.2, and malnutrition was associated with a further decrease (well-nourished vs malnourished patients: 157.0 ± 45.9 vs 142.0 ± 36.5; P < .01). Malnutrition was also associated with decreased social support scores (well-nourished vs malnourished patients: 63.8 ± 10.2 vs 61.6 ± 11.3; P < .01), and patients with malnutrition were more likely to report high perceived stress (well-nourished vs malnourished patients: 71.5% vs 83.1%; P < .01). The 2 groups were similar regarding rates of anxiety and depression, which were all higher than 95%, regardless of nutritional status.
Figure 1.
A and B, Biochemical and sociopsychological profiles among patients with different nutritional statuses. *P < .05; ns, not significant.
Use of Nutrition Support
A total of 421 (41.6%) patients received nutrition support. Malnourished patients were more likely to receive nutrition support than well-nourished patients (well-nourished vs malnourished patients: 38.1%, n = 164 of 512 vs 51.3%, n = 257 of 501; P < .01). Among the 164 well-nourished patients who received nutrition support, 53 patients were assessed as having malnutrition risk according to their NRS-2002 scores. Among the remaining 111 patients without malnutrition risk, the majority (n = 73 of 111, 65.8%) had moderate to severe disease. In total, 305 patients reported the length of nutrition support, with the majority (n = 267 of 305, 87.5%) lasting less than a month, and only 14 (4.6%) patients received relatively long-term support for more than 2 months. Overall, 364 (86.5%) patients reported the routes of nutrition support, including 169 (40.1%) patients who received enteral nutrition, 136 (32.3%) who received parenteral nutrition, and 59 (14.0%) patients who received a combination of enteral and parenteral nutrition.
Patients who received nutrition support were comparable in terms of age, sex, and disease duration to those without nutrition support, but they had lower BMI (18.73 ± 3.10 kg/m2 vs 20.10 ± 2.99 kg/m2; P < .01) and IBDQ (145.5 ± 47.8 vs 152.4 ± 37.3; P = .024) scores. Patients with CD were more likely to receive nutrition support than patients with UC (65.6% vs 53.5%; P < .01). Among patients with CD, the proportion of patients receiving nutrition support was independent of the presence of strictures or fistulas. In addition, 23.5% of patients with strictures or fistulas received parenteral nutrition, which was not significantly higher than this frequency in patients with pure inflammatory disease behaviors (19.1%). In total, 46.3% (n = 184 of 381) of patients with moderate to severe disease received nutrition support, which was higher than this percentage in patients with quiescent or mildly active disease (37.5%, n = 237 of 632). Patients with a more extensive disease were also more likely to receive nutrition support (n = 240 of 537, 44.7% vs n = 176 of 476, 37.0%; P = .013) (Supplementary Table 1).
Risk Factors Associated With Malnutrition
Demographic- and IBD-related features were compared between patients with and without malnutrition. In univariate analyses, malnutrition among patients with CD was associated with younger age, shorter disease duration, presence of either strictures or fistulas, ileocolonic disease, moderate to severe disease, and current use of steroids or immunosuppressants. In addition, CD patients with malnutrition were less likely to receive biologics as treatment. Neither the presence of upper gastrointestinal diseases nor the presence of prior CD-related bowel surgeries was associated with malnutrition among patients with CD. Malnutrition among patients with UC was associated with younger age, female sex, extensive disease, and moderate to severe disease. UC patients with malnutrition were more likely to be treated with steroids (Supplementary Tables 2 and 3). The results of the multivariate logistic regression suggested that ileocolonic disease, moderate to severe disease, presence of strictures or fistulas, current use of steroids, and shorter disease duration were independent risk factors for malnutrition among patients with CD. For patients with UC, extensive disease, active flare, and female sex were independent risk factors for malnutrition, and the risk for malnutrition decreased with increasing age (Table 3).
Table 3.
Multivariate analysis of risk factors associated with malnutrition among patients with CD or UC
| IBD Type | Variable | OR | 95% CI | P Value |
|---|---|---|---|---|
| CD | Ileocolonic disease (yes vs no) | 2.350 | 1.414-3.649 | <.01 |
| Moderate to severe disease (yes vs no) | 5.571 | 2.736-11.346 | <.01 | |
| Presence of strictures (yes vs no) | 3.357 | 2.007-5.616 | <.01 | |
| Presence of fistulas (yes vs no) | 2.090 | 1.136-3.845 | .018 | |
| Disease duration, every increase in 1 y | 0.925 | 0.882-0.971 | <.01 | |
| Current use of glucocorticoids (yes vs no) | 2.407 | 1.247-4.646 | <.01 | |
| UC | Age, every increase in 1 y | 0.958 | 0.942-0.975 | <.01 |
| Female (yes vs no) | 2.851 | 1.765-4.605 | <.01 | |
| Pancolitis (yes vs no) | 2.025 | 1.237-3.317 | <.01 | |
| Moderate to severe disease (yes vs no) | 2.760 | 1.604-4.750 | <.01 |
Abbreviations: CD, Crohn’s disease; CI, confidence interval; IBD, inflammatory bowel disease; OR, odds ratio; UC, ulcerative colitis.
Discussion
In this cross-sectional, multicenter study, we report a malnutrition prevalence of 49.5% among patients with IBD in China. Nutrient deficiencies were present regardless of nutritional status. Malnutrition was associated with impaired quality of life and adverse sociopsychological status among patients with IBD. Moderate to severe disease and extensive disease were independent risk factors for malnutrition, and patients with these risk factors were more likely to receive nutrition support.
Almost half of the patients were diagnosed with malnutrition, which is higher than previously reported rates between 10% and 35%.1,5-7 There are several contributory factors to higher malnutrition prevalence, including a study population comprising mainly hospitalized patients and the use of a mandatory screening process. In hospital-based studies with more proactive screening strategies, malnutrition prevalence rates were reported to be as high as 69.7%, whereas a prevalence of <8% was reported in a population-based study in which universal screening for malnutrition was not performed.1,4-7 In addition, differences in obesity prevalence among patients in Asia-Pacific regions, Latin America, and Europe might also be a contributing factor.11 The Western population is now facing an obesity epidemic, and overnutrition is an emerging issue that affects patients with IBD.10 In our study, <6% of patients were overweight, which is a much lower frequency than in previous reports in which obesity was recorded in up to 31% of patients.10,24 Because the ESPEN criteria were not specifically developed for the Asian population, a unifying BMI for diagnosis of malnutrition across different populations may lead to inaccuracy in the estimation of malnutrition. However, the average BMI of the IBD population in the current study was already lower than the non-IBD population in China back in 2009, which was 23.0 kg/m² in average. In addition, the average BMI of Chinese population has been increasing over the past decades according to a population-based investigation.25 Therefore, results from the current investigation did reflect a generally poorer nutritional status among patients with IBD in China. Use of ESPEN criteria minimized interobserver bias across different study sites, as it adopted a simple and objective evaluation of malnutrition. Given the sample size of our study, and a universal screening strategy being adopted across all participating hospitals, the influence of selection bias on the results were minimal.
In the current study, patients with CD had higher malnutrition prevalence than those with UC; moderate to severe disease and extensive gastrointestinal disease were the 2 major risk factors for malnutrition regardless of IBD subtypes. It was previously speculated that patients with CD were at higher risk of malnutrition compared with those with UC due to more extensive gastrointestinal involvement.8 The presence of fistulas further increases the risk of malnutrition in patients with CD, which was also observed in the current study.1 Active inflammation impairs intestinal absorption and increases protein requirements. In addition, gastrointestinal symptoms lead to altered dietary behaviors, all of which are contributory to malnutrition.1,26,27 Nutrition support is recommended during disease flares and when poor nutritional status cannot be improved via the oral route.8 Likewise, we discovered that patients with CD were more likely to receive nutrition support, possibly as a result of higher malnutrition prevalence and more active disease. Nutrition therapy such as exclusive enteral nutrition is also considered an important treatment for active CD, especially among pediatric patients, and it also effectively decreases the severity and reoccurrence of fistulas.28,29 Accordingly, we suggest that nutrition therapy is of great value in the care of IBD patients, especially of patients with CD.
Although increased age is considered a risk factor for malnutrition due to decreased lean body mass, malnutrition was associated with younger age in the current study.14 In addition, we found a significant association between malnutrition and shorter disease duration, which was similar to findings of previous studies that showed a decrease in malnutrition prevalence with longer disease duration.10,30 It should be noted that disease duration in the current study was defined as the time since an IBD diagnosis had been confirmed. A delayed diagnosis might adversely affect the nutritional status due to a prolonged disease course with no timely treatment, but this issue was not addressed in this study. In the current investigation, the observation that malnutrition was associated with shorter disease duration might suggest less controlled disease courses among newly diagnosed patients compared with patients with a longer disease course who already received treatment and achieved remission.10 Given the fact that malnutrition can affect younger patients with newly diagnosed diseases, we speculate that more active diseases among younger patients might be a contributing factor to such an observation.3 Besides, patients with CD had higher malnutrition prevalence and were generally younger than patients with UC in the current study; therefore, the IBD subtype may also serve as a link between younger age and malnutrition. Nevertheless, the number of older patients was relatively small compared with younger patients, which limited the power of our analysis. In addition, dietary behaviors such as food restriction might also be a contributing factor, as demonstrated by a previous study suggesting that younger patients were more likely to restrict food intake compared with older patients during disease flares.5 Further investigations on how active food restriction affects nutritional status may uncover the etiology behind such observations in the current study population.
We found that malnutrition was associated with the female sex. Previous studies seem to have inconsistent findings regarding sex differences in the risk for malnutrition. Valentini et al31 showed possible trends toward higher malnutrition prevalence among female patients with UC, and Gee et al32 also discovered that protein undernutrition was more prevalent among female patients. However, Nguyen et al1 showed that female sex may be a protective factor against malnutrition. Owing to these inconsistencies across studies, the ESPEN guideline does not recommend a sex-specific strategy for nutritional care, and our results did not identify any sex differences that impact the use of nutrition support.
Malnutrition is associated with multiple adverse events including increased frequency of emergency department attendance and nonelective surgeries, resulting in a higher likelihood of postoperative complications such as infection and prolonged wound healing. Malnutrition also increases the length of hospital stay, results in higher medical expenditure, and increases mortality.1,33-35 Malnourished patients also experience frequent episodes of depression, anxiety, and lower quality of life.12,36 The association between sociopsychological status and malnutrition has been scarcely explored among patients with IBD in Asia. In our study, malnutrition was associated with higher perceived stress and decreased social support; in addition, we discovered a surprisingly high prevalence of depression and anxiety among patients with IBD, similar to a previous report.37 There are multiple interactive relationships among disease flares, malnutrition, and mental health. Decreased appetite directly leads to malnutrition in patients with mood disorders, and for patients with IBD, anxiety is associated with an increase in disabling symptoms such as abdominal pain, which also interferes with food intake.38 In addition, active flares and altered dietary behaviors contribute to malnutrition, causing metabolite dysregulation and possibly resulting in a higher risk for mood disorders.12 The high prevalence of mood disorders and lack of social support in association with malnutrition among patients with IBD suggests that psychological interventions and the establishment of support systems are necessary, especially for patients with malnutrition.
Using mandatory screening protocol, we discovered that prevalence of malnutrition was high. Though several risk factors for malnutrition were identified in our study, patients without these risk factors, such as patients during quiescent or mildly active disease, still faced a malnutrition prevalence of 44.1%. This was consistent with previous studies, in which 26% to 30% of patients in disease remission were diagnosed with malnutrition.6,27,31 Therefore, screening for malnutrition is essential, including screening among patients without identifiable risk factors for malnutrition. In fact, the ESPEN guideline recommends malnutrition screening for all patients with IBD at diagnosis and thereafter.8 However, around 33% of gastroenterologists do not routinely screen patients for malnutrition, and up to two-thirds of patients report unmet dietetic needs during clinic visits, which results in delayed nutrition support.4,9,10 Consistent with previous observations, we found that nutrient deficiencies including anemia and hypoalbuminemia were prevalent, suggesting that a negative malnutrition screening result does not exclude the need for nutrition support.3,31 Nutrition support has been shown to induce mucosal healing and enhance postoperative recovery, which are beneficial even when achieved on a short-term basis.29,39,40 Therefore, universal screening is essential, and the use of nutrition support should be based on multiple perspectives including nutrient profiles, patients’ needs, and potential therapeutic benefits.
This study has several limitations. First, though the majority of patients who received nutrition support were diagnosed with malnutrition, having disease flares, or at increased risk for developing malnutrition, we did not investigate the exact reasons why nutrition support was initiated and the detailed route of nutritional support the patient received. For example, it is not known if the patients were on exclusive enteral nutrition for treatment purpose or were merely receiving oral nutritional products as dietary supplements. Also, we did not investigate if these patients actually met the ESPEN criteria to initiate exclusive enteral nutrition or parenteral nutrition (eg, unable to achieve nutrition requirements by the oral route). However, because duration for nutrition support was short for most of the patients, it was more likely that patients received nutrition product as dietary supplements rather than as part of therapy for CD. In addition, it should be noted that the NRS-2002 system has not been validated among an ambulatory population. Though outpatients consisted a small population in the current study, malnutrition risk screening results among outpatients should be interpreted with caution. Second, dietary behaviors such as food restrictions and dietary consultations provided to patients were not investigated in this study. These are important aspects of nutrition support in patients with IBD, which require more robust investigations in the future. Third, the number of elderly patients in the current study was too small to be representative of this age group, and patients over 75 years of age were excluded. Therefore, the association between aging and reduced malnutrition risk is likely biased and should be interpreted with caution. Future studies investigating the nutritional status in older patients with IBD will provide more pertinent insights into the aging population. Last, owing to the nature of this cross-sectional multicenter study with a large sample size, the consistency of the sociopsychological assessments as well as anthropometry were not fully guaranteed. Detailed instructions for all questionnaires were provided, and all investigators were trained regarding the proper use of the questionnaires prior to the investigations to ensure minimal bias across study sites regarding use of questionnaires.
Conclusions
This was the first study to report the prevalence of and risk factors for malnutrition, as well as the use of nutrition support in Chinese patients with IBD. We showed that malnutrition was highly prevalent. In addition, nutrient deficiencies and adverse sociopsychological status were present even among patients without malnutrition. We also found that malnutrition was associated with the worsening of these adverse events. This study highlights the importance of malnutrition screening and early initiation of nutrition support in patients with IBD.
Supplementary Material
Acknowledgments
We thank Xiaocang Cao, Chunxiao Chen, Jiang Chen, Yan Chen, Baisui Feng, Yubei Gu, Hong Guo, Qin Guo, Huige Hu, Shanshan Hu, Yiqun Hu, Xiaoxi Huang, Yuhong Huang, Yan Jia, Shu Jin, Xue Jing, Guoxun Li, Hongguang Li, Hui Li, Junxia Li, Mingsong Li, Xiuling Li, Ya Li, Yuqin Li, Guanghui Lian, Wangdi Liao, Fei Liu, Xin Liu, Ling Luo, Lin Lv, Xiaoping Lv, Lin Mi, Zhi Pang, Guoqing Qi, Hongmei Qian, Zhiyong Rao, Jun Shen, Yanhong Shi, Xiaomei Sun, Yan Tan, Wen Tang, Chengdang Wang, Junda Wang, Qunying Wang, Ruiling Wang, Xiaoying Wang, Xiaoling Wang, Jing Wu, Xiaoli Wu, Lihong Xu, Xuemei Xu, Xinpeng You, Jing Yu, Hongjie Zhang, Xiaolan Zhang, Feng Zhou, Lanxiang Zhu, and Liangru Zhu for their contributions to patient recruitment and data acquisition.
Contributor Information
Jing Liu, Inflammatory Bowel Disease Center, Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, China; Department of Gastroenterology, Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, China.
Xiaolong Ge, Inflammatory Bowel Disease Center, Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, China; Department of General Surgery, Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, China.
Chunhui Ouyang, Department of Gastroenterology, The Second Xiangya Hospital, Central South University, Changsha, China.
Dongxu Wang, Department of Gastroenterology, Shengjing Hospital, China Medical University, Shenyang, China.
Xiaoqi Zhang, Department of Gastroenterology, Nanjing Drum Tower Hospital, School of Medicine, Nanjing University, Nanjing, China.
Jie Liang, Department of Gastroenterology, Xijing Hospital, School of Medicine, Fourth Military Medical University, Xi’an, China.
Weiming Zhu, Department of General Surgery, Jinling Hospital, Nanjing University, Nanjing, China.
Qian Cao, Inflammatory Bowel Disease Center, Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, China; Department of Gastroenterology, Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, China.
Author Contributions
All authors have contributed to the following: (1) conception and design of the study, data acquisition, analysis, and interpretation; (2) drafting and revising of the article; and (3) final approval of the version to be submitted. J.Liu, X.G.: study design, data analysis and interpretation, writing up the first draft, draft revision. C.O., D.W., X.Z., J.Liang: study design, patient recruitment, data acquisition and interpretation, draft revision. W.Z., Q.C: study design, patient recruitment, data interpretation, writing up the first draft, draft revision.
Funding
This study received no funding.
Supplement Sponsorship
This supplement was sponsored by the China Health Promotion Foundation.
Conflicts of Interest
The authors declare that they have no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1. Nguyen GC, Munsell M, Harris ML.. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm Bowel Dis. 2008;14:1105-1111. [DOI] [PubMed] [Google Scholar]
- 2. Rocha R, Sousa UH, Reis TLM, Santana GO.. Nutritional status as a predictor of hospitalization in inflammatory bowel disease: a review. World J Gastrointest Pharmacol Ther. 2019;10:50-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Geerling BJ, Badart-Smook A, Stockbrügger RW, Brummer RJ.. Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur J Clin Nutr. 2000;54:514-521. [DOI] [PubMed] [Google Scholar]
- 4. Mijac DD, Janković GL, Jorga J, Krstić MN.. Nutritional status in patients with active inflammatory bowel disease: prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med. 2010;21:315-319. [DOI] [PubMed] [Google Scholar]
- 5. Casanova MJ, Chaparro M, Molina B, et al. Prevalence of malnutrition and nutritional characteristics of patients with inflammatory bowel disease. J Crohns Colitis. 2017;11:1430-1439. [DOI] [PubMed] [Google Scholar]
- 6. Filippi J, Al-Jaouni R, Wiroth JB, et al. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm Bowel Dis. 2006;12:185-191. [DOI] [PubMed] [Google Scholar]
- 7. Vadan R, Gheorghe LS, Constantinescu A, Gheorghe C.. The prevalence of malnutrition and the evolution of nutritional status in patients with moderate to severe forms of Crohn’s disease treated with Infliximab. Clin Nutr. 2011;30:86-91. [DOI] [PubMed] [Google Scholar]
- 8. Bischoff SC, Escher J, Hébuterne X, et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin Nutr. 2020;39:632-653. [DOI] [PubMed] [Google Scholar]
- 9. Tinsley A, Ehrlich OG, Hwang C, et al. Knowledge, attitudes, and beliefs regarding the role of nutrition in IBD among patients and providers. Inflamm Bowel Dis. 2016;22:2474-2481. [DOI] [PubMed] [Google Scholar]
- 10. Lomer MCE, Cahill O, Baschali A, et al. A multicentre study of nutrition risk assessment in adult patients with inflammatory bowel disease attending outpatient clinics. Ann Nutr Metab. 2019;74:18-23. [DOI] [PubMed] [Google Scholar]
- 11. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Addolorato G, Capristo E, Stefanini GF, Gasbarrini G.. Inflammatory bowel disease: a study of the association between anxiety and depression, physical morbidity, and nutritional status. Scand J Gastroenterol. 1997;32:1013-1021. [DOI] [PubMed] [Google Scholar]
- 13. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - an ESPEN Consensus Statement. Clin Nutr. 2015;34:335-340. [DOI] [PubMed] [Google Scholar]
- 14. Kondrup J, Rasmussen HH, Hamberg O, Stanga Z; Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321-336. [DOI] [PubMed] [Google Scholar]
- 15. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] [Google Scholar]
- 16. Truelove SC, Witts LJ.. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang Y, Ding R, Hu D, et al. Reliability and validity of a Chinese version of the HADS for screening depression and anxiety in psycho-cardiological outpatients. Compr Psychiatry. 2014;55:215-220. [DOI] [PubMed] [Google Scholar]
- 18. Ren WH, Lai M, Chen Y, et al. Validation of the mainland Chinese version of the Inflammatory Bowel Disease Questionnaire (IBDQ) for ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2007;13:903-910. [DOI] [PubMed] [Google Scholar]
- 19. Zhou K, Li H, Wei X, et al. Reliability and validity of the multidimensional scale of perceived social support in Chinese mainland patients with methadone maintenance treatment. Compr Psychiatry. 2015;60:182-188. [DOI] [PubMed] [Google Scholar]
- 20. Yang TZ, Huang HT.. An epidemiological study on stress among urban residents in social transition period. Zhonghua Liu Xing Bing Xue Za Zhi. 2003;24:760-764. [PubMed] [Google Scholar]
- 21. Slonim-Nevo V, Sarid O, Friger M, et al. ; Israeli IBD Research Nucleus (IIRN). Effect of social support on psychological distress and disease activity in inflammatory bowel disease patients. Inflamm Bowel Dis. 2018;24:1389-1400. [DOI] [PubMed] [Google Scholar]
- 22. Bennebroek Evertsz F, Thijssens NAM, Stokkers PCF, et al. Do inflammatory bowel disease patients with anxiety and depressive symptoms receive the care they need? J Crohns Colitis. 2012;6:68-76. [DOI] [PubMed] [Google Scholar]
- 23. Cohen S, Kamarck T, Mermelstein R.. A global measure of perceived stress. J Health Soc Behav. 1983;24:385-396. [PubMed] [Google Scholar]
- 24. Seminerio JL, Koutroubakis IE, Ramos-Rivers C, et al. Impact of obesity on the management and clinical course of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:2857-2863. [DOI] [PubMed] [Google Scholar]
- 25. Xi B, Liang Y, He T, et al. Secular trends in the prevalence of general and abdominal obesity among Chinese adults, 1993-2009. Obes Rev. 2012;13:287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hartman C, Marderfeld L, Davidson K, et al. Food intake adequacy in children and adolescents with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2016;63:437-444. [DOI] [PubMed] [Google Scholar]
- 27. Costa CO, Carrilho FJ, Nunes VS, et al. A snapshot of the nutritional status of Crohn’s disease among adolescents in Brazil: a prospective cross-sectional study. BMC Gastroenterol. 2015;15:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yan D, Ren J, Wang G, et al. Predictors of response to enteral nutrition in abdominal enterocutaneous fistula patients with Crohn’s disease. Eur J Clin Nutr. 2014;68:959-963. [DOI] [PubMed] [Google Scholar]
- 29. Swaminath A, Feathers A, Ananthakrishnan AN, et al. Systematic review with meta-analysis: enteral nutrition therapy for the induction of remission in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2017;46:645-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cameron FL, Gerasimidis K, Papangelou A, et al. Clinical progress in the two years following a course of exclusive enteral nutrition in 109 paediatric patients with Crohn’s disease. Aliment Pharmacol Ther. 2013;37:622-629. [DOI] [PubMed] [Google Scholar]
- 31. Valentini L, Schaper L, Buning C, et al. Malnutrition and impaired muscle strength in patients with Crohn’s disease and ulcerative colitis in remission. Nutrition. 2008;24:694-702. [DOI] [PubMed] [Google Scholar]
- 32. Gee MI, Grace MG, Wensel RH, et al. Protein-energy malnutrition in gastroenterology outpatients: increased risk in Crohn’s disease. J Am Diet Assoc. 1985;85:1466-1474. [PubMed] [Google Scholar]
- 33. Chima CS, Barco K, Dewitt ML, et al. Relationship of nutritional status to length of stay, hospital costs, and discharge status of patients hospitalized in the medicine service. J Am Diet Assoc. 1997;97:975-978; quiz 979. [DOI] [PubMed] [Google Scholar]
- 34. Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K.. A novel risk score to stratify severity of Crohn’s disease hospitalizations. Am J Gastroenterol. 2010;105:1799-1807. [DOI] [PubMed] [Google Scholar]
- 35. Weinsier RL, Hunker EM, Krumdieck CL, Butterworth CE Jr. Hospital malnutrition. A prospective evaluation of general medical patients during the course of hospitalization. Am J Clin Nutr. 1979;32:418-426. [DOI] [PubMed] [Google Scholar]
- 36. Norman K, Kirchner H, Lochs H, Pirlich M.. Malnutrition affects quality of life in gastroenterology patients. World J Gastroenterol. 2006;12:3380-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walker JR, Ediger JP, Graff LA, et al. The Manitoba IBD cohort study: a population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am J Gastroenterol. 2008;103:1989-1997. [DOI] [PubMed] [Google Scholar]
- 38. Goodhand JR, Wahed M, Mawdsley JE, et al. Mood disorders in inflammatory bowel disease: relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm Bowel Dis. 2012;18:2301-2309. [DOI] [PubMed] [Google Scholar]
- 39. Li G, Ren J, Wang G, et al. Preoperative exclusive enteral nutrition reduces the postoperative septic complications of fistulizing Crohn’s disease. Eur J Clin Nutr. 2014;68:441-446. [DOI] [PubMed] [Google Scholar]
- 40. Mańkowska-Wierzbicka D, Karczewski J, Swora-Cwynar E, et al. The clinical importance of 21-day combined parenteral and enteral nutrition in active inflammatory bowel disease patients. Nutrients. 2019;11:2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.