Abstract
Background:
Keratinocyte carcinomas are amenable to many treatments, including radiotherapy (RT). Electronic skin surface brachytherapy (ESSB) enables the precise delivery of radiation without radioisotopes.
Objectives:
In this prospective multicenter clinical trial, we characterized early outcomes of ESSB prospectively through both patient- and clinician-reported measures. To corroborate the cosmesis observations, we also assessed patient-reported quality of life (QoL) and adverse events.
Methods:
Patients ≥60 years old with stage T1N0M0 keratinocyte carcinoma were treated with ESSB. At 2, 6, and 12 weeks post-treatment, cosmesis from ESSB was assessed by both the patient and a clinician study investigator as either “good,” “fair”, or “bad.” The Skindex-16 and the Skin Cancer Index (SCI) were used to assess patient QoL before and after treatment. Adverse events were assessed using the Common Toxicity Criteria for Adverse Events (CTCAE), version 4.0.
Results:
Cosmesis and QoL were collected at 97% (99/102) of possible follow-up patient-time points. By 12 weeks post-treatment, 93.9% (31/33) of patient-reported and 96.9% (31/32) of clinician-reported cosmesis outcomes were “good.” Compared to baseline, total Skindex-16 score significantly deteriorated at 2 weeks post-treatment (10.5 vs 24.5, p<0.001), but significantly improved at 6 weeks (10.5 vs 4.7, p=.014) and 12 weeks (10.5 vs 2.1, p=.001) post-treatment. Total SCI score significantly improved from baseline to 6 weeks (78.4 vs 89.0, p=.001) post-treatment. The most frequent adverse events were radiation dermatitis, skin pain, and pruritus. All adverse events resolved to Grade ≤1 by 12 weeks post-treatment.
Conclusions:
This prospective, multicenter study demonstrated that ESSB is associated with a high rate of “good” early patient-reported cosmesis and increasing QoL and satisfaction with time. Validated assessments demonstrated a significant improvement in quality of life and resolution of moderate early adverse events by 6–12 weeks after treatment and corroborate the observation of favorable cosmesis.
Introduction
Keratinocyte carcinomas including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are the most common malignancy.1 Early-stage keratinocyte carcinomas are amenable to many treatments including topical medicines, photodynamic therapy, cryotherapy, electrodessication and curettage, surgery, and radiotherapy (RT).2,3 Electronic skin surface brachytherapy (ESSB) enables the precise delivery of radiation without radioisotopes, potentially limiting staff exposure and side effects.4
Prospective studies of the early cosmesis, quality of life (QoL), and adverse events following ESSB are limited. Moreover, little is known about patient-reported QoL as assessed by validated instruments following RT for skin cancer.5–7 These outcomes are crucial to characterize in the context of malignancies that are not life-threatening that have a wide range of effective treatment options available. A better understanding of these issues may help guide shared decision making about treatment options and expectations after treatment.
In this prospective, multicenter clinical trial, we characterized early outcomes of ESSB, hypothesizing that this novel method of delivering RT would afford cosmesis superior to what has been previously reported.8 To corroborate the cosmesis observations, we also prospectively assessed patient reported QoL and adverse events following ESSB for early-stage keratinocyte carcinoma.
Methods
This prospective, multicenter clinical trial was approved by the Institutional Review Board (MSKCC IRB 14-001, NCT02131805) Written patient consent was obtained. Patients ≥60 years old with an American Joint Committee on Cancer clinical stage T1N0M0 BCC or SCC were recruited. Patients were treated with ESSB using Esteya® (Elekta AB, Stockholm). Patients received a total dose of 42 Gy in 6 fractions of 7 Gy, administered over 2 to 3 weeks to a depth of 3 mm below skin surface using the smallest applicator that encompassed the clinical target volume, defined as the gross tumor with a radial expansion of 4 mm on the skin surface.
At 2, 6, and 12 weeks post-treatment, cosmesis was assessed by both the patient and a clinician investigator (CAB or MEK) as either “good,” “fair”, or “bad,” according to a previously reported method.8 The investigator also assessed the effect of ESSB on skin as “slightly visible,” “clearly marked,” or “bad.”8 Patients assessed their satisfaction with treatment using a visual analogue scale with the anchor descriptors “not satisfied” and “satisfied.”
The Skindex-16 and the Skin Cancer Index (SCI), both validated patient-reported outcome instruments, were used to assess patient QoL before and after treatment.9,10 Better QoL is represented by a lower score on the Skindex-16, but by a higher score on the SCI. These were administered at baseline, 2, 6, and 12 weeks post-treatment.
Adverse events, including radiation dermatitis, alopecia, skin atrophy, pain, pruritus, hyperpigmentation, hypopigmentation, ulceration, telangiectasia, and induration, were assessed and graded using the Common Toxicity Criteria for Adverse Events (CTCAE), version 4.0. A clinician investigator (CAB or MEK) performed these assessments each week during treatment and at 2-, 6-, and 12-weeks post-treatment.
A primary endpoint of the study was patient-reported cosmesis, with the hypothesis that ESSB would be associated with a 12-week post-treatment patient-report of “good” cosmesis in ≥90% of patients. A maximum sample size of 34 patients was calculated to determine if the rate of “good” cosmesis was superior to a previously reported 74% rate of “good” cosmesis based on a 90% 2-sided Clopper-Pearson confidence interval of the sample proportion of patients who reported “good” cosmesis.
Descriptive statistics were used to characterize the patient population, skin cancers, and treatments, and summarize survey results. The Wilcoxon signed rank test was used to assess differences in QoL scores from the Skindex-16 and SCI between baseline and each follow up time point. All analyses were performed using IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, N.Y., USA).
Results
Baseline subject characteristics of the 34 participants are summarized in Table 1. Mean age was 75 years (range 59–93). Most patients were white (100%), non-Hispanic (100%), women (58.8%) who were treated for BCC (94.1%), on the face (97.1%), and nose (67.6%). The median tumor diameter was 8 mm (range 3–17 mm).
Table 1.
Subject Characteristics (n=34)
| n | % | |
|---|---|---|
|
| ||
| Sex | ||
| Women | 20 | 58.8 |
| Men | 14 | 41.2 |
| Race | ||
| White | 34 | 100 |
| Ethnicity | ||
| Non-Hispanic | 34 | 100 |
| Histology | ||
| BCC | 32 | 94.1 |
| SCC | 2 | 5.9 |
| Tumor Location | ||
| Face | 33 | 97.1 |
| Cheek | 5 | 14.7 |
| Chin | 1 | 2.9 |
| Forehead | 4 | 11.8 |
| Nose | 23 | 67.6 |
| Leg | 1 | 2.9 |
| Applicator Size | ||
| 15 mm | 8 | 23.5 |
| 20 mm | 14 | 41.2 |
| 25 mm | 10 | 29.4 |
| 30 mm | 2 | 5.9 |
Cosmesis and QoL was collected at 97% (99/102) of possible follow-up patient-time points. In total, 32 (94.1%) patients completed QoL surveys at 2 weeks, 34 (100%) at 6 weeks, and 33 (97.1%) completed surveys at 12 weeks post-treatment (Figure 1). By 12 weeks post-treatment, 93.9% (31/33) of patients reported “good” cosmesis and the clinician reported “good” cosmesis in 96.9% (31/32) of patients, with neither patients nor clinician reporting “bad” cosmesis (Figure 2). Similarly, by 12 weeks post-treatment, the clinician considered the scar to be only “slightly visible” in 96.9% (31/32). The 2-sided Pearson-Clopper 90% confidence interval for patient reported “good” cosmesis was 82–99%; this interval does not include the previously reported “good” cosmesis rate of 74%.
Figure 1.
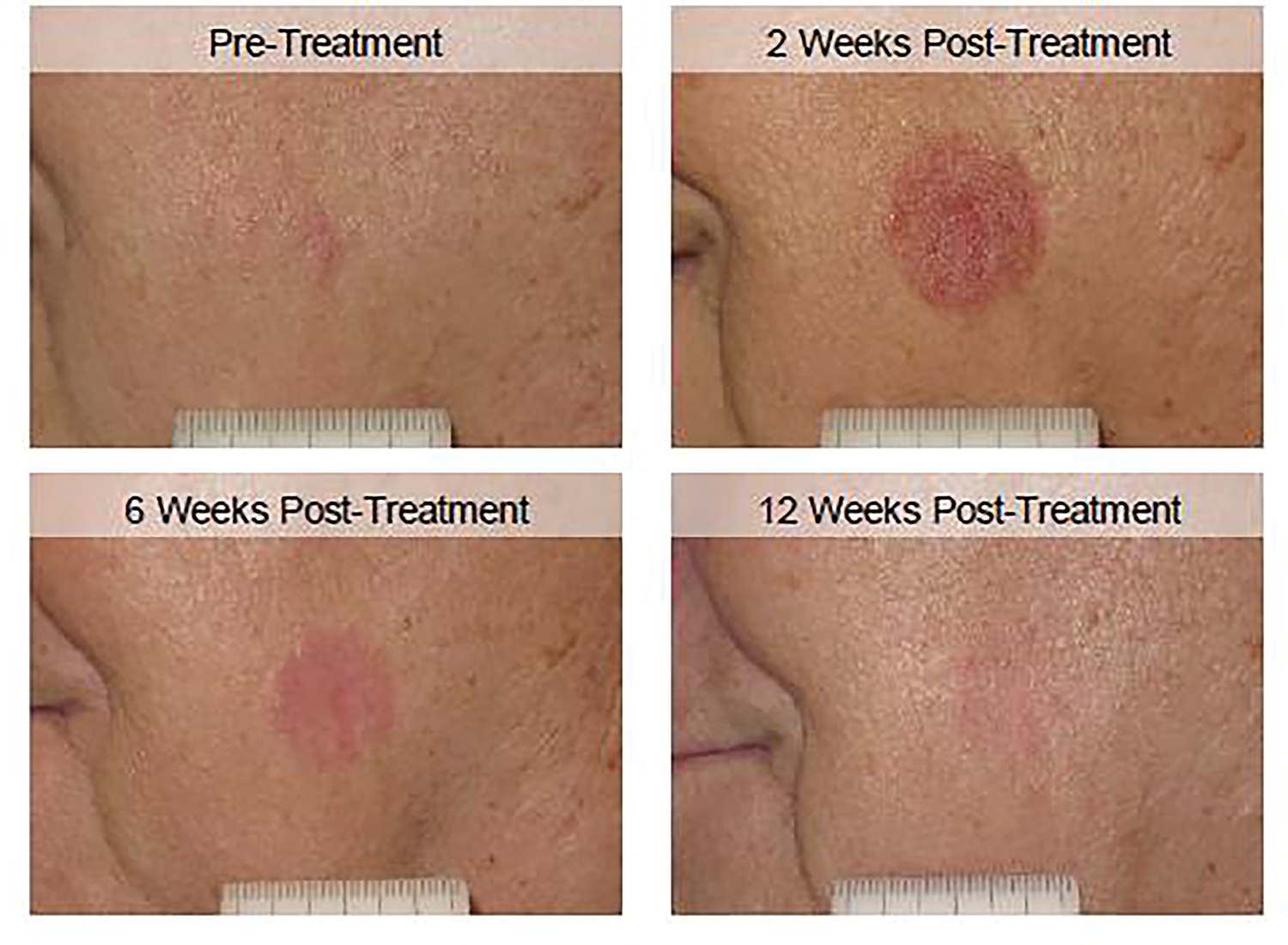
A patient treated with ESSB at pre-treatment and 2, 6, and 12 weeks post-treatment.
Figure 2.
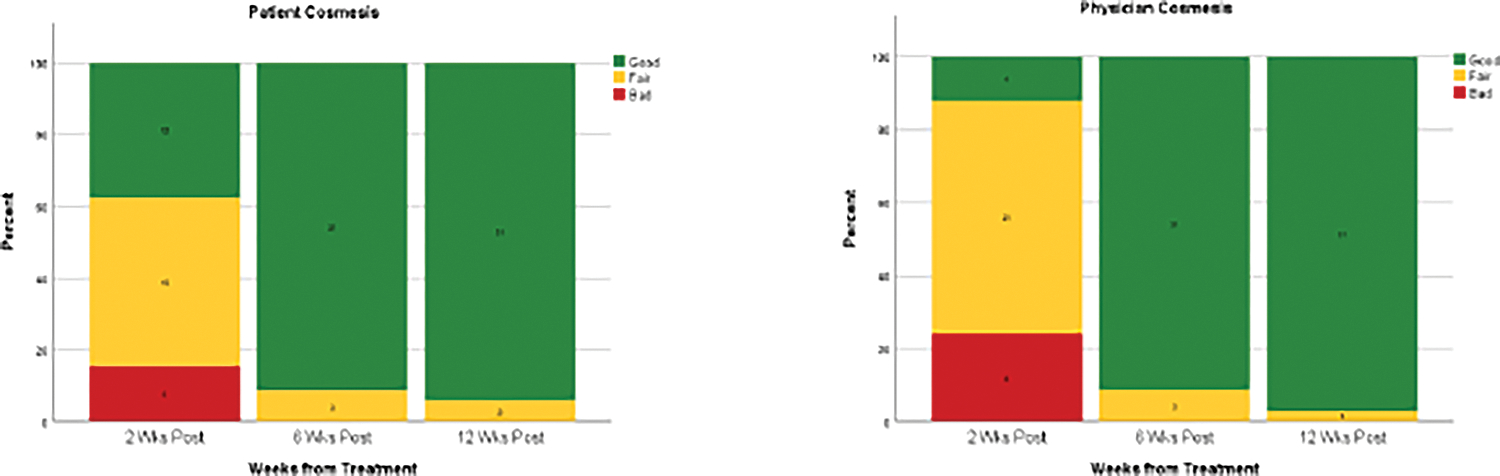
Patient and clinician assessed cosmesis over time.
Table 2 shows the mean Skindex-16 and SCI scores at each timepoint. Compared to baseline, total Skindex-16 score was significantly higher at 2 weeks post-treatment (10.5 vs 24.5, p<.001), representing a deterioration in QoL. There was a significant decrease in the Skindex-16 score at 6 weeks (10.5 vs 4.7, p=.014) and 12 weeks (10.5 vs 2.1, p=.001) post-treatment, representing an improvement in QoL. Total SCI score increased significantly from baseline to 6 weeks (78.4 vs 89.0, p=.001) post-treatment representing an improvement in QoL. Between baseline and 12 weeks post-treatment, the emotions subscale of the Skindex-16 (19.7 vs 3.1, p<.001 and the appearance subscale of the SCI (67.4 vs 87.6, p=.006) showed greatest improvement. Patients completed treatment and follow-up with minimal adverse events from ESSB (Figure 3). The most frequently reported toxicities were radiation dermatitis, skin pain, and pruritus. At any given timepoint, alopecia, hypopigmentation, telangiectasia, ulceration, hyperpigmentation, and induration were reported in ≤20% of patients. No patients experienced skin atrophy. The most severe adverse events were Grade 3 and occurred in week 3 of treatment (painful skin, 6.6%) and 2 weeks post-treatment (radiation dermatitis, 42.4%). By 12 weeks post-treatment, all adverse events had resolved to Grade ≤1. In general, patients were satisfied with the results of ESSB (Figure 4).
Table 2.
QoL over time (Mean±SD).
| Baseline n=34 | 2 Weeks Post-Treatment n=32 | 6 Weeks Post-Treatment n=34 | 12 Weeks Post-Treatment n=33 | |
|---|---|---|---|---|
|
| ||||
| Skindex-16 | ||||
| Symptoms | 7.4±17.7 | 31.4±30.1 a | 5.9±11.7 | 1.6±3.7 |
| Emotions | 19.7± 24.0 | 25.6±30.4 | 6.2±9.1 a | 3.1±6.0 a |
| Functioning | 4.4±10.5 | 16.5±26.6 | 2.2±5.3 | 1.5±7.0 |
| Total | 10.5±14.9 | 24.5±27.0 b | 4.7±7.3 b | 2.1±4.6 b |
| Skin Cancer Index (SCI) | ||||
| Emotional | 77.7±22.2 | 79.6±23.0 | 86.7±15.6 a | 86.3±15.7 |
| Social | 90.1 ±19.1 | 83.0±24.6 | 92.5±13.3 | 92.3±13.4 |
| Appearance | 67.4±33.1 | 76.0±31.7 | 88.0±20.1 a | 87.6±20.3 a |
| Total | 78.4±21.9 | 79.5±24.4 | 89.0±13.3 b | 88.7±13.3 |
significantly different from baseline, p≤.006 (Bonferroni corrected p-value for 9 tests)
significantly different from baseline, p≤.017 (Bonferroni corrected p-value for 3 tests)
Figure 3.
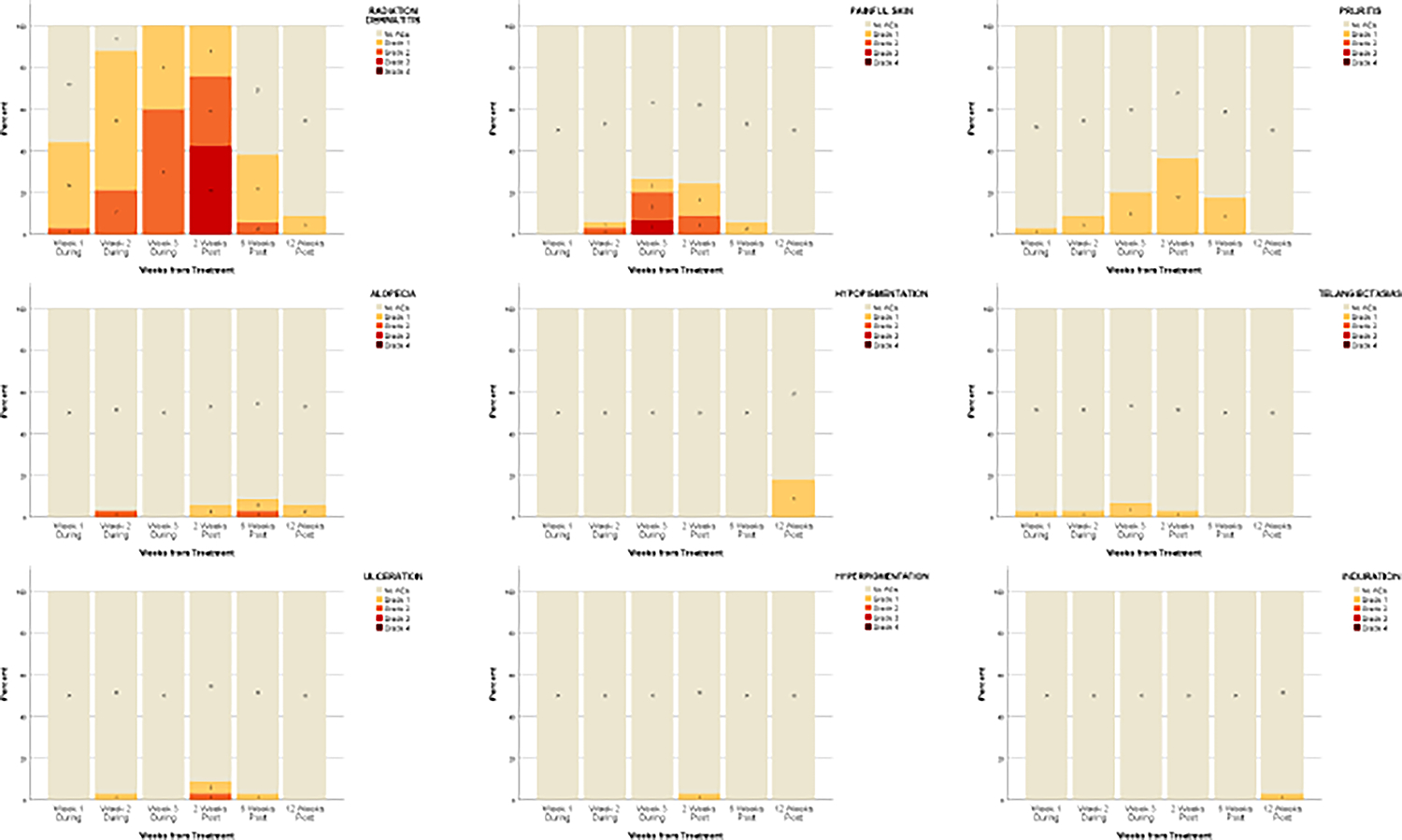
Plots of adverse events graded using CTCAE, version 4.0, over time. No skin atrophy was reported at any time point.
Figure 4.
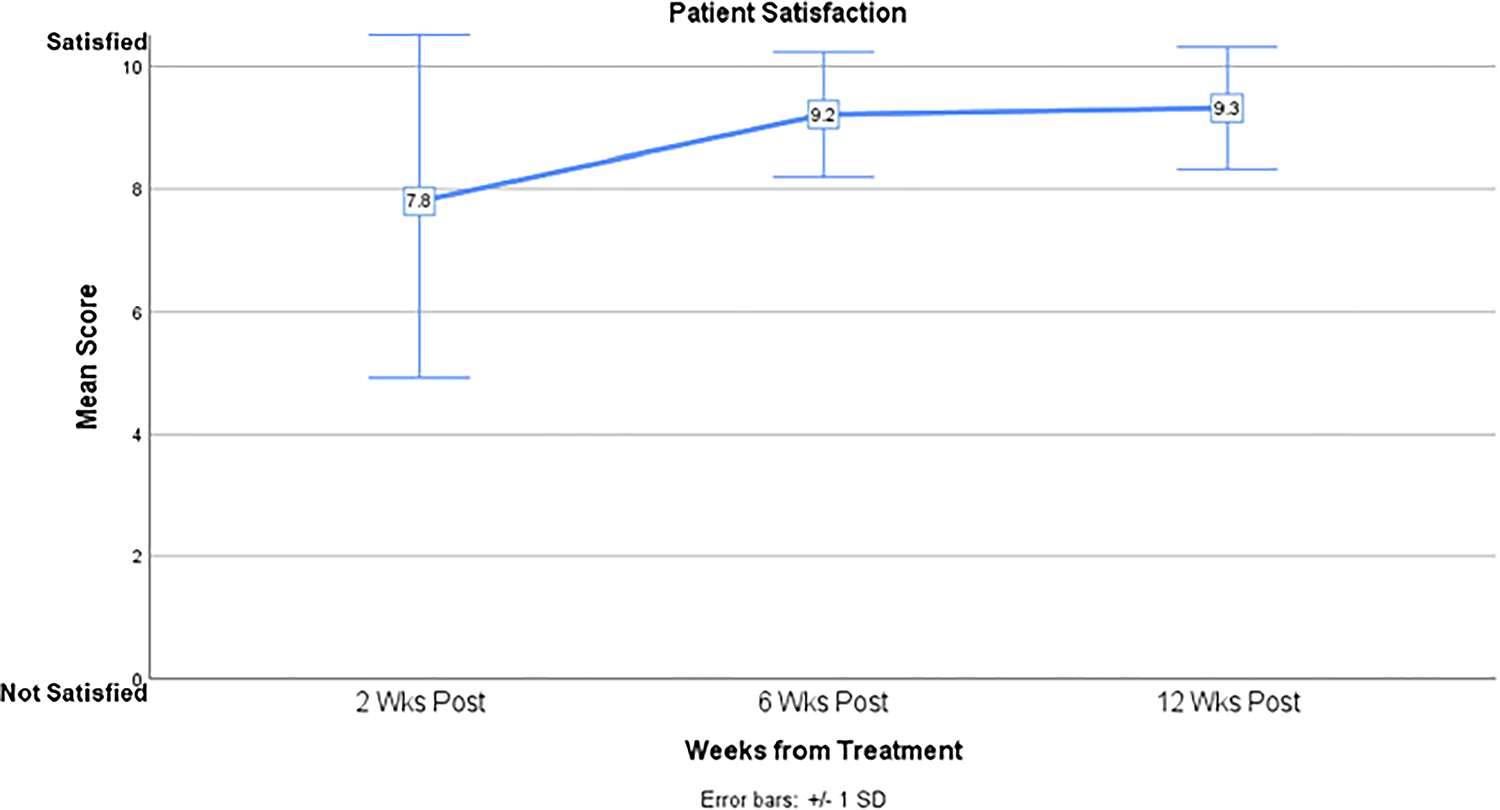
Patient satisfaction over time.
Discussion
This multicenter, prospective study demonstrates that ESSB is associated with a high rate (94%) of early patient-reported “good” cosmesis and that patient and clinician reported cosmesis were congruent. Both patient-reported cosmesis and satisfaction increased with time over the 3 months following treatment. The prospective assessment of QoL using validated instruments was a strength of this study. Although QoL can worsen temporarily at 2 weeks post-treatment, it then improves and becomes significantly better than baseline by 6 to 12 weeks post-treatment. Notably, the temporary deterioration in QoL at 2 weeks post-treatment correlates with the timing of adverse events.
Although keratinocyte carcinomas are rarely lethal, the impact of symptoms, functional limitations, cosmetic burdens, and treatment side effects can significantly disrupt QoL. Many treatment options are available for keratinocyte carcinomas.2,3 For certain patients and keratinocyte carcinomas, ESSB may be a suitable treatment option. ESSB is noninvasive and therefore causes little scarring, a typical concern when the tumor is on the face. Other advantages of brachytherapy include decreased number of treatments and decreased dependence on patient positioning during treatment, which can be especially important in older patients with limited mobility who are commonly afflicted by keratinocyte carcinoma.5 A current limitation to the use of ESSB is the cost, which tends to be higher than other RT modalities.11
At the start of the present study, the only prospective study characterizing patient reported cosmesis after RT for skin cancers was a 1997 publication reporting a “good” cosmesis rate of 74%.8 The prior study predominantly involved radioisotope-based interstitial brachytherapy 8 which suggests that the method employed in this study (ESSB) may be associated with the superior patient-reported “good” cosmesis rate. More recent studies of ESSB have also demonstrated “good” cosmesis at longer term follow-up periods.12–14
No grade ≥3 adverse events were noted after 2 weeks post-treatment, consistent with data from a previous study reporting acute grade 3 events, but not late adverse events.15 Other studies of ESSB have reported no grade ≥3 adverse events, possibly because assessments were made at later time points, after adverse events had resolved.16,17 Adverse events due to RT generally subside with time, but rare side effects such as telangiectasias and pigmentary change may emerge later. Additionally, temporary poor cosmesis may bring distress about physical appearance.18 Recognizing these trends can better prepare patients before they undergo treatment and alleviate worry they may experience immediately post-treatment as cosmesis appears to worsen.
Several studies, summarized in Supplemental Table A, have begun to examine the use of ESSB in the treatment of keratinocyte carcinomas. None assessed early patient-reported cosmesis and QoL. Current American Brachytherapy Society guidelines recommend limiting the use of ESSB for keratinocyte cancers as part of clinical trials or registries and calls for prospective studies to provide a better understanding acute and chronic toxicity profiles with ESSB in addition to clinical outcomes.4 The present study answers this call and provides valuable insight to the early outcomes of treatment.
A recent study comparing ESSB to Mohs micrographic surgery showed similar cosmesis between the two treatments, as reported by both clinicians and patients. The present study does not directly compare Skindex-16 and SCI scores between ESSB and other treatment modalities for keratinocyte carcinoma. However, it is notable that QoL as measured by these validated instruments is equivalent or superior to previously published studies of surgery and other treatments for keratinocyte carcinoma.18–20 This suggests an opportunity for studies that directly compare patient reported QoL following different treatments for keratinocyte carcinomas.
This study has some limitations. While the sample size seems small, it was prospectively derived using careful biostatistical calculations. Another limitation is the relative homogeneity of the patient population that self-identified as white and non-Hispanic, reflective of the population predominantly affected by keratinocyte carcinomas. Nevertheless, the results may not be generalizable to other patient populations. While the instruments used to assess patient-reported quality of life have been extensively validated, the visual analogue scale and the cosmesis assessment have not and may be subject to bias. Additionally, only the effects of ESSB were assessed; future studies of interest would compare outcomes among RT techniques or other modalities used to treat keratinocyte carcinoma. Finally, the follow up of 12 weeks only provides information about the early effects of treatment. Future analyses will characterize 5 years of prospective follow-up and a valuable opportunity to characterize longer-term outcomes.
This study has many strengths. To our knowledge, it is one of few studies to prospectively assess patient cosmesis and QoL after RT for keratinocyte carcinoma.6,7 It is the first to longitudinally measure patient QoL following treatment of BCC and SCC with ESSB. QoL was evaluated using both the Skindex-16, a validated QoL questionnaire that measures how various skin conditions affect patients, and the Skin Cancer Index, a QoL instrument which was designed and validated expressly for patients with keratinocyte carcinoma. Our study population was highly representative of the population most frequently affected by BCCs and SCCs. Finally, meticulous data collection before, during, and after treatment at many time points with very few missing data points allows for a complete and thorough understanding of how cosmesis, patient satisfaction, adverse events, QoL progress over time. This information may enable better patient counseling and guide patient expectations of ESSB.
Conclusions
This prospective, multicenter study demonstrated that ESSB is associated with a high rate of “good” early patient-reported cosmesis and increasing QoL and patient satisfaction with time. Validated assessments demonstrated a significant improvement in quality of life and resolution of moderate early adverse events by 6–12 weeks after treatment and support the observation of favorable cosmesis. Knowledge of the evolution of these trends can help patients make decisions regarding ESSB, prepare for treatment, anticipate the early outcomes.
Supplementary Material
Funding Statement
This study was supported by Elekta and NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of Interest Statement for All Authors
EHL has received grants from the American College of Mohs Surgery Foundation and royalties from UpToDate. AR is a consultant for Evolve CME, Almirall, Merz, Dynamed, Canfield Scientific, Evolus, Biofrontera, Quantia MD, Lam Therapeutics, Regeneron, and Cutera, has received travel support from Mavig and L’Oreal, is an advisor for Skinfix and Allergan Inc, is the founder of DAR companies, has received grants and research funding from ASLMS, Skin Cancer Foundation, Regen, LeoPharma, and Biofrontera, is on the editorial board of Lasers in Surgery and Medicine, CUTIS, Journal of the American Academy of Dermatology (JAAD), Dermatologic Surgery, and is a board member of ASDS and committee member and/or chair of AAD, ASDS, and ASLMS. GC has received MSKCC funding from Alpha Tau Medical in support of his post-doctoral research. MZ has received consulting fees from Alpha Tau Medical and serves as Editor in Chief for Brachytherapy. MEK discloses that his institution was loaned an early version of the ESTEYA machine for use during the trial, which was promptly returned and no patients were treated off trial. CAB has received honoraria from Regeneron.
AMK, KN, MC, AS, ML, and ZZ have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Nehal KS, Bichakjian CK. Update on Keratinocyte Carcinomas. N Engl J Med 2018;379(4):363–374. DOI: 10.1056/NEJMra1708701. [DOI] [PubMed] [Google Scholar]
- 2.Thomson J, Hogan S, Leonardi-Bee J, Williams HC, Bath-Hextall FJ. Interventions for basal cell carcinoma of the skin. Cochrane Database Syst Rev 2020;11:CD003412. DOI: 10.1002/14651858.CD003412.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lansbury L, Bath-Hextall F, Perkins W, Stanton W, Leonardi-Bee J. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ 2013;347:f6153. DOI: 10.1136/bmj.f6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah C, Ouhib Z, Kamrava M, et al. The American Brachytherapy society consensus statement for skin brachytherapy. Brachytherapy 2020;19(4):415–426. DOI: 10.1016/j.brachy.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Albert A, Knoll MA, Conti JA, Zbar RIS. Non-Melanoma Skin Cancers in the Older Patient. Curr Oncol Rep 2019;21(9):79. DOI: 10.1007/s11912-019-0828-9. [DOI] [PubMed] [Google Scholar]
- 6.Arts LPJ, Waalboer-Spuij R, de Roos KP, et al. Health-Related Quality of Life, Satisfaction with Care, and Cosmetic Results in Relation to Treatment among Patients with Keratinocyte Cancer in the Head and Neck Area: Results from the PROFILES Registry. Dermatology 2020;236(2):133–142. DOI: 10.1159/000502033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kharofa J, Currey A, Wilson JF. Patient-reported outcomes in patients with nonmelanomatous skin cancers of the face treated with orthovoltage radiation therapy: a cross-sectional survey. Int J Radiat Oncol Biol Phys 2013;87(4):636–7. DOI: 10.1016/j.ijrobp.2013.06.2061. [DOI] [PubMed] [Google Scholar]
- 8.Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? Results of a randomized study. Br J Cancer 1997;76(1):100–6. DOI: 10.1038/bjc.1997.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chren MM, Lasek RJ, Sahay AP, Sands LP. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg 2001;5(2):105–10. DOI: 10.1177/120347540100500202. [DOI] [PubMed] [Google Scholar]
- 10.Rhee JS, Matthews BA, Neuburg M, Logan BR, Burzynski M, Nattinger AB. Validation of a quality-of-life instrument for patients with nonmelanoma skin cancer. Arch Facial Plast Surg 2006;8(5):314–8. DOI: 10.1001/archfaci.8.5.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cognetta AB Jr., Wolfe CM, Goldberg DJ, Hong HG. Practice and Educational Gaps in Radiation Therapy in Dermatology. Dermatol Clin 2016;34(3):319–33. DOI: 10.1016/j.det.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Guix B, Finestres F, Tello J, et al. Treatment of skin carcinomas of the face by high-dose-rate brachytherapy and custom-made surface molds. Int J Radiat Oncol Biol Phys 2000;47(1):95–102. DOI: 10.1016/s0360-3016(99)00547-7. [DOI] [PubMed] [Google Scholar]
- 13.Delishaj D, Laliscia C, Manfredi B, et al. Non-melanoma skin cancer treated with high-dose-rate brachytherapy and Valencia applicator in elderly patients: a retrospective case series. J Contemp Brachytherapy 2015;7(6):437–44. DOI: 10.5114/jcb.2015.55746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauden R, Pracy M, Avery AM, Hodgetts I, Gauden S. HDR brachytherapy for superficial non-melanoma skin cancers. J Med Imaging Radiat Oncol 2013;57(2):212–7. DOI: 10.1111/j.1754-9485.2012.02466.x. [DOI] [PubMed] [Google Scholar]
- 15.Paravati AJ, Hawkins PG, Martin AN, et al. Clinical and cosmetic outcomes in patients treated with high-dose-rate electronic brachytherapy for nonmelanoma skin cancer. Pract Radiat Oncol 2015;5(6):e659–64. DOI: 10.1016/j.prro.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Bhatnagar A Nonmelanoma skin cancer treated with electronic brachytherapy: results at 1 year. Brachytherapy 2013;12(2):134–40. DOI: 10.1016/j.brachy.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Ballester-Sanchez R, Pons-Llanas O, Candela-Juan C, et al. Two years results of electronic brachytherapy for basal cell carcinoma. J Contemp Brachytherapy 2017;9(3):251–255. DOI: 10.5114/jcb.2017.68191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Miller CJ, O’Malley V, Etzkorn JR, Shin TM, Sobanko JF. Patient quality of life fluctuates before and after Mohs micrographic surgery: A longitudinal assessment of the patient experience. J Am Acad Dermatol 2018;78(6):1060–1067. DOI: 10.1016/j.jaad.2018.02.065. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Montero P, de Galvez-Aranda MV, Blazquez-Sanchez N, et al. Quality of Life During Treatment for Cervicofacial Non-melanoma Skin Cancer. J Cancer Educ 2020. DOI: 10.1007/s13187-020-01781-7. [DOI] [PubMed] [Google Scholar]
- 20.Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol 2007;127(6):1351–7. DOI: 10.1038/sj.jid.5700740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.


