Abstract
We describe the immunologic and virologic impact of monkeypox (mpox) infection in a woman with human immunodeficiency virus (HIV) whose plasma HIV viremia was suppressed by clinically effective antiretroviral therapy. Extensive phenotypic analyses of B and T cells in peripheral blood and biomarkers in plasma showed significant immunologic perturbations despite the presence of mild mpox disease. Dramatic shifts were noted in the frequencies of total B cells, plasmablasts, and plasmablast immunoglobulin isotypes. Flow cytometric analyses showed a dramatic increase in the frequency of CD38+HLA-DR+ CD8+ T cells after mpox infection. Our data offer guidance for future studies involving mpox infection in affected populations.
Keywords: HIV, monkeypox, T cells, B cells, Biomarkers
We describe the immunologic and virologic impact of monkeypox infection in a woman with human immunodeficiency virus receiving antiretroviral therapy. Extensive phenotypic analyses of B and T cells and biomarkers showed significant immunologic perturbations despite mild monkeypox infection.
The recent outbreak of monkeypox (mpox) has generated a unique set of challenges for affected populations and the medical community [1–3]. The majority of reported cases have been in men who have sex with men, including people living with human immunodeficiency virus (HIV) (PLWH), who are at a particularly increased risk [4, 5]. The effects of mpox on the immune system are not well understood and are important to delineate in immune-compromised individuals such as PLWH [3]. To this end, we evaluated several immunologic and HIV reservoir parameters before and after a mild and self-limited case of mpox in a woman with HIV whose HIV viremia was suppressed.
METHODS
Consent Statement
The study participant provided informed consent. Blood samples were collected in accordance with protocols approved by the Institutional Review Board of the National Institutes of Health (NIH).
B-Cell Analysis
B-cell phenotyping was performed as described elsewhere [6]. Briefly, cryopreserved peripheral blood mononuclear cells were thawed and 2 × 106 cells were stained with Zombie NIR Fixable Viability Dye (Biolegend) at room temperature for 10 minutes, followed by incubation with a cocktail containing 11 monoclonal antibodies against CD45-BUV805 (clone HI30), CD3-BV570 (clone UCHT1), CD19-BV650 (clone SJ25-C1), CD20–allophycocyanin (APC)–H7 (clone 2H7), CD38-APC/Fire810 (clone HB-7), CD27-BV785 (clone O323), CD21-phycoerythrin (PE)/Dazzle594 (clone BU32), immunoglobulin (Ig) D-BV605 (clone IA6-2), IgM-BV711 (clone MHM-88), IgG PE–cyanine (Cy) 7 (clone G18-145) and IgA-VioBlue (clone IS11-8E10) at 4°C for 30 minutes. Stained cells were fixed (Lysing Solution; BD Biosciences), acquired using an Aurora cytometer and SpectroFlo software version 3.0.1 (Cytek Biosciences), and analyzed using FlowJo software version 10 (BD Biosciences).
T-Cell Analysis
Peripheral blood mononuclear cells were stained with viability reagent Zombie NIR along with the following fluorophore-conjugated antibodies in Brilliant Stain Buffer Plus: CD3-BUV805 (clone SK7), CD4-BUV395 (clone M-T477), CD8-BUV737 (clone SK1), CD152-PE-Cy5 (clone BNI3), CD28-BUV496 (clone CD28.2), CD226-BV480 (clone DX11), CD134-BV786 (clone ACT35), CD27–Alexa Fluor 700 (clone M-T271), CXCR5-BV510 (clone RF8B2), CD96-BV711 (clone 6F9), CD45RA–peridinin chlorophyll protein (PerCP)–Cy5.5 (clone HI100), CD278-BV421 (clone DX29), CD197-V450 (clone 150503), CD38-BB515 (clone HIT2), CD160–Alexa Fluor 647 (clone BY55), CD179-PE-Cy7 (clone EH12.1), HLA-DR-SuperBright 436 (clone LN3), T cell immunoreceptor with Ig and ITIM domains (TIGIT)-PE-eFluor610 (clone MBSA43), CD244-PE (clone C1.7), and CD137-APC (clone C-7). Acquisition was performed with an Aurora cytometer using SpectroFlo software version 3.0.1 (Cytek BioSciences), and analyzed using FlowJo software version 10 (BD Biosciences).
Measurements of Biomarkers
Levels of granzyme B, perforin, CCL5, CCL3, interferon-gamma-inducible protein 10, interleukin-2 receptor α (IL-2Rα), interleukin 6 (IL-6), and programmed cell death ligand 1 (PD-L1) in plasma were determined using the ELLA platform (ProteinSimple), according to the manufacturer's instructions.
Measurements of Intact HIV Proviral DNA
The level of CD4+ T cells carrying intact HIV proviruses was assessed using the intact proviral DNA assay [6] and the QIAcuity digital polymerase chain reaction system (Qiagen). Genomic DNA was isolated using QIAamp DNA mini kit (Qiagen), per instructions. Digital polymerase chain reaction was performed using 2 sets of primers and probes specific for HIV gag and envelope and 2 fragments of housekeeping gene RPP30 in 2 independent, separate reactions.
For HIV quantification, 910 ng of DNA was used, with the following primers and probes: HIV gag specific [7]—gag forward (5′-GACTAGCGGAGGCTAGAAGGAGAGA-3′; nucleotides 764–788), gag reverse (5′-CTAATTCTCCCCCGCTTAATAYTGACG-3′; nucleotides 829–803), and gag LNA probe (5′-6FAM-A+T+GGG+TG+CGAGA-IABkFQ-3′) and HIV env specific—env forward (5′-AGTGGTGCAGAGAGAAAAAAGAGC-3′; nucleotides 7736–7759), env reverse (5′-GTCTGGCCTGTACCGTCAGC-3′; nucleotides 7851–7832), and env probes (5′-VIC-CCTTGGGTTCTTGGGA-MGB-3′ [nucleotides 7781–7798] and unlabeled hypermutated probe 5′-CCTTAGGTTCTTAGGAGC-MGB-3′). For RPP30 quantification, 9.1 ng of DNA was used, with the following primers and probes: RPP30-1 forward (5′-GATTTGGACCTGCGAGCG-3′; nucleotides 51–68), RPP30-1 reverse (5′-GCGGCTGTCTCCACAAGT-3′; nucleotides 112–95), RPP30-1 probe (5′-6FAM-TTCTGACCTGAAGGCTCTGCGC-IABkFQ-3′; nucleotides 71–92), RPP30-2 forward (5′-GTGTGAGTCAATCACTAGACAGAA-3′; nucleotides 7034–7057), RPP30-2 reverse (5′-AAACTGCAACAACATCATAGAGC-3′; nucleotides 7157–7135), and RPP30-2 probe (5′-HEX-AGAGAGCAACTTCTTCAAGGGCCC-IABkFQ-3′; nucleotides 7110–7113).
All reactions were performed in quadruplicate. Intact HIV DNA copy numbers (gag and env double-positive partitions) were normalized per 1 × 106 CD4+ T cells and adjusted for DNA shearing using the DNA shearing index, based on a ratio of double-positive RPP30 partitions [6].
RESULTS
Clinical Presentation
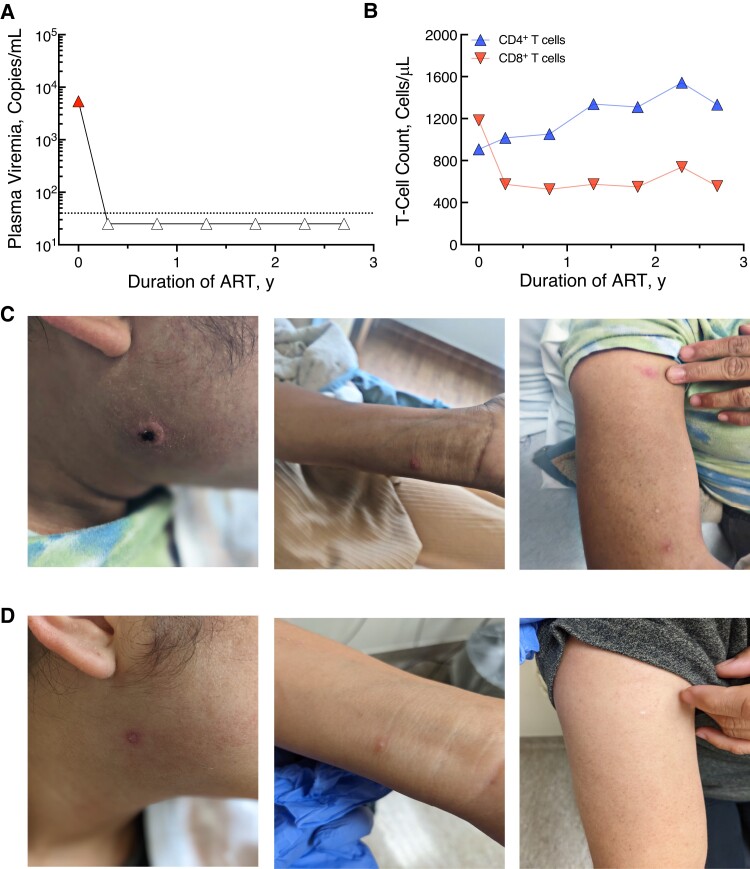
Our study participant was a 27-year-old woman who tested positive for HIV at a local health department on 21 October 2019. Her risk factor was heterosexual contact. Before starting antiretroviral therapy (ART) on 28 October 2019, her CD4+ T-cell count was 907/µL, with plasma HIV viremia of 6290 copies/mL (Figure 1A and 1B). After her HIV diagnosis, she was referred to the NIH and enrolled in a longitudinal natural history study (ClinicalTrials.gov identifier NCT00039689). She started treatment with Biktarvy (bictegravir, emtricitabine, and tenofovir alafenamide) and continued with sustained virologic suppression (Figure 1A).
Figure 1.
Clinical presentation and characteristic in a woman with human immunodeficiency virus (HIV) and mild mpox infection. A, Plasma HIV viremia since diagnosis of HIV, by duration of antiretroviral therapy (ART). B, Total CD4+ and CD8+ T-cell counts since diagnosis of HIV, by duration of ART. C, D, Appearance of mpox lesions at initial presentation on 6 September 2022 (C) and at follow-up clinic visit on 28 September 2022 (D).
On 16 August 2022, the study participant attended her prescheduled visit to the NIH in good health and with no complaints. However, soon thereafter, a nonhealing papule developed on her face and progressively became larger, leading her to seek evaluation at a local emergency room on 31 August 2022. She was labeled as having cellulitis and was discharged but had a swab sample collected from the lesion, which was sent for mpox testing. Subsequently, she was notified of a positive mpox result, but as she had no other symptoms, she contacted the NIH for additional testing and follow-up.
The study participant was seen at the NIH on 6 September 2022. She was afebrile and hemodynamically stable. On review of symptoms, she was asymptomatic. Physical examination revealed a circular, raised lesion with well-demarcated borders bordering on the parotid and submental region (Figure 1C). Two additional lesions were also observed, one on the study participant’s right forearm and the other on her left wrist (Figure 1C). No inguinal, genital, or other eruptions were noticed elsewhere. Bloodwork revealed a white blood cell count of 9.38 × 103/µL with 54% neutrophils (absolute neutrophil count, 5.07 × 103/µL) and 39% lymphocytes (absolute lymphocyte count, 3.66 × 103/µL). Her electrolyte levels and renal function were normal. The only aberration noted was an elevation in her liver function test results; her alanine aminotransferase level was elevated to 106 U/L (normal range 0–55 U/L) and her aspartate aminotransferase level was 49 U/L (normal range 5–34 U/L).
On 6 September 2022, the lesions were swabbed, and they tested positive for mpox. The study participant denied any exposure to mpox and was sexually active only with her husband, and no household contacts had any symptoms suggestive of mpox. She works as a restaurant cook and denied any sick contacts. Given the mild nature of her symptoms and per the CDC guidelines at that time, the decision was made to not proceed with any mpox treatment strategies but to continue monitoring. The study participant agreed with this plan, and follow-up was conducted using phone interviews. When she returned on 28 September 2022, her facial lesion had dramatically improved (Figure 1D), with similar improvement in her wrist and forearm lesions.
There are sparse data pertaining to the dynamics of immunologic and HIV virologic changes following mpox infection in PLWH. Of note, a recent study demonstrated that mpox is characterized by an early expansion of activated effector CD4+ and CD8+ T cells [2]; however, longitudinal analyses of B cells, plasma biomarkers, and the HIV reservoir before, during, and after mpox have been lacking. Therefore, we conducted immunologic analyses of our study participant's peripheral blood lymphocytes (B and T cells) and plasma biomarkers before, during, and after her mpox infection.
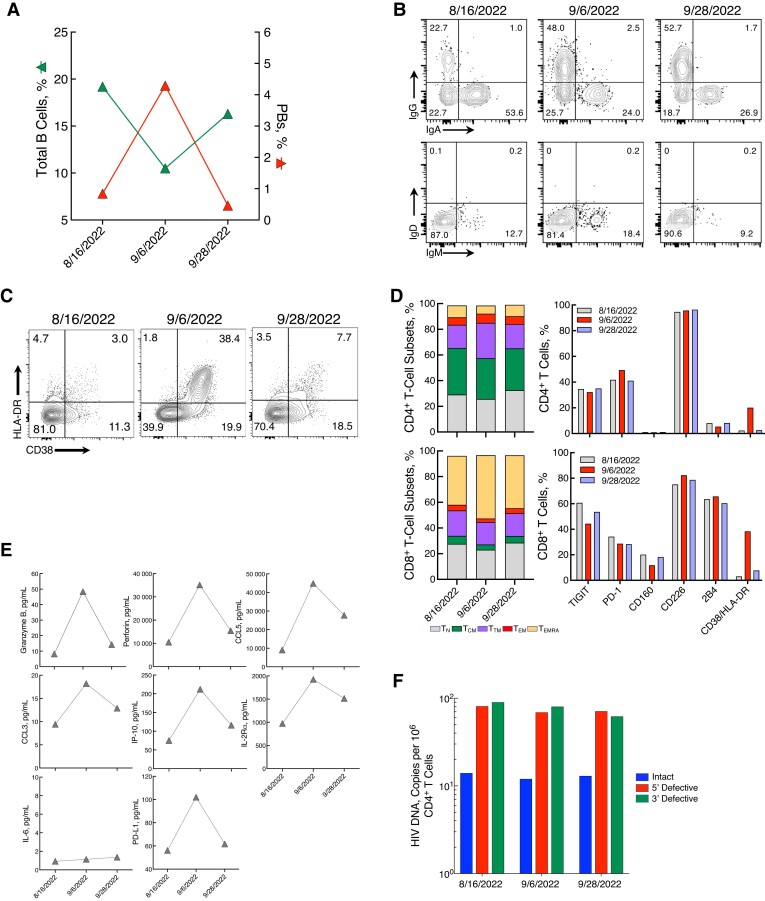
There was a marked decrease (8.7%) in circulating total B cells after mpox, with a concomitant increase in plasmablasts (PBs), followed by normalization of both total B cells and PBs by week 4 (Figure 2A). Further analysis of PBs revealed a predominantly IgA isotype before mpox infection (Figure 2B), consistent with immunoglobulin distribution at steady state in healthy donors and in PLWH with aviremia [8]. Following mpox, there was a strong and persistent shift to IgG among circulating PBs (Figure 2B), consistent with the induction of an immune response to viral antigens [9, 10].
Figure 2.
Dynamics of immunologic and virologic parameters in a woman with human immunodeficiency virus (HIV) and mild mpox infection. A, Changes in total B cells and plasmablasts (PBs) over the course of mpox infection. (Throughout figure, dates appear in month/date/year format.) B, Flow cytometric plots of cell surface immunoglobulin (Ig) isotype distribution among PBs at each time point. C, Frequencies of CD38+/HLA-DR+ on CD8+ T cells before (left), during (middle), and after (right) mpox infection. D, Frequencies of CD4+ and CD8+ T-cell subsets: naïve (TN), central memory (TCM), transitional memory (TTM), effector memory (TEM), and effector memory RA (TEMRA). The frequencies of TIGIT, programmed cell death 1 (PD-1), CD160, CD226, 2B4 and CD38/HLA-DR on CD4+ and CD8+ T cells are shown in parallel. E, Plasma levels of granzyme B, perforin, CCL5, CCL3, interferon-gamma-inducible protein 10 (IP-10), interleukin-2 receptor α (IL-2Rα), interleukin 6 (IL-6), and programmed cell death ligand 1 (PD-L1). F, Levels of intact, 5′ defective, and 3′ defective HIV proviral DNA before, during, and after mpox infection.
The proportion of T-cell subsets and phenotypic markers of CD4+ and CD8+ T cells also underwent changes during the course of infection (Figure 2C and 2D). Of interest, there was a dramatic increase in the level of CD38+HLA-DR+ CD8+ T cells after mpox infection (Figure 2C and 2D). In addition, levels of plasma biomarkers—including granzyme B, perforin, RANTES (regulated on activation, normal T cell expressed and secreted), CCL3, CXCL10, IL-2Rα, PD-L1, but not IL-6 increased markedly with mpox infection (Figure 2E).
Finally, we examined the impact of mpox on the size of HIV reservoirs carrying intact proviral DNA in highly enriched CD4+ T cells of the study participant (Figure 2F). No significant changes were noted in the level of intact or defective proviral HIV DNA, suggesting that a mild case of mpox infection, while inducing a strong immunologic response, did not lead to any substantial change in the size of the persistent HIV reservoir in this participant.
DISCUSSION
We present a woman with HIV receiving ART, in whom we conducted comprehensive immunologic and virologic analyses before and after mild mpox. It has been shown recently in a global series that clinical features of mpox in women are similar to those described in men [11]. To our knowledge, this is one of the first detailed reports of a woman with HIV with mpox that included extensive analyses of lymphocytes and HIV reservoirs.
The majority of mpox cases to date have been described in men who have sex with men. In this regard, our findings highlight an atypical presentation of mpox in a cis female without any inguinal/groin lesions. Although HIV has been associated with worse clinical outcomes [12], our study participant had a very mild presentation. However, comprehensive analyses of phenotypic cell surface markers and biomarkers in plasma identified several immunologic perturbations.
There has been a paucity of comprehensive and longitudinal analyses of B cells in mpox-infected humans since the current outbreak began. Of note, it has been shown that while serum IgG/IgM antibodies to mpox can help identify exposures and delineate response to vaccination, their diagnostic value remain uncertain unless longitudinal monitoring is performed [13]. In our study participant, mpox was associated with dramatic shifts in frequencies of total B cells, PBs, and PB immunoglobulin isotypes, suggesting a robust B-cell response. Given the strong and persistent shift from IgA to IgG PBs, this could be used as a means of evaluating exposure to mpox even when longitudinal samples are limited. However, additional studies will be necessary to further characterize the immune responses in diverse cohorts with varied clinical manifestations.
T-cell responses in this mpox outbreak are currently being investigated. It has been shown that mpox leads to an early expansion of activated effector T cells [2]. Our data also showed an increase in CD8+ effector memory RA cells at the time of mpox, with a decrease in naïve and effector memory CD8+ T cells. For CD4+ T cells, we found an increase in effector memory and transitional memory cells during mpox, with a decrease in the naïve T-cell subset. Flow cytometric analyses showed a dramatic increase in the frequency of CD38+HLA-DR+ CD8+ T cells after the diagnosis of mpox in our participant. It has been shown that an elevated level of HLA-DR+CD38+CD8+ T cells is one of the immunologic hallmarks of HIV infection in viremic PLWH [14]. Interestingly, the expression of TIGIT, PD-1, and CD-160 decreased on CD8+ T cells during mpox, before normalizing back to pre-mpox levels.
The elevation in certain biomarkers in plasma samples from our study participant after mpox infection largely agrees with a cross-sectional study [15], although her levels of IL-6 remained unchanged after mpox infection. Further investigation in a larger study of PWLH is needed, especially given the variable kinetics of IL-6 that has been reported after mpox infection [2] and the unknown effect of HIV infection on these kinetics. Collectively, our data show that a robust immune response occurred despite a mild and self-limiting mpox infection. Of interest, we observed no significant change in the size of HIV-infected CD4+ T cells carrying intact proviral DNA, despite dynamic changes in immune activation and inflammation.
Major caveats include the sample size, which was limited to a single study participant, and the lack of mpox antigen-specific immune analyses of B and T cells. A larger study involving comprehensive analyses of immunologic and virologic parameters will be necessary to better understand the effect of mpox infection on cellular activation and inflammation in PLWH receiving ART. Nonetheless, the detailed immunologic and virologic data obtained from our study participant receiving ART with a mild and self-limited mpox infection offer guidance for future pathogenesis and therapeutic studies involving affected populations.
Contributor Information
M Ali Rai, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Victoria Shi, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Brooke D Kennedy, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Jesse S Justement, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Maegan R Manning, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Lauren Praiss, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Esther J Kang, Clinical Center, National Institutes of Health, Bethesda, Maryland, USA.
Kathleen Gittens, Clinical Center, National Institutes of Health, Bethesda, Maryland, USA.
Lela Kardava, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Jana Blazkova, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Susan Moir, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Tae-Wook Chun, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Notes
Acknowledgments. We are grateful to the study participant for their participation in this study. We thank the National Institute of Allergy and Infectious Diseases HIV Outpatient Clinic staff for their assistance with the study’s execution.
Financial support. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
References
- 1. Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N Engl J Med 2022; 387:679–91. [DOI] [PubMed] [Google Scholar]
- 2. Agrati C, Cossarizza A, Mazzotta V, et al. Immunological signature in human cases of monkeypox infection in 2022 outbreak: an observational study. Lancet Infect Dis 2023; 23:320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lum FM, Torres-Ruesta A, Tay MZ, et al. Monkeypox: disease epidemiology, host immunity and clinical interventions. Nat Rev Immunol 2022; 22:597–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tarin-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet 2022; 400:661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martinez J I, Montalban E G, Bueno S J, et al. Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Euro Surveill 2022; 27:2200471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bruner KM, Wang Z, Simonetti FR, et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019; 566:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levy CN, Hughes SM, Roychoudhury P, et al. A highly multiplexed droplet digital PCR assay to measure the intact HIV-1 proviral reservoir. Cell Rep Med 2021; 2:100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buckner CM, Moir S, Ho J, et al. Characterization of plasmablasts in the blood of HIV-infected viremic individuals: evidence for nonspecific immune activation. J Virol 2013; 87:5800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellebedy AH, Jackson KJ, Kissick HT, et al. Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat Immunol 2016; 17:1226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mei HE, Yoshida T, Sime W, et al. Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood 2009; 113:2461–9. [DOI] [PubMed] [Google Scholar]
- 11. Thornhill JP, Palich R, Ghosn J, et al. Human monkeypox virus infection in women and non-binary individuals during the 2022 outbreaks: a global case series. Lancet 2022; 400:1953–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis 2019; 19:872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karem KL, Reynolds M, Hughes C, et al. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin Vaccine Immunol 2007; 14:1318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giorgi JV, Ho HN, Hirji K, et al. ; the Multicenter AIDS Cohort Study Group . CD8+ lymphocyte activation at human immunodeficiency virus type 1 seroconversion: development of HLA-DR+ CD38-CD8+ cells is associated with subsequent stable CD4+ cell levels. J Infect Dis 1994; 170:775–81. [DOI] [PubMed] [Google Scholar]
- 15. Johnston SC, Johnson JC, Stonier SW, et al. Cytokine modulation correlates with severity of monkeypox disease in humans. J Clin Virol 2015; 63:42–5. [DOI] [PMC free article] [PubMed] [Google Scholar]