Abstract
Background
Prospective evaluations of long COVID in outpatients with coronavirus disease 2019 (COVID-19) are lacking. We aimed to determine the frequency and predictors of long COVID after treatment with the monoclonal antibody bamlanivimab in ACTIV-2/A5401.
Methods
Data were analyzed from participants who received bamlanivimab 700 mg in ACTIV-2 from October 2020 to February 2021. Long COVID was defined as the presence of self-assessed COVID symptoms at week 24. Self-assessed return to pre-COVID health was also examined. Associations were assessed by regression models.
Results
Among 506 participants, median age was 51 years. Half were female, 5% Black/African American, and 36% Hispanic/Latino. At 24 weeks, 18% reported long COVID and 15% had not returned to pre-COVID health. Smoking (adjusted risk ratio [aRR], 2.41 [95% confidence interval {CI}, 1.34– 4.32]), female sex (aRR, 1.91 [95% CI, 1.28–2.85]), non-Hispanic ethnicity (aRR, 1.92 [95% CI, 1.19–3.13]), and presence of symptoms 22–28 days posttreatment (aRR, 2.70 [95% CI, 1.63–4.46]) were associated with long COVID, but nasal severe acute respiratory syndrome coronavirus 2 RNA was not.
Conclusions
Long COVID occurred despite early, effective monoclonal antibody therapy and was associated with smoking, female sex, and non-Hispanic ethnicity, but not viral burden. The strong association between symptoms 22–28 days after treatment and long COVID suggests that processes of long COVID start early and may need early intervention.
Clinical Trials Registration
Keywords: bamlanivimab, clinical trial, long COVID, postacute sequelae of SARS-CoV-2 infection (PASC), symptom
An uncertain proportion of persons with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection experience symptoms after acute infection, often termed “long COVID.” The reported prevalence of long COVID varies widely, depending on the study design, population studied, symptoms investigated, and duration of observation following the acute infection [1].
Clinical manifestations of long COVID show a wide spectrum of severity and affect multiple body systems [2, 3]. Several demographic and clinical characteristics have been associated with increased prevalence of long COVID, including female sex [4, 5], obesity [6], and poor pre–coronavirus disease 2019 (COVID-19) health [5]. Severity of COVID-19 illness, duration of hospitalization, and requirement for intensive care have also been associated with risk of long COVID [7]. Notably, most currently available data on long COVID were not collected in rigorous prospective COVID-19 interventional trials but include cross-sectional, retrospective, or observational data describing heterogenous populations, often with variable or unknown time since acute COVID-19 at enrollment. In addition, to our knowledge, previous studies have not investigated the prevalence of long COVID in cohorts with mild to moderate illness receiving monoclonal antibody (mAb) therapies as outpatients.
In November 2020, the United States (US) Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the use of bamlanivimab 700 mg for the treatment of COVID-19 in nonhospitalized adult and pediatric patients at high risk for progressing to severe COVID-19 [8]. This EUA remained active until Eli Lilly and Company voluntarily asked the FDA to revoke the EUA for bamlanivimab alone in April 2021 after resistant variants had become widespread [9]. Here, we characterized the prevalence and predictors of long COVID following bamlanivimab treatment for acute COVID-19 in the ACTIV-2/A5401 study during a time period when bamlanivimab had clinical activity against circulating variants in the community, which comprised almost entirely the original Alpha and B.1 variants [10, 11].
METHODS
Study Design and Participants
ACTIV-2/A5401 evaluates the safety and efficacy of investigational agents for the treatment of nonhospitalized adults with mild-to-moderate COVID-19 in a phase 2/3 adaptive platform trial (NCT04518410). The protocol was approved by a central institutional review board (IRB), Advarra (Pro00045266), with additional local IRB review and approval as required by participating sites. All participants provided written informed consent. Eligible participants were outpatient adults (≥18 years of age) within 10 days of symptom onset with a positive SARS-CoV-2 antigen or nucleic acid test within 7 days prior to study entry and ongoing COVID-19 symptoms within 48 hours prior to study entry. Participants included in this analysis were enrolled in either a phase 2 randomized (1:1), blinded, placebo-controlled evaluation of 1-time intravenous (IV) infusion of the SARS-CoV-2 mAb bamlanivimab 700 mg (n = 111) or saline placebo (n = 112), or the subsequent open-label, single-arm uncontrolled evaluation of bamlanivimab 700 mg (n = 1051) that was introduced after EUA of bamlanivimab by the US FDA (Supplementary Figure 1). The analysis was restricted to participants who received bamlanivimab (n = 1162) and excluded placebo-assigned participants (n = 112) due to the small number who received placebo and the absence of a contemporaneous control group during the open-label portion of the trial (see Supplementary Table 1 for summary of placebo recipients not included in the analysis). The analysis population was further restricted to participants with available long-term symptom diaries at study week 24 (n = 506), which were introduced partway through the trial as a protocol-required assessment for all participants (see Supplementary Table 2 for comparison of those with and without assessments). Participants were enrolled at 35 sites in the US from October 2020 to February 2021.
Assessments
Participant-Completed Symptom Diaries
Participants completed an “acute viral illness” 13-symptom diary daily from enrollment on day 0 through day 28, as previously described [12]. Participants graded each symptom as “absent,” “mild,” “moderate,” or “severe”, and were instructed to report the worst severity over the preceding 24 hours by self-assessment. Total symptom scores were calculated as the sum of the individual scores for the 13 symptoms, with absent scored as 0, mild as 1, moderate as 2, and severe as 3, allowing for a total symptom score range of 0 to 39 for a given day. A long-term symptom diary was completed at 24 weeks after study entry and included the same 13 symptoms present in the acute viral illness daily diary plus 14 additional long COVID symptoms selected based on available literature at the time [13] (see Supplementary Appendix for the long-term diary). The long-term symptom diary instructed participants to report the overall severity of their symptoms over the past 4 weeks by self-assessment. For both diaries, participants were not asked to assess relatedness of symptoms to COVID-19. The long-term diary also included 3 global assessment questions evaluating (1) overall severity of COVID-19 symptoms over the previous 4 weeks, graded by the participant as no symptoms, mild, moderate, or severe; (2) general physical health over the previous 4 weeks, graded as excellent, very good, good, fair, or poor; and (3) return to usual (pre-COVID) health at time of diary completion, with response options of “yes” or “no,” all by participant self-assessment.
Virology
Participant-collected anterior nasal swabs were obtained using standardized swabs and collection procedures daily on days 0 through 14 and days 21 and 28 in phase 2, and on days 0, 3, 7, 10, 14, 21, and 28 in the open-label evaluation of bamlanivimab for quantitative SARS-CoV-2 RNA polymerase chain reaction (PCR) (collection and analysis methods described previously) [12]. The assay limit of detection (LoD) was 1.4 log10 copies/mL, the lower limit of quantification (LLoQ) was 2 log10 copies/mL, and the upper limit of quantification (ULoQ) was 7 log10 copies/mL. For samples with RNA levels >ULoQ, the assay was rerun with dilutions to obtain a quantitative value.
Serum Inflammation and Coagulation Biomarkers
Inflammatory and coagulation markers including C-reactive protein (CRP) (Tina-quant C-Reactive Protein IV, cobas analyzer, Roche Diagnostics), ferritin (Elecsys Ferritin, cobas analyzer, Roche Diagnostics), lactate dehydrogenase (LDH) (LDHI2, cobas analyzer, Roche Diagnostics), activated partial thromboplastin time (aPTT) (HemosIL SynthASil, ACL TOP 500 analyzer, Instrumentation Laboratory), and fibrinogen (HemosIL Fibrinogen-C, ACL TOP 500 analyzer, Instrumentation Laboratory) were measured in real time by a central clinical laboratory (PPD Laboratory Services Global Central Labs) at days 0 and 28, per the manufacturers’ protocols.
Outcome Measures
The primary outcome measure, which defines long COVID for this exploratory analysis, was presence of overall COVID-19 symptoms (any of mild, moderate, or severe) at week 24, as recorded by participants in response to the first global assessment question. Additional outcome measures included self-assessed general physical health at week 24, self-assessed return to usual (pre-COVID-19) health, and presence of 27 individual symptoms, each graded in severity from absent to severe.
Statistical Analysis
Associations with the primary outcome were assessed using univariable and multivariable modified Poisson regression models with robust variance estimation to reflect the fact that the outcome was binary [14]. Coagulation and inflammation markers were categorized as high versus normal based on laboratory reference ranges; normal values were further divided into 3 equally sized tertiles for analysis as possible predictors of long COVID. Results are presented as risk ratios (RRs), 95% confidence intervals (CIs), and P values from Wald tests, with global P values provided for predictors with >2 categories. Statistical significance was based on a 2-sided 5% type I error rate without adjustment for multiple comparisons. Analyses were performed using SAS version 9.4 software (SAS Institute, Cary, North Carolina).
Potential Predictors of Long COVID
Pretreatment characteristics examined as potential predictors of long COVID included age, sex, race (Black, White, and other), ethnicity (Hispanic/Latino or non-Hispanic/Latino), body mass index (BMI), cigarette smoking status at study enrollment (current, former, never), presence of comorbidities conferring increased risk for severe COVID-19 (analyzed as at least 1 vs none), and anterior nasal SARS-CoV-2 RNA level, serum inflammatory and coagulation biomarkers, and total symptom score on day 0.
Postentry measures assessed included viral burden defined as the area under the curve (AUC) of log10 SARS-CoV-2 RNA levels from anterior nasal swabs collected from day 0 to 28, presence of symptoms on study days 22–28 (the last week of the acute symptom diary), serum inflammatory and coagulation biomarkers at day 28, and receipt of COVID-19 vaccination at any point prior to week 24.
RESULTS
The analysis population included 506 participants, which comprised 44% (506/1162) of those who received 700 mg of bamlanivimab in ACTIV-2 (Supplementary Figure 1). Median (Q1) age was 51 (40, 60) years, 50% were female, 5% identified as Black/African American, and 36% identified as Hispanic/Latino (Table 1). At enrollment, median (Q3s) BMI was 28.4 (25.2, 33.5) kg/m2, 5% were current smokers, 17% were previous smokers, and 54% reported ≥1 high-risk comorbidity. Only 2 participants had previously received at least 1 dose of a SARS-CoV-2 vaccine prior to study entry; 107 (21%) participants received a SARS-CoV-2 vaccine between day 0 and week 24. Postbaseline characteristics are shown in Supplementary Table 3.
Table 1.
Participant Characteristics Prior to Receiving Bamlanivimab Treatment by Participants Who Reported Presence (n = 91) or Absence (n = 415) of Overall Coronavirus Disease 2019 Symptoms on Global Assessment at Week 24
| Characteristic | Global Assessment: Overall COVID-19 Symptoms | ||
|---|---|---|---|
| Present (n = 91) | Absent (n = 415) | Total (N = 506) | |
| Age, y, median (quartiles) | 52 (43, 60) | 50 (40, 61) | 51 (40, 60) |
| Female sex | 59 (65) | 196 (47) | 255 (50) |
| Cisgender | 89 (98) | 413 (100) | 502 (99) |
| Race | |||
| White | 82 (90) | 370 (89) | 452 (89) |
| Asian | 3 (3) | 15 (4) | 18 (4) |
| Black or African American | 5 (5) | 19 (5) | 24 (5) |
| American Indian or Alaska Native | 0 (0) | 1 (0) | 1 (0) |
| Native Hawaiian or other Pacific Islander | 1 (1) | 1 (0) | 2 (0) |
| Multiple | 0 (0) | 2 (0) | 2 (0) |
| Other | 0 (0) | 7 (2) | 7 (1) |
| Hispanic/Latino ethnicity | 20 (22) | 164 (40) | 184 (36) |
| BMI, kg/m2, median (quartiles) | 30.4 (26.0, 34.8) | 28.2 (25.1, 32.7) | 28.4 (25.2, 33.5) |
| Cigarette smoking status | |||
| Current | 10 (11) | 15 (4) | 25 (5) |
| Former | 15 (16) | 71 (17) | 86 (17) |
| Never | 66 (73) | 329 (79) | 365 (78) |
| Reported ≥1 high-risk comorbiditya | 55 (60) | 217 (5) | 272 (54) |
| SARS-CoV-2 vaccination | 0 (0) | 2 (<0.5) | 2 (<0.5) |
| Symptom duration at enrollment, d, median (quartiles) | 5 (4, 7) | 5 (4, 7) | 5 (4, 7) |
| ≤5 d | 51 (56) | 225 (54) | 276 (55) |
| >5 d | 40 (44) | 190 (46) | 230 (45) |
| SARS-CoV-2 RNA from AN swabs, log10 copies/mL, median (quartiles) | 5.4 (3.3, 6.8) | 5.6 (3.2, 6.9) | 5.5 (3.3, 6.9) |
| <LLoQ | 12 (13) | 56 (14) | 68 (14) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AN, anterior nasal; BMI, body mass index; COVID-19, coronavirus disease 2019; LLoQ, lower limit of quantification; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
High-risk comorbidities include active cancer, moderate to severe asthma, chronic kidney disease, chronic liver disease, history of cirrhosis, chronic lung disease, current smoker, cardiovascular disease, diabetes, hypertension, treatment with biologics/immunomodulators/cancer chemotherapy within 90 days of entry, human immunodeficiency virus with CD4 count <200 cells/μL, receiving corticosteroids within 30 days of entry, and obesity.
Long COVID Outcomes and Internal Validity of Study Long COVID Definition
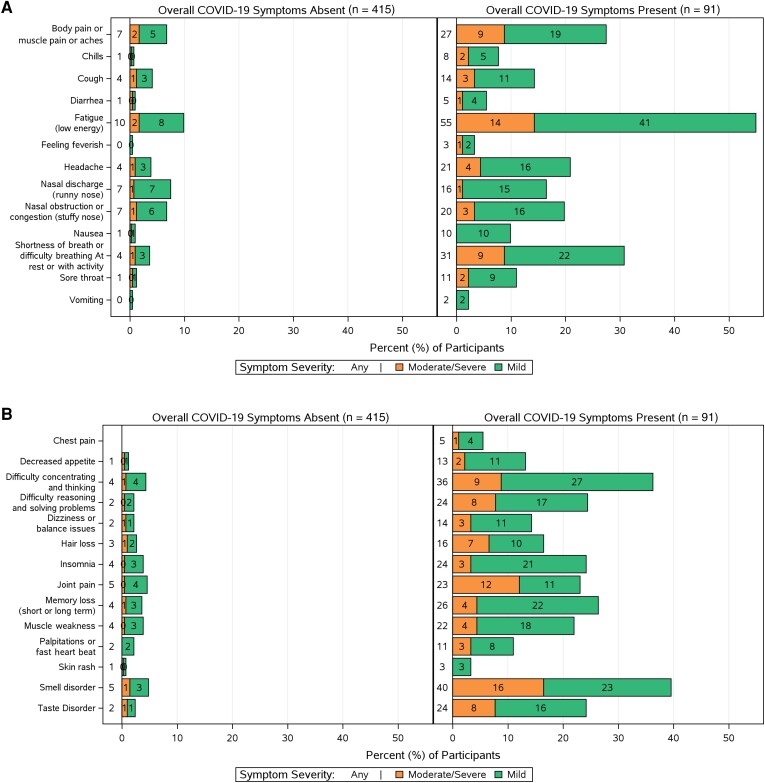
At 24 weeks after bamlanivimab treatment, 18% (91/506) of participants reported long COVID (ie, the presence of COVID-19 symptoms of at least mild severity in the preceding 4 weeks). Prevalence of long COVID was similar for participants treated within 5 days and >5 days from symptom onset (18% [51/276] and 17% [40/230], respectively). Distributions of the frequency of individually reported symptoms by the presence and absence of long COVID show marked differences in the report of individual targeted symptoms between the groups (Figure 1). All 27 symptoms assessed in the long-term diary were reported with greater frequency among participants reporting presence of overall COVID-19 symptoms on the global assessment than in those reporting absence of overall COVID-19 symptoms.
Figure 1.
Frequency and severity of 13 viral illness long COVID symptoms (A) and 14 additional long COVID symptoms (B) reported in participants’ long-term diaries at week 24 by participants who reported presence or absence of overall coronavirus disease 2019 (COVID-19) symptoms on global assessment. All individual targeted symptoms assessed in the long-term diary were reported with greater frequency among participants who reported presence of overall COVID-19 symptoms by global assessment. Bars labeled as “0” represent some small value between 0 and 0.5; a true zero value would not have a bar drawn in these plots.
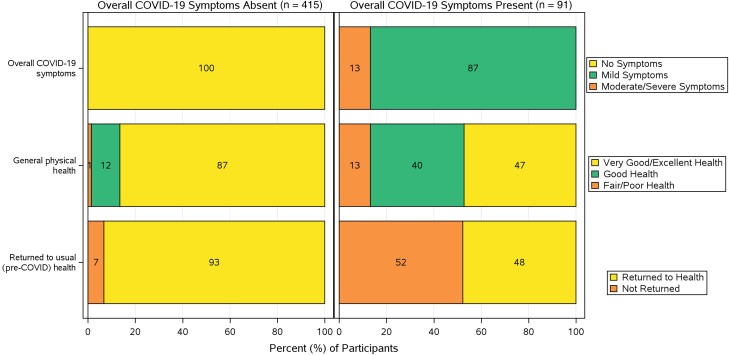
Among participants reporting long COVID at week 24 (n = 91), the most common symptoms (reported by ≥20% of 91 participants) were fatigue (55%), smell disorder (40%), difficulty concentrating/thinking (36%), breathing difficulties (31%), musculoskeletal pain (27%), weakness (22%), memory loss (26%), taste disorders (24%), difficulty reasoning and solving problems (24%), insomnia (24%), joint pain (23%), headache (21%), and nasal obstruction or congestion (20%) (Figure 1). Eighty-seven percent reported their overall symptoms as mild and 13% as moderate/severe (Figure 2), and 53% reported at least 4 symptoms present of mild or worse severity (compared with only 7% [30/415] among those without long COVID) (Supplementary Table 4).
Figure 2.
Responses to global assessment questions in long-term diary at week 24 by participants who reported presence or absence of overall coronavirus disease 2019 (COVID-19) symptoms. The health status (general physical health and whether or not they had returned to usual pre-COVID-19 health) reported by participants was notably different between the 2 groups.
While 18% reported long COVID at week 24, a slightly smaller percentage (15% [76/506]) reported that they had not returned to their usual pre-COVID health in response to the third global assessment question on the long-term diary. Participants who reported long COVID being present at week 24 were more likely to report not having returned to their usual pre-COVID health than participants without COVID-19 symptoms at week 24 (52% [47/91] vs 7% [29/415]) (Figure 2). These differences, along with the greater frequency of participants reporting ≥4 symptoms and higher frequency of all 27 individual targeted long COVID symptoms, supported the internal validity of presence of overall COVID-19 symptoms by global assessment as our definition of long COVID for subsequent regression analyses exploring predictors of long COVID.
Models Evaluating Predictors of Long COVID at Week 24
We first evaluated pretreatment demographic and clinical characteristics (age, BMI, cigarette smoking status, ethnicity, race, sex, presence of high-risk comorbidities, and total symptom score at day 0) as potential predictors of long COVID at week 24 in univariable models and a multivariable model (base model) including all covariates (Table 2). In the univariable model, we observed an increased risk of long COVID across increasing categories of BMI; however, this finding did not retain statistical significance in the multivariable model adjusting for baseline demographics, cigarette smoking status, presence of high-risk comorbidities, and severity of symptoms at entry. In both univariable and multivariable models, female sex (adjusted RR [aRR], 1.91 [95% CI, 1.28–2.85]) and current versus never smoking (aRR, 2.41 [95% CI, 1.34–4.32]) were associated with increased risk, and Hispanic/Latino ethnicity with lower risk (aRR, 0.52 [95% CI, .32–.84]) of long COVID at week 24.
Table 2.
Univariable and Multivariable (Model 0) Analysis Evaluating the Association of Baseline Clinical and Demographic Characteristics With Long COVID at Week 24 (Presence of Overall Coronavirus Disease 2019 Symptoms at Week 24 by Global Assessment)
| Variable | Univariable Model | Multivariable Model (Base Model)a | ||||||
|---|---|---|---|---|---|---|---|---|
| Risk Ratio | (95% CI) | P Value | Global P Valueb | Adjusted Risk Ratio | (95% CI) | P Value | Global P Valueb | |
| Age (per 10 y) at day 0 | 1.00 | (.89–1.12) | .96 | 0.99 | (.87–1.14) | .93 | ||
| BMI (kg/m2) at day 0 | ||||||||
| 25–29.9 vs <25 [ref] | 0.76 | (.43–1.36) | .36 | .04 | 0.90 | (.49–1.63) | .72 | .11 |
| 30–34.9 vs <25 [ref] | 1.42 | (.81–2.47) | .22 | 1.65 | (.93–2.93) | .09 | ||
| ≥35 vs < 25 [ref] | 1.51 | (.85–2.67) | .16 | 1.40 | (.75–2.64) | .29 | ||
| Cigarette smoking status at day 0 | ||||||||
| Current vs never [ref] | 2.39 | (1.41–4.06) | .001 | .10 | 2.41 | (1.34–4.32) | .003 | .07 |
| Former vs never [ref] | 1.04 | (.63–1.74) | .87 | 0.85 | (.49–1.47) | .56 | ||
| Ethnicity | ||||||||
| Hispanic or Latino vs not [ref] | 0.49 | (.31–.78) | .003 | 0.52 | (.32–.84) | .007 | ||
| High-risk comorbiditiesc | ||||||||
| At least 1 vs non/not reported [ref] | 1.31 | (.90–1.93) | .16 | 1.15 | (.72–1.84) | .56 | ||
| Race | ||||||||
| Black or African American vs White [ref] | 1.15 | (.51–2.57) | .74 | .71 | 1.14 | (.52–2.50) | .75 | .85 |
| Other vs White [ref] | 0.73 | (.29–1.87) | .52 | 0.81 | (.31–2.12) | .67 | ||
| Sex | ||||||||
| Female vs male [ref] | 1.81 | (1.22–2.69) | .003 | 1.91 | (1.28–2.85) | .001 | ||
| Total symptom score at day 0 (per unit higher) | 1.03 | (1.00–1.06) | .09 | 1.01 | (.98–1.05) | .41 | ||
Abbreviations: BMI, body mass index; CI, confidence interval; Non, none; ref=reference.
Multivariable model includes all variables together in 1 model.
Global P values provided for categorical variables with >2 categories.
High-risk comorbidities include active cancer, moderate to severe asthma, chronic kidney disease, chronic liver disease, history of cirrhosis, chronic lung disease, current smoker, cardiovascular disease, diabetes, hypertension, treatment with biologics/immunomodulators/cancer chemotherapy within 90 days of entry, human immunodeficiency virus with CD4 count <200 cells/μL, receiving corticosteroids within 30 days of entry, and obesity.
We next evaluated whether symptoms at the end of acute infection (days 22–28; model 1), SARS-CoV-2 vaccination preenrollment to week 24 (model 2), anterior nasal SARS-CoV-2 RNA level on day 0 (model 3), or day 0–28 AUC of anterior nasal SARS-CoV-2 RNA days 0–28 (model 4) (Supplementary Table 3) were associated with risk of long COVID at week 24 in univariable and multivariable models (Table 3), with each multivariable model being the base model plus the additional covariate. The number of participants with any symptom presence from day 22 to 28 was 271 (54%). Of those, 26% (71/271) reported the presence of long COVID at week 24 compared to 74% (200/271) reporting the absence of long COVID at the same time point. In comparison, the number of participants with no symptoms reported from day 22 to 28 was 235. Of those, 9% (20/235) reported the presence of long COVID at week 24 compared to 92% (215/235) reporting the absence of long COVID. In both univariable and multivariable analysis, the presence of any acute viral symptom during days 22–28 was strongly associated with increased risk of long COVID at week 24 (aRR, 2.70 [95% CI, 1.63–4.46]). There was no significant association of SARS-CoV-2 vaccination or anterior nasal SARS-CoV-2 RNA levels with risk of long COVID, although only 0.4% of participants had received a vaccination.
Table 3.
Univariable and Multivariable Analysis Evaluating the Association of Acute Symptoms, Coronavirus Disease 2019 Vaccination, and Nasal Severe Acute Respiratory Syndrome Coronavirus 2 RNA Levels With Long COVID at Week 24
| Model | Univariable Model | Multivariable Modelsa | ||||
|---|---|---|---|---|---|---|
| Risk Ratio | (95% CI) | P Value | Adjusted Risk Ratio | (95% CI) | P Value | |
| Model 1: any symptoms present day 22 to 28 (yes vs no) [ref] | 3.08 | (1.93–4.90) | <.001 | 2.70 | (1.63–4.46) | <.001 |
| Model 2: COVID-19 vaccineb received at any time prior to week 24 (yes vs no) [ref] | 1.23 | (.81–1.88) | .33 | 1.34 | (.87–2.08) | .19 |
| Model 3: SARS-CoV-2 RNA (per 1 log10 copies/mL higher than the LLoQ) on day 0 | 0.97 | (.86–1.08) | .53 | 0.99 | (.88–1.12) | .88 |
| Model 4: SARS-CoV-2 RNA AUC and >LLoQ (per 1 log10 copies/mL × day) from day 0 to day 28 | 1.00 | (.98–1.01) | .69 | 1.00 | (.98–1.02) | .98 |
Abbreviations: AUC, area under the curve; CI, confidence interval; COVID-19, coronavirus disease 2019; LLoQ, lower limit of quantification (2 log10 copies/mL); SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Each model adjusted for age (years), sex, race, ethnicity, smoking status, body mass index (kg/m2), presence of high-risk comorbidities, and total symptom score at day 0.
COVID-19 vaccine refers to any single dose of COVID-19 vaccination regardless of brand.
Finally, we evaluated associations between day 0 or day 28 inflammatory and coagulation markers and risk of long COVID. In univariable analyses, higher day 28 CRP was associated with higher risk of long COVID and higher day 28 ferritin levels with lower risk of long COVID (RRs, 1.76 [95% CI, 1.01–3.06] and 0.31 [95% CI, .10–.99], respectively) (Supplementary Table 5), but the associations did not persist in multivariable analyses adjusted for pretreatment characteristics (Supplementary Table 5). Other biomarker levels at day 0 were not associated with increased risk of long COVID (Supplementary Table 5).
DISCUSSION
In this prospective study of outpatients with mild-to-moderate COVID-19 treated with bamlanivimab during a time when it was expected to be active against circulating variants [8, 10–12], 18% of those completing the long-term diary reported the presence of COVID-19 symptoms and 15% reported that they had not returned to their usual pre-COVID health 24 weeks after treatment. The presence of self-reported COVID-19 symptoms defined long COVID in our analysis. We found that this definition distinguished 2 populations with widely differing self-reported health status and symptom experience across 27 long COVID symptoms. Consistent with other large long COVID studies, predominant reported symptoms included fatigue, dysosmia, dysgeusia, breathing difficulties, musculoskeletal, and cognitive complaints [15–19]. While symptoms were generally graded as mild, >60% of those with long COVID reported ≥3 symptoms.
Overall, our findings suggest a somewhat lower but still significant rate of long COVID when compared to other prospective cohort studies employing questionnaires at similar time points following COVID-19, although our population in which all individuals received bamlanivimab treatment has no direct comparator in the literature. In a prospective cohort study of individuals with COVID-19 (21% hospitalized), 61% (189/312) had persistent symptoms at 6 months that were associated with several factors, including severity of acute illness [20]. In another prospective study of 177 participants (85% outpatients with mild illness) who completed follow-up surveys a median of 5.6 months after acute COVID-19, persistent symptoms were reported by approximately 30% [21]. In the absence of a placebo group, it remains unknown if early mAb antiviral therapy can reduce the incidence of long COVID. A recently published retrospective cohort study using the healthcare databases of the US Department of Veterans Affairs demonstrated a 26% reduced risk of long COVID at 180 days in outpatients who received the oral antiviral nirmatrelvir within 5 days after a positive test for SARS-CoV-2 when compared to an untreated control group [22]. Similarly, the randomized controlled COVID-OUT trial reported a 41% reduction in the cumulative incidence of long COVID in participants randomized to receive the antidiabetic agent metformin during acute COVID-19 when compared to placebo [23]. Substantive differences in study design, long COVID definition, and duration of observation make direct comparisons with our study challenging. Regardless, our data demonstrate that early antiviral therapy, at least with mAbs, does not eliminate the risk of long COVID, and additional interventions are needed.
We identified a number of risk factors for long COVID after bamlanivimab treatment, including female sex, current cigarette smoking, and non-Hispanic ethnicity. Female participants were approximately 90% more likely to report ongoing symptoms than male participants. This association has been reported in a number of other studies [4, 5] but not universally [24]. Various biologic hypotheses include sex-based differences in innate and adaptive immunity [19, 25, 26]. We did not find an association of age or presence of comorbidities with risk of long COVID, which has been found in some cohorts [5]. Our finding of an association between active smoking and risk of long COVID has also been previously reported [4] and may be caused by concurrent chronic illnesses like lung disease or cardiovascular disease that are associated with both smoking and complications of COVID-19, but may also relate to upregulation of angiotensin-converting enzyme 2 in the lung [27]. Our finding that Hispanic ethnicity was associated with lower risk of long COVID differs from that of other reports [28–30] and is not readily explained. The association may be driven by unrecognized or unmeasured confounders and additional research in cohorts that are racially and ethnically diverse is warranted, acknowledging that analyses grouped by racial/ethnic identity are likely evaluating structural inequities in access to both medical care and clinical trials, rather than long COVID differing biologically by racial/ethnic identity [31].
Since viral persistence has been proposed as a possible pathogenic mechanism in long COVID [32], we evaluated viral measures collected from day 0 to day 28. We found no associations between nasal viral shedding pretreatment or over 28 days and risk of long COVID. It is possible that viral measures taken from the upper respiratory tract do not reflect the presence of virus in other compartments, precluding our ability to identify an association between viral burden and long COVID [33, 34]. In addition, any potential role of persistent viral replication in long COVID in our cohort may have been abrogated by bamlanivimab, which has been demonstrated to reduce nasopharyngeal SARS-CoV-2 RNA levels more than placebo, although these reductions are modest relative to the natural rate of viral decline in the nose [12]. Our finding that the presence of acute viral illness symptoms at 22–28 days after bamlanivimab treatment was strongly associated with increased risk of long COVID at week 24 was robust and may provide clinicians with a valuable metric for the identification of patients for increased monitoring for long COVID, which may become more valuable if effective therapies for long COVID are identified. This suggests that long COVID likely starts early and that interventions at interrupting its disease process may be needed earlier. While the inclusion criteria of 10 days since a positive PCR test could have the potential to bias the results toward the null if early treatment is needed for protection against long COVID, the median number of days of symptom duration at enrollment was the same in our study between those with and without long COVID. Furthermore, when stratified by symptom duration of ≤5 versus >5 days at enrollment, we did not observe higher rates of long COVID in those enrolled later in their symptom course. Disturbance of inflammatory and coagulation pathways have been implicated in the pathogenesis of SARS-CoV-2 infection and long COVID [35], but our findings did not identify an association between these markers during acute COVID and risk of long COVID.
Limitations of this study include the introduction of the long-term diary while study follow-up was ongoing, resulting in a study population that is a subset of all who received bamlanivimab in ACTIV-2. The predominance of participants identifying as White may also limit the generalizability of our findings to other racial groups. The large difference in reported rates of symptoms between those who indicated that they still had COVID-19 symptoms versus those who did not suggests that the participants largely attributed their week 24 targeted symptoms to COVID-19, but the absence of a control group without infection or data about the prevalence of these symptoms in our cohort prior to infection hinders our ability to definitively attribute reported symptoms to COVID-19. We also acknowledge that in a population of persons with a high rate of comorbidities, symptoms experienced during any 4-week period could be attributable to causes other than COVID-19. In addition, all participants received bamlanivimab and in the absence of a placebo group we are unable to formally conclude that the rates of long COVID in our cohort have been impacted by early antiviral therapy. This study is also limited by being performed earlier in the COVID-19 pandemic and before widespread vaccination, which may limit the generalizability of our findings to risk of long COVID with current and future variants and following vaccination or reinfection, particularly as there is evidence of decreased long COVID prevalence with Omicron variants (compared with earlier variants) [36, 37] as well as emerging evidence of a protective effect of COVID vaccination on long COVID prevalence [38, 39].
In summary, our study is the first of its kind to investigate long COVID prospectively within a clinical trial after treatment of acute COVID-19 with an effective anti-SARS-CoV-2 monoclonal antibody in a large, geographically diverse cohort of outpatients. Although generally mild, multiple symptoms of long COVID were commonly reported, and our findings suggest early mAb or other antiviral therapy for acute COVID-19 is unlikely to broadly prevent long COVID across an at-risk population. Additional prospective studies are needed to define long COVID. For the greatest impact, future randomized controlled interventional trials should be conducted in the outpatient setting in the context of current circulating variants, enroll racially and ethnically diverse individuals, and include those groups that have been demonstrated to potentially be at increased risk for long COVID (eg, the elderly, women, and individuals with comorbidities including smoking). In addition, a significant proportion of the cohort should be vaccinated at baseline and exhibit a range of BMIs. Such studies will be useful to determine predictors of and mechanisms of long COVID; understand the impact of different types of antiviral therapies, prior infection, vaccination, and variant subtype on long COVID development; and identify effective interventions, whether given early or late, for the prevention and treatment of long COVID.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Teresa H Evering, Department of Medicine, Weill Cornell Medicine, New York, New York.
Carlee B Moser, Center for Biostatistics in AIDS Research, Harvard T. H. Chan School of Public Health.
Nikolaus Jilg, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts.
Eunice Yeh, Center for Biostatistics in AIDS Research, Harvard T. H. Chan School of Public Health.
Busola Sanusi, Center for Biostatistics in AIDS Research, Harvard T. H. Chan School of Public Health.
David A Wohl, Department of Medicine, School of Medicine, University of North Carolina at Chapel Hill.
Eric S Daar, Lundquist Institute, Harbor-UCLA Medical Center, Torrance, California.
Jonathan Z Li, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts.
Paul Klekotka, Eli Lilly and Company, San Diego, California.
Arzhang Cyrus Javan, National Institutes of Health, Rockville, Maryland.
Joseph J Eron, Department of Medicine, School of Medicine, University of North Carolina at Chapel Hill.
Judith S Currier, Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles.
Michael D Hughes, Center for Biostatistics in AIDS Research, Harvard T. H. Chan School of Public Health; Department of Biostatistics, Harvard T. H. Chan School of Public Health, Boston, Massachusetts.
Davey M Smith, Department of Medicine, University of California, San Diego, La Jolla.
Kara W Chew, Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles.
for the ACTIV-2/A5401 Study Team:
Lara Hosey, Jhoanna Roa, Nilam Patel, Robert Coombs, Alexander Greninger, Emily Degli-Angeli, Erin Goecker, Glenda Daza, Socorro Harb, Joan Dragavon, Grace Aldrovandi, William Murtaugh, Marlene Cooper, Howard Gutzman, Kevin Knowles, Rachel Bowman, Bill Erhardt, Lorraine Waring, Diane Hessinger, Stacey Adams, and Asha R Kallianpur
Notes
Acknowledgments. We thank the study participants, site staff, site investigators, and the entire ACTIV-2/A5401 study team; the AIDS Clinical Trials Group (ACTG), including Lara Hosey, Jhoanna Roa, and Nilam Patel; the University of Washington Virology Specialty Laboratory, including Robert Coombs, MD, PhD, Alexander Greninger, MD, PhD, Emily Degli-Angeli, Erin Goecker, Glenda Daza, Socorro Harb, and Joan Dragavon; the ACTG Laboratory Center, including Grace Aldrovandi, MD, and William Murtaugh; Frontier Science, including Marlene Cooper, Howard Gutzman, Kevin Knowles, and Rachel Bowman; the Harvard Center for Biostatistics in AIDS Research and ACTG Statistical and Data Analysis Center; the ACTIV-2 Community Advisory Board; the Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID); Bill Erhardt, MD, Lorraine Waring, and Diane Hessinger; the Foundation for the National Institutes of Health (NIH) and the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership, including Stacey Adams; and the PPD clinical research business of Thermo Fisher Scientific. We also thank Asha R. Kallianpur, MD, MPH, for her insights. Lilly voluntarily asked the FDA to revoke the Emergency Use Authorization for bamlanivimab 700 mg alone in April 2021. This request was not due to any new safety concerns.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Financial support. This work was supported by the NIAID/NIH (award numbers UM1AI068634 to M. D. H., UM1 AI068636 to J. S. C., and UM1AI106701 to G. A.). Bamlanivimab was provided by Eli Lilly.
Supplement sponsorship. This article appears as part of the supplement “Findings From the ACTIV-2/AIDS Clinical Trials Group A5401 Adaptive Platform Trial of Investigational Therapies for Mild-to-Moderate COVID-19,” sponsored by the National Institutes of Health through a grant to the University of California, Los Angeles.
References
- 1. Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open 2021; 4:e2128568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deer RR, Rock MA, Vasilevsky N, et al. Characterizing long COVID: deep phenotype of a complex condition. EBioMedicine 2021; 74:103722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hope AA, Evering TH. Postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. Infect Dis Clin North Am 2022; 36:379–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bai F, Tomasoni D, Falcinella C, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect 2022; 28:611.e9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021; 27:626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aminian A, Bena J, Pantalone KM, Burguera B. Association of obesity with postacute sequelae of COVID-19. Diabetes Obes Metab 2021; 23:2183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun 2021; 12:6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Food and Drug Administration . FDA authorizes monoclonal antibodies for treatment of COVID-19. 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19-0. Accessed 5 August 2022.
- 9. Eli Lilly and Company . Lilly requests revocation of emergency use authorization for bamlanivimab alone to complete transition to bamlanivimab and etesevimab together for treatment of COVID-19 in the U.S. 2021. https://investor.lilly.com/news-releases/news-release-details/lilly-requests-revocation-emergency-use-authorization. Accessed 5 August 2022.
- 10. Hodcroft E. CoVariants version 0.1.0. Enabled by data from GISAID. 2020–2023. https://covariants.org/per-country?region=United+States. Accessed 5 August 2022.
- 11. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 2021; 384:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chew KW, Moser C, Daar ES, et al. Antiviral and clinical activity of bamlanivimab in a randomized trial of non-hospitalized adults with COVID-19. Nat Commun 2022; 13:4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021; 38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–6. [DOI] [PubMed] [Google Scholar]
- 15. Margalit I, Yelin D, Sagi M, et al. Risk factors and multidimensional assessment of long COVID fatigue: a nested case-control study. Clin Infect Dis 2022; 75:1688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verveen A, Wynberg E, van Willigen HDG, et al. Severe fatigue in the first year following SARS-CoV-2 infection: a prospective cohort study. Open Forum Infect Dis 2022; 9:ofac127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whiteside DM, Basso MR, Naini SM, et al. Outcomes in post-acute sequelae of COVID-19 (PASC) at 6 months post-infection part 1: cognitive functioning. Clin Neuropsychol 2022; 36:806–28. [DOI] [PubMed] [Google Scholar]
- 18. Nolen LT, Mukerji SS, Mejia NI. Post-acute neurological consequences of COVID-19: an unequal burden. Nat Med 2022; 28:20–3. [DOI] [PubMed] [Google Scholar]
- 19. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16:626–38. [DOI] [PubMed] [Google Scholar]
- 20. Blomberg B, Mohn KG, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med 2021; 27:1607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 2021; 4:e210830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xie Y, Choi T, Al-Aly Z. Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition. JAMA Intern Med 2023; 183:554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bramante CT, Buse JB, Liebovitz DM, et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial [manuscript published online ahead of print 8 June 2023]. Lancet Infect Dis 2023. doi: 10.1016/S1473-3099(23)00299-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carfi A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group . Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohamed MS, Moulin TC, Schioth HB. Sex differences in COVID-19: the role of androgens in disease severity and progression. Endocrine 2021; 71:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zeng F, Dai C, Cai P, et al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between sex. J Med Virol 2020; 92:2050–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leung JM, Yang CX, Tam A, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J 2020; 55:2000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bell ML, Catalfamo CJ, Farland LV, et al. Post-acute sequelae of COVID-19 in a non-hospitalized cohort: results from the Arizona CoVHORT. PLoS One 2021; 16:e0254347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoo SM, Liu TC, Motwani Y, et al. Factors associated with post-acute sequelae of SARS-CoV-2 (PASC) after diagnosis of symptomatic COVID-19 in the inpatient and outpatient setting in a diverse cohort. J Gen Intern Med 2022; 37:1988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hirschtick JL, Titus AR, Slocum E, et al. Population-based estimates of post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) prevalence and characteristics. Clin Infect Dis 2021; 73:2055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andrasik MP, Maunakea AK, Oseso L, et al. Awakening: the unveiling of historically unaddressed social inequities during the COVID-19 pandemic in the United States. Infect Dis Clin North Am 2022; 36:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brodin P, Casari G, Townsend L, et al. Studying severe long COVID to understand post-infectious disorders beyond COVID-19. Nat Med 2022; 28:879–82. [DOI] [PubMed] [Google Scholar]
- 33. Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nat Med 2021; 591:639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tejerina F, Catalan P, Rodriguez-Grande C, et al. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect Dis 2022; 22:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bryce C, Grimes Z, Pujadas E, et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol 2021; 34:1456–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ. Risk of long COVID associated with Delta versus Omicron variants of SARS-CoV-2. Lancet 2022; 399:2263–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goldstein A, Keating D. Long-covid symptoms are less common now than earlier in the pandemic. 2023. https://www.washingtonpost.com/health/2023/03/18/long-covid-less-likely/. Accessed 25 June 2023.
- 38. Watanabe A, Iwagami M, Yasuhara J, Takagi H, Kuno T. Protective effect of COVID-19 vaccination against long COVID syndrome: a systematic review and meta-analysis. Vaccine 2023; 41:1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Azzolini E, Levi R, Sarti R, et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA 2022; 328:676–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.