Abstract
The morbidity and mortality of myocardial infarction (MI) are increasing worldwide. Mesenchymal stem cells (MSCs) are multipotent stem cells with self-renewal and differentiation capabilities that are essential in tissue healing and regenerative medicine. However, the low implantation and survival rates of transplanted cells hinder the widespread clinical use of stem cells. Exosomes are naturally occurring nanovesicles that are secreted by cells and promote the repair of cardiac function by transporting noncoding RNA and protein. In recent years, MSC-derived exosomes have been promising cell-free treatment tools for improving cardiac function and reversing cardiac remodeling. This review describes the biological properties and therapeutic potential of exosomes and summarizes some engineering approaches for exosomes optimization to enhance the targeting and therapeutic efficacy of exosomes in MI.
1. Introduction
Myocardial infarction (MI) involves the irreversible death of cardiomyocytes due to prolonged oxygen deprivation by an obstructed blood supply (ischemia), which results in contractile dysfunction and cardiac remodeling. Although significant progress has been made in medical treatments, such as thrombolytic therapy, percutaneous coronary intervention, and coronary artery bypass grafting surgery, MI is still the leading cause of cardiovascular disease (CVD)-associated death [1]. The high prevalence and mortality of MI suggest that it is essential to continue the search for suitable treatments. Stem cells have powerful regenerative potential and are expected to be some of the best candidates for MI treatment [2, 3]. Mesenchymal stem cells (MSCs) are multipotent stem cells that can self-renew and differentiate. They have received much attention due to their strong proliferative capacity, multidirectional differentiation potential, immunomodulatory properties, low immunogenicity, and ease of isolation and expansion. MSCs may protect the myocardium by reducing inflammation, promoting angiogenesis around infarcted areas, increasing resistance to apoptosis, and inhibiting fibrosis [4]. In recent years, some pretreatment methods, such as hypoxia pretreatment, drug pretreatment, cytokine pretreatment, and gene modification, have been used to enhance the functional benefits of transplanted stem cells to promote cardiac regeneration and angiogenesis and inhibit fibrosis progression. However, poor engraftment and survival rates of MSCs in the myocardium inhibit their therapeutic efficacy. Exosomes, which are products of MSCs, have been prioritized in acellular therapy for cardiac rehabilitation after MI and other infections, and they may yield significant economic and social benefits [5]. Numerous studies have indicated that MSC-derived exosomes (MSC-Exos) can promote cardiomyocyte survival and angiogenesis, inhibit fibrosis, and regulate the cardiac microenvironment, which are indispensable therapeutic strategies for alleviating ischemic heart disease [6–8]. Exosome targeting has also been used to enhance the capability of exosomes to target homologous receptors in specific tissues and organs [7]. MSC-Exos are attractive alternatives for acellular therapy after MI [9]. This review aims to discuss the biological roles and mechanisms of exosomes, as well as the best strategies to improve the efficacy of exosome-based treatment, and reveal the remarkable potential of exosomes in the treatment of MI.
2. Biological Properties of MSCs
Currently, the stem cells used for MI therapy include MSCs, embryonic stem cells (ESCs), placental-derived stem cells, and cord blood-derived stem cells. MSCs are multipotent and nonhematopoietic adult stem cells with self-renewal and differentiation abilities [10, 11]. MSCs can be isolated from a very diverse range of tissues or organs, including adipose tissue, bone marrow, the placenta, the umbilical cord blood and umbilical cord, skin, skeletal muscle, tendons, synovial membranes, the endometrium, amniotic fluid, the amniotic membrane, peripheral blood, menstrual blood, salivary glands, pulp, and periodontal ligaments [12–15]. Clinically, MSCs are derived mainly from bone marrow, umbilical cord blood, and adipose tissue [3, 16, 17]. In 2006, the International Society for Cell Therapy proposed the following minimal criteria for defining MSCs: adherent growth in stable culture; CD105, CD73, and CD90 expression but no expression of CD45, CD34, CD14 or CD11b, CD79α or CD19, and HLA-DR; and an in vitro ability to differentiate into osteoblasts, adipocytes, and chondrocytes [18]. MSCs are capable of secreting a range of cytokines, such as vascular endothelial growth factor (VEGF) and pigment epithelial-derived factor [19, 20]. Preclinical findings in rat and porcine MI models have indicated that MSCs can significantly reduce MI size, restore myocardial contractility, and improve the structure and function of infarcted hearts through the synergistic effects of myogenesis and angiogenesis [21, 22]. Hare et al. [23] conducted the first clinical trial of MSCs for MI in 2005 and demonstrated the safety of allogeneic human bone marrow-derived MSCs (hBMMSCs) for treating MI. In addition, studies have shown that intravenous, intracoronary, or myocardial injection of autologous or allogeneic MSCs is safe, and beneficial effects have been observed over 12 months of long-term follow-up [24, 25]. While some clinical trials have demonstrated the effectiveness of MSC treatment, some studies have not observed a benefit from MSC treatment [26, 27]. The differential therapeutic effects of MSCs may be related to the method of cell acquisition, the transplantation step, the survival of cells after transplantation, poor homing, and the high dose needed to maintain the therapeutic effect [28–31]. Moreover, numerous studies in recent years have demonstrated that MSCs help restore cardiac function mainly through their paracrine effects, especially those involving exosomes [32, 33]. Exosomes derived from MSCs (MSC-Exos) have the potential to promote cardiomyocyte survival, proliferation, and angiogenesis and limit the inflammatory response, which can be used as a practicable strategy for cell-free heart repair [34, 35]. These biological activities of MSCs provide new ideas for treating CVDs.
3. Biological Properties of Paracrine Exosomes from MSCs
Extracellular vesicles (EVs) mediate the communication of cell-to-cell, which are membrane vesicles (MVs) released by cells [36]. EVs contain a range of bioactive substances, including DNA, RNA, lipids, metabolites, and cytosolic and cell-surface proteins [37, 38]. The International Extracellular Vesicle Society classifies EVs into the following categories: exosomes, apoptotic bodies, and MVs [36, 39, 40]. Exosomes are extracellular membranous nanovesicles with sizes ranging from 30 to 150 nm [29, 41]. Exosomes are formed by inward germination of the multivesicular body membrane and subsequent fusion with the plasma membrane to release intraluminal vesicles into the extracellular space [38, 40, 42]. In addition, there is evidence that exosomes germinate directly from plasma membranes [43]. To date, we have found that ultracentrifugation, precipitation, density gradient centrifugation, immune affinity capture, microfluidic technologies, and size-exclusion chromatography can all be used to isolate exosomes [44–47]. The biomarkers of exosomes are heat shock proteins, ALG-2-interacting protein X (Alix), and integral membrane tetraspanin proteins (CD81, CD63, and CD9), which are widespread in all exosomes [48–51]. In addition, the cargo of exosomes includes many biologically active substances, such as proteins, lipids, and genetic material (mRNAs, miRNAs, long noncoding RNAs, and DNA) [52]. The Exo-Carta exosome database (http://www.exocarta.org) is a collection of 9,769 proteins, 3,408 mRNAs, 2,838 miRNAs, and 1,116 lipids identified in exosomes from diverse cell types and different species. Exosomes play a critical role in cell-to-cell communication by delivering biologically active molecules [41, 53–56]. Recently, exosomes have been shown to participate in many processes, including cell survival, angiogenesis, and immune reactions, by altering the communication among cells/organs [55]. Studies have shown that MSC-Exos have significant advantages over their parental cells [57], such as long-term stability, more accessible storage, lower immune rejection, less tumorigenicity, and lower production costs. Moreover, the smaller size of exosomes relative to their parental cells enables them to pass through capillaries without clogging them [29, 58]. Lai et al. [59] confirmed the role of human ESC-derived MSC-Exos in decreasing myocardial fibrosis and enhancing cardiac function for the first time in 2010. Since then, research on MSC-Exos has reached a new zenith. The role of MSC-Exos in cardioprotection has been heavily investigated, laying the groundwork for future treatments of ischemic heart disease.
4. The Therapeutic Potential of MSC-Exos in MI
After MI, the ventricle exhibits a range of responses, such as cardiac hypertrophy, interstitial fibrosis, and inflammation (Figure 1). Programmed cell death (PCD) pathways are activated after ischemia or hypoxia, and they are the major causes of heart failure [45]. Many studies have shown that MSC-Exos can inhibit PCD and fibrosis, promote angiogenesis, and improve the microenvironment of the ischemic myocardium [60, 61]. As some of the most common components of exosomes, microRNAs (miRNAs) play crucial roles in the physiology of the cardiovascular system and CVD progression [62]. Next, we will elaborate on the role of exosomes in MI from several perspectives.
Figure 1.
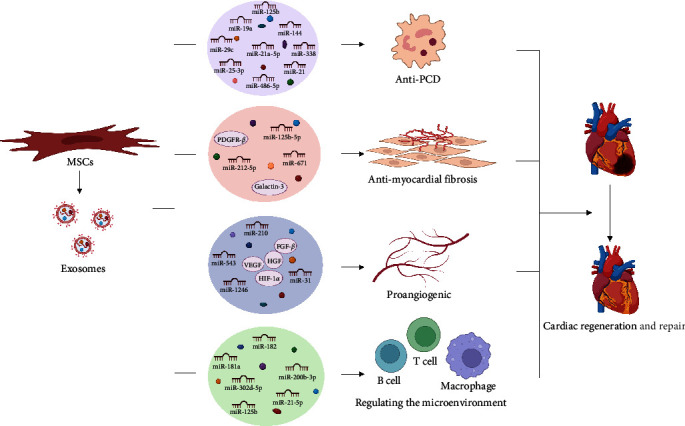
Reparative effect of MSC-Exos on ischemic hearts after MI. After myocardial infarction (MI), the intramuscular injection of exosomes derived from mesenchymal stem cells (MSC-Exos) had an anti-programmed cell death (PCD) effect by releasing miR-19a, miR-144, miR-486-5p, miR-21, miR-21a-5p, miR-25-3p, and other miRNAs. Galectin-3, miR-212-5p, miR-125b-5p, PDGFR-β, and miR-671 in MSC-Exos promote ischemic myocardial repair by antimyocardial fibrosis. The hepatocyte growth factor (HGF), angiogenic fibroblast growth factor-β (FGF-β), vascular endothelial growth factor (VEGF), miR-31, miR-543, and miR-1246 in MSC-Exos can promote the generation of myocardial vascular endothelial cells and maintain myocardial blood flow. Substances such as miR-181a, miR-125b, miR-182, miR-21-5p, miR-302d-5p, and miR-200b-3p released by MSC-Exos can regulate the cardiac microenvironment after myocardial infarction, reduce the inflammatory response and promote myocardial repair.
4.1. Inhibiting PCD
PCD has a variety of forms, such as apoptosis, autophagy, pyroptosis, and ferroptosis (Figure 2). MSC-Exos can protect the myocardium from ischemic and hypoxic injury by inhibiting apoptosis, autophagy, pyroptosis, and ferroptosis. Exosomes from human umbilical cord-derived MSCs (hUCMSCs) protect cardiomyocytes from acute MI damage and reduce apoptosis by transferring miR-19a to target SOX6, which activates AKT and inhibits JNK3/caspase-3 activation [63]. The PI3K/AKT pathway is an important signaling pathway that regulates apoptosis and survival. Both miR-144 and miR-486-5p in BMMSC-Exos significantly inhibit apoptosis in hypoxic cardiomyocytes by targeting the PTEN/PI3K/AKT signaling pathway [64, 65]. miR-21 in endometrial MSC-Exos can also exert significant antiapoptotic effects by interacting with the PTEN/PI3K/AKT signaling pathway [66]. Similarly, miR-21a-5p and miR-25-3p in BMMSC-Exos can reduce apoptosis in hypoxic cardiomyocytes by downregulating the expression of proapoptotic genes, including FasL, PDPD4, Peli1, and PTEN [67, 68]. In addition, miR-129-5p and miR-338 are enriched in BMMSC-Exos and inhibit myocardial apoptosis and improve cardiac function in MI rats by regulating the TRAF3/NF-κB and MAP3K2/JNK signaling pathways, respectively [69, 70]. Furthermore, Lee et al. [71] found that MSC-derived EVs upregulate the expression of survivin by the miR-199a-3p-AKT-Sp1/p53 signaling pathway, which protects cardiomyocytes from injury in doxorubicin-induced cardiomyopathy.
Figure 2.
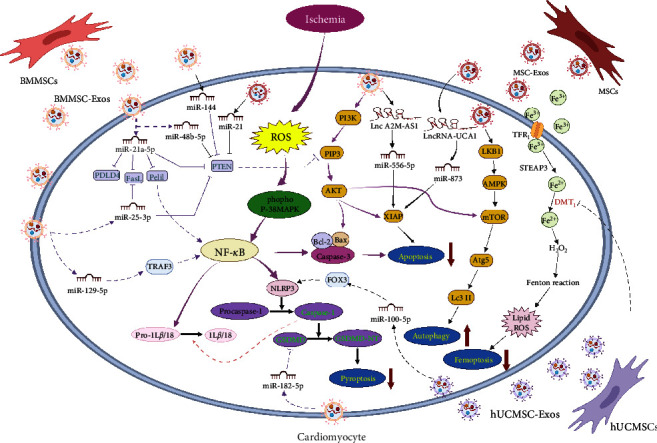
Exosomes from different MSCs mediate PCD in myocardial cells after myocardial infarction. MiR-144 and miR-486-5p in exosomes derived from bone marrow mesenchymal stem cells (BMMSC-Exos) and miR-21 in endometrial exosomes derived from mesenchymal stem cells (MSC-Exos) can inhibit apoptosis of hypoxic cardiomyocytes by targeting the PTEN/PI3K/AKT signaling pathway. MiR-21a-5p and miR-25-3p in BMMSC-Exos can reduce the apoptosis of hypoxic cardiomyocytes by downregulating the expression of proapoptotic genes such as FasL, PDPD4, Peli1, and PTEN. MiR-129-5p in BMMSC-Exos inhibits cardiomyocyte apoptosis by regulating the TRAF3/NF-κB signaling pathway. BMMSC-Exos induce autophagy in cardiomyocytes by activating the AMPK/mTOR and AKT/mTOR signaling pathways. Human BMMSC-Exos inhibit pyroptosis by inhibiting the expression of Caspase-1 and NLRP3. Moreover, miR-182-5p in BMMSC-Exos inhibit pyroptosis by inhibiting the expression of GSDMD. MiR-100-5p in exosomes derived from human umbilical cord mesenchymal stem cells (hUCMSC-Exos) inhibit pyroptosis by inhibiting the expression of FOXO3. HUCMSC-Exos reduce ischemic hypoxia-induced iron death in cardiomyocytes by targeting DMT1. LncRNA-UCA1 in human MSC-Exos and Lnc A2M-AS1 in human BMMSC-Exos inhibit myocardial cell apoptosis by adjusting the miR-873-5p/XIAP axis and miR-556-5p/XIAP axis, respectively.
Autophagy is the process by which lysosomes engulf organelles and other factors to remove unnecessary or dysfunctional cellular components [72]. Recent research has shown that a certain level of autophagy plays a positive role in maintaining the structure and function of cardiomyocytes and that increased autophagy helps protect the heart during myocardial ischemia and hypoxia [73]. Zou et al. [74] found that BMMSC-Exos regulate the postinfarction cardiac microenvironment by promoting autophagy in infarcted hearts. The mechanism of action may be related to increases in the expression of autophagy-related protein 13 in H9c2 cells after MI modeling. It has been found that hUCMSC-Exos attenuate coxsackievirus B3-induced myocarditis by activating the AMPK/mTOR-mediated autophagic flux pathway, thereby attenuating cardiomyocyte apoptosis [75]. Liu et al. [76] found that BMMSC-Exos reduce apoptosis by activating the AMPK/mTOR and AKT/mTOR signaling pathways to induce autophagy in cardiomyocytes. Basal levels of autophagy are critical for cardiac protection, while excessive autophagy promotes cell death and ventricular remodeling [51]. miR-29c and miR-125b in BMMSC-Exos inhibit excessive autophagy by regulating the PTEN/AKT/mTOR and p53/Bnip3 signaling pathways, respectively, thereby reducing the inflammatory response after myocardial I/R injury [77, 78].
The nod-like receptor protein 3 (NLRP3) inflammasome is a multiprotein signaling complex that mediates the maturation of proinflammatory cytokines such as IL-1β and IL-18 through interactions with caspase 1 [79]. Pyroptosis is a type of PCD characterized by inflammatory cytokine (caspase-1, NLRP3) release [80, 81]. Tang et al. [81] found that hBMMSC-Exos exhibit significantly reduced expression of the pyroptosis-related proteins caspase-1 and NLRP3, thereby protecting the myocardium from ischemia/reperfusion (I/R) damage. Liang et al. [82] further found that miR-100-5p is enriched in hUCMSC-Exos and inhibits activation of the NLRP3 inflammasome by inhibiting the expression of FOXO3, thus preventing the release of cytokines and blocking pyroptosis. Furthermore, miR-182-5p in BMMSC-Exos can attenuate ischemic myocardial injury and pyroptosis by targeting GSDMD and reducing its expression [83]. DMT1 overexpression promotes ferroptosis induced by hypoxia/reoxygenation (H/R). Song et al. [84] found that hUCMSC-Exos decrease H/R-induced ROS levels, iron deposition, and Fe2+ and MDA levels in cardiomyocytes by targeting DMT1. In addition to the above-mentioned models of cell death, cuproptosis is a novel cell death mechanism that is closely related to mitochondrial respiration [85]. Whether the mechanism by which exosomes inhibit cell death is related to cuproptosis has not been confirmed, which may be a new idea for future exosome research.
It has also been found that some lncRNAs are involved in the cardioprotective effects of exosomes. The lncRNA KLF3-AS1 in hMSC-Exos can inhibit the viability, the inflammatory response, and pyroptosis in cardiomyocytes by regulating the miR-138-5p/SIRT1 axis, thus inhibiting the progression of MI [86]. LncRNA-UCA1 in hypoxia-pretreated hMSC-Exos plays a protective role in cardiac injury repair by targeting the miR-873-5p/XIAP axis and increasing the antiapoptotic protein Bcl2 levels [87]. In addition, human BMMSC-Exo-mediated Lnc A2M-AS1 inhibits H/R-induced myocardial injury by the miR-556-5p/XIAP axis [88]. As mentioned above, the cardioprotective effects of stem cell-derived exosomal miRNAs and lncRNAs have been confirmed by in vivo studies in animal models. New therapeutic approaches based on exosomal miRNAs will lay the foundation for the clinical treatment of patients with CVD.
4.2. Inhibiting Myocardial Fibrosis
The cardiac repair response after MI can be divided into three stages: inflammation, proliferation, and maturation [89]. Fibroblasts are involved in different stages of heart repair and play different roles in these stages. Fibroblasts exhibit a proinflammatory phenotype during the inflammatory phase and degrade extracellular matrix (ECM) components [90]. At this point, the differentiation of cardiac fibroblasts (CFs) into myofibroblasts is inhibited. When the dead cells are removed, cardiac repair enters the proliferative stage. During this stage, the inflammatory response is suppressed, and most fibroblasts differentiate into myofibroblasts, exhibiting an anti-inflammatory phenotype and producing an ECM that enhances the contractile capacity of the myocardium to maintain the structural and functional integrity of the heart [91]. Therefore, it may be beneficial for the increase of myocardial fibroblasts during the inflammatory period after MI to cardiac repair after MI [89]. Shi et al. [92] found that exosomes derived from hUCMSCs in the inflammatory phase after MI promote the differentiation of fibroblasts into myofibroblasts by increasing the density of myofibroblasts in the infarcted area, thus reducing the inflammatory response of cardiomyocytes. Activation of the Wnt/β-catenin signaling pathway is associated with fibrosis in various tissues and organs [93]. It has been found that activation of the Wnt/β-catenin signaling pathway can affect myofibroblast generation and promote myocardial repair [94]. Moreover, galectin-3 is a key protein associated with modulation of the Wnt/β-catenin signaling pathway. Guo et al. [91] observed in a rat MI model that galectin-3 in hUCMSC-Exos promoted the differentiation of CFs into myofibroblasts in an inflammatory environment by upregulating β-catenin levels in fibroblasts. Notably, an appropriate increase in the Wnt signaling pathway can promote the repair of necrotic myocardial tissue after MI, reduce the size of MI, and improve ventricular function [95, 96]. However, persistent overactivation of the Wnt signaling pathway can lead to severe myocardial fibrosis and impair myocardial function [95].
Transforming growth factor β (TGF-β) molecules are multipotent cytokines that cause tissue fibrosis [97]. TGF-β1 is essential in most stages of wound healing and scar formation [98]. Smad2 and Smad3 are generally considered the key downstream mediators of TGF-β1, and they play a very important role in the expression of matrix collagen and tissue fibrosis [99, 100]. Wu et al. [101] found that BMMSC-Exo-derived miR-212-5p alleviates MI-induced fibrosis by modulating the NLRC5/VEGF/TGF-β1/Smad axis. The reduction in TGF-β1 directly alleviates ECM deposition and fibrosis. In addition, miR-671 in exosomes derived from adipose-derived mesenchymal stem cell (ADMSC-Exos) can directly target the TGFBR2/Smad2 axis to attenuate myocardial tissue fibrosis in mice with MI, thus alleviating ischemic myocardial injury [102]. Smad7 is an important negative modulator of TGF-β/Smad signaling and protects against myocardial damage. It inhibits downstream gene transcription by inhibiting the phosphorylation of Smad2/3 by TGF-β1 and interfering with the interactions between receptors and other Smad proteins [103]. Studies have shown that exosomes derived from hUCMSCs can enhance myocardial repair by promoting the expression of Smad7 through inhibition of miR-125b-5p [104] (Figure 3).
Figure 3.
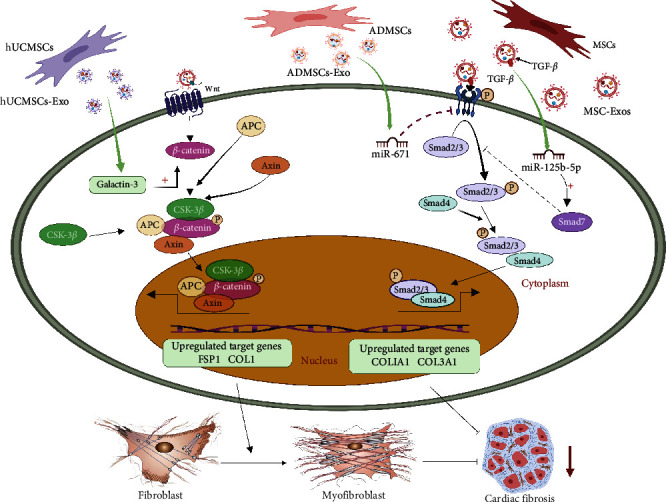
Anti-myocardial fibrosis effect of MSC-Exos. Galactin-3 in exosomes derived from mesenchymal stem cells (MSC-Exos) promotes the differentiation of cardiac fibroblasts into myoblasts in an inflammatory environment by upregulating β-catenin levels in fibroblasts. MiR-212-5p and miR-671 in MSC-Exos reduce myocardial infarct-induced fibrosis by regulating the NLRC5/VEGF/TGF-β1/Smad axis and TGFBR2/Smad2 axis, respectively. MSC-Exos promote Smad7 expression by inhibiting miR-125b-5p, inhibiting Smad2/3 phosphorylation, and further inhibiting myocardial fibrosis.
Furthermore, Kore et al. [105] found that BMMSC-Exos treatment of MI significantly reduced interstitial and perivascular fibrosis in the ischemic heart and the expression of fibronectin in the infarct and peri-infarct regions; the underlying mechanisms involved suppression of the activation of p-38MAPK and NF-κB, which inhibited fibronectin, collagen 1, and collagen 3 expression. Wang et al. [106] found in a rat myocardial I/R model and an in vitro myocardial microvascular endothelial cell H/R model that BMMSC-Exos promoted microvascular regeneration under stress conditions by regulating platelet-derived growth factor receptor β (PDGFR-β), thereby alleviating myocardial fibrosis after I/R and ultimately improving cardiac function. They also found that early activation of PDGF-BB/PDGFR-β promoted the renewal of functional tissues. By contrast, excessive activation of PDGF-BB/PDGFR-β led to fibrosis of functional tissues.
4.3. Proangiogenic Effects
Angiogenesis is the physiological process by which new blood vessels form and develop from the existing vascular system. Myocardial angiogenesis after MI is limited. Severe angiogenic dysfunction may lead to systolic dysfunction after heart failure [107]. In a rat model of acute MI, we found that, compared to PBS injection, intramyocardial injection of MSC-EVs significantly enhanced blood flow restoration and reduced infarct size without altering cardiac systolic and diastolic function [61]. In a Sprague‒Dawley rat-induced AMI model, Teng et al. [108] found that compared to PBS, BMMSC-Exos significantly enhanced new functional capillary density and inhibited cell proliferation to impair T-cell function and reduce apoptosis, thus promoting blood flow recovery. Likewise, Xu et al. [17] found that exosomes derived from UCMSCs, ADMSCs, and BMMSCs reduced apoptosis in cardiomyocytes and promoted angiogenesis by increasing the levels of hepatocyte growth factor, angiogenic fibroblast growth factor-β, and VEGF in a rat model of MI. The study also showed that the proangiogenic genes Ang1 and Flk1 expression were upregulated in ADMSC-Exo-treated HUVECs, while the antiangiogenic genes Vash1 and TSP1 expression were downregulated [109]. Furthermore, Hu et al. [110] found that human amniotic fluid MSC-Exos promoted angiogenesis by increasing hypoxia-inducible factor 1-α (HIF-1α) and VEGF expression in rats with isoproterenol-induced cardiac fibrosis. One study in a swine model of MI showed that intramyocardial injection of BMMSC-Exos increased capillary density and blood flow to ischemic myocardial tissue by upregulating the MAPK and AKT/eNOS pathways, resulting in increased cardiac output [111]. In addition, a study on a Yorkshire pig model of metabolic syndrome and chronic myocardial ischemia showed that intramyocardial administration of human BMMSC-Exos increased vascular density and blood flow and improved cardiac function in ischemic myocardial tissue [112]. In a porcine model of MI, we found that placement of decellularized pericardial scaffolds filled with peptide hydrogels and cardiac adipose tissue MSC-EVs on the myocardium resulted in an improvement in cardiac function, a significant increase in right ventricular ejection fraction, marked reductions in myocardial fibrosis and scar tissue, a twofold increase in vascular density, and a reduction in macrophage infiltration observed after 30 days, whereas the expression of anti-inflammatory CD73 was increased by 5.8-fold [113].
Wang et al. [114] found that a single intravenous injection of EVs secreted by BMMSCs in mice could promote angiogenesis and improve cardiac function in infarcted hearts. The mechanism by which angiogenesis was promoted may have been related to the miR-210-Efna3 pathway. Transplanting BMMSC-Exos loaded with miR-132 mimics into the ischemic hearts of mice significantly promotes neovascularization around the infarct area by regulating RASAI gene expression [115]. miR-125a and miR-31 in ADMSC-Exos promote endothelial cell angiogenesis by inhibiting the expression of the angiogenic inhibitor delta-like 4 and activating the FIH1/HIF-1α pathway, respectively [109, 116]. An increase in miR-29b-3p secreted by BMMSC-Exos can promote angiogenesis and ventricular remodeling in MI rats by targeting A Disintegrin and Metalloproteinase with Thrombospondin Motifs 16 (ADAMTS16) expression [117]. miR-543 in human MSC-Exos promotes proliferation, migration, and angiogenesis in cardiac microvascular endothelial cells through the downregulation of COL4A1 expression [118]. miR-1246 in hUCMSC-Exos promotes HUVEC angiogenesis by targeting the PRSS23/Snail/α-SMA axis [119]. These results demonstrate the therapeutic potential of MSC-Exos in ischemic heart disease (Figure 4).
Figure 4.
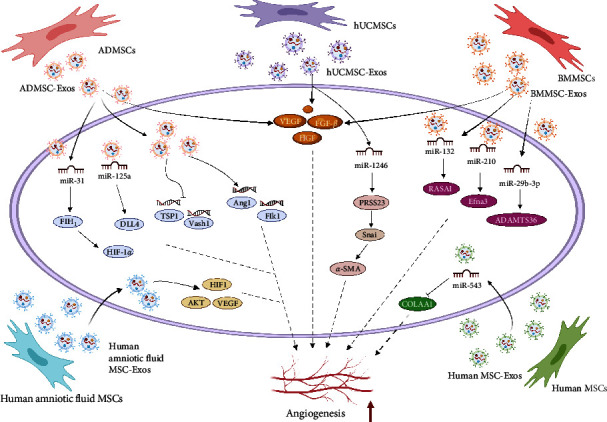
Angiogenic effect of exosomes released by different MSCs after MI. Exosomes derived from umbilical cord mesenchymal stem cells (UCMSCs), adipose-derived mesenchymal stem cells (ADMSCs), and bone marrow mesenchymal stem cells (BMMSCs) promote angiogenesis by increasing levels of hepatocyte growth factor (HGF), angiogenic fibroblast growth factor-β (FGF-β), and vascular endothelial growth factor (VEGF). Exosomes derived from adipose-derived mesenchymal stem cells (ADMSC-Exos) can upregulate the expression of the angiogenic genes Ang1 (also known as ANGPT1) and Flk1 (also known as KDR) and downregulate the expression of the antiangiogenic genes Vash1 and TSP1 (also known as THBS1). Human amniotic fluid exosomes derived from mesenchymal stem cells (MSC-Exos) promote angiogenesis by increasing the expression of HIF-1α and VEGF in fibrotic hearts. Exosomes derived from bone marrow mesenchymal stem cells (BMMSC-Exos) increase capillary density and blood flow in ischemic myocardial tissue by upregulating the MAPK and AKT/eNOS pathways. Extracellular vesicles (EVs) secreted by BMMSCs can promote the angiogenesis of infarcted hearts by regulating the miR-210-Efna3 pathway. MiR-132 mimics and miR-29b-3p loaded in BMMSC-Exos significantly promote neovasculature around the infarcted heart by regulating RASAI gene expression and A Disintegrin and Metalloproteinase with Thrombospondin Motifs 16 (ADAMTS16) expression, respectively. MiR-125a and miR-31 in ADMSC-Exos promote endothelial cell angiogenesis by inhibiting the expression of the angiogenic inhibitor delta-like 4 (DLL4) and activating the FIH1/HIF-1α pathways, respectively. MiR-543 in human MSC-Exos promotes the formation of cardiac microvascular endothelial cells by downregulating the expression of COL4A1. miR-1246 in exosomes derived from human umbilical cord mesenchymal stem cells (hUMSC-Exos) promotes angiogenesis by targeting the PRSS23/Snail/α-SMA axis.
Notably, some studies have shown that although intravenous injection of BMMSC-Exos upregulates some proangiogenic signaling pathway factors, it does not increase the vascular density in the ischemic myocardium, possibly because the effect of intravenous injection on angiogenic signaling is different from that of direct myocardial injection [120]. In addition, the dose of exosomes and the timing of exosome transplantation after MI can affect the outcomes. In conclusion, MSC-Exos undoubtedly exhibit strong potential to improve cardiac function and promote angiogenesis.
4.4. Regulating the Microenvironment after MI
Chronic and excessive proinflammatory responses after MI produce adverse left ventricular remodeling [121], which is strongly associated with worsening clinical outcomes after MI; therefore, the management of inflammation after MI is critical for limiting infarct size. Although the entire mechanism of action is not fully understood, it is known that MSC-Exos exert potent immunosuppressive and anti-inflammatory effects [5, 56]. After MI, ATP and NADH depletion is increased, while oxidative stress and cell death are increased [122]. HMSC-Exos can promote cardiac function after I/R injury and reduce myocardial apoptosis by increasing NADH and ATP levels in the I/R heart, reducing oxidative stress, increasing the phosphorylation of GSK-3β and AKT, and decreasing the phosphorylation of c-JNK [9]. miR-200b-3p in EVs secreted by MSCs inhibits the activation of NLRP1 by inhibiting the expression of Bcl-2-like protein 11, effectively inhibiting the inflammatory response and oxidative stress in MI mice and improving cardiac function [123]. In addition, Liu et al. [124] found that miR-302d-3p in BMMSC-derived EVs regulates the inflammatory microenvironment by mediating the BCL6/MD2/NF-κB axis to alleviate ventricular remodeling after AMI. In addition, miR-25-3p in BMMSC-Exos inhibits SOCS3 expression by downregulating EZH2 and further inhibits the inflammatory response of the ischemic myocardium [68] (Figure 5(a)).
Figure 5.
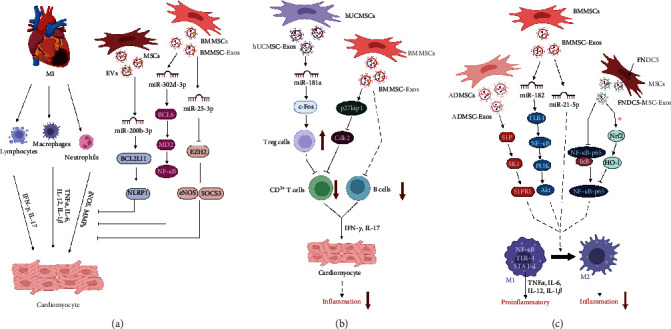
Exosomes secreted by different sources of MSCs regulate the inflammatory microenvironment after myocardial infarction. (a) MiR-200b-3p in extracellular vesicles (EVs) secreted by mesenchymal stem cells (MSCs) inhibits the activation of NLRP1 by inhibiting the expression of Bcl-2-like protein 11 (Bcl2L11), which effectively inhibits the inflammatory response after myocardial infarction (MI). MiR-302d-3p in EVs derived from bone marrow mesenchymal stem cells (BMMSCs) regulates the post-MI inflammatory microenvironment by mediating the BCL6/MD2/NF-κB axis. MiR-25-3p in exosomes derived from bone marrow mesenchymal stem cells (BMMSC-Exos) inhibits the inflammatory response of ischemic myocardial injury by downregulating enhancer of zest homolog 2 (EZH2) and inhibiting suppressor of cytokine signaling 3 (SOCS3) expression. (b) BMMSC-Exos regulate the postinfarction cardiac microenvironment by upregulating p27kip1 and downregulating CDK2 to inhibit the proliferation of CD3+ T cells after MI and inhibit the proliferation and differentiation of B cells. MiR-181a in exosomes derived from human umbilical cord mesenchymal stem cells (hUCMSC-Exos) can significantly inhibit the inflammatory response and increase the proportion of Treg cells by targeting the inflammatory transcription factor c-fos, thereby inhibiting T-cell proliferation. (c) MiR-182 in BMMSC-Exos targets the TLR4/NF-κB/PI3K/AKT signaling cascade to promote M1-to-M2 polarization of macrophages. Meanwhile, BMMSC-Exos can promote macrophage differentiation to the M2 phenotype through miR-21-5p, thereby reducing inflammation. Exosomes derived from adipose-derived mesenchymal stem cells (ADMSC-Exos) promote macrophage M2 polarization and cardiac repair by activating S1P/SK1/S1PR1 signal transduction. BMMSC-Exos overexpressing fibronectin type III domain-containing protein 5 (FNDC5) play an anti-inflammatory role by inhibiting the NF-κB signaling pathway and promoting the polarization of M2 macrophages.
Studies have shown that MSC-Exos inhibit the invasion and proliferation of immune cells in MI and reduce inflammatory infiltration of myocardial tissue [108]. In addition, the proliferation of CD3+ T cells is significantly inhibited following treatment with BMMSC-Exos. The mechanism may be associated with upregulation of p27kip1 and downregulation of CDK2 to induce cell cycle arrest in T cells [125]. Similar results have been reported for previous in vitro research on the interaction of BMMSC-Exos with peripheral blood mononuclear cells. In that study, the researchers found that MSCs induced apoptosis in CD3+ T cells and inhibited CpG-stimulated B-cell proliferation and differentiation and the production of IgG, IgA, and IgM [126]. Some studies have shown that BMMSC-Exos treatment significantly reduces the levels of the inflammatory regulators IL-1b, phospho-p38-MAPK, NF-κB, and the NLRP3 inflammasome [105]. Wei et al. [127] found in a mouse myocardial I/R injury model that hUCMSC-Exos overexpressing miR-181a could significantly inhibit the inflammatory response and increase the proportion of Treg cells by targeting the inflammatory transcription factor c-Fos. BMMSC-Exos, which carry miR-125b, restore cardiac function in I/R rats by inhibiting inflammation and apoptosis in I/R cardiomyocytes by targeting SIRT7 [128] (Figure 5(b)).
Macrophages are central mediators of inflammation in the heart and are involved in its development and regression. M1 macrophages show proinflammatory effects and generate inflammatory factors such as IL-6, IL-1β, and TNF-α, whereas M2 macrophages mediate repair, which is conducive to the activation of CFs, reconstitution of the ECM, and angiogenesis [51, 129]. Zhao et al. [130] demonstrated that miR-182 in BMMSC-Exos targets the TLR4/NF-κB/PI3K/AKT signaling cascade and reduces myocardial I/R damage by polarizing inflammatory macrophages into anti-inflammatory macrophages in the heart. Similarly, Deng et al. [131] found that ADMSC-Exos ameliorate cardiac injury after MI by activating the signaling passway of S1P/SK1/S1PR1 and promoting M2 polarization of macrophages. Another study has shown that intramyocardial injection of BMMSC-Exos in mice with myocardial ischemia can reduce inflammation by promoting the differentiation of macrophages into the M2 phenotype through miR-21-5p, thereby promoting cardiac repair [132]. Correspondingly, BMMSC-Exos overexpressing fibronectin type III domain-containing protein 5 exert anti-inflammatory effects by inhibiting the NF-κB signaling pathway and upregulating the Nrf2/HO-1 axis to promote M2 macrophage polarization [133] (Figure 5(c)). In a mouse dilated cardiomyopathy model, Sun et al. [134] found that MSC-Exos mediated macrophage polarization by modulating the JAK2-STAT6 signaling pathway, thereby improving the inflammatory microenvironment in and reducing inflammatory cell infiltration. Thus, MSC-Exos have a modulatory effect on the microenvironment after MI, which lays an essential foundation for subsequent modification and optimization of MSCs for improved cardioprotective outcomes.
5. Pretreatment and Engineering Strategies to Improve the Efficacy of MSC-Derived Exosomes in Cell-Free Therapy
Exosomes have low immunogenicity, low toxicity, and high engineering potential and are expected to become cell-free therapeutics for a variety of diseases [48]. The biomolecules encapsulated in exosomes fluctuate according to the surrounding microenvironment and the state of MSCs. Although the transplantation of MSC-Exos has shown significant advantages in restoring cardiac function after MI, how to collect more exosomes with strong reparative effects to target the ischemic myocardium effectively still needs to be further explored [135–137]. Many attempts have been made to address this issue. Here, we summarize two possible strategies for improving the therapeutic activity of MSC-Exos: pretreatment and the use of engineered exosomes (Figure 6).
Figure 6.
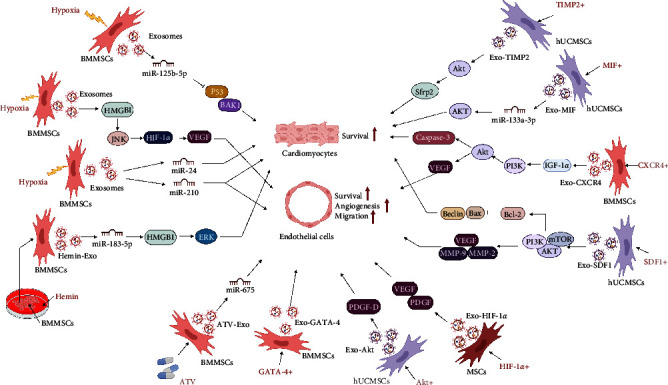
Repair effect of engineered MSC-Exos on ischemic heart. Exosomes secreted by bone marrow mesenchymal stem cells (BMMSCs) preconditioned with moderate hypoxia promote angiogenesis or anti-myocardial apoptosis through upregulation of miR-24, miR-125b-5p, miR-210, HMGB1, etc. HIF-1α-overexpressing mesenchymal stem cells (MSCs) secreted exosomes (Exo-HIF-1α), SDF1-overexpressing human umbilical cord mesenchymal stem cells (hUCMSCs) secreted exosomes (Exo-SDF1), CXCR4-overexpressing bone marrow mesenchymal stem cells (BMMSCs) secreted exosomes (Exo-CXCR4), TIMP2-modified hUCMSCs secreted exosomes (Exo-TIMP2), and MIF-overexpressing hUCMSCs secreted exosomes (Exo-MIF), AKT-modified hUCMSCs secreted exosomes (Exo-AKT), and GATA-4 overexpressing BMMSCs secreted exosomes (Exo-GATA-4), atorvastatin pretreated BMMSCs secreted exosomes (ATV-Exo), hemin pretreated BMMSCs secreted exosomes (Hemin-Exo) played a better role in promoting angiogenesis or antimyocardial apoptosis in infarcted hearts. HMGB1, high mobilitygroup box 1 protein; SDF1, stromal-derived factor 1; Bax, Bcl-2-associated X protein; Bcl-2, B-Cell CLL/Lymphoma 2; MMP-2, matrix metalloprotein-2; MMP-9, matrix metalloprotein-9; VEGF, vascular endothelial growth factor; AKT, protein kinase B; PDGF-D, platelet-derived growth factor D; HIF-1α, hypoxia-inducible factor 1α; IGF-1α, insulin-like growth factor-1α; MIF, macrophage migration inhibitory factor; Sfrp2, secreted frizzled-related protein 2; TIMP2, recombinant tissue inhibitors of metalloproteinase 2; GATA-4, GATA binding protein 4; ATV, atorvastatin.
5.1. Pretreatment of Exosomes
5.1.1. Hypoxia-Pretreated Exosomes
Hypoxic preconditioning is a common method used in vitro. In general, oxygen tension is considered an essential factor that affects the biological behaviors of stem cell cultures [138, 139]. Several studies have demonstrated that hypoxic preconditioning promotes the survival, proliferation, and migration of MSCs in the context of MI, which enhances the efficacy of transplanted MSCs after MI [140, 141]. The beneficial effect of low oxygen tension on MSCs and the secretion of exosomes from MSCs cultured in a hypoxic environment has gradually attracted extensive attention [142]. Hypoxia preconditioning (0.5% O2 for 24 hr) promotes paracrine proangiogenic effects of BMMSCs, increases vascular density and decreases endogenous cell apoptosis [142]. Zhang et al. [143] found that BMMSC-Exos that were pretreated with hypoxia inhibited apoptosis in cardiomyocytes in AMI rats by upregulating microRNA-24. Similarly, Zhu et al. [144] found that hypoxia-pretreated BMMSC-Exos (1% O2 for 72 hr) inhibited myocardial apoptosis and promoted ischemic heart repair via miR-125b-5p. The mechanism may have involved inhibiting the proapoptotic genes p53 and BAK1 expression. Some studies have shown that hypoxia-pretreated BMMSC-Exos (0.5% O2 for 24 hr) promote miR-210 production and NSMase2 activation via the action of HIF-1α, significantly improving the biological characteristics and therapeutic effects of exosomes by increasing vascular density, decreasing cardiomyocyte apoptosis, and reducing fibrosis in the infarcted heart [145]. Gao et al. [146] further confirmed that treatment with hypoxia (5% O2 for 6 days) increased HMGB1 expression in BMMSC-Exos, which activated the JNK/HIF-1α/VEGF pathway, thereby promoting angiogenesis in HUVECs. Another study showed that hypoxia-BMMSC-Exos (1% O2 for 48 hr) were more readily absorbed by cells than normal MSC-Exos [147], suggesting that hypoxia treatment can indirectly improve exosome utilization. Therefore, moderate hypoxic pretreatment is a safe, natural, and effective method to optimize the therapeutic effects of MSC-Exos.
5.1.2. Genetically Modified Exosomes
HIF-1α is a key transcriptional regulator of the hypoxia response that regulates the expression of many genes, including those encoding angiogenic cytokines [148]. Sun et al. [149] observed that HIF-1α-overexpressing exosomes (Exos-HIF-1α) exert proangiogenic and cardioprotective effects on the ischemic heart via VEGF- and PDGF-mediated phenotypes and that Exos-HIF-1α can rescue angiogenesis, proliferation, and migration in hypoxia-injured HUVECs. Studies have shown that overexpression of SDF1 in hUCMSCs increases the levels of SDF1 in hUCMSC-Exos and can inhibit cardiomyocyte autophagy, promoting endothelial microangiogenesis by activating the PI3K pathway [150]. Exosomes derived from BMMSCs overexpressing the chemokine receptor CXCR4, another major regulator of stem/progenitor cell activities, initiate the signaling pathway of IGF-1/PI3K/AKT in cardiomyocytes, thereby decreasing myocardial apoptosis, promoting angiogenesis, and preventing ventricular remodeling post-MI [151]. Ni et al. [152] found that exosomes derived from TIMP2-modified hUCMSCs can repair the ischemic myocardium by activating the prosurvival AKT/Sfrp2 pathway to inhibit cardiomyocyte apoptosis, remodel the ECM, and promote angiogenesis. Exosomes released by UCMSCs infected with lentiviruses containing macrophage migration inhibitory factor (MIF), a proinflammatory cytokine that is widely expressed in MSCs, show improved cardioprotective effects in promoting angiogenesis and cardiac function and inhibiting apoptosis and fibrosis. The mechanism of MIF-Exos involves miR-133a-3p and the downstream AKT signaling pathway [153]. Liu et al. [154] also confirmed that MIF-overexpressing BMMSC-Exos inhibit mitochondrial fission induced by hypoxia and serum deprivation by activating the AMPK signaling pathway, which can reduce apoptosis and mitochondrial fragmentation of cardiomyocytes and promote cardiac function. Ma et al. [155] found that exosomes derived from AKT-modified hUCMSCs secreted more platelet-derived growth factor D to promote angiogenesis and improve cardiac function in mice than hUCMSC-Exos. Moreover, He et al. [156] found that exosomes secreted by GATA-4-expressing BMMSCs could increase myocardial vascular density and improve cardiac function in a mouse MI model. These reports suggest that genetic modifications can effectively increase the overall functional performance of MSC-Exos in MI injuries. These preclinical findings thus provide an important foundation for clinical application and transgene-optimized exosome transduction.
5.1.3. Drug-Pretreated Exosomes
MSC-Exos that are pretreated with drugs or cytokines have also been shown to have excellent cardioprotective effects. Huang et al. [157] demonstrated that exosomes derived from BMMSCs that were pretreated with atorvastatin (BMMSCATV-Exos) exhibited an improved ability to enhance angiogenesis and protect cardiomyocytes while improving cardiac function following infarction. Mechanistically, lncRNA H19 in BMMSCATV-Exos activated the expression of miR-675 and promoted angiogenesis. Some studies have shown that hBMMSC-Exos pretreated with hemin are superior to nonpretreated hBMMSC-Exos in improving cardiac function after infarction, and miR-183-5p enriched in hemin-pretreated MSC-Exos inhibits ischemia-induced cardiomyocyte senescence via inhibition of the HMGB1/ERK pathway [158]. In a murine model of MI, Xu et al. [159] observed that pretreatment of BMMSC-Exos with low concentrations of LPS promoted M2 macrophage polarization in vitro and attenuated postinfarct inflammation and cardiomyocyte apoptosis through mediation of macrophage polarization. These drug-pretreated exosomes have been shown to potentially mitigate transplant rejection, providing a strong basis for improved functioning of exosomes in vivo.
In addition, exosomes derived from hUCMSCs encapsulated in functional peptide hydrogels can augment myocardial function by decreasing inflammation, fibrosis, apoptosis, and angiogenesis of the adhesions [160]. Various in vitro exosome pretreatment methods have improved the transplantation rate and survival of exosomes in vivo, providing more possibilities for future MI treatment.
5.2. Exosome Targeting
In the previous section, we described the beneficial effects of MSC-Exos on the heart. However, accurate targeting of exosomes to recipient cells is still faced with serious challenges. Homing peptides or ligand fragments discovered by in vivo biopanning methods and phage display with fusion to enriched molecules outside exosomes have been used to improve the ability of exosomes carrying cognate receptors to target specific tissues or organs. Exosomal surface ligands or homing peptides improve drug delivery targeting and efficiency [161]. Wang et al. [162] demonstrated that the ischemic myocardial targeting peptide CSTSMLKAC can directly target the ischemic myocardium, thereby increasing the targeting and utilization of exosomes. In another study, Vandergriff et al. [163] performed targeted injection of exosomes bound to the cardiac homing peptide into infarcted hearts and found that exosome retention increased in the cardiac sections of isolated rat cardiomyocytes and promoted functional recovery in animal models by inducing cardiomyocyte proliferation, reducing fibrosis, and promoting angiogenesis. The cardiac-targeting peptide (CTP)-Lamp2b has been modified to yield exosomes expressing CTP-Lamp2b on the exosome membrane (CTP-Exos). Compared with native exosomes, CTP-Exos deliver exosomes to heart cells and cardiac tissues significantly more effectively [164]. Moreover, peptide libraries and phage displays have identified many peptides located in the cardiovascular system, such as normal cardiomyocytes, myocardial cells injured by I/R, heart failure, atherosclerotic plaques, and vasculature [165]. Targeted peptide-modified MSC-Exos undoubtedly provide new possibilities with which to improve the efficiency of targeted therapy for MI.
6. Human Induced Pluripotent Stem Cell-Derived Exosomes in MI
The limited sources of MSCs have greatly hindered clinical research and applications. In recent years, MSCs derived from induced pluripotent stem cells (iMSCs) have attracted widespread attention. Studies have found that induced pluripotent stem cells (iPSCs) show similar capacity and morphology to ESCs for self-renewal and differentiation without ethical concerns [166, 167]. Compared with MSCs, iMSCs have been shown to have greater advantages in immunomodulation, microenvironmental regulation, and secretion of bioactive factors [167]. In addition, iMSCs have better proliferation ability and lower immunogenicity than MSCs [168]. Notably, studies have shown that the donor age of MSCs plays an important role in regenerative capacity, where MSCs from young donors have better regenerative capacity than those from older donors. By contrast, iMSCs can bypass tissue- and age-related heterogeneity problems [169, 170]. The therapeutic potential of iMSC-derived exosomes in ischemic heart disease has been heavily investigated. Gao et al. [136] found in an animal infarction model that exosomes from human iPSC-derived cardiomyocytes were also cardioprotective and that they improved the recovery of the ischemic myocardium without increasing the incidence of arrhythmic complications. In addition, exosomes from iMSCs ameliorate myocardial injury induced by severe acute pancreatitis through activation of the AKT/Nrf2/HO-1 axis [166]. Another study has shown that iMSC-derived exosomes regulate autophagy by regulating the PI3K-AKT-mTOR and MAPK signaling pathways to improve cardiac function after MI [171]. These exosomes also play very significant roles in the regulation of apoptosis, inflammation, fibrosis, and angiogenesis [172–173]. Currently, the study of exosomes in CVD is progressing toward iMSC-derived exosomes and enriched miRNA candidates. It is promising to use iMSC-derived exosomes under certain conditions. However, the limited amount of evidence for clinical applications and the absence of evidence at the clinical trial level means that research on iMSC-derived exosomes is still at a nascent stage. The clinical use of iMSC-derived exosomes in the treatment of CVD still needs to be investigated in further detail.
7. Clinical Trial of MSCs in the Treatment of MI
To date, there is abundant preclinical evidence for the efficacy of exosomes in animal models of MI [112, 151]. MSC-Exos have shown great potential in numerous studies [60, 61]. The safety of exosomes has been tentatively demonstrated in certain clinical trials, although most exosome studies have been preclinical [174]. In addition, there are already clinical trials with MSC-Exos for the treatment of MI that are in the recruitment stage (NCT05669144), which means that new data on immunogenicity should be available soon. The safety and efficacy of exosome therapy still need to be confirmed by further clinical studies. Although the results of clinical studies on the use of MSC-Exos for the treatment of MI or other heart-related problems have not yet been published, clinical studies on the use of MSC-Exos in the treatment of other systemic diseases, including cerebrovascular disorders, diabetes mellitus type 1, and Alzheimer's disease, are already underway (see Table 1).
Table 1.
Mesenchymal stem cell-derived exosomes in clinical trials.
| NCT number | Condition or disease | Status | Phase | Brief summary | Sponsor |
|---|---|---|---|---|---|
| NCT03384433 | Cerebrovascular disorders | Unknown status | Phase 1, Phase 2 | Allogenic mesenchymal stem cell-derived exosome in patients with acute ischemic stroke | Isfahan University of Medical Sciences |
|
| |||||
| NCT05813379 | Antiaging | Recruiting | Phase 1, Phase 2 | Mesenchymal stem cells derived exosomes in skin rejuvenation | Isfahan University of Medical Sciences |
|
| |||||
| NCT04544215 | Drug-resistant | Unknown status | Phase 1, Phase 2 | A clinical study of mesenchymal progenitor cell exosomes nebulizer for the treatment of pulmonary infection | Ruijin Hospital |
|
| |||||
| NCT05871463 | Decompensated liver cirrhosis | Recruiting | Phase 2 | Effect of mesenchymal stem cells-derived exosomes in decompensated liver cirrhosis | Research Institute for Gastroenterology and Liver Diseases (RIGLD) |
|
| |||||
| NCT05523011 | Psoriasis | Completed | Phase 1 | Safety and tolerability study of MSC exosome ointment | Paracrine Therapeutics Dermatology Pte. Ltd |
|
| |||||
| NCT04356300 | Multiple organ failure | Not yet recruiting | Not applicable | Exosome of mesenchymal stem cells for multiple organ dysfunction syndrome after surgical repair of acute type A aortic dissection | Fujian Medical University |
|
| |||||
| NCT05261360 | Knee; injury, meniscus (lateral) (medial)/meniscus tear/meniscus lesion/6 more | Recruiting | Phase 2 | Clinical efficacy of exosome in degenerative meniscal injury | Eskisehir Osmangazi University |
|
| |||||
| NCT05499156 | Perianal fistula in patients with Crohn's disease | Unknown status | Phase 1, Phase 2 | Safety of injection of placental mesenchymal stem cell-derived exosomes for treatment of resistant perianal fistula in Crohn's patients | Tehran University of Medical Sciences |
|
| |||||
| NCT04276987 | Coronavirus | Completed | Phase 1 | A pilot clinical study on inhalation of mesenchymal stem cells exosomes treating severe novel coronavirus pneumonia | Ruijin Hospital |
|
| |||||
| NCT05808400 | Long COVID-19 syndrome | Recruiting | Early Phase 1 | Safety and efficacy of umbilical cord mesenchymal stem cell exosomes in treating chronic cough after COVID-19 | Huazhong University of Science and Technology |
|
| |||||
| NCT05402748 | Fistula perianal | Recruiting | Phase 1, Phase 2 | Safety and efficacy of injection of human placenta mesenchymal stem cells derived exosomes for treatment of complex anal fistula | Tehran University of Medical Sciences |
|
| |||||
| NCT04313647 | Healthy | Completed | Phase 1 | A tolerance clinical study on aerosol inhalation of mesenchymal stem cells exosomes in healthy volunteers | Ruijin Hospital |
|
| |||||
| NCT05413148 | Retinitis pigmentosa | Recruiting | Phase 2, Phase 3 | The effect of stem cells and stem cell exosomes on visual functions in patients with retinitis pigmentosa | TC Erciyes University |
|
| |||||
| NCT05216562 | SARS-CoV2 infection | Recruiting | Phase 2, Phase 3 | Efficacy and safety of EXOSOME-MSC therapy to reduce hyperinflammation in moderate COVID-19 patients (EXOMSC-COV19) | Dermama Bioteknologi Laboratorium |
|
| |||||
| NCT03437759 | Macular holes | Unknown status | Early Phase 1 | MSC-Exos promote healing of MHs (MSCs) | Tianjin Medical University |
|
| |||||
| NCT04602104 | Acute respiratory distress syndrome | Unknown status | Phase 1, Phase 2 | A clinical study of mesenchymal stem cell exosomes nebulizer for the treatment of ARDS | Ruijin Hospital |
|
| |||||
| NCT04213248 | Dry eye | Recruiting | Phase 1, Phase 2 | Effect of UMSCs derived exosomes on dry eye in patients With cGVHD | Zhongshan Ophthalmic Center, Sun Yat-sen University |
|
| |||||
| NCT05669144 | Myocardial infarction/myocardial ischemia/myocardial stunning | Recruiting | Phase 1, Phase 2 | Cotransplantation of mesenchymal stem cell-derived exosomes and autologous mitochondria for patients candidate for CABG surgery | Tehran University of Medical Sciences |
|
| |||||
| NCT05787288 | COVID-19 pneumonia | Recruiting | Early Phase 1 | A clinical study on the safety and effectiveness of mesenchymal stem cell exosomes for the treatment of COVID-19 | First Affiliated Hospital of Wenzhou Medical University |
|
| |||||
| NCT02138331 | Diabetes mellitus type 1 | Unknown status | Phase 2, Phase 3 | Effect of microvesicles and exosomes therapy on β-cell mass in type I diabetes mellitus (T1DM) | General Committee of Teaching Hospitals and Institutes, Egypt |
|
| |||||
| NCT04173650 | Dystrophic epidermolysis bullosa | Not yet recruiting | Phase 1, Phase 2 | MSC EVs in dystrophic epidermolysis bullosa | Aegle Therapeutics |
|
| |||||
| NCT05354141 | Acute respiratory distress syndrome/ARDS | Recruiting | Phase 3 | Extracellular vesicle treatment for acute respiratory distress syndrome (ARDS) (EXTINGUISH ARDS) | Direct Biologics, LLC |
|
| |||||
| NCT04493242 | COVID-19 ARDS | Completed | Phase 2 | Extracellular vesicle infusion treatment for COVID-19-associated ARDS | Direct Biologics, LLC |
|
| |||||
| NCT04998058 | Bone loss, osteoclastic/bone loss, alveolar/alveolar bone loss/2 more | Not yet recruiting | Phase 1, Phase 2 | Autogenous mesenchymal stem cell culture-derived signaling molecules as enhancers of bone formation in bone grafting | Pontificia Universidade Católica do Rio Grande do Sul |
|
| |||||
| NCT05387278 | COVID-19 acute respiratory distress syndrome/respiratory distress syndrome | Recruiting | Phase 1 | Safety and effectiveness of placental derived exosomes and umbilical cord mesenchymal stem cells in moderate to severe acute respiratory distress syndrome (ARDS) associated with the novel coronavirus infection (COVID-19) | Vitti Labs, LLC |
|
| |||||
| NCT04388982 | Alzheimer disease | Unknown status | Phase 1, Phase 2 | The safety and the efficacy evaluation of allogenic adipose MSC-Exos in patients with Alzheimer's disease | Ruijin Hospital |
Note. Searched by ClinicalTrials.gov (https://clinicaltrials.gov/, accessed on 1 October 2023).
8. Conclusion
In this review, we investigated the therapeutic effects of exosomes on MI. As cell-free substitutes for stem cell therapy, exosomes have the potential to improve myocardial fibrosis and apoptosis, regulate the cardiac microenvironment after infarction, promote myocardial regeneration, and increase neovascularization after I/R injury. Although recent studies have demonstrated the outstanding performance of exosomes in attenuating myocardial injury, the specific effects of exosome cargoes on particular signaling pathways need to be further explored. Meanwhile, due to the limitations of exosome extraction technology in the past, most of the early studies on the mechanism of MSC-Exo-mediated myocardium repair were carried out via miRNA-related sequencing analysis or detection; relatively few studies involved the detection of proteins, lipids, and other components. The mechanisms of action of the other components of exosomes in the treatment of infarction need to be further studied. Moreover, the heterogeneity of donor cells, the cell growth environment in vitro, and the low targeting and low retention rates of exosomes in recipient cells may affect the function of exosomes. Engineering technology for exosomes makes them natural nanocarriers for the delivery of molecular drugs, as well as through surface modification enhances the target specificity of exosomes, increasing their value for clinical applications. However, exosomes obtained by pretreating parental cells are usually less efficient in terms of drug loading, and the drug levels may be nonuniform. In contrast, direct loading of drugs into exosomes enables better control of drug loading. The development of engineered exosomes is still in the preliminary stage. Although, as drug carriers, the effectiveness of exosomes has been preliminarily established in preclinical studies, further studies are still needed to verify their long-term safety in the future. In addition, standardized methods of exosome isolation and purification need to be further explored. The cardiac muscle repair ability of exosomes obtained from different MSC sources may vary, but there are few studies that are conclusive as to which type of MSC source is most effective and suitable for clinical use. The nature of MSCs depends largely on their tissue origin and the conditions of cellular culture, which suggests that the most effective types of transplanted cells should be selected according to disease characteristics and the characteristics of the different MSCs in future clinical use. Thus, in future clinical applications, the most effective types of transplanted cells should be chosen according to the characteristics of the disease and the different MSCs. We believe that addressing these challenges will lead to the widespread application of exosomes in clinical settings, thereby revealing new clinical strategies for exosomes and enabling the exploration of new clinical therapeutic approaches to benefit patients with MI.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 82000269), the Surface Project of China Postdoctoral Science Foundation (No. 2022M711323), the Tai Shan Young Scholar Foundation of Shandong Province (No. tsqn201909192), and the Shandong Provincial Natural Science Foundation (No. ZR2020YQ59).
Contributor Information
Bin Zhang, Email: zhb861109@163.com.
Cheng Shen, Email: shenc1988@mail.jnmc.edu.cn.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
Bin Zhang and Cheng Shen are responsible for the conception of the idea and the drafting of the paper. Xiaorong Yin, Lizhi Lin, and Fang Fang are responsible for the writing, review, and revision of the manuscript. Xiaorong Yin and Lizhi Lin are responsible for the figures. All authors reviewed the manuscript and approved the final manuscript.
References
- 1.Csöbönyeiová M., Beerová N., Klein M., Debreová-Čeháková M., Danišovič Ľ. Cell-based and selected cell-free therapies for myocardial infarction: how do they compare to the current treatment options? International Journal of Molecular Sciences . 2022;23(18) doi: 10.3390/ijms231810314.10314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho G.-S., Fernandez L., Kwon C. Regenerative medicine for the heart: perspectives on stem-cell therapy. Antioxidants & Redox Signaling . 2014;21(14):2018–2031. doi: 10.1089/ars.2014.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu L., Liu Y., Sun Y., et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Research & Therapy . 2017;8(1) doi: 10.1186/s13287-017-0716-x.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Y., Yu Y., Hu S., Chen Y., Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death & Disease . 2020;11(5) doi: 10.1038/s41419-020-2542-9.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong Y.-Y., Gong Z.-T., Tang R.-J., Yang Y.-J. The pivotal roles of exosomes derived from endogenous immune cells and exogenous stem cells in myocardial repair after acute myocardial infarction. Theranostics . 2021;11(3):1046–1058. doi: 10.7150/thno.53326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen Z., Zheng S., Zhou C., Wang J., Wang T. Repair mechanisms of bone marrow mesenchymal stem cells in myocardial infarction. Journal of Cellular and Molecular Medicine . 2011;15(5):1032–1043. doi: 10.1111/j.1582-4934.2010.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shafei A. E.-S., Ali M. A., Ghanem H. G., et al. Mesenchymal stem cell therapy: a promising cell-based therapy for treatment of myocardial infarction. The Journal of Gene Medicine . 2017;19(12) doi: 10.1002/jgm.2995.e2995 [DOI] [PubMed] [Google Scholar]
- 8.Jiao W., Hao J., Xie Y., Meng M., Gao W. EZH2 mitigates the cardioprotective effects of mesenchymal stem cell-secreted exosomes against infarction via HMGA2-mediated PI3K/AKT signaling. BMC Cardiovascular Disorders . 2022;22(1) doi: 10.1186/s12872-022-02533-9.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arslan F., Lai R. C., Smeets M. B., et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Research . 2013;10(3):301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Fu X., Liu G., Halim A., Ju Y., Luo Q., Song A. G. Mesenchymal stem cell migration and tissue repair. Cells . 2019;8(8) doi: 10.3390/cells8080784.784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song N., Scholtemeijer M., Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends in Pharmacological Sciences . 2020;41(9):653–664. doi: 10.1016/j.tips.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mushahary D., Spittler A., Kasper C., Weber V., Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry Part A . 2018;93(1):19–31. doi: 10.1002/cyto.a.23242. [DOI] [PubMed] [Google Scholar]
- 13.Ridge S. M., Sullivan F. J., Glynn S. A. Mesenchymal stem cells: key players in cancer progression. Molecular Cancer . 2017;16(1) doi: 10.1186/s12943-017-0597-8.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deo D., Marchioni M., Rao P. Mesenchymal stem/stromal cells in organ transplantation. Pharmaceutics . 2022;14(4) doi: 10.3390/pharmaceutics14040791.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai R. C., Yeo R. W. Y., Lim S. K. Mesenchymal stem cell exosomes. Seminars in Cell & Developmental Biology . 2015;40:82–88. doi: 10.1016/j.semcdb.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Bosch J., Houben A. P., Radke T. F., et al. Distinct differentiation potential of “MSC” derived from cord blood and umbilical cord: are cord-derived cells true mesenchymal stromal cells? Stem Cells and Development . 2012;21(11):1977–1988. doi: 10.1089/scd.2011.0414. [DOI] [PubMed] [Google Scholar]
- 17.Xu H., Wang Z., Liu L., Zhang B., Li B. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. Journal of Cellular Biochemistry . 2020;121(3):2089–2102. doi: 10.1002/jcb.27399. [DOI] [PubMed] [Google Scholar]
- 18.Dominici M., Le Blanc K., Mueller I., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy . 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 19.Ratajczak M. Z., Kucia M., Jadczyk T., et al. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia . 2012;26(6):1166–1173. doi: 10.1038/leu.2011.389. [DOI] [PubMed] [Google Scholar]
- 20.Ju C., Li Y., Shen Y., et al. Transplantation of cardiac mesenchymal stem cell-derived exosomes for angiogenesis. Journal of Cardiovascular Translational Research . 2018;11(5):429–437. doi: 10.1007/s12265-018-9824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang J., Xie Q., Pan G., Wang J., Wang M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. European Journal of Cardio-Thoracic Surgery . 2006;30(2):353–361. doi: 10.1016/j.ejcts.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 22.Yang K., Xiang P., Zhang C., et al. Magnetic resonance evaluation of transplanted mesenchymal stem cells after myocardial infarction in swine. Canadian Journal of Cardiology . 2011;27(6):818–825. doi: 10.1016/j.cjca.2011.07.633. [DOI] [PubMed] [Google Scholar]
- 23.Hare J. M., Traverse J. H., Henry T. D., et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. Journal of the American College of Cardiology . 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S. H., Cho J. H., Lee Y. H., et al. Improvement in left ventricular function with intracoronary mesenchymal stem cell therapy in a patient with anterior wall ST-segment elevation myocardial infarction. Cardiovascular Drugs and Therapy . 2018;32(4):329–338. doi: 10.1007/s10557-018-6804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathiasen A. B., Qayyum A. A., Jørgensen E., et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: final 4-year follow-up of the MSC-HF trial. European Journal of Heart Failure . 2020;22(5):884–892. doi: 10.1002/ejhf.1700. [DOI] [PubMed] [Google Scholar]
- 26.Gastl M., Sürder D., Corti R., et al. Effect of intracoronary bone marrow-derived mononuclear cell injection early and late after myocardial infarction on CMR-derived myocardial strain. International Journal of Cardiology . 2020;310:108–115. doi: 10.1016/j.ijcard.2020.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Telukuntla K. S., Suncion V. Y., Schulman I. H., Hare J. M. The advancing field of cell-based therapy: insights and lessons from clinical trials. Journal of the American Heart Association . 2013;2(5) doi: 10.1161/JAHA.113.000338.e338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L., Chen X., Wang W. E., Zeng C. How to improve the survival of transplanted mesenchymal stem cell in ischemic heart? Stem Cells International . 2016;2016:14. doi: 10.1155/2016/9682757.9682757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joladarashi D., Kishore R. Mesenchymal stromal cell exosomes in cardiac repair. Current Cardiology Reports . 2022;24(4):405–417. doi: 10.1007/s11886-022-01660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen M., Chen T. Mesenchymal stem cell-derived exosomes and their potential agents in hematological diseases. Oxidative Medicine and Cellular Longevity . 2021;2021:13. doi: 10.1155/2021/4539453.4539453 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Yu K., Zeng Z., Cheng S., et al. TPP1 enhances the therapeutic effects of transplanted aged mesenchymal stem cells in infarcted hearts via the MRE11/AKT pathway. Frontiers in Cell and Developmental Biology . 2020;8 doi: 10.3389/fcell.2020.588023.588023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology . 2014;30(1):255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 33.Bei Y., Das S., Rodosthenous R. S., et al. Extracellular vesicles in cardiovascular theranostics. Theranostics . 2017;7(17):4168–4182. doi: 10.7150/thno.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dougherty J. A., Mergaye M., Kumar N., Chen C.-A., Angelos M. G., Khan M. Potential role of exosomes in mending a broken heart: nanoshuttles propelling future clinical therapeutics forward. Stem Cells International . 2017;2017:14. doi: 10.1155/2017/5785436.5785436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung J.-H., Fu X., Yang P. C. Exosomes generated from iPSC-derivatives: new direction for stem cell therapy in human heart diseases. Circulation Research . 2017;120(2):407–417. doi: 10.1161/CIRCRESAHA.116.309307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato S., Weaver A. M. Extracellular vesicles: important collaborators in cancer progression. Essays in Biochemistry . 2018;62(2):149–163. doi: 10.1042/EBC20170080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z., Yang J., Yan W., Li Y., Shen Z., Asahara T. Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. Journal of the American Heart Association . 2016;5(1) doi: 10.1161/JAHA.115.002856.e002856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalluri R., LeBleu V. S. The biology, function, and biomedical applications of exosomes. Science . 2020;367(6478) doi: 10.1126/science.aau6977.eaau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lötvall J., Hill A. F., Hochberg F., et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. Journal of Extracellular Vesicles . 2014;3(1) doi: 10.3402/jev.v3.26913.26913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jayaraman S., Gnanasampanthapandian D., Rajasingh J., Palaniyandi K. Stem cell-derived exosomes potential therapeutic roles in cardiovascular diseases. Frontiers in Cardiovascular Medicine . 2021;8 doi: 10.3389/fcvm.2021.723236.723236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong P., Yang H., Wu Y., Li K., Tang Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Research & Therapy . 2019;10(1) doi: 10.1186/s13287-019-1358-y.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowal J., Tkach M., Théry C. Biogenesis and secretion of exosomes. Current Opinion in Cell Biology . 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Pegtel D. M., Gould S. J. Exosomes. Annual Review of Biochemistry . 2019;88(1):487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 44.Kang I. S., Kwon K. Potential application of biomimetic exosomes in cardiovascular disease: focused on ischemic heart disease. BMB Reports . 2022;55(1):30–38. doi: 10.5483/BMBRep.2022.55.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y., Bi J., Huang J., Tang Y., Du S., Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. International Journal of Nanomedicine . 2020;15:6917–6934. doi: 10.2147/IJN.S264498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gheinani A. H., Vögeli M., Baumgartner U., et al. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Scientific Reports . 2018;8(1) doi: 10.1038/s41598-018-22142-x.3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou R., Li Y., Sui Z., et al. Advances in exosome isolation methods and their applications in proteomic analysis of biological samples. Analytical and Bioanalytical Chemistry . 2019;411(21):5351–5361. doi: 10.1007/s00216-019-01982-0. [DOI] [PubMed] [Google Scholar]
- 48.Liang Y., Duan L., Lu J., Xia J. Engineering exosomes for targeted drug delivery. Theranostics . 2021;11(7):3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song Y., Shao L., Zhang Y., et al. Exosomes derived from embryonic stem cells as potential treatment for cardiovascular diseases. Advances in Experimental Medicine and Biology . 2017;998:187–206. doi: 10.1007/978-981-10-4397-0_13. [DOI] [PubMed] [Google Scholar]
- 50.Joo H. S., Suh J. H., Lee H. J., Bang E. S., Lee J. M. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. International Journal of Molecular Sciences . 2020;21(3) doi: 10.3390/ijms21030727.727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang J., Zhang Y., Chen D., Zheng Y., Jiang J. Exosomes and exosomal cargos: a promising world for ventricular remodeling following myocardial infarction. International Journal of Nanomedicine Volume . 2022;17:4699–4719. doi: 10.2147/IJN.S377479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balbi C., Vassalli G. Exosomes: beyond stem cells for cardiac protection and repair. Stem Cells . 2020;38(11):1387–1399. doi: 10.1002/stem.3261. [DOI] [PubMed] [Google Scholar]
- 53.Huang P., Wang L., Li Q., et al. Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Research & Therapy . 2019;10(1) doi: 10.1186/s13287-019-1353-3.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sahoo S., Losordo D. W. Exosomes and cardiac repair after myocardial infarction. Circulation Research . 2014;114(2):333–344. doi: 10.1161/CIRCRESAHA.114.300639. [DOI] [PubMed] [Google Scholar]
- 55.Yáñez-Mó M., Siljander P. R.-M., Andreu Z., et al. Biological properties of extracellular vesicles and their physiological functions. Journal of Extracellular Vesicles . 2015;4(1) doi: 10.3402/jev.v4.27066.27066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan W., Zhu Y., Meng X., Zhang C., Yang Y., Bei Y. Immunomodulation by exosomes in myocardial infarction. Journal of Cardiovascular Translational Research . 2019;12(1):28–36. doi: 10.1007/s12265-018-9836-7. [DOI] [PubMed] [Google Scholar]
- 57.Shao L., Zhang Y., Lan B., et al. miRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. Biomed Research International . 2017;2017:9. doi: 10.1155/2017/4150705.4150705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Y.-Q., Zhang Y., Li X., et al. insensitivity of human iPS cells-derived mesenchymal stem cells to interferon-Γ-induced HLA expression potentiates repair efficiency of hind limb ischemia in immune humanized NOD Scid gamma mice. Stem Cells . 2015;33(12):3452–3467. doi: 10.1002/stem.2094. [DOI] [PubMed] [Google Scholar]
- 59.Lai R. C., Arslan F., Lee M. M., et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research . 2010;4(3):214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Sun S.-J., Wei R., Li F., Liao S.-Y., Tse H.-F. Mesenchymal stromal cell-derived exosomes in cardiac regeneration and repair. Stem Cell Reports . 2021;16(7):1662–1673. doi: 10.1016/j.stemcr.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bian S., Zhang L., Duan L., Wang X., Min Y., Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. Journal of Molecular Medicine . 2014;92(4):387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 62.Navickas R., Gal D., Laucevičius A., Taparauskaitė A., Zdanytė M., Holvoet P. Identifying circulating microRNAs as biomarkers of cardiovascular disease: a systematic review. Cardiovascular Research . 2016;111(4):322–337. doi: 10.1093/cvr/cvw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang L., Yang L., Ding Y., Jiang X., Xia Z., You Z. Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle . 2020;19(3):339–353. doi: 10.1080/15384101.2019.1711305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wen Z., Mai Z., Zhu X., et al. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Research & Therapy . 2020;11(1):p. 36. doi: 10.1186/s13287-020-1563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun X.-H., Wang X., Zhang Y., Hui J. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thrombosis Research . 2019;177:23–32. doi: 10.1016/j.thromres.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Wang K., Jiang Z., Webster K. A., et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA-21. Stem Cells Translational Medicine . 2017;6(1):209–222. doi: 10.5966/sctm.2015-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luther K. M., Haar L., McGuinness M., et al. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. Journal of Molecular and Cellular Cardiology . 2018;119:125–137. doi: 10.1016/j.yjmcc.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 68.Peng Y., Zhao J.-L., Peng Z.-Y., Xu W.-F., Yu G.-L. Exosomal miR-25-3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death & Disease . 2020;11(5) doi: 10.1038/s41419-020-2545-6.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan F., Cui W., Chen Z. Mesenchymal stem cell-derived exosome-loaded microRNA-129-5p inhibits TRAF3 expression to alleviate apoptosis and oxidative stress in heart failure. Cardiovascular Toxicology . 2022;22(7):631–645. doi: 10.1007/s12012-022-09743-9. [DOI] [PubMed] [Google Scholar]
- 70.Fu D.-L., Jiang H., Li C.-Y., Gao T., Liu M.-R., Li H.-W. microRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. European Review for Medical and Pharmacological Sciences . 2020;24(19):10107–10117. doi: 10.26355/eurrev_202010_23230. [DOI] [PubMed] [Google Scholar]
- 71.Lee J. Y., Chung J., Byun Y., Kim K. H., An S. H., Kwon K. Mesenchymal stem cell-derived small extracellular vesicles protect cardiomyocytes from doxorubicin-induced cardiomyopathy by upregulating survivin expression via the miR-199a-3p-Akt-Sp1/p53 signaling pathway. International Journal of Molecular Sciences . 2021;22(13) doi: 10.3390/ijms22137102.7102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ikeda S., Zablocki D., Sadoshima J. The role of autophagy in death of cardiomyocytes. Journal of Molecular and Cellular Cardiology . 2022;165:1–8. doi: 10.1016/j.yjmcc.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sciarretta S., Maejima Y., Zablocki D., Sadoshima J. The role of autophagy in the heart. Annual Review of Physiology . 2018;80(1):1–26. doi: 10.1146/annurev-physiol-021317-121427. [DOI] [PubMed] [Google Scholar]
- 74.Zou L., Ma X., Lin S., Wu B., Chen Y., Peng C. Bone marrow mesenchymal stem cell-derived exosomes protect against myocardial infarction by promoting autophagy. Experimental and Therapeutic Medicine . 2019;18(4):2574–2582. doi: 10.3892/etm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gu X., Li Y., Chen K., et al. Exosomes derived from umbilical cord mesenchymal stem cells alleviate viral myocarditis through activating AMPK/mTOR-mediated autophagy flux pathway. Journal of Cellular and Molecular Medicine . 2020;24(13):7515–7530. doi: 10.1111/jcmm.15378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu L., Jin X., Hu C.-F., Li R., Zhou Z., Shen C.-X. Exosomes derived from mesenchymal stem cells rescue myocardial ischaemia/reperfusion injury by inducing cardiomyocyte autophagy via AMPK and Akt pathways. Cellular Physiology and Biochemistry . 2017;43(1):52–68. doi: 10.1159/000480317. [DOI] [PubMed] [Google Scholar]
- 77.Xiao C., Wang K., Xu Y., et al. Transplanted mesenchymal stem cells reduce autophagic flux in infarcted hearts via the exosomal transfer of miR-125b. Circulation Research . 2018;123(5):564–578. doi: 10.1161/CIRCRESAHA.118.312758. [DOI] [PubMed] [Google Scholar]
- 78.Li T., Gu J., Yang O., Wang J., Wang Y., Kong J. Bone marrow mesenchymal stem cell-derived exosomal miRNA-29c decreases cardiac ischemia/reperfusion injury through inhibition of excessive autophagy via the PTEN/Akt/mTOR signaling pathway. Circulation Journal . 2020;84(8):1304–1311. doi: 10.1253/circj.CJ-19-1060. [DOI] [PubMed] [Google Scholar]
- 79.Toldo S., Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nature Reviews Cardiology . 2018;15(4):203–214. doi: 10.1038/nrcardio.2017.161. [DOI] [PubMed] [Google Scholar]
- 80.Audia J. P., Yang X.-M., Crockett E. S., et al. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Research in Cardiology . 2018;113(5) doi: 10.1007/s00395-018-0692-z.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang J., Jin L., Liu Y., et al. Exosomes derived from mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through inhibiting pyroptosis. Drug Design, Development and Therapy . 2020;14:3765–3775. doi: 10.2147/DDDT.S239546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liang C., Liu Y., Xu H., et al. Exosomes of human umbilical cord MSCs protect against hypoxia/reoxygenation-induced pyroptosis of cardiomyocytes via the miRNA-100-5p/FOXO3/NLRP3 pathway. Frontiers in Bioengineering and Biotechnology . 2021;8 doi: 10.3389/fbioe.2020.615850.615850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yue R., Lu S., Luo Y., et al. Mesenchymal stem cell-derived exosomal microRNA-182-5p alleviates myocardial ischemia/reperfusion injury by targeting GSDMD in mice. Cell Death Discovery . 2022;8(1) doi: 10.1038/s41420-022-00909-6.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song Y., Wang B., Zhu X., et al. Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biology and Toxicology . 2021;37(1):51–64. doi: 10.1007/s10565-020-09530-8. [DOI] [PubMed] [Google Scholar]
- 85.Yuan H. J., Xue Y. T., Liu Y. Cuproptosis, the novel therapeutic mechanism for heart failure: a narrative review. Cardiovascular Diagnosis and Therapy . 2022;12(5):681–692. doi: 10.21037/cdt-22-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mao Q., Liang X.-L., Zhang C.-L., Pang Y.-H., Lu Y.-X. LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Research & Therapy . 2019;10(1) doi: 10.1186/s13287-019-1522-4.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun L., Zhu W., Zhao P., et al. Long noncoding RNA UCA1 from hypoxia-conditioned hMSC-derived exosomes: a novel molecular target for cardioprotection through miR-873-5p/XIAP axis. Cell Death & Disease . 2020;11(8) doi: 10.1038/s41419-020-02783-5.696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu H., Pan Y., Dai M., Wang X., Chen H. Mesenchymal stem cell-originated exosomal Lnc a2M-as1 alleviates hypoxia/reperfusion-induced apoptosis and oxidative stress in cardiomyocytes. Cardiovascular Drugs and Therapy . 2023;37:891–904. doi: 10.1007/s10557-022-07339-7. [DOI] [PubMed] [Google Scholar]
- 89.Ma Y., Iyer R. P., Jung M., Czubryt M. P., Lindsey M. L. Cardiac fibroblast activation post-myocardial infarction: current knowledge gaps. Trends in Pharmacological Sciences . 2017;38(5):448–458. doi: 10.1016/j.tips.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shinde A. V., Frangogiannis N. G. Fibroblasts in myocardial infarction: a role in inflammation and repair. Journal of Molecular and Cellular Cardiology . 2014;70:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo Q., Zhao Y., Li J., et al. Galectin-3 derived from HucMSC exosomes promoted myocardial fibroblast-to-myofibroblast differentiation associated with β-catenin upregulation. International Journal of Stem Cells . 2021;14(3):320–330. doi: 10.15283/ijsc20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi Y., Yang Y., Guo Q., et al. Exosomes derived from human umbilical cord mesenchymal stem cells promote fibroblast-to-myofibroblast differentiation in inflammatory environments and benefit cardioprotective effects. Stem Cells and Development . 2019;28(12):799–811. doi: 10.1089/scd.2018.0242. [DOI] [PubMed] [Google Scholar]
- 93.Ge Z., Li B., Zhou X., Yang Y., Zhang J. Basic fibroblast growth factor activates β-catenin/RhoA signaling in pulmonary fibroblasts with chronic obstructive pulmonary disease in rats. Molecular and Cellular Biochemistry . 2016;423(1-2):165–174. doi: 10.1007/s11010-016-2834-7. [DOI] [PubMed] [Google Scholar]
- 94.Duan J., Gherghe C., Liu D., et al. Wnt1/βcatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO Journal . 2012;31(2):429–442. doi: 10.1038/emboj.2011.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hahn J.-Y., Cho H.-J., Bae J.-W., et al. β-Catenin overexpression reduces myocardial infarct size through differential effects on cardiomyocytes and cardiac fibroblasts. Journal of Biological Chemistry . 2006;281(41):30979–30989. doi: 10.1074/jbc.M603916200. [DOI] [PubMed] [Google Scholar]
- 96.Zhao X., Hua Y., Chen H., et al. Aldehyde Dehydrogenase-2 protects against myocardial infarction-related cardiac fibrosis through modulation of the Wnt/β-catenin signaling pathway. Therapeutics and Clinical Risk Management . 2015;11:1371–1381. doi: 10.2147/TCRM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dobaczewski M., Chen W., Frangogiannis N. G. Transforming growth factor (TGF)-β signaling in cardiac remodeling. Journal of Molecular and Cellular Cardiology . 2011;51(4):600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li C. W., Wang Q., Li J., et al. Silver nanoparticles/Chitosan oligosaccharide/poly(vinyl alcohol) nanofiber promotes wound healing by activating TGFβ1/Smad signaling pathway. International Journal of Nanomedicine . 2016;11:373–386. doi: 10.2147/IJN.S91975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meng X. M., Huang X. R., Chung A. C. K., et al. Smad2 protects against TGF-β/Smad3-mediated renal fibrosis. Journal of the American Society of Nephrology . 2010;21(9):1477–1487. doi: 10.1681/ASN.2009121244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu J., Chen Y., Huang Y., Su Y. Human umbilical cord mesenchymal stem cell-derived exosomes suppress dermal fibroblasts-myofibroblats transition via inhibiting the TGF-β1/Smad 2/3 signaling pathway. Experimental and Molecular Pathology . 2020;115 doi: 10.1016/j.yexmp.2020.104468.104468 [DOI] [PubMed] [Google Scholar]
- 101.Wu Y., Peng W., Fang M., Wu M., Wu M. MSCs-derived extracellular vesicles carrying miR-212-5p alleviate myocardial infarction-induced cardiac fibrosis via NLRC5/VEGF/TGF-β1/SMAD axis. Journal of Cardiovascular Translational Research . 2022;15(2):302–316. doi: 10.1007/s12265-021-10156-2. [DOI] [PubMed] [Google Scholar]
- 102.Wang X., Zhu Y., Wu C., Liu W., He Y., Yang Q. Adipose-derived mesenchymal stem cells-derived exosomes carry microRNA-671 to alleviate myocardial infarction through inactivating the TGFBR2/Smad2 axis. Inflammation . 2021;44(5):1815–1830. doi: 10.1007/s10753-021-01460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miyazawa K., Miyazono K. Regulation of TGF-β family signaling by inhibitory Smads. Cold Spring Harbor Perspectives in Biology . 2017;9(3) doi: 10.1101/cshperspect.a022095.a022095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang X. L., Zhao Y. Y., Sun L., et al. Exosomes derived from human umbilical cord mesenchymal stem cells improve myocardial repair via upregulation of Smad7. International Journal of Molecular Medicine . 2018;41(5):3063–3072. doi: 10.3892/ijmm. [DOI] [PubMed] [Google Scholar]
- 105.Kore R. A., Wang X., Henson J. C., Ding Z., Jamshidi-Parsian A., Mehta J. L. Proteomic basis of modulation of postischemic fibrosis by MSC exosomes. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology . 2021;321(5):R639–R654. doi: 10.1152/ajpregu.00124.2021. [DOI] [PubMed] [Google Scholar]
- 106.Wang X., Bai L., Liu X., et al. Cardiac microvascular functions improved by MSC-derived exosomes attenuate cardiac fibrosis after ischemia-reperfusion via PDGFR-β modulation. International Journal of Cardiology . 2021;344:13–24. doi: 10.1016/j.ijcard.2021.09.017. [DOI] [PubMed] [Google Scholar]
- 107.Yan W., Lin C., Guo Y., et al. N-Cadherin overexpression mobilizes the protective effects of mesenchymal stromal cells against ischemic heart injury through a β-catenin-dependent manner. Circulation Research . 2020;126(7):857–874. doi: 10.1161/CIRCRESAHA.119.315806. [DOI] [PubMed] [Google Scholar]
- 108.Teng X., Chen L., Chen W., Yang J., Yang Z., Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cellular Physiology and Biochemistry . 2015;37(6):2415–2424. doi: 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- 109.Liang X., Zhang L., Wang S., Han Q., Zhao R. C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. Journal of Cell Science . 2016;129(11):2182–2189. doi: 10.1242/jcs.170373. [DOI] [PubMed] [Google Scholar]
- 110.Hu J., Chen X., Li P., et al. Exosomes derived from human amniotic fluid mesenchymal stem cells alleviate cardiac fibrosis via enhancing angiogenesis in vivo and in vitro. Cardiovascular Diagnosis and Therapy . 2021;11(2):348–361. doi: 10.21037/cdt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Potz B. A., Scrimgeour L. A., Pavlov V. I., Sodha N. R., Abid M. R., Sellke F. W. Extracellular vesicle injection improves myocardial function and increases angiogenesis in a swine model of chronic ischemia. Journal of the American Heart Association . 2018;7(12) doi: 10.1161/JAHA.117.008344.e008344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scrimgeour L. A., Potz B. A., Aboul G. A., et al. Extracellular vesicles promote arteriogenesis in chronically ischemic myocardium in the setting of metabolic syndrome. Journal of the American Heart Association . 2019;8(15) doi: 10.1161/JAHA.119.012617.e12617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Monguió-Tortajada M., Prat-Vidal C., Martínez-Falguera D., et al. Acellular cardiac scaffolds enriched with MSC-derived extracellular vesicles limit ventricular remodelling and exert local and systemic immunomodulation in a myocardial infarction porcine model. Theranostics . 2022;12(10):4656–4670. doi: 10.7150/thno.72289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang N., Chen C., Yang D., et al. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease . 2017;1863(8):2085–2092. doi: 10.1016/j.bbadis.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 115.Ma T., Chen Y., Chen Y., et al. microRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells International . 2018;2018:11. doi: 10.1155/2018/3290372.3290372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu D., Wang Y., Thomas M., et al. Exosomes from adipose-derived stem cells alleviate myocardial infarction via microRNA-31/FIH1/HIF-1α pathway. Journal of Molecular and Cellular Cardiology . 2022;162:10–19. doi: 10.1016/j.yjmcc.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zheng J., Zhang X., Cai W., et al. Bone marrow mesenchymal stem cell-derived exosomal microRNA-29b-3p promotes angiogenesis and ventricular remodeling in rats with myocardial infarction by targeting ADAMTS16. Cardiovascular Toxicology . 2022;22(8):689–700. doi: 10.1007/s12012-022-09745-7. [DOI] [PubMed] [Google Scholar]
- 118.Yang M., Liu X., Jiang M., Li J., Tang Y., Zhou L. miR-543 in human mesenchymal stem cell-derived exosomes promotes cardiac microvascular endothelial cell angiogenesis after myocardial infarction through COL4A1. IUBMB Life . 2021;73(7):927–940. doi: 10.1002/iub.2474. [DOI] [PubMed] [Google Scholar]
- 119.Wang Z., Gao D., Wang S., Lin H., Wang Y., Xu W. Exosomal microRNA-1246 from human umbilical cord mesenchymal stem cells potentiates myocardial angiogenesis in chronic heart failure. Cell Biology International . 2021;45(11):2211–2225. doi: 10.1002/cbin.11664. [DOI] [PubMed] [Google Scholar]
- 120.Scrimgeour L. A., Potz B. A., Aboul G. A., et al. Intravenous injection of extracellular vesicles to treat chronic myocardial ischemia. PLoS One . 2020;15(9) doi: 10.1371/journal.pone.0238879.e238879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ong S.-B., Hernández-Reséndiz S., Crespo-Avilan G. E., et al. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacology & Therapeutics . 2018;186:73–87. doi: 10.1016/j.pharmthera.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tian X. F., Cui M. X., Yang S. W., Zhou Y. J., Hu D. Y. Cell death, dysglycemia and myocardial infarction. Biomedical Reports . 2013;1(3):341–346. doi: 10.3892/br.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wan J., Lin S., Yu Z., et al. Protective effects of microRNA-200b-3p encapsulated by mesenchymal stem cells-secreted extracellular vesicles in myocardial infarction via regulating BCL2L11. Journal of the American Heart Association . 2022;11(12) doi: 10.1161/JAHA.121.024330.e24330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu Y., Guan R., Yan J., Zhu Y., Sun S., Qu Y. Mesenchymal stem cell-derived extracellular vesicle-shuttled microRNA-302d-3p represses inflammation and cardiac remodeling following acute myocardial infarction. Journal of Cardiovascular Translational Research . 2022;15(4):754–771. doi: 10.1007/s12265-021-10200-1. [DOI] [PubMed] [Google Scholar]
- 125.Lee S., Kim S., Chung H., Moon J. H., Kang S. J., Park C.-G. Mesenchymal stem cell-derived exosomes suppress proliferation of T cells by inducing cell cycle arrest through p27kip1/Cdk2 signaling. Immunology Letters . 2020;225:16–22. doi: 10.1016/j.imlet.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 126.Budoni M., Fierabracci A., Luciano R., Petrini S., Di Ciommo V., Muraca M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplantation . 2013;22(2):369–379. doi: 10.3727/096368911X582769b. [DOI] [PubMed] [Google Scholar]
- 127.Wei Z., Qiao S., Zhao J., et al. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia-reperfusion injury. Life Sciences . 2019;232 doi: 10.1016/j.lfs.2019.116632.116632 [DOI] [PubMed] [Google Scholar]
- 128.Chen Q., Liu Y., Ding X., et al. Bone marrow mesenchymal stem cell-secreted exosomes carrying microRNA-125b protect against myocardial ischemia reperfusion injury via targeting SIRT7. Molecular and Cellular Biochemistry . 2020;465(1-2):103–114. doi: 10.1007/s11010-019-03671-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kologrivova I., Shtatolkina M., Suslova T., Ryabov V. Cells of the immune system in cardiac remodeling: main players in resolution of inflammation and repair after myocardial infarction. Frontiers in Immunology . 2021;12 doi: 10.3389/fimmu.2021.664457.664457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao J., Li X., Hu J., et al. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovascular Research . 2019;115(7):1205–1216. doi: 10.1093/cvr/cvz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Deng S., Zhou X., Ge Z., et al. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. International Journal of Biochemistry & Cell Biology . 2019;114 doi: 10.1016/j.biocel.2019.105564.105564 [DOI] [PubMed] [Google Scholar]
- 132.Shen D., He Z. Mesenchymal stem cell-derived exosomes regulate the polarization and inflammatory response of macrophages via miR-21-5P to promote repair after myocardial reperfusion injury. Annals of Translational Medicine . 2021;9(16) doi: 10.21037/atm.1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ning H., Chen H., Deng J., et al. Exosomes secreted by FNDC5-BMMSCs protect myocardial infarction by anti-inflammation and macrophage polarization via NF-κB signaling pathway and Nrf2/HO-1 axis. Stem Cell Research & Therapy . 2021;12(1) doi: 10.1186/s13287-021-02591-4.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun X., Shan A., Wei Z., Xu B. Intravenous mesenchymal stem cell-derived exosomes ameliorate myocardial inflammation in the dilated cardiomyopathy. Biochemical and Biophysical Research Communications . 2018;503(4):2611–2618. doi: 10.1016/j.bbrc.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 135.Yan W., Guo Y., Tao L., et al. C1q/tumor necrosis factor-related protein-9 regulates the fate of implanted mesenchymal stem cells and mobilizes their protective effects against ischemic heart injury via multiple novel signaling pathways. Circulation . 2017;136(22):2162–2177. doi: 10.1161/CIRCULATIONAHA.117.029557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao L., Wang L., Wei Y., et al. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Science Translational Medicine . 2020;12(561) doi: 10.1126/scitranslmed.aay1318.eaay1318 [DOI] [PubMed] [Google Scholar]
- 137.Hu M., Guo G., Huang Q., et al. The harsh microenvironment in infarcted heart accelerates transplanted bone marrow mesenchymal stem cells injury: the role of injured cardiomyocytes-derived exosomes. Cell Death & Disease . 2018;9(3) doi: 10.1038/s41419-018-0392-5.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ferreira J. R., Teixeira G. Q., Santos S. G., Barbosa M. A., Almeida-Porada G., Gonçalves R. M. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Frontiers in Immunology . 2018;9 doi: 10.3389/fimmu.2018.02837.2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Estrada J. C., Albo C., Benguría A., et al. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death and Differentiation . 2012;19(5):743–755. doi: 10.1038/cdd.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mao C., Li D., Zhou E., et al. Extracellular vesicles from anoxia preconditioned mesenchymal stem cells alleviate myocardial ischemia/reperfusion injury. Aging . 2021;13(4):6156–6170. doi: 10.18632/aging.202611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang J.-W., Qiu Y.-R., Fu Y., Liu J., He Z.-J., Huang Z.-T. Transplantation with hypoxia-preconditioned mesenchymal stem cells suppresses brain injury caused by cardiac arrest-induced global cerebral ischemia in rats. Journal of Neuroscience Research . 2017;95(10):2059–2070. doi: 10.1002/jnr.24025. [DOI] [PubMed] [Google Scholar]
- 142.Hu X., Xu Y., Zhong Z., et al. A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in nonhuman primates: paracrine activity without remuscularization. Circulation Research . 2016;118(6):970–983. doi: 10.1161/CIRCRESAHA.115.307516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang C.-S., Shao K., Liu C.-W., Li C.-J., Yu B.-T. Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. European Review for Medical and Pharmacological Sciences . 2019;23(15):6691–6699. doi: 10.26355/eurrev_201908_18560. [DOI] [PubMed] [Google Scholar]
- 144.Zhu L.-P., Tian T., Wang J.-Y., et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics . 2018;8(22):6163–6177. doi: 10.7150/thno.28021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhu J., Lu K., Zhang N., et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artificial Cells Nanomedicine and Biotechnology . 2018;46(8):1659–1670. doi: 10.1080/21691401.2017.1388249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gao W., He R., Ren J., et al. Exosomal HMGB1 derived from hypoxia-conditioned bone marrow mesenchymal stem cells increases angiogenesis via the JNK/HIF-1α pathway. FEBS Open Bio . 2021;11(5):1364–1373. doi: 10.1002/2211-5463.13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu W., Rong Y., Wang J., et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. Journal of Neuroinflammation . 2020;17(1) doi: 10.1186/s12974-020-1726-7.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ke Q., Costa M. Hypoxia-inducible factor-1 (HIF-1) Molecular Pharmacology . 2006;70(5):1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 149.Sun J., Shen H., Shao L., et al. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Research & Therapy . 2020;11(1) doi: 10.1186/s13287-020-01881-7.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gong X.-H., Liu H., Wang S.-J., Liang S.-W., Wang G.-G. Exosomes derived from SDF1-overexpressing mesenchymal stem cells inhibit ischemic myocardial cell apoptosis and promote cardiac endothelial microvascular regeneration in mice with myocardial infarction. Journal of Cellular Physiology . 2019;234(8):13878–13893. doi: 10.1002/jcp.28070. [DOI] [PubMed] [Google Scholar]
- 151.Kang K., Ma R., Cai W., et al. Exosomes secreted from CXCR4 overexpressing mesenchymal stem cells promote cardioprotection via AKT signaling pathway following myocardial infarction. Stem Cells International . 2015;2015:14. doi: 10.1155/2015/659890.659890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ni J., Liu X., Yin Y., Zhang P., Xu Y.-W., Liu Z. Exosomes derived from TIMP2-modified human umbilical cord mesenchymal stem cells enhance the repair effect in rat model with myocardial infarction possibly by the Akt/Sfrp2 pathway. Oxidative Medicine and Cellular Longevity . 2019;2019:19. doi: 10.1155/2019/1958941.1958941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhu W., Sun L., Zhao P., et al. Macrophage migration inhibitory factor facilitates the therapeutic efficacy of mesenchymal stem cells derived exosomes in acute myocardial infarction through upregulating miR-133a-3p. Journal of Nanobiotechnology . 2021;19(1) doi: 10.1186/s12951-021-00808-5.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu X., Li X., Zhu W., et al. Exosomes from mesenchymal stem cells overexpressing MIF enhance myocardial repair. Journal of Cellular Physiology . 2020;235(11):8010–8022. doi: 10.1002/jcp.29456. [DOI] [PubMed] [Google Scholar]
- 155.Ma J., Zhao Y., Sun L., et al. Exosomes derived from Akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Translational Medicine . 2017;6(1):51–59. doi: 10.5966/sctm.2016-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.He J.-G., Li H.-R., Han J.-X., et al. GATA-4-expressing mouse bone marrow mesenchymal stem cells improve cardiac function after myocardial infarction via secreted exosomes. Scientific Reports . 2018;8(1) doi: 10.1038/s41598-018-27435-9.9047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang P., Wang L., Li Q., et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovascular Research . 2020;116(2):353–367. doi: 10.1093/cvr/cvz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zheng H., Liang X., Han Q., et al. Hemin enhances the cardioprotective effects of mesenchymal stem cell-derived exosomes against infarction via amelioration of cardiomyocyte senescence. Journal of Nanobiotechnology . 2021;19(1) doi: 10.1186/s12951-021-01077-y.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu R., Zhang F., Chai R., et al. Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. Journal of Cellular and Molecular Medicine . 2019;23(11):7617–7631. doi: 10.1111/jcmm.14635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Han C., Zhou J., Liang C., et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomaterials Science . 2019;7(7):2920–2933. doi: 10.1039/C9BM00101H. [DOI] [PubMed] [Google Scholar]
- 161.Chen H., Wang L., Zeng X., et al. Exosomes, a new star for targeted delivery. Frontiers in Cell and Developmental Biology . 2021;9 doi: 10.3389/fcell.2021.751079.751079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang X., Chen Y., Zhao Z., et al. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. Journal of the American Heart Association . 2018;7(15) doi: 10.1161/JAHA.118.008737.e8737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vandergriff A., Huang K., Shen D., et al. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics . 2018;8(7):1869–1878. doi: 10.7150/thno.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim H., Yun N., Mun D., et al. Cardiac-specific delivery by cardiac tissue-targeting peptide-expressing exosomes. Biochemical and Biophysical Research Communications . 2018;499(4):803–808. doi: 10.1016/j.bbrc.2018.03.227. [DOI] [PubMed] [Google Scholar]
- 165.Lin Y., Lu Y., Li X. Biological characteristics of exosomes and genetically engineered exosomes for the targeted delivery of therapeutic agents. Journal of Drug Targeting . 2020;28(2):129–141. doi: 10.1080/1061186X.2019.1641508. [DOI] [PubMed] [Google Scholar]
- 166.Chen M., Chen J., Huang W., et al. Exosomes from human induced pluripotent stem cells derived mesenchymal stem cells improved myocardial injury caused by severe acute pancreatitis through activating Akt/Nrf2/HO-1 axis. Cell Cycle . 2022;21(15):1578–1589. doi: 10.1080/15384101.2022.2057762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhou A. K., Jou E., Lu V., et al. Using pre-clinical studies to explore the potential clinical uses of exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells. Tissue Engineering and Regenerative Medicine . 2023;20(6):793–809. doi: 10.1007/s13770-023-00557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhou M., Xi J., Cheng Y., et al. Reprogrammed mesenchymal stem cells derived from iPSCs promote bone repair in steroid-associated osteonecrosis of the femoral head. Stem Cell Research & Therapy . 2021;12(1) doi: 10.1186/s13287-021-02249-1.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wruck W., Graffmann N., Spitzhorn L.-S., Adjaye J. Human induced pluripotent stem cell-derived mesenchymal stem cells acquire rejuvenation and reduced heterogeneity. Frontiers in Cell and Developmental Biology . 2021;9 doi: 10.3389/fcell.2021.717772.717772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Spitzhorn L.-S., Megges M., Wruck W., et al. Human iPSC-derived MSCs (iMSCs) from aged individuals acquire a rejuvenation signature. Stem Cell Research & Therapy . 2019;10(1) doi: 10.1186/s13287-019-1209-x.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Santoso M. R., Ikeda G., Tada Y., et al. Exosomes from induced pluripotent stem cell-derived cardiomyocytes promote autophagy for myocardial repair. Journal of the American Heart Association . 2020;9(6) doi: 10.1161/JAHA.119.014345.e14345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Adamiak M., Cheng G., Bobis-Wozowicz S., et al. Induced pluripotent stem cell (iPSC)-derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circulation Research . 2018;122(2):296–309. doi: 10.1161/CIRCRESAHA.117.311769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang A. Y. L. Human induced pluripotent stem cell-derived exosomes as a new therapeutic strategy for various diseases. International Journal of Molecular Sciences . 2021;22(4) doi: 10.3390/ijms22041769.1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chu M., Wang H., Bian L., et al. Nebulization therapy with umbilical cord mesenchymal stem cell-derived exosomes for COVID-19 pneumonia. Stem Cell Reviews and Reports . 2022;18(6):2152–2163. doi: 10.1007/s12015-022-10398-w. [DOI] [PMC free article] [PubMed] [Google Scholar]


