Abstract
Objective
Sodium-glucose co-transporter-2 inhibitors (SGLT2is), such as dapagliflozin, have a diuretic effect, and their early initiation to treat acute heart failure (AHF) may improve outcomes; however, the significance of the timing of starting dapagliflozin after hospital admission remains unclear.
Methods
We performed a post hoc analysis of a prospective, observational registry. Participants were divided into the early (E) group and late (L) group using the median time to the initiation of dapagliflozin (6 days) as the cut-off. We evaluated the relationship between the time to the initiation of dapagliflozin after hospital admission and patient characteristics and the length of the hospital stay.
Patients
Study subjects were 118 patients with AHF admitted between January 2021 and April 2022 who were started on dapagliflozin treatment (10 mg/day).
Results
Patients were divided into the E group (n=63) and L group (n=55). The HF severity as evaluated by the New York Heart Association class and the N-terminal pro-brain natriuretic peptide level was not significantly different between the groups. The time to the initiation of dapagliflozin and length of hospital stay showed a significant positive correlation (p<0.001, r=0.46). The hospital stay was significantly shorter in group E [median, 16.5 days; interquartile range (IQR): 13-22 days] than in group L (median, 22 days; IQR: 17-27 days; p=0.002). A multivariate logistic regression analysis showed that the early initiation of dapagliflozin was independently associated with a shorter hospital stay, even after multiple adjustments.
Conclusion
Early initiation of dapagliflozin after hospital admission is associated with a shorter hospital stay, suggesting it is a key factor for shortening hospital stays.
Keywords: heart failure, SGLT2 inhibitor, dapagliflozin, length of hospital stay
Introduction
Acute heart failure (AHF) is a major health problem worldwide. It is the leading cause of hospitalization and has high mortality (1). Nearly 20% of patients discharged with a diagnosis of HF are readmitted within 30 days (2), and 44% are readmitted for any cause within 6 months (3). Furthermore, a longer hospital stay for HF is associated with short-term readmission and mortality risk (4).
Decongestion with diuretics and optimization of guideline-directed medical therapies are major objectives during hospitalization for AHF. Dapagliflozin, a sodium-glucose co-transporter-2 inhibitor (SGLT2i), has been shown to reduce hospital admissions for HF, kidney disease progression, and cardiovascular mortality among patients with stable chronic HF with a reduced ejection fraction (HFrEF) (5-7). Furthermore, adding an SGLT2i to treatment with loop diuretics was shown to cause beneficial natriuresis, which resulted in an improved congestive status (8).
Recently, researchers have become increasingly interested in the effects of early initiation of SGLT2is in AHF, but the associated benefits remain unclear, and the clinical effects of the early initiation of dapagliflozin in addition to standard treatment with loop diuretics have not been fully evaluated (9,10). In AHF, the early initiation of diuretics has beneficial effects on the management of congestion and the early initiation of loop diuretics reduces in-hospital mortality (11). Furthermore, a recent study showed that the early initiation of the vasopressin V2 receptor antagonist tolvaptan was associated with a shortened hospital stay (12). However, the effects of the early initiation of dapagliflozin on the length of the hospital stay in patients with AHF remain to be investigated.
Therefore, the present study examined the association between the early initiation of dapagliflozin treatment and patient characteristics and the length of the hospital stay in patients hospitalized for AHF.
Materials and Methods
Patients and study protocol
The present study used data from the SAKURA HF REGISTRY-2 (UMIN 000043852), a single-center, prospective, observational cohort registry. This registry enrolled consecutive patients with AHF admitted to Nihon University Itabashi Hospital, Tokyo, Japan, who agreed to be followed for the collection of outcome data. The diagnosis of AHF was based on the Framingham criteria (13). All patients provided their informed consent.
To evaluate potential prognostic factors, demographic, laboratory and echocardiographic data were obtained at admission and discharge. We assessed clinical scenarios at admission; a clinical scenario is a classification system considering the systolic blood pressure and other symptoms: (CS1) dyspnea and/or congestion with systolic blood pressure >140 mm Hg; (CS2) dyspnea and/or congestion with systolic blood pressure 100-140 mm Hg; (CS3) dyspnea and/or congestion with systolic blood pressure <100 mm Hg; (CS4) dyspnea and/or congestion with signs of acute coronary syndrome; and (CS5) isolated right ventricular failure (14).
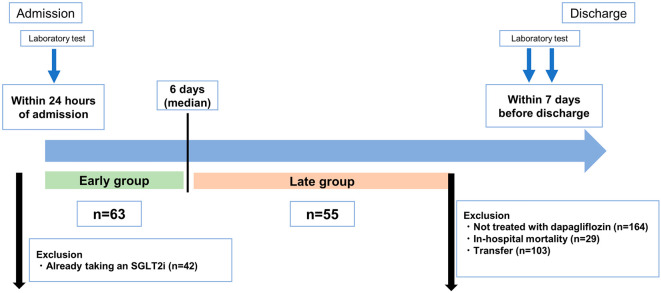
We screened all patients enrolled in the registry between January 2021 and April 2022. The total number of patients was 463, but we excluded 29 who died during the hospital stay, 103 who were transferred to other hospitals, 42 who were already taking an SGLT2i before admission, 164 who were not treated with dapagliflozin, and 7 who started dapagliflozin but then discontinued it. We therefore ultimately analyzed data from 118 patients who were continuously treated with dapagliflozin (10 mg once daily) after hospital admission. There were no patients who were treated with 5 mg of dapagliflozin. Study participants were divided into an early group (group E) and late group (group L) using the median time to the initiation of dapagliflozin after hospital admission as a cut-off.
The study complied with the principles of the Declaration of Helsinki, and the use of patient information was approved by the Nihon University Itabashi Hospital Ethics Committee (RK-180612-2).
Laboratory tests and the evaluation of the volume status
Laboratory tests, including assessments of hemoglobin, sodium, total bilirubin, creatinine, blood urea nitrogen (BUN), and N-terminal pro-brain natriuretic peptide (NT-pro BNP) values, were performed within 24 hours of admission and within 7 days before discharge. When performing laboratory tests at discharge, all patients had improved congestion and were not on intravenous therapy. The estimated glomerular filtration rate (eGFR) was calculated from the serum creatinine value. The estimated plasma volume status (ePVS) was calculated using the Strauss-derived Duarte formula with the hematocrit and hemoglobin values at admission and discharge, as follows: ePVS (dL/g)=[100-hematocrit (%)]/hemoglobin (g/dL) (15). The change in the ePVS from admission to discharge was calculated and expressed as the ΔePVS. In addition, the Strauss-Davis-Rosenbaum formula was used as another calculation method to represent changes in the plasma volume, as follows: %ΔPV=[((Hb1/Hb2)×((100-Hct2)/(100-Hct1)))-1]×100, where Hb1 is the hemoglobin level at admission, Hb2 that at discharge, Hct1 the hematocrit value at admission, and Hct2 that at discharge (16). The study flow is presented in Fig. 1.
Figure 1.
Study flow of the present study.
Echocardiography
On admission, echocardiography was performed by experienced sonographers according to the American Society of Echocardiography guidelines (17). The left ventricular (LV) diastolic diameter (LVDd) and left atrial diameter (LAD) were measured on the parasternal long-axis view, and the LV ejection fraction (LVEF) was calculated by the modified Simpson method or the Teichholz method. The right ventricular (RV) end-diastolic diameter (RVDd) was measured at the basal ventricular level of the right ventricle. The LV diastolic function was calculated as the ratio of the early transmitral flow velocity to the mitral annular velocity (E/e'), which was assessed by transmitral Doppler flow and tissue Doppler imaging. Valve regurgitations were graded on a four-point scale (trivial, mild, moderate, or severe) from color flow Doppler images. The tricuspid regurgitation pressure gradient was measured by continuous-wave Doppler imaging. The diameter of the inferior vena cava in its long axis was measured from the subcostal view.
Introduction of an SGLT2i during hospitalization
To improve patient outcomes, starting in April 2021 [the time of release of the JCS/JHFS 2021 Guideline Focused Update on Diagnosis and Treatment of Acute and Chronic Heart Failure (18)], through lectures and/or ward rounds, each physician treating patients in our registry was fully educated on new medications for HFrEF, including angiotensin receptor-neprilysin inhibitors (ARNIs), SGLT2is, and ivabradine, and was motivated to use them. Use of dapagliflozin 10 mg once daily was indicated in patients with HFrEF with stabilized hemodynamic conditions after relief of pulmonary congestion by the standard treatment for acute decompensated HF, such as vasodilation or loop diuretics; in patients receiving guideline-directed medical therapy; and in patients with an eGFR >25 mL/min/1.73 m2. For de novo AHF, with reference to the proposed algorithm of the American Heart Association (19), our drug introduction procedure is to initiate a small dose of an angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin receptor blocker (ARB), β-blocker, and SGLT-2i and then switch to an ARNI from ACE-Is or ARBs and initiate mineralocorticoid receptor antagonist (MRA). In patients with HF with a preserved ejection fraction (HFpEF), for insurance indication reasons in Japan, dapagliflozin is introduced to patients with type 2 diabetes and/or chronic kidney disease. However, dapagliflozin has also been shown to be safe in HFpEF patients without type 2 diabetes and chronic kidney disease (20).
Evaluations and study outcomes
We evaluated the relationship between the time to the initiation of dapagliflozin after hospital admission and patient characteristics and the length of the hospital stay. The median length of the hospital stay in the study population was 19 days, so we defined a long hospital stay as that of ≥19 days. In addition, a composite event of rehospitalization for HF and all-cause death within 30 and 90 days was evaluated.
Statistical analyses
Continuous data were expressed as the mean±standard deviation (SD) if they were symmetrically distributed or as the median [interquartile range (IQR)] if they were asymmetrically distributed. Comparisons were performed with Student's t-test or Wilcoxon's rank sum test. Categorical data were compared with the chi-squared test and expressed as numbers and percentages. Univariate and multivariate logistic regression analyses were performed to evaluate the association between the time to the initiation of dapagliflozin after admission and the length of the hospital stay. In the multivariate logistic regression analysis, we constructed a multivariate model to adjust for the factors related to the severity of HF; the model included the age, sex, New York Heart Association (NYHA) class, natural log (ln) NT-pro BNP (NT-pro BNP data were subjected to natural log transformation to meet model assumptions), eGFR at admission, and HF type (de novo AHF or worsening HF). Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. To determine the association between the early initiation of dapagliflozin and the length of the hospital stay, propensity score matching was performed on key baseline characteristics (age, sex, hypertension, ischemic etiology, and ePVS and hemoglobin values at admission) to balance the two groups with regard to possible confounding factors. Matching was achieved by a 1:1 nearest neighbor approach (without replacement) within a caliper of 0.05 of the SD.
Statistical analyses were performed with the JMP Pro software program, ver. 16.1.0 (SAS Institute, Cary, USA). A p value <0.05 was considered statistically significant.
Results
Baseline characteristics
In the present study, the median time from hospital admission to the initiation of dapagliflozin was six days. Thus, patients who started treatment with dapagliflozin within 6 days after admission were assigned to group E (n=63), and those who started treatment with dapagliflozin on day 7 or later were assigned to group L (n=55).
Table 1 shows the baseline characteristics of the groups. The severity of HF evaluated by the NYHA class and NT-pro BNP level was not significantly different between the two groups. In addition, there were no significant differences in the sex; body mass index; systolic blood pressure; rates of type 2 diabetes, chronic obstructive pulmonary disease, and atrial fibrillation, ischemic etiology; classification of HF according to the ejection fraction; other medications at admission and discharge; or CS classification. Participants in group E were significantly younger than those in group L [median age, 68 (IQR: 55-77) years old vs. 77 (IQR: 62-82) years old, respectively; p=0.033], and group E had significantly fewer patients with hypertension than group L [n=42 (66.6%) vs. 47 (85.4%), respectively; p=0.016]. Regarding the laboratory findings, hemoglobin and alanine aminotransferase levels at admission and the hemoglobin and hematocrit levels at discharge were significantly higher in group E than in group L. The ePVS was significantly lower in group E than in group L at both admission [median, 4.46 (IQR: 3.53-5.50) vs. 4.93 (IQR: 4.28-6.20); p=0.043] and discharge [median, 4.37 (IQR: 3.30-5.12) vs. 5.01 (IQR: 3.80-6.08); p=0.010]. Of note, the ΔePVS and %ΔePVS, i.e. the change in the ePVS from admission to discharge, were similar in the two groups. There were no significant differences in the other laboratory data. At admission, the mean echocardiographic LVDd was greater in group E than in group L (58±9 vs. 53±9, respectively; p=0.008), but the other echocardiographic data were not significantly different between the groups.
Table 1.
Baseline Characteristics of Patients Divided into Two Groups by the Median Time to Initiation of Dapagliflozin after Hospital Admission (Early Initiation, i.e., by Day 6, Group E; Late Initiation, i.e., on Day 7 or Later, Group L).
| Group E (n=63) | Group L (n=55) | p value | ||||
|---|---|---|---|---|---|---|
| Demographic variables | ||||||
| Age, median (IQR), y | 68 (55, 77) | 77 (62, 82) | 0.033 | |||
| Male, n (%) | 44 (69.8) | 38 (69.0) | 0.92 | |||
| Body mass index at admission, median (IQR), kg/m2 | 26.6 (23.4, 30.4) | 24.6 (22.6, 28.8) | 0.24 | |||
| Systolic BP at admission, median (IQR), mmHg | 142 (123, 178) | 147 (128, 170) | 0.86 | |||
| Type 2 diabetes, n (%) | 27 (42.8) | 21 (38.1) | 0.60 | |||
| Hypertension, n (%) | 42 (66.6) | 47 (85.4) | 0.016 | |||
| COPD, n (%) | 5 (7.94) | 4 (7.55) | 0.93 | |||
| Atrial fibrillation, n (%) | 24 (38.1) | 25 (45.4) | 0.41 | |||
| Ischemic etiology, n (%) | 13 (20.6) | 20 (36.3) | 0.057 | |||
| HFpEF, n (%) | 20 (31.7) | 20 (36.3) | 0.59 | |||
| NYHA class ≥III, n (%) | 40 (63.4) | 42 (76.3) | 0.12 | |||
| HF type | 0.43 | |||||
| De novo AHF, n (%) | 40 (63.5) | 31 (56.4) | ||||
| Worsening HF, n (%) | 23 (36.5) | 24 (43.6) | ||||
| Clinical scenario classification | 0.31 | |||||
| CS 1, n (%) | 28 (44.4) | 32 (58.1) | 0.13 | |||
| CS 2, n (%) | 31 (49.2) | 18 (32.79) | 0.068 | |||
| CS 3, n (%) | 1 (1.59) | 2 (3.64) | 0.47 | |||
| CS 4, n (%) | 3 (4.76) | 3 (5.45) | 0.86 | |||
| CS 5, n (%) | 0 (0) | 0 (0) | - | |||
| Laboratory variables at admission | ||||||
| Hemoglobin, mean±SD, g/dL | 13.2±2.4 | 12.3±2.3 | 0.038 | |||
| Hematocrit, mean±SD, % | 40.8±6.9 | 38.4±6.6 | 0.053 | |||
| Total bilirubin, median (IQR), mg/dL | 0.81 (0.60, 1.29) | 0.88 (0.59, 1.31) | 0.89 | |||
| AST, median (IQR), U/L | 32 (24, 55) | 29 (22, 47) | 0.24 | |||
| ALT, median (IQR), U/L | 27 (22, 47) | 20 (13, 37) | 0.011 | |||
| Albumin, median (IQR), g/dL | 3.6 (3.3, 3.9) | 3.5 (3.1, 3.8) | 0.16 | |||
| Sodium, median (IQR), mEq/L | 141 (139, 143) | 141 (138, 143) | 0.76 | |||
| BUN, median (IQR), mg/dL | 19.0 (15.5, 25.1) | 20.5 (14.9, 26.6) | 0.82 | |||
| Cr, median (IQR), mg/dL | 1.05 (0.87, 1.38) | 0.97 (0.85, 1.38) | 0.53 | |||
| eGFR, mean±SD, mL/min/1.73 m2 | 50.7±17.6 | 50.6±16.4 | 0.95 | |||
| C-reactive protein, median (IQR) mg/dL | 0.38 (0.16, 1.08) | 0.76 (0.23, 3.13) | 0.012 | |||
| NT pro-BNP, median (IQR), pg/mL | 3,696 (2,161, 9,220) | 4,680 (2,411, 9,090) | 0.52 | |||
| ePVS, median (IQR), dL/g | 4.46 (3.53, 5.50) | 4.93 (4.28, 6.20) | 0.043 | |||
| Echocardiographic variables at admission | ||||||
| LVDd, mean±SD, mm | 58±9 | 53±9 | 0.008 | |||
| LVEF, median (IQR), % | 38 (28, 57) | 46 (31, 58) | 0.30 | |||
| LAD, median (IQR), mm | 46 (42, 51) | 45 (42, 51) | 0.71 | |||
| E, mean±SD, m/s | 92±30 | 95±29 | 0.54 | |||
| E/e’ ratio, median (IQR) | 16.9 (13.0, 23.5) | 18.1 (15.0, 23.3) | 0.51 | |||
| RVDd, mean±SD, mm | 36±8 | 35±6 | 0.35 | |||
| Moderate or severe TR, n (%) | 15 (25.0) | 18 (36.7) | 0.18 | |||
| TRPG, median (IQR), mmHg | 25 (20, 36) | 29 (22, 38) | 0.16 | |||
| Maximal IVC diameter, mean±SD, mm | 18±4 | 18±5 | 0.99 | |||
| Medications at admission | ||||||
| ACE-I or ARB, n (%) | 27 (42.8) | 20 (36.3) | 0.47 | |||
| ARNI, n (%) | 1 (1.59) | 3 (5.45) | 0.23 | |||
| Beta-blocker, n (%) | 26 (41.2) | 20 (36.3) | 0.89 | |||
| Ivabradine, n (%) | 1 (1.59) | 0 (0) | 0.26 | |||
| Aldosterone antagonist, n (%) | 9 (14.2) | 9 (16.3) | 0.75 | |||
| Loop diuretic, n (%) | 53 (84.1) | 44 (80.0) | 0.55 | |||
| Tolvaptan, n (%) | 4 (6.35) | 7 (12.7) | 0.23 | |||
| DPP4-I, n (%) | 13 (20.6) | 6 (10.9) | 0.14 | |||
| SGLT-2 inhibitor, n (%) | 0 (0) | 0 (0) | - | |||
| Laboratory variables at discharge | ||||||
| Hemoglobin, median (IQR), g/dL | 13.4 (12.1, 15.7) | 12.2 (10.7, 14.0) | 0.011 | |||
| Hematocrit, median (IQR), % | 42.4 (38.2, 48.0) | 38 (34.6, 44.8) | 0.016 | |||
| Total bilirubin, median (IQR), mg/dL | 0.62 (0.47, 0.86) | 0.57 (0.45, 0.74) | 0.29 | |||
| AST, median (IQR), U/L | 22 (18, 32) | 23 (18, 31) | 0.58 | |||
| ALT, median (IQR), U/L | 20 (14, 35) | 21 (12, 30) | 0.40 | |||
| Albumin, mean±SD, g/dL | 3.6±0.4 | 3.4±0.4 | 0.11 | |||
| Sodium, median (IQR), mEq/L | 140 (139, 142) | 140 (138, 142) | 0.61 | |||
| BUN, median (IQR), mg/dL | 21.4 (16.4, 26.1) | 25.0 (17.6, 30.3) | 0.24 | |||
| Cr, median (IQR), mg/dL | 1.08 (0.90, 1.41) | 1.12 (0.87, 1.46) | 0.72 | |||
| eGFR, median (IQR), mL/min/1.73 m2 | 46.2 (34.6, 59.0) | 47.6 (34.4, 57.3) | 0.65 | |||
| C-reactive protein, median (IQR) mg/dL | 0.19 (0.10, 0.51) | 0.29 (0.10, 0.78) | 0.95 | |||
| NT pro-BNP, median (IQR), pg/mL | 1,032 (286, 2,072) | 1,107 (397, 2,211) | 0.63 | |||
| ePVS, median (IQR), dL/g | 4.37 (3.30, 5.12) | 5.01 (3.80, 6.08) | 0.010 | |||
| ΔePVS, median (IQR), dL/g | 0.37 (-0.16, 0.82) | 0.39 (-0.58, 0.94) | 0.74 | |||
| %ΔePVS, median (IQR), % | -8.8 (-18.0, 4.65) | -7.55 (-19.6, 9.54) | 0.71 | |||
| Medications at discharge | ||||||
| ACE-I or ARB, n (%) | 34 (53.9) | 25 (45.4) | 0.35 | |||
| ARNI, n (%) | 26 (41.2) | 20 (36.3) | 0.58 | |||
| Beta-blocker, n (%) | 58 (92.0) | 51 (92.7) | 0.89 | |||
| Ivabradine, n (%) | 7 (11.1) | 3 (5.45) | 0.26 | |||
| Aldosterone antagonist, n (%) | 48 (76.1) | 36 (65.4) | 0.19 | |||
| Loop diuretic, n (%) | 53 (84.1) | 44 (80.0) | 0.55 | |||
| Tolvaptan, n (%) | 22 (34.9) | 20 (36.3) | 0.87 | |||
| DPP4-I, n (%) | 16 (25.4) | 10 (18.1) | 0.34 |
Significant p values are written in bold.
For multiple comparisons, analysis of variance was used for symmetrical continuous variables, the Kruskal-Wallis test was used for asymmetric continuous variables, and the chi-squared test was used for categorical variables.
ACE-I: angiotensin converting enzyme inhibitor, AHF: acute heart failure, ALT: alanine aminotransferase, ARB: angiotensin II receptor blocker, ARNI: angiotensin receptor-neprilysin inhibitor, AST: aspartate aminotransferase, BP: blood pressure, BUN: blood urea nitrogen, COPD: chronic obstructive pulmonary disease, Cr: creatinine, CS: clinical scenario, DPP4-I: dipeptidyl peptidase-4 inhibitor, E: peak early diastolic transmitral flow velocity, e’: peak early diastolic mean mitral annular velocity, eGFR: estimated glomerular filtration rate, ePVS: estimated plasma volume status, HFpEF: heart failure with preserved ejection fraction, IQR: interquartile range, IVC: inferior vena cava, LAD: left atrial dimension, LVDd: left ventricular end-diastolic dimension, LVEF: left ventricular ejection fraction, NT pro-BNP: N-terminal pro-brain natriuretic peptide, NYHA: New York Heart Association, RVDd: right ventricular end-diastolic dimension, TR: tricuspid regurgitation, TRPG: tricuspid regurgitation pressure gradient
Time to the initiation of dapagliflozin and length of hospital stay
We constructed a regression curve depicting the relationship between the time to the initiation of dapagliflozin after hospital admission and the length of the hospital stay (Fig. 2). The two variables showed a significant positive correlation (r=0.46, p<0.001), and the length of the hospital stay was significantly shorter in group E than in group L [median, 16.5 (IQR: 13-22) days vs. 22 (IQR: 17-27) days, respectively; p=0.002] (Fig. 3). Composite events within 30 and 90 days between groups E and L did not differ significantly (p=0.26, p=0.83, Supplementary material 1).
Figure 2.
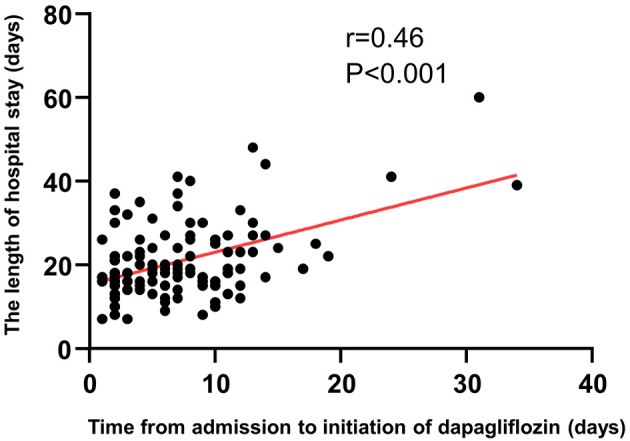
Regression curve of the relationship between the time to the initiation of dapagliflozin after hospital admission and the length of the hospital stay. The two variables showed a significant positive correlation (r=0.46, p<0.001).
Figure 3.
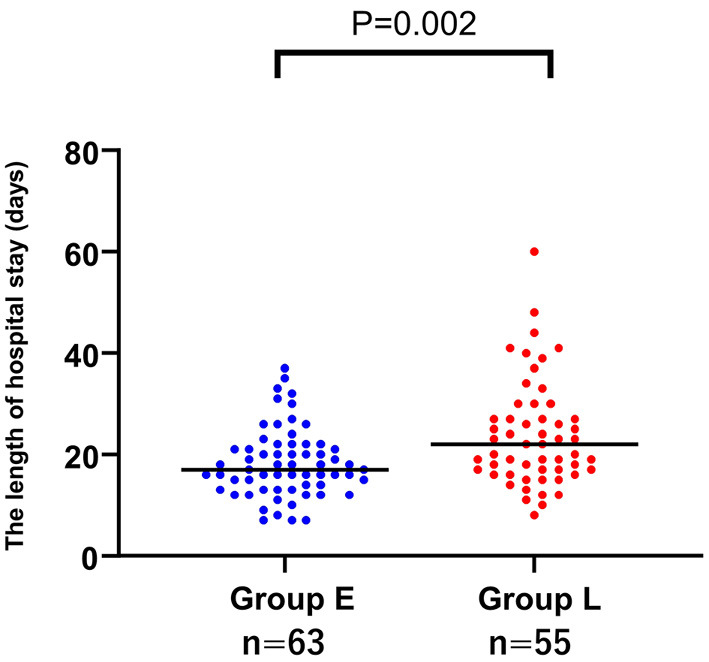
A comparison of the length of the hospital stay in the patients with early and late initiation of dapagliflozin treatment. The length of the hospital stay was significantly shorter in the group with early initiation (group E) than in the group with late initiation [group L; median, 16.5 (interquartile range (IQR): 13-22) days and 22 (IQR: 17-27) days; p=0.001].
Univariate and multivariate analyses to identify factors associated with a longer hospital stay
The median length of the hospital stay was 19 days, so we defined a longer stay as ≥19 days and performed univariate and multivariate analyses to evaluate the associated factors. A univariate logistic regression analysis revealed that both the time to the initiation of dapagliflozin after admission and the early initiation of dapagliflozin (within 6 days) were significantly associated with a shorter hospital stay, as was the use of dobutamine and tolvaptan (all p<0.05, Table 2).
Table 2.
Univariate Logistic Regression Analyses of Factors Associated with a Longer Hospital Stay (≥19 Days) in Patients with Early or Late Initiation of Dapagliflozin Treatment after Hospital Admission.
| Item | Odds ratio | 95% CI | p value | |||
|---|---|---|---|---|---|---|
| Demographic variables | ||||||
| Age (per 1-year increase) | 0.97 | 0.95-1.00 | 0.09 | |||
| Male sex | 2.11 | 0.94-4.69 | 0.067 | |||
| Body mass index at admission (per 1 kg/m2 increase) | 1.05 | 0.99-1.12 | 0.08 | |||
| Type 2 diabetes | 2.43 | 1.13-5.18 | 0.021 | |||
| Hypertension | 1.44 | 0.62-3.34 | 0.39 | |||
| Atrial fibrillation | 0.95 | 0.45-1.98 | 0.90 | |||
| Ischemic etiology | 1.96 | 0.86-4.50 | 0.10 | |||
| NYHA class ≥III | 1.29 | 0.59-2.83 | 0.51 | |||
| HF type (denovo HF vs. worsening HF) | 1.20 | 0.57-2.51 | 0.62 | |||
| Early initiation of dapagliflozin (≤6 days) | 0.40 | 0.19-0.84 | 0.016 | |||
| Time to initiation of dapagliflozin after admission | 1.14 | 1.04-1.25 | <0.001 | |||
| Use of dobutamine | 4.85 | 1.00-23.5 | 0.049 | |||
| Laboratory variables at admission | ||||||
| Hemoglobin (per 1-g/dL increase) | 1.06 | 0.91-1.23 | 0.41 | |||
| Total bilirubin (per 0.1-mg/dL increase) | 1.01 | 0.96-1.06 | 0.56 | |||
| Albumin (per 1-g/dL increase) | 0.94 | 0.45-1.99 | 0.89 | |||
| Sodium (per 1-mmol/L increase) | 0.99 | 0.90-1.08 | 0.86 | |||
| BUN (per 1-mg/dL increase) | 1.00 | 0.96-1.04 | 0.87 | |||
| Cr (per 0.1-mg/dL increase) | 1.05 | 0.97-1.13 | 0.15 | |||
| eGFR (per 1-mL/min/1.73 m2) | 0.99 | 0.97-1.01 | 0.45 | |||
| ln [NT-pro BNP] (per 1-increase) | 0.90 | 0.65-1.24 | 0.54 | |||
| Echocardiographic variables at admission | ||||||
| LVDd (per 1-mm increase) | 1.00 | 0.96-1.04 | 0.75 | |||
| LVEF (per 10% increase) | 1.12 | 0.89-1.41 | 0.31 | |||
| LAD (per 1-mm increase) | 1.02 | 0.97-1.07 | 0.37 | |||
| E/e’ (per 1 increase) | 0.97 | 0.91-1.02 | 0.28 | |||
| RVDd (per 1-mm increase) | 1.01 | 0.96-1.06 | 0.62 | |||
| TRPG (per 1-mmHg increase) | 0.98 | 0.95-1.02 | 0.40 | |||
| Medications at discharge | ||||||
| ACE-I or ARB | 0.62 | 0.29-1.28 | 0.19 | |||
| ARNI | 1.03 | 0.49-2.17 | 0.93 | |||
| Beta-blocker | 1.37 | 0.34-5.37 | 0.65 | |||
| Ivabradine | 0.59 | 0.15-2.23 | 0.44 | |||
| Aldosterone antagonist | 0.66 | 0.29-1.49 | 0.32 | |||
| Loop diuretic | 1.21 | 0.47-3.13 | 0.68 | |||
| Tolvaptan | 2.60 | 1.18-5.71 | 0.016 |
Significant p values are written in bold.
ACE-I: angiotensin converting enzyme inhibitor, ARB: angiotensin II receptor blocker, ARNI: angiotensin receptor-neprilysin inhibitor, BUN: blood urea nitrogen, Cr: creatinine, E: peak early diastolic transmitral flow velocity, e’: peak early diastolic mean mitral annular velocity, eGFR: estimated glomerular filtration rate, LAD: left atrial dimension, ln: natural log, LVDd: left ventricular end-diastolic dimension, LVEF: left ventricular ejection fraction, NT pro-BNP: N-terminal pro-brain natriuretic peptide, NYHA: New York Heart Association, RVDd: right ventricular end-diastolic dimension, TRPG: tricuspid regurgitation pressure gradient
A multivariate logistic regression analysis showed that the time to the initiation of dapagliflozin after hospital admission was significantly associated with a longer hospital stay and that the early initiation of dapagliflozin (within 6 days) was significantly associated with a shorter hospital stay, even after adjusting for the age, sex, NYHA class, ln NT-pro BNP, and eGFR at admission (all p<0.05, Table 3, model 1). Furthermore, in the multivariable linear regression analysis, the time from admission to the initiation of dapagliflozin was significantly associated with the length of hospital stay, even after adjusting for potential confounders (age, ln NT-pro BNP, eGFR at admission) (Supplementary material 2).
Table 3.
Multivariate Logistic Regression Analyses for the Association between Longer Hospital Stay (≥19 Days) and the Time to Initiation of Dapagliflozin Treatment after Hospital Admission.
| Early initiation of dapagliflozin (≤6 days) |
Time from admission to initiation of dapagliflozin (per 1-day increase) | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | |
| Model 1 | 0.28 | 0.12-0.66 | 0.003 | 1.19 | 1.06-1.32 | <0.001 |
| Model 2 | 0.33 | 0.15-0.74 | 0.007 | 1.16 | 1.05-1.28 | <0.001 |
| Model 3 | 0.28 | 0.12-0.66 | 0.003 | 1.18 | 1.06-1.32 | <0.001 |
CI: confidence interval
Model 1: Age; sex, New York Heart Association class ≥III, natural log of N-terminal pro-brain natriuretic peptide level at admission, estimated glomerular filtration rate at admission
Model 2: Age; sex, HF type (de novo AHF or worsening HF)
Model 3: Age; sex, New York Heart Association class ≥III, natural log of N-terminal pro-brain natriuretic peptide level at admission, estimated glomerular filtration rate at admission, HF type (de novo AHF or worsening HF)
Propensity score matching
Propensity score matching resulted in 33 patient pairs, as indicated in Table 4. An analysis of these pairs also showed that the length of hospital stay was significantly shorter in group E than in group L [median, 18 (IQR: 15-22) days and 24 (IQR: 17-27) days, respectively; p=0.038].
Table 4.
Propensity Score Matching of Baseline Characteristics of Patients Divided into Two Groups by the Median Time to Initiation of Dapagliflozin after Hospital Admission (Early Initiation, i.e., by Day 6, Group E; Late Initiation, i.e., on Day 7 or Later, Group L).
| Group E (n=33) | Group L (n=33) | p value | ||||
|---|---|---|---|---|---|---|
| Demographic variables | ||||||
| Age, mean±SD, y | 66±14 | 67±15 | 0.69 | |||
| Male sex, n (%) | 23 (69.7) | 24 (72.7) | 0.78 | |||
| Body mass index at admission, median (IQR), kg/m2 | 26.6 (23.9, 31.6) | 26.1 (23.5, 30.1) | 0.67 | |||
| Systolic BP at admission, mean±SD, mmHg | 153±41 | 153±32 | 0.94 | |||
| Type 2 diabetes, n (%) | 12 (36.3) | 13 (39.3) | 0.79 | |||
| Hypertension, n (%) | 29 (87.8) | 27 (81.8) | 0.49 | |||
| COPD, n (%) | 3 (9.0) | 4 (12.5) | 0.65 | |||
| Atrial fibrillation, n (%) | 15 (45.4) | 18 (54.5) | 0.45 | |||
| Ischemic etiology, n (%) | 7 (21.2) | 10 (30.3) | 0.39 | |||
| HFpEF, n (%) | 21 (63.6) | 19 (57.5) | 0.61 | |||
| NYHA class ≥III, n (%) | 23 (69.7) | 26 (78.7) | 0.39 | |||
| Medications at discharge | ||||||
| ACE-I or ARB, n (%) | 18 (54.5) | 17 (51.5) | 0.80 | |||
| ARNI, n (%) | 14 (42.4) | 10 (30.3) | 0.30 | |||
| Beta-blocker, n (%) | 30 (90.9) | 32 (96.9) | 0.29 | |||
| Ivabradine, n (%) | 1 (3.03) | 1 (3.03) | 1.00 | |||
| Aldosterone antagonists, n (%) | 27 (81.8) | 21 (63.6) | 0.09 | |||
| Loop diuretic, n (%) | 27 (81.8) | 25 (75.7) | 0.54 | |||
| Tolvaptan, n (%) | 11 (33.3) | 9 (27.2) | 0.59 | |||
| DPP4-I, n (%) | 7 (21.2) | 5 (15.1) | 0.52 | |||
| Laboratory variables at admission | ||||||
| Hemoglobin, mean±SD, g/dL | 12.6±2.5 | 12.6±2.4 | 0.95 | |||
| Total bilirubin, median (IQR), mg/dL | 0.77 (0.58, 1.35) | 0.96 (0.65, 1.54) | 0.43 | |||
| AST, median (IQR), U/L | 31 (24, 54) | 37 (22, 65) | 0.90 | |||
| ALT, median (IQR), U/L | 26 (19, 50) | 25 (12, 48) | 0.43 | |||
| Albumin, median (IQR), g/dL | 3.5 (3.2, 3.8) | 3.5 (3.1, 3.9) | 0.76 | |||
| Sodium, median (IQR), mEq/L | 141 (138, 143) | 141 (138, 143) | 0.90 | |||
| BUN, median (IQR), mg/dL | 18.0 (13.1, 26.2) | 19.6 (14.5, 24.9) | 0.74 | |||
| Cr, median (IQR), mg/dL | 1.04 (0.85, 1.30) | 1.05 (0.87, 1.38) | 0.93 | |||
| eGFR, mean±SD, mL/min/1.73 m2 | 53.0±19.4 | 51.1±17.6 | 0.69 | |||
| NT pro-BNP, median (IQR), pg/mL | 3,783 (2,791, 9,643) | 3,455 (1,799, 8,767) | 0.56 | |||
| ePVS, median (IQR), dL/g | 4.80 (3.79, 6.58) | 4.77 (4.03, 5.32) | 0.89 | |||
| Laboratory variables at discharge | ||||||
| Hemoglobin, median (IQR), g/dL | 13.0 (11.8, 15.3) | 12.4 (11.1, 14.7) | 0.47 | |||
| Total bilirubin, median (IQR), mg/dL | 0.62 (0.47, 0.81) | 0.58 (0.44, 0.80) | 0.95 | |||
| AST, median (IQR), U/L | 24 (20, 35) | 24 (18, 31) | 0.56 | |||
| ALT, median (IQR), U/L | 20 (15, 43) | 21 (13, 37) | 0.64 | |||
| Albumin, mean±SD, g/dL | 3.5±0.3 | 3.4±0.5 | 0.39 | |||
| Sodium, median (IQR), mEq/L | 140 (139, 141) | 140 (138, 142) | 0.97 | |||
| BUN, median (IQR), mg/dL | 21.7 (16.8, 27.0) | 22.0 (16.8, 27.3) | 0.98 | |||
| Cr, median (IQR), mg/dL | 1.08 (0.85, 1.42) | 1.13 (0.91, 1.46) | 0.67 | |||
| eGFR, median (IQR), mL/min/1.73 m2 | 45.7 (33.5, 61.5) | 49.4 (35.3, 63.0) | 0.94 | |||
| NT pro-BNP, median (IQR), pg/mL | 983 (385, 1,719) | 993 (307, 2,198) | 0.79 | |||
| ePVS, median (IQR), dL/g | 4.56 (3.34, 5.24) | 4.83 (3.74, 5.81) | 0.41 | |||
| ΔePVS, median (IQR), dL/g | 0.48 (0.00, 0.99) | 0.32 (-0.78, 1.00) | 0.34 | |||
| Echocardiographic variables at admission | ||||||
| LVDd, mean±SD, mm | 58±9 | 54±10 | 0.12 | |||
| LVEF, median (IQR), % | 42 (32, 61) | 46 (32, 58) | 0.73 | |||
| LAD, median (IQR), mm | 45 (42, 50) | 47 (43, 53) | 0.31 | |||
| E, mean±SD, m/s | 93±29 | 98±30 | 0.58 | |||
| E/e’ ratio, median (IQR) | 14.5 (12.3, 23.2) | 17.8 (13.4, 20.7) | 0.61 | |||
| RVDd, mean±SD, mm | 37±8 | 36±7 | 0.68 | |||
| Moderate or severe TR, n (%) | 9 (28.1) | 10 (33.3) | 0.65 | |||
| TRPG, median (IQR), mmHg | 29 (22, 39) | 30 (22, 40) | 0.75 | |||
| Maximal IVC diameter, mean±SD, mm | 18±4 | 18±5 | 0.81 |
For multiple comparisons, analysis of variance was used for symmetrical continuous variables, the Kruskal-Wallis test was used for asymmetric continuous variables, and the chi-squared test was used for categorical variables. Abbreviations as in Table 1.
For the sensitivity analysis, we also performed propensity score matching on age, sex, LVEF, NT-pro BNP, eGFR, and NYHA class. The length of hospital stay was also significantly shorter in group E (n=28) than in group L (n=28) [median, 17 (IQR: 14-26) days and 23 (IQR: 17-32) days, respectively; p=0.031] (Supplementary material 3).
HF type and the length of hospital stay
As a sensitivity analysis, we also examined the association between HF types (de novo AHF or worsening HF) and the length of stay. With de novo AHF, the length of the hospital stay was significantly shorter in group E than in group L [median, 16 (IQR: 13-22) days vs. 22 (IQR: 17-30) days, p=0.01]. Conversely, with worsening HF, there were no significant differences between the groups [median, 18 (IQR: 13-21) days vs. 21 (IQR: 16-26) days, p=0.08]. However, in the whole population, the length of the hospital stay was significantly shorter in group E (n=63) than in group L (n=55) (p=0.002), and the difference remained significant even after adjusting for the HF type (p<0.001). Furthermore, in the multivariate logistic analysis, the association between the time to the initiation of dapagliflozin and the length of hospital stay was consistent, even after adjusting for the HF type (all p<0.05, Table 3, model 2 and model 3).
Discussion
The present study is the first to report an association between the timing of starting dapagliflozin administration after hospital admission and the length of the hospital stay in patients with AHF. There were four major findings. First, patients who started dapagliflozin treatment earlier after admission had a shorter hospital stay than those who started later. Second, the early initiation of dapagliflozin was significantly and independently associated with a shorter hospital stay. Third, even after propensity score matching, the early initiation group had a shorter hospital stay than the late initiation group. Finally, the changes in the plasma volume during hospitalization were similar in the early and late initiation groups.
Recently, SGLT2is have been recommended as a fundamental therapy for patients with HF because of the beneficial effect of these drugs on the clinical prognosis (21). Although clinical practice guidelines recommend dapagliflozin or empagliflozin for HFrEF, the timing of the initiation of these drugs is not well defined (22). Recently, several clinical studies have reported favorable effects of early, upfront initiation of these drugs in patients hospitalized with HF. For example, the SOLOIST-WHF (Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure) trial enrolled patients either during hospitalization for AHF or within three days after hospital discharge who had received intravenous diuretic therapy and were transitioning from intravenous to oral diuretic therapy. In that trial, sotagliflozin therapy resulted in a significantly lower total number of cardiovascular deaths and hospitalizations and urgent visits for HF in patients with type 2 diabetes and AHF than placebo (7). The EMPULSE trial (a study to test the effect of empagliflozin in patients who are in hospital for acute HF) enrolled patients with AHF who were stabilized between 24 hours and 5 days after admission, including during administration of intravenous diuretics, and examined outcomes such as mortality and worsening HF 90 days after discharge (23). That trial showed that empagliflozin reduced the primary composite endpoints of death, number of HF events, time to first HF event, and change from baseline in the Kansas City Cardiomyopathy Questionnaire total symptom score, as evaluated by the win ratio. More recently, the clinical benefits and safety of the early initiation of dapagliflozin in AHF has become a topic of high interest, and a large clinical trial is currently ongoing (10).
Our results revealed that patients who started dapagliflozin treatment earlier during hospitalization had a shorter hospital stay than those who started later, even though there was no marked difference between the two groups in concomitant medications, including tolvaptan, the use of inotropic drugs, EF, and the severity of HF. Furthermore, the late initiation of dapagliflozin was significantly and independently associated with a longer hospital stay. In the multivariate analysis adjusted for factors related to the length of hospital stay, the early initiation of dapagliflozin was also significantly associated with a shorter hospital stay. Even after propensity score matching, the group with the early initiation of dapagliflozin had a shorter hospital stay than the group with late initiation of the drug. These results suggest that earlier initiation of dapagliflozin may be beneficial in AHF.
The rehospitalization rate for AHF was reported to be higher after a longer initial hospital stay (4). Therefore, the early initiation of dapagliflozin may reduce the length of the hospital stay, which may lead to a lower readmission rate. Regarding the HF type (de novo AHF or worsening HF), it should be noted that the length of hospital stay was shorter in patients who started dapagliflozin treatment earlier in the case of de novo AHF; however, there were no significant differences between the groups in the case of worsening HF. Despite performing additional analyses, including a multivariate logistic analysis, we cannot exclude the possibility of some effect of the HF type on our findings.
Our results also showed that the changes in plasma volume during hospitalization were not inferior in the early initiation group compared with the late initiation group. Recently, the early initiation of empagliflozin in addition to standard treatment was reported to be safe and to increase urine output without affecting the renal function, suggesting that SGLT2is may have some beneficial effects on decongestion in AHF (24,25). Our results suggest that, although the early initiation group was discharged earlier, their decongestion therapy had been completed because their congestion status was not worse than that of the late initiation group.
SGLT2is decreases sodium and glucose reabsorption in the early proximal tubule and induce natriuresis and an osmotic diuretic effect (26). Adding an SGLT2i to treatment with loop diuretics was shown to cause beneficial natriuresis, which resulted in improved congestive status. This effect has already begun manifesting by three hours after SGLT2i administration (8). In addition, SGLT2is immediately decreases the systolic blood pressure and cause improvements in the systemic endothelial function and arterial stiffness via oxidative stress reduction (27). Given these previous findings, we speculate that the early introduction of dapagliflozin in this study may have led to an early improvement in the plasma volume and reduction in the afterload, which may have resulted in early discharge.
Several limitations associated with the present study warrant mention. First, it was a single-center study with a relatively small sample size. Second, the ePVS was significantly higher in group L than in group E at both admission and discharge, so there is a possibility that the severity of congestion at admission is related to the time until the initiation of dapagliflozin. In the present study, the timing of the initiation of dapagliflozin was left to the discretion of the attending physician. However, the severity of HF, prevalence of diabetes, body mass index, renal function, and other factors related to the timing of initiation of dapagliflozin were similar between the two groups, and we confirmed that the result was consistent, even after propensity score matching. Third, in cases of HFpEF, for insurance indication reasons in Japan, dapagliflozin is limited to patients with type 2 diabetes or chronic kidney disease and may not be applicable to all HFpEF patients. However, as noted earlier, the benefits of SGLT2is have also been demonstrated in HFpEF without diabetes or chronic kidney disease. Fourth, regarding the effect of dapagliflozin by different types of HF (de novo AHF or worsening HF), because of the small sample size, we cannot draw concrete conclusions from our study alone. More large-scale studies are needed. Fifth, patients who were transferred to other hospitals were excluded, so the study may not have included patients who might have had very long hospital stays. In addition, six patients with CS4 were included. Therefore, we cannot exclude the possibility of selection bias in our cohort. Finally, this study investigated only the use of dapagliflozin, and the effects of other SGLT2is, including empagliflozin, were not considered. Further large-scale, multicenter studies are needed to confirm the results of the present study and examine the effects of the timing of the initiation of other SGLT2is.
Conclusion
The present study revealed that the early initiation of dapagliflozin after hospital admission was associated with a shorter hospital stay, suggesting that the early initiation of treatment with dapagliflozin is a key factor for shortening hospital stays.
The authors state that they have no Conflict of Interest (COI).
Supplementary Material
Comparison of composite events risk between patients divided into two groups by the median time to initiation of dapagliflozin after hospital admission (early initiation, i.e., by day 6, group E; late initiation, i.e., on day 7 or later, group L).
Association between time from admission to initiation of dapagliflozin and the length of hospital stay.
Propensity score matching of baseline characteristics of patients divided into two groups by the median time to initiation of dapagliflozin after hospital admission (early initiation, i.e., by day 6, group E; late initiation, i.e., on day 7 or later, group L)
References
- 1.Tromp J, Ferreira JP, Janwanishstaporn S, et al. Heart failure around the world. Eur J Heart Fail 21: 1187-1196, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Au AG, McAlister FA, Bakal JA, Ezekowitz J, Kaul P, van Walraven C. Predicting the risk of unplanned readmission or death within 30 days of discharge after a heart failure hospitalization. Am Heart J 164: 365-372, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Krumholz HM, Parent EM, Tu N, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med 157: 99-104, 1997. [PubMed] [Google Scholar]
- 4.Reynolds K, Butler MG, Kimes TM, Rosales AG, Chan W, Nichols GA. Relation of acute heart failure hospital length of stay to subsequent readmission and all-cause mortality. Am J Cardiol 116: 400-405, 2015. [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381: 1995-2008, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383: 1413-1424, 2020. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 384: 117-128, 2021. [DOI] [PubMed] [Google Scholar]
- 8.Griffin M, Rao VS, Ivey-Miranda J, et al. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation 142: 1028-1039, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomasoni D, Fonarow GC, Adamo M, et al. Sodium-glucose co-transporter 2 inhibitors as an early, first-line therapy in patients with heart failure and reduced ejection fraction. Eur J Heart Fail 24: 431-441, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox ZL, Collins SP, Aaron M, et al. Efficacy and safety of dapagliflozin in acute heart failure: rationale and design of the DICTATE-AHF trial. Am Heart J 232: 116-124, 2021. [DOI] [PubMed] [Google Scholar]
- 11.Matsue Y, Damman K, Voors AA, et al. Time-to-furosemide treatment and mortality in patients hospitalized with acute heart failure. J Am Coll Cardiol 69: 3042-3051, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Kiuchi S, Hisatake S, Kabuki T, et al. The relationship between the time until commencement of tolvaptan and the length of hospital stay in heart failure patients. Heart Vessels 33: 367-373, 2018. [DOI] [PubMed] [Google Scholar]
- 13.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 285: 1441-1446, 1971. [DOI] [PubMed] [Google Scholar]
- 14.Mebazaa A, Gheorghiade M, Pina IL, et al. Practical recommendations for prehospital and early in-hospital management of patients presenting with acute heart failure syndromes. Crit Care Med 36: S129-S139, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Duarte K, Monnez JM, Albuisson E, Pitt B, Zannad F, Rossignol P. Prognostic value of estimated plasma volume in heart failure. JACC Heart Fail 3: 886-893, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Bilchick KC, Chishinga N, Parker AM, et al. Plasma volume and renal function predict six-month survival after hospitalization for acute decompensated heart failure. Cardiorenal Med 8: 61-70, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440-1463, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsui H, Ide T, Ito H, et al. JCS/JHFS 2021 guideline focused update on diagnosis and treatment of acute and chronic heart failure. J Card Fail 27: 1404-1444, 2021. [DOI] [PubMed] [Google Scholar]
- 19.McMurray JJV, Packer M. How Should we sequence the treatments for heart failure and a reduced ejection fraction?: a redefinition of evidence-based medicine. Circulation 143: 875-877, 2021. [DOI] [PubMed] [Google Scholar]
- 20.Solomon SD, de Boer RA, DeMets D, et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail 23: 1217-1225, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 79: e263-e421, 2022. [DOI] [PubMed] [Google Scholar]
- 22.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42: 3599-3726, 2021. [DOI] [PubMed] [Google Scholar]
- 23.Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med 28: 568-574, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulze PC, Bogoviku J, Westphal J, et al. Effects of early empagliflozin initiation on diuresis and kidney function in patients with acute decompensated heart failure (EMPAG-HF). Circulation 146: 289-298, 2022. [DOI] [PubMed] [Google Scholar]
- 25.Tamaki S, Yamada T, Watanabe T, et al. Effect of empagliflozin as an add-on therapy on decongestion and renal function in patients with diabetes hospitalized for acute decompensated heart failure: a prospective randomized controlled study. Circ Heart Fail 14: e007048, 2021. [DOI] [PubMed] [Google Scholar]
- 26.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 15: 853-862, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solini A, Giannini L, Seghieri M, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol 16: 138, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of composite events risk between patients divided into two groups by the median time to initiation of dapagliflozin after hospital admission (early initiation, i.e., by day 6, group E; late initiation, i.e., on day 7 or later, group L).
Association between time from admission to initiation of dapagliflozin and the length of hospital stay.
Propensity score matching of baseline characteristics of patients divided into two groups by the median time to initiation of dapagliflozin after hospital admission (early initiation, i.e., by day 6, group E; late initiation, i.e., on day 7 or later, group L)