Abstract
Amplification of the mesenchymal-epithelial transition (MET) gene plays an important role in anticancer drug resistance to anaplastic lymphoma kinase-tyrosine kinase inhibitors (ALK-TKIs) in echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK)-rearranged lung cancer cells. We encountered an ALK-rearranged lung cancer patient who developed MET amplification after alectinib treatment and showed an effective response to fifth-line crizotinib. First-line alectinib treatment was effective for 2.5 years; however, liver metastases exacerbated. Liver biopsy specimens revealed MET and human epidermal growth factor receptor 2 (HER2) amplifications. Switching to the MET inhibitor crizotinib improved liver metastases. Crizotinib may be effective in ALK-positive patients with MET amplification.
Keywords: lung cancer, echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK), mesenchymal-epithelial transition (MET) amplification, human epidermal growth factor receptor 2 (HER2), crizotinib, alectinib
Introduction
The echinoderm microtubule associated protein like 4 (EML4)-anaplastic lymphoma kinase (ALK) fusion gene (EML4-ALK), identified in 2007, is known as a driver gene in patients with lung cancer and can be found in approximately 5% of non-small-cell lung cancer patients (1).
Drug resistance, including that to anticancer tyrosine kinase inhibitors (TKIs; such as ALK inhibitors), is a serious clinical problem in non-small-cell lung cancer treatment (2,3). Mesenchymal-epithelial transition (MET) amplification is a main drug resistance mechanism after TKI treatment. MET is a proto-oncogene that encodes c-MET, a receptor-type tyrosine kinase. c-MET binding to its ligand, hepatocyte growth factor (HGF), activates various intracellular signaling cascades that promote tumor cell proliferation, migration, invasion, and angiogenesis (4,5). MET activation occurs upon MET gene amplification, MET protein overexpression, overstimulation by HGF, and MET exon 14 skipping mutations (6).
MET amplification is one of the main drug resistance mechanisms after alectinib treatment. In addition, MET amplification is also associated with resistance to other TKIs in non-small-cell lung cancer treatment, such as epidermal growth factor receptor (EGFR)-TKIs (7). Therefore, combined EGFR-TKIs and MET inhibitors may help treat non-small-cell lung cancer patients with resistance to EGFR inhibitors promoted by MET amplification (8). MET amplification was detected in approximately 10% of tumor biopsies from patients that relapsed after TKI treatment (9). Several case reports (10-14) and one study (15) have suggested that MET amplification can mediate resistance to ALK-TKIs, and the reported rate of MET amplification is 15% among tumor biopsies from patients that relapse after treatment with selective ALK inhibitors (15). Overall, sequential therapy using MET inhibitors may also be effective in treating non-small-cell lung cancers, but proper screening of genetic mutations is essential for achieving effective treatment strategies in these patients.
Crizotinib was first approved in Japan for the treatment of ALK fusion gene-positive lung cancers. In addition to ALK, crizotinib is also a kinase inhibitor of MET and c-ros oncogene 1 (ROS-1). Alectinib was approved for the treatment of ALK fusion gene-positive lung cancer patients in Japan in 2014 (16) and is often administered as the first-line treatment, since it has a reduced rate of adverse effects and greater efficacy than other ALK inhibitors (17-21). Several reports have demonstrated the efficacy of crizotinib as a MET inhibitor (10-15).
We herein report a Japanese patient with ALK-positive lung cancer that recurred after transiently successful alectinib treatment, showing confirmation of MET amplification in metastatic liver lesions, that was successfully treated with crizotinib.
Case Report
A non-smoking Japanese woman in her 40s was diagnosed with lung adenocarcinoma (cT1cN3M1c, clinical stage IVB, 8th edition of the International Union against Cancer/American Joint Committee on Cancer TNM staging system) in August 20XX-3. Her initial general health condition was poor, with pericardial effusion, hoarseness associated with recurrent laryngeal nerve palsy, and multiple liver metastases. Biopsy specimens were taken from the mediastinal lymph nodes, and the diagnosis of ALK-positive lung adenocarcinoma was made based on immunohistochemical (IHC) analysis and break-apart fluorescence in-situ hybridization (FISH) results.
Alectinib (300 mg twice daily) was used as first-line therapy, and her general condition and radiological findings improved. One year after initiating alectinib treatment, a single brain metastasis was observed in the cerebellum, and brain radiotherapy was added to the treatment plan (36 Gy/3 fractions). Approximately 2.5 years after alectinib administration (January 20XX), the number of liver metastases rapidly increased, and alectinib was switched to lorlatinib as second-line treatment, resulting in no response and exacerbated liver metastases on computed tomography (CT) in March 20XX (Fig. 1).
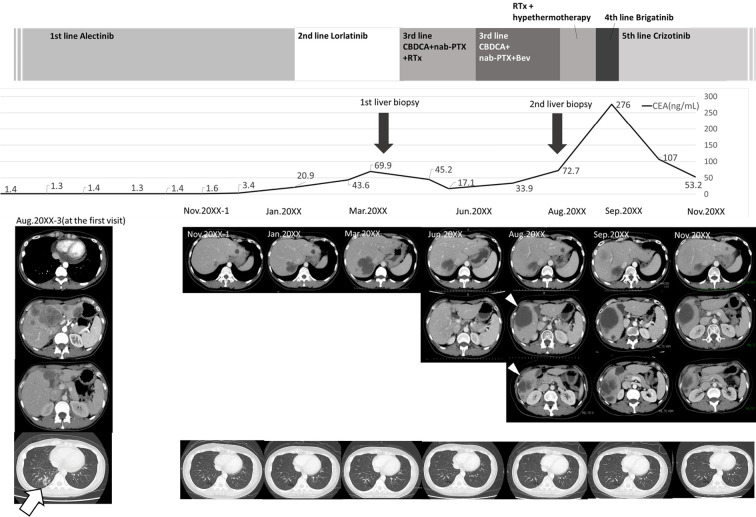
Figure 1.
Clinical course of the patient. The patient was diagnosed with stage IVB ALK-positive lung adenocarcinoma in August 20XX-3 and given alectinib as first-line treatment. A positive treatment effect continued for about 2.5 years. However, in January 20XX, liver metastases rapidly developed, and the patient was switched to lorlatinib as second-line treatment. Nevertheless, the liver metastases increased further, and levels of tumor markers also tended to increase. A liver biopsy was performed to confirm the resistance mechanism, but an adequate specimen could not be obtained. The treatment regimen was changed to third-line CBDCA+nab-PTX in April 20XX, and BEV was started in June 20XX. However, computed tomography in August 20XX revealed new liver metastases (white arrowheads), and a second liver biopsy of these metastases was performed. The biopsied specimens were subjected to next-generation sequencing. In September 20XX, before obtaining the results of the analysis, palliative radiotherapy and hyperthermia therapy were administered, and brigatinib was started as fourth-line treatment. Despite these treatments, growth of the liver metastases and peritoneal dissemination were observed. After the second liver biopsy confirmed MET amplification, crizotinib was administered as fifth-line treatment, and all liver metastases were reduced in size. A marked reduction in the CEA level was also observed. During the entire treatment period, the primary lesion in the lower right lobe (white arrow) did not increase. ALK: anaplastic lymphoma kinase, BEV: bevacizumab, CBDCA: carboplatin, CEA: carcinoembryonic antigen, MET: mesenchymal-epithelial transition gene, nab-PTX: nanoparticle albumin-bound paclitaxel, RTx: radiation therapy
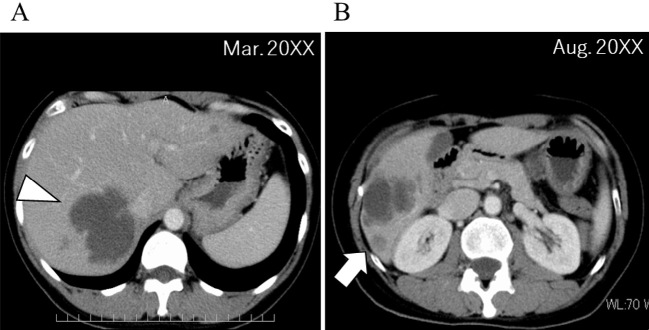
Since the liver metastases increased rapidly, while the primary lesions did not, we considered the acquisition of a resistance mechanism to ALK-TKI in the liver metastases. In addition, there were mixed areas of both increasing and decreasing liver metastases in size, indicating tumor heterogeneity. A liver biopsy was performed to analyze gene alterations of the tumor (Fig. 2A, white arrowhead, Fig. 3A-ii) in March 20XX, but the sample volume was not sufficient to perform next-generation sequencing (NGS). Other organs did not exhibit signs of metastases, but the liver metastases were rapidly growing; palliative radiotherapy (30 Gy) was therefore performed in April 20XX.
Figure 2.
Location of the liver biopsy. (A) An initial liver biopsy was performed, but the specimen collected at the cystic metastatic site (white arrowhead) was in a liquid state, indicating necrosis, and adequate tissue sample could not be obtained. (B) For the second liver biopsy, a large area was selected within the vicinity of emerging liver metastases (white arrow) and evaluated by abdominal ultrasonography, resulting in sufficient tissue sampling.
Figure 3.
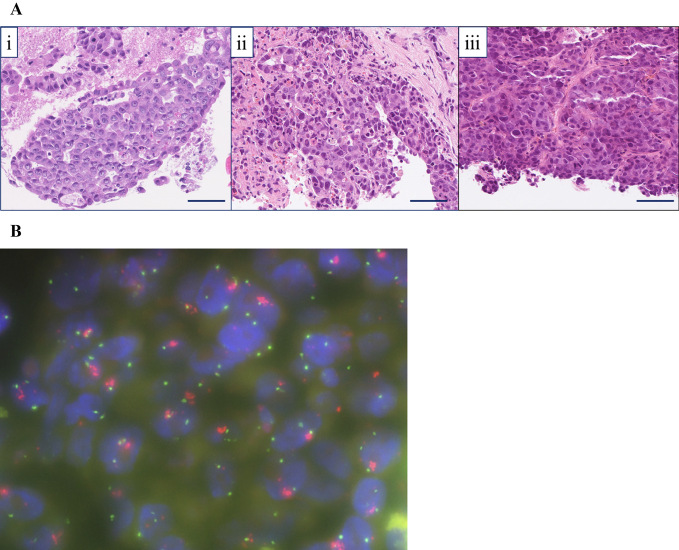
(A) Hematoxylin and Eosin (H&E) staining sample of the metastatic tumor. Each tissue sample had similar tumor cell characteristics. i) Lymph node metastasis of lung adenocarcinoma (EBUS-TBNA, H&E staining, ×200): the specimen showed the proliferation of atypical epithelial cells arranged in a tubular or cribriform growth fashion. ii) Liver metastasis of lung adenocarcinoma (needle biopsy in March 20XX, H&E staining, ×200): atypical epithelial cells arranged in a tubular or cribriform growth fashion were seen (immunohistochemically, the atypical cells were diffusely positive for TTF-1, Napsin A, and ALK and negative for synaptophysin and INSM-1). iii) Liver metastasis of lung adenocarcinoma (needle biopsy in Aug 20XX, H&E staining, ×200): the specimen revealed a proliferation of atypical epithelial cells arranged in nests, sheets, or a trabecular growth fashion (immunohistochemically, the atypical cells were diffusely positive for TTF-1, Napsin A, and ALK and negative for synaptophysin and INSM-1). Scale bar=50 μm. (B) A MET/CEP7 FISH assay (red signal: MET gene; green signal: CEP7). This representative case with MET/CEP7 5.6 (≥5) was classified as having high-level MET amplification. EBUS-TBNA: endobronchial ultrasound-guided transbronchial needle aspiration, TTF-1: thyroid transcription factor-1, INSM-1: insulinoma-associated protein 1, MET/CEP7: ratio of MET to centromere chromosome 7 probe, CEP7: centromere of chromosome 7, FISH: fluorescence in-situ hybridization
Subsequent systemic chemotherapy with carboplatin (CBDCA) and nanoparticle albumin-bound paclitaxel (nab-PTX) were administered as third-line chemotherapy, and bevacizumab (BEV) was also initiated in June 20XX. However, these systemic chemotherapy and radiotherapy rounds were ineffective, and new liver metastases (Fig. 1, white arrowheads) and paratracheal lymph node enlargement were observed, on CT in August 20XX. At this time, a second liver biopsy of the new liver metastases was performed (Fig. 2B, white arrow, Fig. 3A-iii), and biopsied specimens were submitted to the Lung Cancer Genomic Screening Project for Individualized Medicine Molecular Testing for Resistant Tumors to Systemic Therapy (LC-SCRUM-TRY), a project for the genomic screening of cancers, for the analysis of chemotherapy resistance genes using NGS (22). The genetic analysis was conducted using the Oncomine™ Precision Assay (Thermo Fisher Scientific, Waltham, USA). Before obtaining the results of the genetic analysis, a second round of palliative radiotherapy (30 Gy) and hyperthermia therapy were administered, and brigatinib was started as fourth-line treatment in September 20XX. Despite these treatments, the growth of liver metastases and peritoneal dissemination was observed. Opioid analgesics were started for abdominal pain due to the peritoneal metastasis dissemination.
A genetic analysis (conducted using the Oncomine™ Precision Assay; Thermo Fisher Scientific) revealed amplification of the MET (9.91 gene copies) and human epidermal growth factor receptor type 2 (HER2) genes (4.26 gene copies), but no point mutations in ALK [EML4-ALK fusion (variant 2: E20; A20) was detected]. Furthermore, FISH images of biopsies showed a high copy number of MET (MET/CEP7 ratio 5.6, Fig. 3B) (23).
Crizotinib (250 mg twice daily) was introduced in October 20XX to target MET amplification, and her abdominal pain gradually improved. Abdominal echography performed 11 days after crizotinib administration showed a decreased size of the liver metastases. Abdominal CT performed 30 days after the start of crizotinib showed shrinkage of liver metastases and para-aortic lymph nodes (Fig. 1, November 20XX). Grade 2 dysgeusia and appetite loss appeared as adverse effects of crizotinib, but these symptoms abated over time. Crizotinib was effective for four months until a re-increase in the liver metastases occurred.
Discussion
Mechanisms of resistance to ALK inhibitors include secondary mutations in their receptors, such as L1196M, C1156Y, L1152R, and G1202R (3); activation of bypass pathways; overexpression of P-glycoproteins (2,24,25); and cancer cell transformations, such as conversion to small-cell carcinoma (26,27). First- and second-generation ALK inhibitors (crizotinib, alectinib, ceritinib) exhibit different types of inhibitory activities to counter secondary mutations (3). The activation of c-KIT, EGFR, and MET is known to activate the bypass pathways of tumor proliferation (3). Crizotinib, a multi-kinase inhibitor, including targeting MET, is suggested to be effective in downregulating MET activation, and in vitro studies have also demonstrated the therapeutic potential of crizotinib for inhibiting MET activation (28).
In the present patient, MET amplification was suggested to be the mechanism responsible for the development of alectinib resistance, as 1) the third-generation ALK-TKIs lorlatinib and brigatinib were ineffective in inhibiting the effects of ALK mutations; 2) no secondary mutations in ALK were observed; 3) the coexistence of ALK and MET mutations is rarely observed prior to ALK-TKI administration, especially in high-level MET amplification cases (29-32); and 4) an improvement in the clinical condition of our patient was observed after crizotinib administration.
Lower levels of MET amplification were reported in ALK-positive lung cancer patients receiving crizotinib therapy than in those treated with other ALK-TKIs (15). This finding highlights the importance of MET amplification in the development of drug resistance to alectinib, which is being used increasingly frequently as the first-line treatment in ALK-positive lung cancer patients. Our patient developed resistance to alectinib after showing a favorable anticancer response for 2.5 years, with a rapid increase in the number of hepatic metastases and consequent carcinomatous peritonitis. In a previous report on MET amplification in a patient with ALK-positive lung cancer, disease aggravation was accompanied by an extremely progressive and deteriorating clinical condition, such as one characterized by pericardial effusion and disseminated intravascular coagulation (Table). In these severe disease cases, it may not be possible to confirm the resistance mechanism by a biopsy and rescue the patient with systemic anticancer therapy in time. Therefore, it is essential to confirm the type of drug resistance mechanism as soon as the symptoms of progressive disease (PD) appear for the proper selection of therapeutic modalities. In addition, the primary lung lesions were not consistently enlarged in this case, and only the liver metastases were noticeably enlarged. This suggests tumor heterogeneity, and it is important to search for resistance mechanisms targeting tumors that tend to increase in size.
Table.
Case Reports of MET Gene Amplification in ALK-positive Lung Cancer.
| Case | Age | Sex | Prior chemotherapy | Duration time between starting other prior ALK-TKI and receiving crizotinib | Recurrent sites and complications | Outcome | Reference | |
|---|---|---|---|---|---|---|---|---|
| Duration of administration | Response | |||||||
| 1 | 43 | F | CDDP+PEM, DOC, CBDCA+GEM, alectinib | Alectinib (10.5 months) | Multiple liver metastases, DIC | ND | ND | 10 |
| 2 | 44 | F | Alectinib, lorlatinib | Alectinib (9 months), lorlatinib (4 weeks) | Pericardial effusion, pulmonary embolism | 1 month | PR | 11 |
| 3 | 40 | F | Crizotinib, CBDCA+PEM, alectinib, DOC, lorlatinib | Lorlatinib (7 months) | Brain metastasis, right adrenal gland metastasis | 14 months | PR | 13 |
| 4 | ND | ND | Alectinib, CBDCA+PEM | Alectinib (9 months) | Chest wall metastasis, liver metastasis, bone metastasis | 10 weeks | PR | 15 |
| 5 | 64 | F | Alectinib, CBDCA+PTX | Alectinib (6 months) | Right adrenal gland metastasis, bone metastasis, abdominal and retroperitoneal lymph nodes metastasis | 10 months | PR | 14 |
| Present case | 42 | F | Alectinib, CBDCA+nab-PTX+Bev, lorlatinib, brigatinib | Alectinib (2.5 years), lorlatinib (2 months), brigatinib (1 month) | Brain metastasis, liver metastasis, peritoneal dissemination | 4 months | PR | - |
CDDP: cisplatin, PEM: pemetrexed, DOC: docetaxel, CBDCA: carboplatin, GEM: gemcitabine, PTX: paclitaxel, nab-PTX: nanoparticle albumin-bound paclitaxel, Bev: bevacizumab, DIC: disseminated intravascular coagulation, PR: partial response, PD: progressive disease, ND: not described
The FISH method was used to evaluate the copy number of the MET gene in our patient. FISH evaluates the copy number by assessing the ratio of the total number of signals in the MET gene and the centromere region of chromosome 7 (MET/CEP7 ratio). Unlike the polymerase chain reaction method, it can also distinguish between MET gene amplification and polysomy of chromosome 7. Although there is still no international consensus on the MET gene copy number and MET/CEP7 ratio thresholds, we used a high amplification threshold of the MET gene when the MET/CEP7 ratio was ≥5, based on previous reports (23,33,34). In cases of MET amplification in the EGFR-TKI resistance mechanism, the higher the amplified copy number, the stronger the combination effect of EGFR-TKI and MET inhibitor (35). Therefore, determining the burden of severe MET amplification may help determine the drug resistance mechanism.
HER2 amplification was simultaneously detected with MET amplification in our patient. HER2 amplification is observed in EGFR mutation-positive patients as a drug resistance mechanism (36), and reports suggest that HER2 amplification can also contribute to ALK-TKI resistance (37). Thus, HER2 amplification might also have been responsible for the anticancer drug resistance in our patient. Effective treatment strategies to suppress HER2 amplification in EGFR- and ALK-positive cancers have not yet been established. Nevertheless, the administration of HER2-TKI to a patient with HER2-positive lung cancer (without respective driver mutation) was effective in alleviating symptoms (38). HER2-TKIs might therefore be an alternative effective strategy of treatment in cancer patients with drug resistance developed due to HER2 amplification.
Conclusions
We encountered a patient with ALK-positive lung cancer who became refractory to ALK-TKI treatment, possibly due to MET amplification confirmed by re-biopsy specimens after 2.5 years of successful treatment, and in whom crizotinib was clinically effective. The present findings suggest the importance of the early detection of gene alterations responsible for drug resistance using re-biopsy specimens.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448: 561-566, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med 4: 120ra17, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov 6: 1118-1133, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376: e768-e771, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Sattler M, Salgia R. c-Met and hepatocyte growth factor: potential as novel targets in cancer therapy. Curr Oncol Rep 9: 102-108, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol 3: S7-S19, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316: 1039-1043, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Scagliotti G, Moro-Sibilot D, Kollmeier J, et al. A randomized-controlled phase 2 study of the MET antibody emibetuzumab in combination with erlotinib as first-line treatment for EGFR mutation-positive NSCLC patients. J Thorac Oncol 15: 80-90, 2020. [DOI] [PubMed] [Google Scholar]
- 9.Coleman N, Hong L, Zhang J, et al. Beyond epidermal growth factor receptor: MET amplification as a general resistance driver to targeted therapy in oncogene-driven non-small-cell lung cancer. ESMO Open 6: 100319, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toyokawa G, Takenoyama M, Watanabe S, et al. Dramatic response to crizotinib in an ALK-positive adenocarcinoma patient with disseminated intravascular coagulation. J Thorac Oncol 8: e96-e98, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Mazzotta M, Filetti M, Rossi A, et al. Is there a place for crizotinib in c-MET alterations? A case of efficacy in ALK positive NSCLC patient with secondary c-MET amplification. Ann Oncol 31: 440-441, 2020. [DOI] [PubMed] [Google Scholar]
- 12.Gouji T, Takashi S, Mitsuhiro T, Yukito I. Crizotinib can overcome acquired resistance to CH5424802: is amplification of the MET gene a key factor? J Thorac Oncol 9: e27-e28, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Sakakibara-Konishi J, Kitai H, Ikezawa Y, et al. Response to crizotinib re-administration after progression on lorlatinib in a patient with ALK-rearranged non-small-cell lung cancer. Clin Lung Cancer 20: e555-e559, 2019. [DOI] [PubMed] [Google Scholar]
- 14.Ji J, Mitra A, Camidge DR, Riess JW. Early alectinib resistance from MET amplification in ALK-rearranged NSCLC: response to crizotinib with re-response to alectinib and crizotinib. Clin Lung Cancer 22: e851-e855, 2021. [DOI] [PubMed] [Google Scholar]
- 15.Dagogo-Jack I, Yoda S, Lennerz JK, et al. MET alterations are a recurring and actionable resistance mechanism in ALK-positive lung cancer. Clin Cancer Res 26: 2535-2545, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol 14: 590-598, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 390: 29-39, 2017. [DOI] [PubMed] [Google Scholar]
- 18.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 377: 829-838, 2017. [DOI] [PubMed] [Google Scholar]
- 19.Zhou C, Kim SW, Reungwetwattana T, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med 7: 437-446, 2019. [DOI] [PubMed] [Google Scholar]
- 20.Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 31: 1056-1064, 2020. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa K, Hida T, Nokihara H, et al. Final progression-free survival results from the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer 139: 195-199, 2020. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto S. Development of molecular-targeted therapy through large-scale genome screening for rare oncogene driver-positive lung cancer. Jpn J Lung Cancer 58: 252-257, 2018(in Japanese, Abstract in English). [Google Scholar]
- 23.Tong JH, Yeung SF, Chan AW, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res 22: 3048-3056, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 363: 1734-1739, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Katayama R, Sakashita T, Yanagitani N, et al. P-glycoprotein mediates ceritinib resistance in anaplastic lymphoma kinase-rearranged non-small cell lung cancer. EBioMedicine 3: 54-66, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cha YJ, Cho BC, Kim HR, Lee HJ, Shim HS. A case of ALK-rearranged adenocarcinoma with small cell carcinoma-like transformation and resistance to crizotinib. J Thorac Oncol 11: e55-e58, 2016. [DOI] [PubMed] [Google Scholar]
- 27.Zhu YC, Liao XH, Wang WX, et al. Patients harboring ALK rearrangement adenocarcinoma after acquired resistance to crizotinib and transformation to small-cell lung cancer: a case report. OncoTargets Ther 10: 3187-3192, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isozaki H, Ichihara E, Takigawa N, et al. Non-small cell lung cancer cells acquire resistance to the ALK inhibitor alectinib by activating alternative receptor tyrosine kinases. Cancer Res 76: 1506-1516, 2016. [DOI] [PubMed] [Google Scholar]
- 29.Jordan EJ, Kim HR, Arcila ME, et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov 7: 596-609, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong JH, Yeung SF, Chan AWH, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res 22: 3048-3056, 2016. [DOI] [PubMed] [Google Scholar]
- 31.Le X. Heterogeneity in MET-aberrant NSCLC. J Thorac Oncol 16: 504-506, 2021. [DOI] [PubMed] [Google Scholar]
- 32.Kron A, Scheffler M, Heydt C, et al. Genetic heterogeneity of MET-aberrant NSCLC and its impact on the outcome of immunotherapy. J Thorac Oncol 16: 572-582, 2021. [DOI] [PubMed] [Google Scholar]
- 33.Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol 6: 942-946, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Cappuzzo F, Jänne PA, Skokan M, et al. MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann Oncol 20: 298-304, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo R, Luo J, Chang J, et al. MET-dependent solid tumors - molecular diagnosis and targeted therapy. Nat Rev Clin Oncol 17: 569-587, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol 29: i10-i19, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minari R, Gnetti L, Lagrasta CA, et al. Emergence of a HER2-amplified clone during disease progression in an ALK-rearranged NSCLC patient treated with ALK-inhibitors: a case report. Transl Lung Cancer Res 9: 787-792, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li BT, Smit EF, Goto Y, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med 386: 241-251, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]