Abstract
A woman in her 70s presented with gallbladder carcinoma with liver metastases and peritoneal dissemination. After standard chemotherapy failed, a liver biopsy was performed. A FoundationOne CDx analysis showed that the tumor mutational burden (TMB) was high (34 mutations/megabase). Treatment with pembrolizumab, which is an immune checkpoint inhibitor (ICI), resulted in a partial response, and there were no significant immune-related adverse events. According to recently published reports, the frequency of TMB-high biliary tract cancer (BTC) is 3.4-4%, which makes it extremely rare. In conclusion, ICIs may be effective in patients with TMB-high BTC.
Keywords: tumor mutational burden-high, gallbladder carcinoma, biliary tract cancer, immune checkpoint inhibitor, pembrolizumab
Introduction
Biliary tract cancer (BTC) is one of the most aggressive type of cancer (1). Systemic chemotherapy is the standard treatment option for patients with BTC with unresectable or recurrent disease. Based on the results of a phase III clinical trial (ABC-02 trial) (2), and a phase II clinical trial (BT-22 trial) (3), gemcitabine plus cisplatin (GC) is the standard first-line chemotherapeutic regimen for advanced BTC in Japan and elsewhere. Subsequently, gemcitabine plus S-1 (GS) therapy proved to be non-inferior to GC therapy in terms of the overall survival (OS) (4). Nevertheless, the median OS after GC or GS administration for advanced BTC is 11.2-15.1 months, and this requires improvement.
More recently, combination therapy using GC and S-1 (GCS) or GC plus the anti-programmed cell death-ligand 1 (PD-L1) antibody durvalumab (GCD) was shown to be more effective than GC alone in terms of improving the OS. Furthermore, a median OS of 13.5 months was reported for GCS by the KHBO1401-MITSUBA study (5), and a median OS of 12.8 months was reported for GCD by the TOPAZ-1 study (6).
The phase II KEYNOTE-158 study revealed that tumors with a high mutational burden (TMB-high) have positive responses to immune checkpoint inhibitors (ICIs), regardless of their primary site (7). In February 2022, pembrolizumab (Pembro), an anti-programmed cell death protein 1 (PD-1) monoclonal antibody, was approved in Japan for TMB-high cases, including BTC, that were refractory to standard chemotherapy. However, the KEYNOTE-158 study did not include TMB-high BTC cases, and information about these patients is lacking.
We herein report a case of TMB-high gallbladder carcinoma (GBC) with liver metastases and peritoneal dissemination where Pembro was found to be effective.
Case Report
A woman in her 70s developed sudden upper abdominal pain in February 202X and was admitted to a nearby hospital. As a teenager, she had undergone appendectomy and had no other medical history. Her family history included a mother who had stomach and lung cancer and a brother who had laryngeal cancer. After hospital admission, contrast-enhanced computed tomography (CT) and magnetic resonance cholangiopancreatography were performed. Based on these investigations, GBC with liver metastases and an omental disseminated nodule were suspected. Blood tests revealed elevated hepatobiliary enzymes and direct bilirubin levels, suggesting obstructive jaundice. She underwent endoscopic biliary drainage. In March 202X, she was referred to a university hospital and underwent diagnostic laparoscopy, which revealed liver metastasis in segment six and an omental disseminated nodule in the anterior pylorus of the stomach.
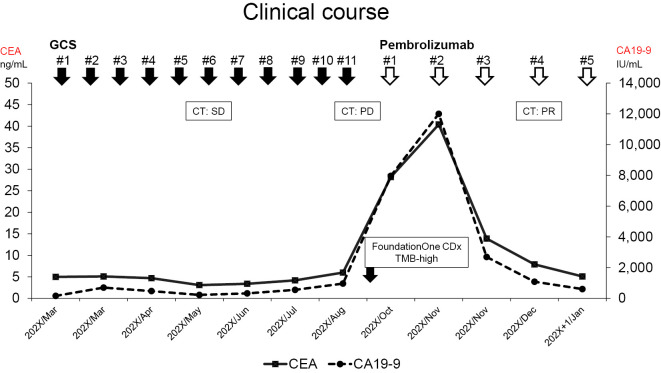
A histopathological analysis of the tumor biopsy specimen revealed adenocarcinoma. Based on this result, standard chemotherapy with GCS was started for unresectable advanced gallbladder cancer. Fig. 1 shows the course of treatment and changes in tumor markers. Gemcitabine and cisplatin (1,000 and 25 mg/m2, respectively) were administered intravenously on day 1. Furthermore, S-1 was administered orally twice a day for seven consecutive days and repeated every two weeks. She was administered 120 mg/day of S-1 (body surface area; 1.53 m2) (5). Before treatment, the carcinoembryonic antigen (CEA) level was 5.0 ng/mL, and the carbohydrate antigen 19-9 (CA19-9) level was 165 U/mL. After the first course of treatment, she was referred to our hospital (first visit in the same month).
Figure 1.
Clinical course showing the use of cancer pharmacotherapy and changes in tumor markers for gallbladder cancer. GCS: gemcitabine plus cisplatin and S-1, CT: computed tomography, SD: stable disease, PD: progressive disease, PR: partial response, TMB: tumor mutational burden, CEA: carcinoembryonic antigen, CA19-9: carbohydrate antigen 19-9
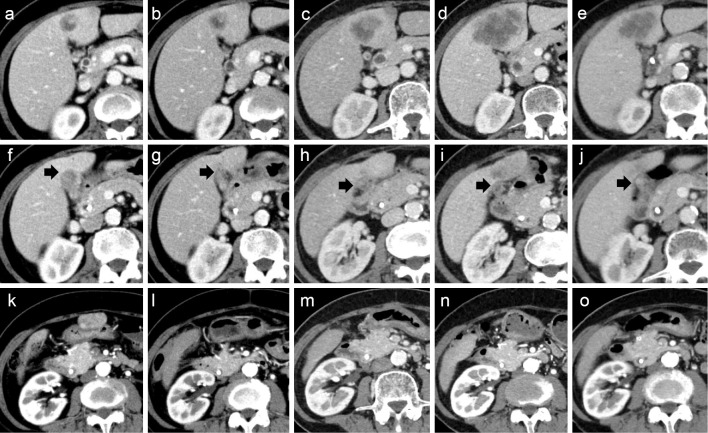
At the first visit, her general condition was good, with an Eastern Cooperative Oncology Group Performance Status of 0 and body mass index of 22.5. She was able to continue the GCS therapy with a full dose. After 4 courses (10 weeks), CT revealed that her GBC had shrunk, and the omental disseminated nodule had disappeared. However, there was no significant change in liver metastasis (Fig. 2b, g, l). Notably, after the 10th course (22 weeks), CT revealed a marked increase in liver metastasis (Fig. 2c), indicating progressive disease.
Figure 2.
Contrast-enhanced computed tomography image showing a liver metastasis (a-e), the primary site of gallbladder cancer (f-j, black arrows), and an omental disseminated nodule (k-o). Computed tomography images immediately before the introduction of gemcitabine plus cisplatin and S-1 (GCS) (a, f, k). Computed tomography images at 10 weeks after the introduction of GCS (b, g, l). Computed tomography images at 22 weeks after the introduction of GCS (c, h, m). Computed tomography images just before starting pembrolizumab (Pembro) (d, i, n). Computed tomography images at eight weeks after starting Pembro (e, j, o). Increase in liver metastasis after GCS (d). Liver metastasis was reduced after Pembro use (e). Before the introduction of GCS, an omental disseminated nodule was observed on the anterior surface of the gastric antrum (k). However, the omental disseminated nodule disappeared after the introduction of GCS (l-o).
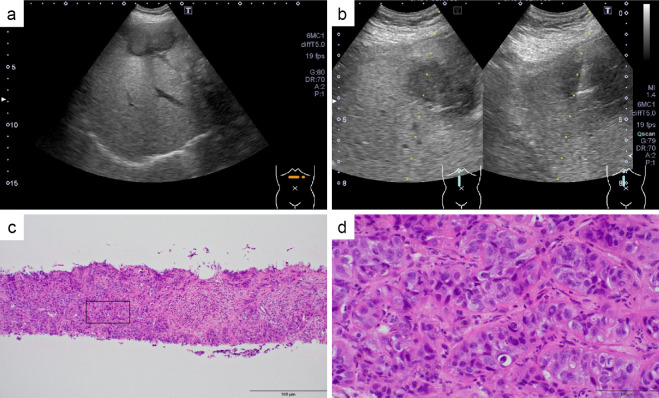
Standard treatment had already been completed, and after consulting with the patient and her family, a biopsy of the liver metastasis was performed (Fig. 3). This was followed by FoundationOne CDx (F1CDx; Foundation Medicine, Cambridge, USA), which is a next-generation sequencing-based panel testing (8,9). This testing indicated that the tumor mutation burden was rather high [34 mutations/megabase (Muts/Mb)], although her microsatellite instability (MSI) status was stable (Table 1). No MLH1, MSH2, MSH3, MSH6, or PMS2 genetic mutations were found. No mutations or amplifications of PD-L1 were observed. Based on these results, Pembro (200 mg every 3 weeks) was introduced in October of the same year, as her organ function was well-maintained.
Figure 3.
Hepatic metastatic nodule with a maximum diameter of 40 mm visualized on abdominal sonography by lateral scanning of the epigastric region (a). An echo-guided liver tumor biopsy (b). Hematoxylin and Eosin (H&E) staining (×20) (c). H&E staining (×100) (d).
Table 1.
Results of FoundationOne CDx.
| Biomarkers or some of the genes analysed | Result |
|---|---|
| TMB | 34 Muts/Mb |
| MSI | Stable |
| MLH1 | No genetic mutations |
| MSH2 | No genetic mutations |
| MSH3 | No genetic mutations |
| MSH6 | No genetic mutations |
| PMS2 | No genetic mutations |
| PD-L1 also known as CD274 | No genetic mutations |
TMB: tumor mutational burden, Muts: mutations, Mb: megabase, MSI: microsatellite instability
Table 2 shows the laboratory data obtained before Pembro treatment. The tumor markers seemed to increase two weeks after starting Pembro treatment. However, these markers decreased from their peak values (CEA 40.4 → 13.9 ng/mL; CA19-9>12,000 → 2,698 U/mL) at 6 weeks after the introduction of Pembro (Fig. 1). To assess the effect of treatment, CT was performed, which showed shrinkage of the liver metastases, and a partial response was obtained (Fig. 2e). The patient had been doing well without any immune-related adverse events (irAEs) at least 12 weeks after starting Pembro.
Table 2.
Laboratory Data before Pembrolizumab Treatment.
| Result | |||
|---|---|---|---|
| Hematological analyses | |||
| WBC | 6,200 | /μL | |
| RBC | 377×104 | /μL | |
| Hb | 11.8 | g/dL | |
| Hct | 36.6 | % | |
| Platelet count | 22.5×104 | /μL | |
| Coagulation test | |||
| PT (%) | 94.0 | % | |
| PT-INR | 1.04 | ||
| Blood chemistry | |||
| TP | 7.0 | g/dL | |
| Alb | 4.1 | g/dL | |
| T-Bil | 0.92 | mg/dL | |
| AST | 20 | U/L | |
| ALT | 14 | U/L | |
| LDH | 205 | U/L | |
| ALP | 304 | U/L | |
| γ-GT | 68 | U/L | |
| AMY | 109 | U/L | |
| BUN | 18.1 | mg/dL | |
| UA | 4.8 | mg/dL | |
| Cr | 0.49 | mg/dL | |
| Na | 143 | mEq/L | |
| K | 4.1 | mEq/L | |
| Cl | 104 | mEq/L | |
| T-Cho | 228 | mg/dL | |
| TG | 104 | mg/dL | |
| CRP | 1.64 | mg/dL | |
| Hepatitis viral markers | |||
| HBsAg | Negative | ||
| HBc-Ab | Negative | ||
| HBs-Ab | Negative | ||
| HCV-Ab | Negative | ||
| Tumor markers | |||
| CEA | 28.2 | ng/dL | |
| CA19-9 | 7,967 | U/mL | |
RBC: red blood cell, WBC: white blood cell, Hb: hemoglobin, PT: prothrombin time, INR: international normalized ratio, TP: total protein, T-Bil: total bilirubin, Alb: albumin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GT: γ-glutamyltransferase, BUN: blood urea nitrogen, UA: uric acid, Cr: creatinine, T-Cho: total cholesterol, TG: triglyceride, HBs-Ag: hepatitis B surface antigen, HBs-Ab: anti-hepatitis B surface antibody, HBc-Ab: anti-hepatitis B core antibody, HCV-Ab: hepatitis C virus antibody, CEA: carcinoembryonic antigen, CA19-9: carbohydrate antigen 19-9
Discussion
In February 2022, Pembro became available in Japan as a treatment for advanced/recurrent solid tumors with a high mutation burden that was exacerbated after cancer chemotherapy. In solid tumors with a high mutation burden, the accumulation of somatic mutations produces a large number of tumor-specific antigens that induce an immune response, making it easier for T cells to recognize them (10). In the KEYNOTE-158 study, which was an international collaborative phase II study that served as the basis for insurance approval, 689 cases of solid tumors were included, of which 63 were BTCs (7). However, this study did not include TMB-high BTC cases, and as such, there is a lack of information regarding the effect of Pembro on patients with TMB-high BTC. In this context, the present report of a patient with TMB-high BTC who was successfully treated using Pembro is noteworthy.
In principle, Japanese patients with unresectable or recurrent BTC cannot undergo comprehensive genomic profiling (CGP) before the initiation of first-line chemotherapy. This principle is based on Japanese public insurance policies and not on scientific evidence. For patients with unresectable or metastatic BTC, eight molecular markers (NTRK fusion, MSI-high, TMB-high, BRAF V600E, FGFR2 fusions/rearrangement, IDH1 mutations, HER2 overexpression, and RET fusions) and the corresponding therapies are listed in the latest National Cancer Center Network guidelines as routine workups to perform before initiating first-line treatment (11).
In Japan, it is expected that F1CDx will be performed before first-line chemotherapy for BTC, with the results facilitating selection of a suitable therapy in the future. In addition, the current medical insurance system in Japan permits CGP testing only once in a lifetime. Therefore, it is necessary to make a careful decision about whether and when to perform a CGP test. The results of MSI-H/TMB-H/copy number variations obtained by F1CDx Liquid are not approved for clinical use by the Ministry of Health, Labour, and Welfare in Japan (12), so caution is required. The European Society for Medical Oncology Precision Medicine Working Group also recommends the routine use of tissue-based CGP tests in BTC and three other types of cancer (non-small-cell lung, prostate, and ovarian cancer). This recommendation is based on the high prevalence of druggable markers in these four types of cancer (13).
As described above, various gene mutations have been reported in BTC and are expected to serve as therapeutic targets. However, cases with each gene mutation are rare, and after the failure of standard chemotherapy, only a few patients with BTC can be treated using other drugs. The frequency of TMB-high BTC is approximately 3.4-4% (12,14). In December 2018, Pembro was approved for use in Japan for MSI-high solid cancers, including BTC, that were refractory to standard chemotherapy. In the KEYNOTE-158 study, which only enrolled patients with MSI-high tumors, the results of the treatment of 22 patients with advanced BTC using Pembro were as follows: objective response rate, 40.9%; median progression-free survival, 4.2 months [95% confidence interval (CI) 2.1 to not reached (NR)]; and median OS, 24.3 months (95% CI: 6.5 to NR) (15). It is likely that TMB-high BTC will have results similar to those of MSI-high BTC after treatment with Pembro. Up to 75% of MSI-high solid tumors overlapped with TMB-high solid tumors (14). Therefore, the total number of patients with TMB-high tumors but without MSI-high tumors is estimated to be less than the total number of patients with TMB-high and MSI-high tumors (14). In several cancer types, the occurrence of TMB-high tumors cannot be used to predict a good response to ICIs (16,17). Therefore, the results from future studies of TMB-high BTC are eagerly awaited.
Generally, a therapeutic effect of ICIs can be expected when the TMB is ≥10 Muts/Mb. However, the TMB (Muts/Mb) and effect of ICIs may differ depending on the type of cancer (18). TMB cut-offs for individual histologies may not represent the ideal values for use clinically. Therefore, in the future, it will be necessary to consider the TMB threshold at which an ICI can be expected to be effective against BTC.
BTC is a heterogeneous disease that can be divided into intrahepatic, perihilar, and distal subtypes based on the anatomical location. Different types of BTCs with the same TMB may have different susceptibilities to ICI, so further research on this point is also required.
Based on the results of the TOPAZ-1 trial, GCD was approved in December 2022 as first-line chemotherapy in Japan. Whether or not patients who have failed GCD and are found to be TMB-high after an F1CDx analysis will benefit from the re-use of Pembro is unclear at present. Further studies are therefore required to confirm the clinical benefits in such cases.
The toxicity profile of ICIs differs substantially from that of the classic cytotoxic agents. Although the patient in the present case developed no irAEs, the possibility of irAEs should always be considered during ICI treatment.
To our knowledge, this is the first report of Pembro being effective in treating a patient with TMB-high BTC in Japan. More such cases need to be reported to confirm the effectiveness of Pembro in TMB-high BTCs.
The patient has given her written informed consent for the publication of the details of her case.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet 397: 428-444, 2021. [DOI] [PubMed] [Google Scholar]
- 2.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362: 1273-1281, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 103: 469-474, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morizane C, Okusaka T, Mizusawa J, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol 30: 1950-1958, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Ioka T, Kanai M, Kobayashi S, et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- MITSUBA). J Hepatobiliary Pancreat Sci 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh DY, He AR, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid 1: 2022. [DOI] [PubMed] [Google Scholar]
- 7.Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 21: 1353-1365, 2020. [DOI] [PubMed] [Google Scholar]
- 8.Foundation Medicine. FoundationOne CDx™ Technical Information [Internet]. [cited 2022 Dec 26]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170019C.pdf
- 9.Summary of Safety and Effectiveness Data (SSED) [Internet]. [cited 2022 Dec 26]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170019S016B.pdf
- 10.Fancello L, Gandini S, Pelicci PG, Mazzarella L. Tumor mutational burden quantification from targeted gene panels: major advancements and challenges. J Immunother Cancer 7: 183, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NCCN Clinical Practice Guideline: Biliary Tract Cancers. Ver 4. 2022 [Internet]. [cited 2022 Dec 29]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
- 12.Shao C, Li G, Huang L, et al. Prevalence of high tumor mutational burden and association with survival in patients with less common solid tumors. JAMA Netw Open 3: e2025109, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosele F, Remon J, Mateo J, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol 31: 1491-1505, 2020. [DOI] [PubMed] [Google Scholar]
- 14.Vanderwalde A, Spetzler D, Xiao N, Gatalica Z, Marshall J. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med 7: 746-756, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 38: 1-10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rousseau B, Foote MB, Maron SB, et al. The spectrum of benefit from checkpoint blockade in hypermutated tumors. N Engl J Med 384: 1168-1170, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGrail DJ, Pilié PG, Rashid NU, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol 32: 661-672, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 51: 202-206, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]