Abstract
The aim of this study was to compare the intra and postoperative analgesic effects of sacrococcygeal epidural levobupivacaine with those of lumbosacral levobupivacaine in feline ovariohysterectomy. Thirty-six cats were premedicated with intramuscular acepromazine (0.05 mg/kg) and meperidine (6 mg/kg). Anesthesia was induced with intravenous propofol and maintained with isoflurane in oxygen. The cats were randomly assigned one of the three treatments receiving 0.33% levobupivacaine (0.3 mL/kg) into the sacrococcygeal (S-C group, n=12) or lumbosacral (L-S group, n=12) epidural space, or the same volume of 0.9% saline solution into one of the epidural approaches (Control group, n=12). Intraoperatively, cardiorespiratory variables, end-tidal isoflurane concentration (FE´ISO), and fentanyl requirements were recorded. Postoperative pain was assessed by the UNESP (Universidade Estadual Paulista)-Botucatu multidimensional composite pain scale and the Glasgow feline composite measure pain scale up to 8 hr post-extubation. Morphine was administered as rescue analgesia. Overall FE´ISO and fentanyl requirements were lower in the L-S and S-C compared to the Control (P=0.002–0.048, respectively). There was no significant difference in the cardiorespiratory variables during anesthesia, postoperative pain and rescue analgesia among groups. The time to standing after anesthesia was prolonged in the L-S and S-C groups than in the Control (P<0.001). Lumbosacral and sacrococcygeal epidural levobupivacaine resulted in similar decreases in isoflurane requirements and intraoperative fentanyl supplementation in the cats, with no postoperative benefits.
Keywords: analgesia, extradural, feline, isoflurane, local anesthetic
The combination of epidural analgesia with general anesthesia has been recommended for dogs and cats undergoing different types of surgical procedures, not only to reduce perioperative opioid requirements and stress response [1, 5, 13, 34], but also to decrease the consumption of anesthetic drugs [15, 36], improving anesthesia safety.
The lumbosacral epidural space is the most frequently approached by the insertion of a needle between the spinous process of the seventh lumbar vertebrae (L7) and the first sacral vertebrae (S1) in small animals [37, 40]. In dogs, various volumes of local anesthetic have been administered in the L7-S1 space, resulting in different areas of desensitization. As the rostral spread of local anesthetic is influenced mainly by the volume, smaller volumes are commonly used to provide sensory blockade of the pelvis, perineum, and pelvic limbs, while to desensitize the abdominal organs, larger volumes are required [40, 41]. As an alternative to the lumbosacral approach, the anesthetic solution can also be applied between the spinous process of the third sacral vertebrae (S3) and the first coccygeal vertebrae (Co1), into the sacrococcygeal (S3-C1) space [25]. This approach confers the advantage of reducing the incidence of inadvertent intrathecal anesthesia and the risk of neurological injuries, especially in cats, in which the spinal cord ends at the level of the S1 [28].
To date, few studies have reported the use of S3-Co1 epidural to provide perioperative analgesia in cats, involving surgical procedures in the pelvic limbs, perineum, and testicular structures [12, 29, 39]. However, no studies have evaluated the analgesic benefits of this epidural approach for abdominal surgeries in small animals. Data from a computer tomographic study showed similar rostral spread of the contrast comparing the L7-S1 with the S3-Co1 approach in dog cadavers, where the rostral spread with 0.4 mL/kg injected in the L7-S1 and S3-Co1 spaces extended to the 12th and 11th thoracic vertebrae, respectively, whereas 0.2 mL/kg extended to the 7th lumbar vertebrae in the two approaches [42]. Although these data cannot be extrapolated to the feline species, it is possible that S3-Co1 epidural injection of local anesthetic at a volume of 0.4 mL/kg or less could be sufficient to provide effective inhibition of nociceptive signs in abdominal surgical procedures, improving perioperative analgesia. A previous study in cats reported a significant correlation between volume and rostral spread, following lumbosacral epidural injection of new methylene blue, where the injection of 0.3 and 0.4 mL/kg provided similar and greater rostral spread than those provided by the volumes of 0.1 and 0.2 mL/kg [21]. Nevertheless, it is important to emphasize that no studies have compared the spread of different injected volumes into L7-S1 and S3-Co1 space in cats.
Bupivacaine is the most commonly used long-lasting local anesthetic for epidural anesthesia in dogs and cats [1, 5, 13, 29, 39]. Levobupivacaine is an S (−) isomer of bupivacaine racemic and is also a long-acting anesthetic [4]. Clinical and experimental studies demonstrated that levobupivacaine provides similar analgesic effects to bupivacaine racemic [5, 16], with a reduced risk of cardiac and systemic toxicity, which increases its margin of safety [22]. Despite these advantages, to the authors knowledge, no studies have focused on the effects of epidural levobupivacaine in cats.
The purpose of the current study was to investigate the intra and postoperative analgesic effects of epidural levobupivacaine administered from sacrococcygeal or lumbosacral epidural space in isoflurane-anesthetized cats undergoing elective-ovariohysterectomy (OHE). The primary objective was to evaluate whether the different epidural approaches could decrease anesthetic/analgesic requirements and improve postoperative analgesia. A secondary objective was to monitor the cardiorespiratory effects and occurrence of anesthetic complications produced by epidural levobupivacaine. Our hypothesis was that the sacrococcygeal epidural levobupivacaine could provide similar perioperative analgesic effects produced by the lumbosacral epidural approach, with minimal adverse events.
MATERIALS AND METHODS
Study design and animals
A prospective, randomized, blinded, placebo-controlled clinical trial was designed to compare analgesic and cardiopulmonary effects of epidural levobupivacaine between lumbosacral and sacrococcygeal approaches. This study was approved by the Institutional Animal Care Committee (protocol 7102/2020 CEUA) and was conducted according to the Consolidated Standards of Reporting Trials (CONSORT guidelines, http://www.consort-statement.org/). Informed written consent for the investigation was obtained from all owners. The study involved 36 client-owned cats of different breeds, scheduled for elective OHE. For inclusion, the cats were required to have a normal complete blood count and serum chemistry (blood urea nitrogen, creatinine, gamma-glutamyltransferase and alkaline phosphatase), be aged ≥6 months, and weigh ≥2 kg. Additionally, an abdominal ultrasonography examination was performed in all cats to discard possible pregnancy. The exclusion criteria were: pregnancy, lactation, fearful behaviours, systemic diseases, or skin infection in the lumbosacral or sacrococcygeal areas. Before each experiment, the cats were fasted overnight with free access to water.
Anesthesia, intraoperative monitoring and surgery
The cats were sedated intramuscularly with an intramuscular (IM) acepromazine (0.05 mg/kg; Acepran 0.2%, Vetnil, Louveira, Brazil) in combination with meperidine (6 mg/kg; Dolosal, Cristália Produtos Químicos Farmacêuticos Ltd., Itapira, Brazil). Fifteen min later, a 24-gauge, 1.9 cm catheter (Safelet, NiproMedical Ltd., Sorocaba, Brazil) was aseptically placed in the cephalic vein, followed by an induction of anesthesia with propofol (Propovan 1%, Cristália Produtos Químicos Farmacêuticos Ltd.) in a dose sufficient to permit endotracheal intubation. After instillation of lidocaine (2 mg; Xylestesin 2%, Cristália Produtos Químicos Farmacêuticos Ltd.) on the vocal cords, the cats were intubated with an appropriately sized cuffed-endotracheal tube and attached to a non-rebreathing system (SAT 500, Takaoka, São Paulo, Brazil). Anesthesia was maintained with isoflurane (Isoforine, Cristália Produtos Químicos Farmacêuticos Ltd.) vaporized in 100% oxygen (300 mL/kg/min). The cats were allowed to breathe spontaneously throughout the procedure. Body temperature was maintained between 37 and 38°C, using a forced-air warming blanket (Hefei Longshore, Anhui Province, China), which was applied at the anesthetic induction and maintained until recovery.
Heart rate (HR), percutaneous oxyhemoglobin saturation (SpO2), respiratory rate (RR), end-tidal carbon dioxide concentration (PETCO2), and FE´ISO were continuously measured using a multi-parametric monitor (Gas analyzer module VAMOS plus, Dräger do Brazil, Barueri, Brazil). Before each experiment, the gas analyzer was calibrated with a standard gas mixture (CO2: 5 vol %, N2O: 70 vol %, O2: 24 vol % and is/oflurane: 1 vol %) (White Martins Gases, São Paulo, Brazil). Systolic arterial blood pressure (SAP) was monitored indirectly by sphygmomanometry, using a Doppler ultrasound device (Doppler 841-A; Parks Medical Electronics, Aloha, Beaverton, OR, USA) with the probe placed over the digital artery on the plantar surface and an appropriately sized cuff, with a width of approximately 40% of the circumference of the thoracic limb [32]. Lactated Ringer’s solution was administered IV at 2 mL/kg/hr from anesthetic induction until the surgery initiation, when the infusion rate was increased to 3 mL/kg/hr, and was maintained until extubation, according to the recommendation of intraoperative fluid therapy for cats [8].
Intraoperatively, vaporizer settings were adjusted to maintain surgical depth of anesthesia (rotation of the eyeball, loss of palpebral reflex, and loss of jaw tone) and to prevent autonomic responses to surgical stimulation. The same experienced anesthesiologist (TSB) blinded to the group allocation was responsible for the intraoperative measurements. If systolic arterial blood pressure (SAP) or heart rate (HR) increased by more than 20% compared to the previous time point, FE´ISO was adjusted in 0.1–0.2% increments accordingly. Similarly, if SAP or HR decreased by 20% from previously recorded values, FE´ISO was decreased in 0.1–0.2% increments. Data were recorded at specific time points throughout anesthesia as follows: T0 =before surgical stimulation at 10 min after epidural injection (L-S and S-C groups) or intubation (Control group), anesthetized with 1.1% of FE´ISO, T1=after the skin incision, T2 and T3=after clamping of the first and second ovarian pedicles, respectively, T4=after clamping of the uterine cervix, and T5=after the last skin suture was placed. Additional analgesia was provided with fentanyl (2 µg/kg, IV; Fentanest, Cristália Produtos Químicos Farmacêuticos Ltd.), if an FE´ISO above 1.8% was required. At the end of the surgery, isoflurane administration was immediately discontinued.
The same experienced surgeon (GMN) unaware of the treatment allocation performed all surgeries, using a standard technique through a ventral midline incision laparotomy.
The duration of anesthesia (period elapsed from the administration of propofol to discontinuation of isoflurane) and surgery (period elapsed from the first incision until placement of the last suture), and the time to extubation (defined as the time between isoflurane discontinuation and the presence of palpebral reflex) were recorded. In our clinical practice, extubation is often performed when the palpebral reflex is noted, as reported previously in feline studies [27, 38].
Group allocation
The cats were randomly assigned in one of the three treatments (n=12 per group), which consisted of S3-Co1 (S-C group) or L7-S1 (L-S group) epidural administration of levobupivacaine (1 mg/kg; Novabupi 5 mg/mL, Cristália Produtos Químicos Farmacêuticos Ltd.) or 0.9% saline solution (Samtec Biotecnologia Ltd., Ribeirão Preto, Brazil) in the L7-S1 (n=6) or S3-Co1 (n=6) epidural space (Control group), using an online software program (Research Randomizer, Computer software, http://www.randomizer.org/, Lancaster, PA, USA). In all cats, the fur was clipped around the L7-S1 and S3-Co1 areas, and the skin was aseptically prepared for epidural injection. In both epidural-treated groups 0.5% levobupivacaine was diluted in 0.9% saline solution to obtain a final volume of 0.3 mL/kg. The same volume of 0.9% saline solution was administered to the cats at the L7-S1 or S3-Co1 epidural space in the Control group. The epidural injections were performed immediately after the intubation by the same anesthesiologist (GRZ), with the cats positioned in sternal recumbency and the hind limbs extended forward. The animals were maintained in light anesthesia with 1.1% end-tidal isoflurane concentration (FE’ISO) to preserve the anal sphincter and pelvic limb reflex. The L7-S1 space was identified by the palpation of the depression located immediately caudal to the spinous process of L7 and immediately cranial to the dorsal spinous process of S1 in the LS group. In the S-C group, the S3-Co1 space was identified by the palpation of the spinous process of S3 and Co1 while moving the base of the tail dorsoventrally to help locate the sacrococcygeal articulation. A 22-gauge spinal needle (Becton Dickinson Indústrias Cirúrgicas Ltda, Juiz de Fora, Brazil) was introduced through the skin at midline and advanced until the ligamentum flavum. The correct location of the epidural space was confirmed by the absence of resistance to injection followed by the relaxation of the anal sphincter and absence of reflex responses in the perineal area and pelvic limbs. The epidural injections were administered over a period of 30 sec. The cats were maintained in sternal recumbence for 5 min after epidural injection to facilitate the confirmation of tail and external anal sphincter relaxation. Immediately afterwards, the cats were positioned in dorsal recumbency, followed by the instrumentation for the measurement of cardiopulmonary parameters. The surgical procedures were initiated 15 min after epidural injection. In cases of epidural technique failure, recognized by the absence of anal relaxation and presence of reflex responses, the cats were excluded from the study.
Postoperative analgesia and sedation monitoring
Pain and sedation scores were assessed by a single observer (JOLZ) blinded to group allocation 24 hr prior to surgery (Baseline), 0.5, 1, 2, 4, 6, and 8 hr after extubation, using the Glasgow feline composite measure pain scale (CMPS-Feline; 0, no pain; 20, maximum pain) [31] and the UNESP-Botucatu multidimensional composite pain scale (UFEPS; 0, no pain; 24, maximum pain) [2]. The CMPS-Feline included behavioral categories (scale range=0–16 points) and facial expression changes (scale range=0–4 points), while the UFEPS was based on two domains (pain expression, scale range=0–12 points; psychomotor change, scale range=0–12 points). Sedation scores were measured according to a descriptive numerical scale (ranged from 0–16 points), based on five criteria: spontaneous posture, response to noise (handclap) and noxious stimulus (pressure to a hind paw digit), resistance to being placed in lateral recumbency, and mandibular tonus [30]. Morphine (0.2 mg/kg, IM) was given as rescue analgesia if the UFEPS scores were ≥6 or CMPS-Feline ≥5. At the end of the study (8 hr post-extubation), meloxicam (0.1 mg/kg, IM; Maxicam 0.2%, Ourofino Saúde Animal, Cravinhos, Brazil) was administered to all the cats. Before the beginning of the study, the observer responsible for pain assessments underwent video-based training through a website (www.animalpain.com.br).
The duration of analgesia, defined as the interval between epidural injection and the time to first analgesic supplementation, the total number of morphine doses administered during the study-period, the number of rescued cats, and the duration of motor blockade, defined as the interval between epidural injection and the time to recover the normal ability to stand or walk were recorded for each cat.
Adverse events
The cats were monitored for adverse effects, including seizures, nausea, vomiting, and cardiovascular depression. Bradycardia and hypotension were defined as an HR <100 beats/min and SAP <90 mmHg, respectively, persisting for more than 5 min.
Statistical analysis
A sample size of at least 10 cats per group was estimated to achieve an 80% statistical power to detect a difference of 15% in the overall FE´ISO between the Control (mean of 1.5%) and levobupivacaine-treated groups (mean of 1.28%), and a standard deviation (SD) of 0.18, at an overall alpha level of 0.05. The SD was estimated from a pilot study performed on 12 cats.
A Shapiro-Wilk test was performed to assess the normality of the variables. Body weight, age, dose of propofol, and procedural times were compared among groups using ANOVA and a Tukey post-test. For overall FE´ISO, the mean values recorded from T0 until T5 for each treatment were considered, while percentage variation among groups was assessed by one-way ANOVA with Tukey post-test. For intraoperative cardiorespiratory variables, differences over time within each group, and differences between groups at specific time points were compared using ANOVA followed by the Tukey post-hoc test. A Kruskal-Wallis test was used to compare pain and sedation scores and the number of morphine doses administered postoperatively among groups. A Friedman test was used to compare differences in pain and sedation scores over time within each group. The prevalence of adverse events and rescue analgesia was compared among the three groups using the χ2 test. All analyses were performed using GraphPad Prism7.0 (GraphPad Software Inc., Boston, MA, USA). Differences were considered significant when P<0.05.
RESULTS
There were no significant differences among groups in age, body weight, dose of propofol, duration of anesthesia and surgery, time to extubation and procedural times (Table 1).
Table 1. Demographic and perioperative data recorded in cats undergoing ovariohysterectomy treated with 0.33% levobupivacaine (0.3 mL/kg) administered into the sacrococcygeal epidural space (S-C group, n=12) or lumbosacral epidural space (L-S group, n=12), or 0.9% saline solution into one of the epidural approaches (Control group, n=12).
| Variable | Group |
P value | ||
|---|---|---|---|---|
| S-C | L-S | Control | ||
| Body weight (kg) | 2.6 ± 0.4 | 2.7 ± 0.5 | 2.8 ± 0.3 | 0.65 |
| Age (months) | 15.7 ± 18.0 | 14.7 ± 5.0 | 17.0 ± 13.0 | 0.91 |
| Propofol dose (mg/kg) | 6.6 ± 2.0 | 6.5 ± 1.9 | 7.2 ± 1.8 | 0.70 |
| Surgery time (min) | 20.6 ± 9.0 | 20.4 ± 8.2 | 15.3 ± 4.2 | 0.16 |
| Anesthesia time (min) | 39.7 ± 13.0 | 38.8 ± 9.0 | 43.0 ± 14.0 | 0.21 |
| Extubation time (min) | 6.1 ± 6.0 | 4.9 ± 5.0 | 8.3 ± 5.0 | 0.29 |
| Time from epidural to the skin incision | 15.0 ± 1.0 | 15.0 ± 0.9 | 16.0 ± 1.2 | 0.47 |
| Time from epidural to the clamping of the first ovarian pedicle | 18.0 ± 4.8 | 19.0 ± 2.5 | 19.0 ± 1.7 | 0.46 |
| Time from epidural to the clamping of the second ovarian pedicle | 23.0 ± 2.5 | 24.0 ± 4.5 | 23.0 ± 2.1 | 0.67 |
| Time from epidural to the clamping of the uterine cervix | 26.0 ± 3.0 | 27.0 ± 5.4 | 25.0 ± 2.6 | 0.42 |
| Time from epidural to the skin suture | 33.0 ± 6.5 | 36.0 ± 8.3 | 31.0 ± 3.9 | 0.13 |
| Time from epidural to standing | 99.0 ± 20.0# | 101.0 ± 39.0# | 53.0 ± 10.0 | 0.015 |
Data are presented as mean ± SD. #Significantly different from Control group (Tukey test, P<0.05).
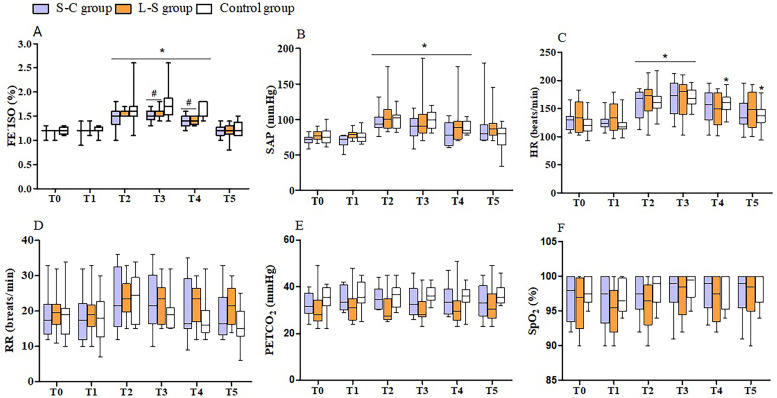
Mean FE´ISO ranged from 1.1 to 1.8%, 1.0 to 1.8% and 0.9 to 1.8% during surgery in the Control, L-S and S-C groups, respectively. Compared with the Control group, the isoflurane requirements were decreased by means of 6.25% in the L-S group and 8.3% in the S-C group (Table 1). The FE´ISO was significantly lower at T3 and T4 in the L-S (P=0.008 and P=0.006) and S-C groups (P=0.009 and P<0.001). Isoflurane requirements increased during surgery from T2 to T4 in all treatment groups (P<0.0001) (Fig. 1a).
Fig. 1.
Mean (min-max) values of end-tidal isoflurane concentration (FE`ISO) (a), systolic arterial blood pressure (SAP) (b), heart rate (HR) (c), respiratory rate (RR) (d), end-tidal carbon dioxide concentration (PETCO2) (e) and percutaneous oxygen saturation (SpO2) (f) recorded during anesthesia in cats undergoing ovariohysterectomy treated with 0.33% levobupivacaine (0.3 mL/kg) administered into the sacrococcygeal epidural space (S-C group, n=12) or lumbosacral epidural space (L-S group, n=12), or 0.9% saline solution into one of the epidural approaches (Control group, n=12). T0 =before surgical stimulation, 10 min after epidural injection, with the cats maintained with 1.1% of FE´ISO, T2 and T3=after the clamping of first and second ovarian pedicles, respectively, T4=after the clamping of the uterine cervix, and T5=after the last skin suture was placed. *Significantly different from baseline values (Tukey test, P<0.0001); #Significantly different from Control group (Tukey test, P=0.0012–0.0055)
The cardiorespiratory parameters did not differ among groups at any time point. Intraoperative hypotension (SAP <90 mmHg) was recorded in 25% (3/12) of the cats in the L-S and S-C groups, and 33.3% (4/12) of the cats in the Control group. Compared to the corresponding baseline values, SAP increased from T2 to T4 in all treatment groups (P<0.0001), while HR increased from T2 to T5 in the Control, from T2 to T3 in the L-S, and from T2 to T3 in the S-C group (P<0.0001) (Fig. 1b and 1c). No significant differences were observed among groups or over time with regards to the RR and PETCO2 (Fig. 1d and 1e).
More cats required intraoperative analgesic supplementation in the Control group compared to the other groups (P=0.048). During the clamping of the ovarian pedicles, a single dose of fentanyl was given in 33.3% (4/12) and 8.3% (1/12) of the cats in the Control and L-S groups, respectively. Fentanyl was not required in the S-C group (Table 2).
Table 2. Number of perioperative analgesic interventions provided in cats undergoing ovariohysterectomy treated with 0.33% levobupivacaine (0.3 mL/kg) administered into the sacrococcygeal epidural space (S-C group, n=12) or lumbosacral epidural space (L-S group, n=12), or 0.9% saline solution into one of the epidural approaches (Control group, n=12).
| Group | Intraoperative time points |
Postoperative time points |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | T5 | 0.5 hr | 1 hr | 2 hr | 4 hr | 6 hr | 8 hr | |
| S-C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 0 |
| L-S | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 0 | 0 |
| Control | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 2 | 3 | 1 | 0 | 0 |
Intraoperative time points: T0 =before surgical stimulation, 10 min after epidural injection, with the cats maintained with 1.1% of end-tidal isoflurane concentration, T2 and T3 =after the clamping of first and second ovarian pedicles, respectively, T4 =after the clamping of the uterine cervix, and T5=after the last skin suture was placed. Postoperative time points: 0.5, 1, 2, 4, 6, and 8 hr post-extubation.
The times to standing were comparable between the L-S and S-C groups, but they were prolonged in comparison with the Control group (P=0.0002) (Table 1).
The number of cats that required postoperative rescue analgesia did not differ statistically among groups (P=0.89) during the study-period. A total of 17 cats required rescue analgesia (S-C group, n=6, 50%; L-S group, n=5, 42%; Control group, n=6, 50%). All cats received only a single dose of morphine at different time points, with exception of one cat in the S-C group that received two doses (Table 2).
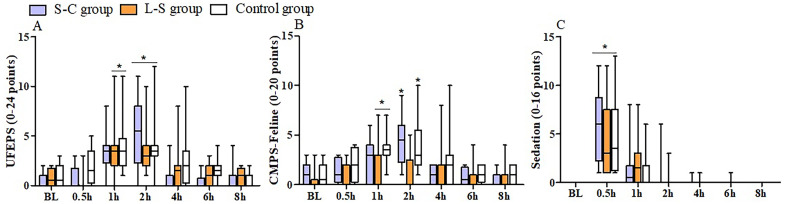
Postoperative pain scores did not differ among groups. Compared with baseline values, UFEPS scores were increased from 1 to 2 hr in the L-S and Control groups, and at 2 hr in the S-C group (P<0.0001) (Fig. 2a), while CMPS-Feline scores were increased from 1 to 2 hr in the Control group, and at 1 and 2 hr in the L-S and S-C groups, respectively (P<0.0001) (Fig. 2b). There were no significant intergroup differences in the sedation scores at any time point. Compared to the baseline, higher scores were recorded at 0.5 hr post-extubation in all treatment groups (P<0.0001) (Fig. 2c).
Fig. 2.
Median (min-max) values of pain (a, b) and sedation (c) scores measured 24 hr prior to anesthesia (baseline=BL) and at 0.5, 1, 2, 4, 6, and 8 hr after ovariohysterectomy in cats treated with 0.33% levobupivacaine (0.3 mL/kg) administered into the sacrococcygeal epidural space (S-C group, n=12) or lumbosacral epidural space (L-S group, n=12), or 0.9% saline solution into one of the epidural approaches (Control group, n=12). UFEPS=UNESP-Botucatu multidimensional composite pain scale, CMPS-Feline=Glasgow feline composite measure pain scale. *Significantly different from baseline values (Friedman test, P<0.0001).
DISCUSSION
The study hypothesis was partially supported, with the results showing that the administration of epidural levobupivacaine decreased anesthetic requirements and intraoperative analgesic supplementation, independent of the approach used. However, no evidence of significant postoperative analgesic benefits was found in comparison with the Control treatment.
Our findings demonstrated that both of the S-C and L-S epidural levobupivacaine provided sensory blockade of the abdominal cavity, resulting in comparable anesthetic and analgesic sparing effects in cats undergoing OHE. Although it was not possible in the current study to determine exactly the distribution of the anesthetic solutions following epidural injection, the similarity between the results found using L-S and S-C approaches suggests that the rostral spread of the levobupivacaine was similar in both groups, which is consistent with previously reported data in canine cadavers [42].
Despite the anesthetic-sparing effect found in the current study, FE´ISO decreased less than 10% in the L-S and S-C groups compared to the Control group. The anesthetic-sparing effects were considered modest when compared with different protocols using intravenous infusion of dexmedetomidine or remifentanil in cats, where isoflurane requirements were reduced by 21–48% [35, 38]. Similarly, a decrease more than 40% in the isoflurane requirements was reported during OHE in cats receiving epidural dexmedetomidine combined with lidocaine, in comparison to those receiving epidural saline solution [36]. Although these findings cannot be directly compared, since the analgesic protocols are not the same, the modest decrease in the isoflurane concentrations recorded in our study suggests that epidural levobupivacaine did not produce an effective inhibition of the sensorial afferent fibers to the surgical noxious stimulus, which could also justify the increase of HR and SAP during the clamping of ovarian pedicles in all treatment groups. As ovaries are richly innervated, the traction of the suspensory ligament and the ligation of the mesovarium are considered the maximum painful stimuli during OHE, representing an important indicator of nociception [19, 20]. Despite the onset of analgesia after epidural levobupivacaine is around 5–10 min [16], it is possible that the interval of around 20 min between the injection of anesthetic solution and the initiation of the ovarian pedicle excision may have been insufficient for complete migration of the anesthetic solution into the epidural space, resulting in partial blockade of the innervation of the ovarian pedicles, which is derived from abdominal prevertebral and paravertebral ganglions located at the levels from the 10th thoracic to 4th lumbar vertebra (T10-L4) [6].
Additionally, the volume injected in the epidural space also plays an important role in the rostral spread of local anesthetic, conferring different levels of sensorial blockade. In the current study, the volume of 0.3 mL/kg was based on previous studies that investigated the effects of lumbosacral and sacrococcygeal epidural techniques in cats [29, 36, 39]. Moreover, data from a previous feline study demonstrated that the injection of 0.3 mL/kg of new methylene blue in the L7-S1 epidural space resulted in a cranial spread that extended until the 7th and 10th thoracic vertebrae (T7 to T10), while 0.2 mL/kg extended until the 1st and 2nd lumbar vertebrae [21]. In view of this, we suppose that 0.3 mL/kg would provide an effective sensory blockade during the surgical manipulation of ovarian pedicles, and reduce the sympathetic response induced by the surgical stimulus. The use of larger volumes (>0.31 mL/kg) was not considered in the current study, due to the increased risk of cardiopulmonary depression [37, 40].
Dose and concentration may also interfere in the action of local anesthetics [10, 17]. Although similar rostral spread has been reported with different concentrations of anesthetic solution, the intensity of sensory blockade may be reduced with low concentrations [40]. Data from a comparative study in dogs showed that 0.25% levobupivacaine injected into the lumbosacral epidural space provided a weaker analgesic effect than higher concentrations (0.50% and 0.75%) [16]. In the present study, to obtain a volume of 0.3 mL/kg, the final concentration of levobupivacaine after the dilution was 0.33%, which may have affected the intensity of sensory blockade. It is likely that the use of a more concentrated anesthetic solution could improve the antinociceptive effect, attenuating the cardiovascular responses as well as providing a greater decrease in the isoflurane requirements. However, in the current study, due to concerns regarding toxic effects, the decision was made not to exceed the epidural bupivacaine dose recommended (1 mg/kg) in cats [17], because no studies have reported the effects of epidural levobupivacaine in this species.
Some aspects of the study design may also have interfered in our results. The premedication with acepromazine and meperidine may have contributed to decrease nociceptive responses and anesthetic requirements, justifying the low isoflurane concentrations (lower than the minimum alveolar concentration in cats) to maintain surgical anesthesia in all treatment groups. In addition, intraoperative analgesic supplementation may have biased differences in isoflurane requirements and cardiovascular parameters, because more cats in the Control group required intraoperative fentanyl. Recently, a reduction of around 50% in the minimum alveolar concentration of isoflurane was reported in cats when fentanyl was administered with acepromazine [3]. In our study, isoflurane concentrations were adjusted in steps of 0.2% to maintain surgical plane of anesthesia, up to a maximum of 1.8%, when fentanyl was administered as analgesic supplementation, due to its rapid onset and short action [23], in order to avoid residual analgesic effects that could interfere in the postoperative pain assessments. Other studies have also reported the use of fentanyl to improve analgesia in cats, if during surgery a FE’ISO over 1.8 to 2.0% was required [24, 26].
With regards to postoperative pain assessments, the administration of levobupivacaine into lumbosacral or sacrococcygeal space resulted in similar analgesic effects and did not improve or prolong analgesia compared to the Control group. The time required for the first administration of rescue analgesia was 1 hr post-extubation in all treatment groups, which corresponds to a period of around 1.5 hr after epidural injection, when the effect of levobupivacaine had probably already declined. In dogs treated with lumbosacral epidural of levobupivacaine at 0.25%, 0.50%, and 0.75%, the duration of analgesia was 28 ± 33, 79 ± 55, and 292 ± 133 min, respectively [16]. It is important to emphasize that in the current study, due to ethical concerns, all cats received pre-medication with meperidine, which despite its short duration of action (60–120 min) [33], may have masked the real analgesic duration provided by epidural levobupivacaine. Data from a previous study in cats pre-medicated with meperidine showed that a mean time for rescue analgesia was 298 ± 150 min, with the first rescue analgesic supplementation required at 140 min after OHE [11]. Similarly, in our study, the interval between premedication and the first rescue analgesic supplementation was around 150 min (1 hr post-extubation) in all treatment groups, when the analgesic effect of meperidine had probably already declined.
The time to standing was similar in L-S and S-C groups, and longer in comparison with the Control group, which was already expected, due to the motor blockade induced by epidural levobupivacaine. In the current study, the mean times from epidural to standing were 99 and 101 min in the S-C and L-S groups, which is close to the 83 min found in dogs treated with lumbosacral epidural 0.25% levobupivacaine [16]. A longer duration of motor blockade (180–360 min) has been reported after lumbosacral epidural administration of 0.5% and 0.75% levobupivacaine [14, 16]. Although the residual anesthetic sedative effects can also interfere in the animal’s ability to stand, in the current study, apparently, sedation did not interfere in the evaluation of motor blockade, because the scores did not differ among groups and returned to baseline values at 1 hr post-extubation.
Minimal perioperative complications were found in the current study, suggesting that epidural levobupivacaine at the dose administered is safe for cats. During anesthesia, transitory hypotension was the most frequent adverse event observed in all treatment groups, which may be attributed to the cardiovascular depressant effects provided by general anesthesia [18], and also to the sympathetic blockade induced by local anesthetic [10] in the levobupivacaine-treated cats. Previous studies also reported the occurrence of hypotension in isoflurane-anesthetized cats and dogs treated via an epidural with different local anesthetics [9, 10, 34, 39]. The hypotension was transient in all cats, and was successfully reversed by decreasing the concentration of the isoflurane.
This study has some limitations. In order to facilitate the epidural puncture, levobupivacaine was injected only after intubation, which may have impaired the recognition of the success of the blockade, as it was determined only by reflex responses. The use of a more accurate method to monitor epidural techniques, such as a nerve stimulator test and ultrasound examination, as recently reported [7, 29], could help to confirm the epidural needle placement, reducing the risk of technique failure. In addition, the optimal dose of levobupivacaine for epidural anesthesia is not established in cats. Thus, it is possible that the low dose/concentration of levobupivacaine used in the current study may have interfered in the intensity and duration of sensory blockade, decreasing both intra and postoperative analgesia. Given the limited information regarding epidural levobupivacaine in cats, the decision made was administering a low dose of this local anesthetic (1 mg/kg), due to concerns regarding possible adverse effects. In this way, 0.5% levobupivacaine (1 mg/kg) was diluted in saline solution to obtain a final volume of 0.3 mL/kg, decreasing levobupivacaine concentration to 0.33%, which may have impaired the sensory blockade. Moreover, it is probable that a longer interval between epidural administration and the initiation of surgery (>15 min) would provide a greater cranial spread of the anesthetic solution in the epidural space, and consequently a more effective sensitivity blockade of the innervation of ovarian pedicles. Furthermore, despite the training of the observer to score pain in cats using videos, it is possible that the lack of previous experience with pain research, may also have interfered in our results.
In conclusion, epidural 0.33% levobupivacaine (0.3 mL/kg) is safe for cats and may be considered as part of a multimodal anesthetic protocol for ovariohysterectomy. At the dose/concentration administered, sacrococcygeal or lumbosacral epidural levobupivacaine provided similar antinociceptive effects, decreasing isoflurane requirements and intraoperative analgesic supplementation. However, neither epidural approach provided significant postoperative analgesic benefits compared to the control treatment. Further studies are required to determine the safety and analgesic efficacy of different doses and concentrations of levobupivacaine for epidural anesthesia in cats.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Acknowledgments
The authors thank Dr Rejane Batista Brinholi for technical assistance.
REFERENCES
- 1.Almeida TF, Fantoni DT, Mastrocinque S, Tatarunas AC, Imagawa VH. 2007. Epidural anesthesia with bupivacaine, bupivacaine and fentanyl, or bupivacaine and sufentanil during intravenous administration of propofol for ovariohysterectomy in dogs. J Am Vet Med Assoc 230: 45–51. doi: 10.2460/javma.230.1.45 [DOI] [PubMed] [Google Scholar]
- 2.Brondani JT, Mama KR, Luna SP, Wright BD, Niyom S, Ambrosio J, Vogel PR, Padovani CR. 2013. Validation of the English version of the UNESP-Botucatu multidimensional composite pain scale for assessing postoperative pain in cats. BMC Vet Res 9: 143. doi: 10.1186/1746-6148-9-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosnan RJ, Pypendop BH. 2021. Evaluation of whether acepromazine maleate causes fentanyl to decrease the minimum alveolar concentration of isoflurane in cats. Am J Vet Res 82: 352–357. doi: 10.2460/ajvr.82.5.352 [DOI] [PubMed] [Google Scholar]
- 4.Casati A, Putzu M. 2005. Bupivacaine, levobupivacaine and ropivacaine: are they clinically different? Best Pract Res Clin Anaesthesiol 19: 247–268. doi: 10.1016/j.bpa.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 5.Cerasoli I, Tutunaru A, Cenani A, Ramirez J, Detilleux J, Balligand M, Sandersen C. 2017. Comparison of clinical effects of epidural levobupivacaine morphine versus bupivacaine morphine in dogs undergoing elective pelvic limb surgery. Vet Anaesth Analg 44: 337–345. doi: 10.1016/j.vaa.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 6.Chien CH, Li SH, Shen CL. 1991. The ovarian innervation in the dog: a preliminary study for the base for electro-acupuncture. J Auton Nerv Syst 35: 185–192. doi: 10.1016/0165-1838(91)90096-L [DOI] [PubMed] [Google Scholar]
- 7.Credie L, Luna S. 2018. The use of ultrasound to evaluate sacrococcygeal epidural injections in cats. Can Vet J 59: 143–146. [PMC free article] [PubMed] [Google Scholar]
- 8.Davis H, Jensen T, Johnson A, Knowles P, Meyer R, Rucinsky R, Shafford H. American Association of Feline PracticionersAmerican Animal Hospital Association. 2013. 2013 AAHA/AAFP fluid therapy guidelines for dogs and cats. J Am Anim Hosp Assoc 49: 149–159. doi: 10.5326/JAAHA-MS-5868 [DOI] [PubMed] [Google Scholar]
- 9.DeRossi R, Hermeto LC, Jardim PHA, de Andrade Bicudo N, de Assis KT. 2016. Postoperative pain control in cats: clinical trials with pre-emptive lidocaine epidural co-administered with morphine or methadone. J Feline Med Surg 18: 882–888. doi: 10.1177/1098612X15602738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dias RSG, Soares JHN, Castro DDSE, Gress MAKA, Machado ML, Otero PE, Ascoli FO. 2018. Cardiovascular and respiratory effects of lumbosacral epidural bupivacaine in isoflurane-anesthetized dogs: The effects of two volumes of 0.25% solution. PLoS One 13: e0195867. doi: 10.1371/journal.pone.0195867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evangelista MC, Silva RA, Cardozo LB, Kahvegian MA, Rossetto TC, Matera JM, Fantoni DT. 2014. Comparison of preoperative tramadol and pethidine on postoperative pain in cats undergoing ovariohysterectomy. BMC Vet Res 10: 252. doi: 10.1186/s12917-014-0252-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Parra R, Zilberstein L, Fontaine C, Adami C. 2017. Comparison of intratesticular lidocaine, sacrococcygeal epidural lidocaine and intravenous methadone in cats undergoing castration: a prospective, randomized, investigator-blind clinical trial. Vet Anaesth Analg 44: 356–363. doi: 10.1016/j.vaa.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 13.Floriano D, Sahagian MJ, Chiavaccini L. 2019. Impact of epidural bupivacaine on perioperative opioid requirements, recovery characteristics, and duration of hospitalization in dogs undergoing cystotomy: A retrospective study of 56 cases. Vet Surg 48: 1330–1337. doi: 10.1111/vsu.13290 [DOI] [PubMed] [Google Scholar]
- 14.Franquelo C, Toledo A, Manubens J, Valladares JE, Cristòfol C, Arboix M. 1999. Pharmacokinetics and pharmacologic effects of the S(-) isomer of bupivacaine after intravenous and epidural administration in dogs. Am J Vet Res 60: 832–835. [PubMed] [Google Scholar]
- 15.Frazílio FO, DeRossi R, Jardim PH, Marques BC, Martins AR, Hermeto LC. 2014. Effects of epidural nalbuphine on intraoperative isoflurane and postoperative analgesic requirements in dogs. Acta Cir Bras 29: 38–46. doi: 10.1590/S0102-86502014000100006 [DOI] [PubMed] [Google Scholar]
- 16.Gomez de Segura IA, Menafro A, García-Fernández P, Murillo S, Parodi EM. 2009. Analgesic and motor-blocking action of epidurally administered levobupivacaine or bupivacaine in the conscious dog. Vet Anaesth Analg 36: 485–494. doi: 10.1111/j.1467-2995.2009.00469.x [DOI] [PubMed] [Google Scholar]
- 17.Grubb T, Lobprise H. 2020. Local and regional anaesthesia in dogs and cats: overview of concepts and drugs (Part 1). Vet Med Sci 6: 209–217. doi: 10.1002/vms3.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hikasa Y, Ohe N, Takase K, Ogasawara S. 1997. Cardiopulmonary effects of sevoflurane in cats: comparison with isoflurane, halothane, and enflurane. Res Vet Sci 63: 205–210. doi: 10.1016/S0034-5288(97)90021-7 [DOI] [PubMed] [Google Scholar]
- 19.Höglund OV, Lövebrant J, Olsson U, Höglund K. 2016. Blood pressure and heart rate during ovariohysterectomy in pyometra and control dogs: a preliminary investigation. Acta Vet Scand 58: 80. doi: 10.1186/s13028-016-0263-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kropf J, Hughes JML. 2018. Effects of midazolam on cardiovascular responses and isoflurane requirement during elective ovariohysterectomy in dogs. Ir Vet J 71: 26. doi: 10.1186/s13620-018-0136-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee I, Yamagishi N, Oboshi K, Yamada H. 2004. Distribution of new methylene blue injected into the lumbosacral epidural space in cats. Vet Anaesth Analg 31: 190–194. doi: 10.1111/j.1467-2987.2004.00149.x [DOI] [PubMed] [Google Scholar]
- 22.Leone S, Di Cianni S, Casati A, Fanelli G. 2008. Pharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaine. Acta Biomed 79: 92–105. [PubMed] [Google Scholar]
- 23.Liehmann L, Mosing M, Auer U. 2006. A comparison of cardiorespiratory variables during isoflurane-fentanyl and propofol-fentanyl anaesthesia for surgery in injured cats. Vet Anaesth Analg 33: 158–168. doi: 10.1111/j.1467-2995.2005.00251.x [DOI] [PubMed] [Google Scholar]
- 24.Lima CMS, Segatto CZ, Zanelli GR, Nicácio GM, Cassu RN. 2022. Effects of lidocaine injection at acupuncture points on perioperative analgesia in cats undergoing ovariohysterectomy. J Acupunct Meridian Stud 15: 255–263. doi: 10.51507/j.jams.2022.15.4.255 [DOI] [PubMed] [Google Scholar]
- 25.Maierl J, Reindl S, Knospe C. 1997. [Observations on epidural anesthesia in cats from the anatomical viewpoint]. Tierarztl Prax 25: 267–270. [PubMed] [Google Scholar]
- 26.Mosing M, Reich H, Moens Y. 2010. Clinical evaluation of the anaesthetic sparing effect of brachial plexus block in cats. Vet Anaesth Analg 37: 154–161. doi: 10.1111/j.1467-2995.2009.00509.x [DOI] [PubMed] [Google Scholar]
- 27.Nutt LK, Webb JA, Prosser KJ, Defarges A. 2014. Management of dogs and cats with endotracheal tube tracheal foreign bodies. Can Vet J 55: 565–568. [PMC free article] [PubMed] [Google Scholar]
- 28.O’Hearn AK, Wright BD. 2011. Coccygeal epidural with local anesthetic for catheterization and pain management in the treatment of feline urethral obstruction. J Vet Emerg Crit Care (San Antonio) 21: 50–52. doi: 10.1111/j.1476-4431.2010.00609.x [DOI] [PubMed] [Google Scholar]
- 29.Otero PE, Verdier N, Zaccagnini AS, Fuensalida SE, Tarragona L, Portela DA. 2015. The use of a nerve stimulation test to confirm sacrococcygeal epidural needle placement in cats. Vet Anaesth Analg 42: 115–118. doi: 10.1111/vaa.12173 [DOI] [PubMed] [Google Scholar]
- 30.Ravasio G, Gallo M, Beccaglia M, Comazzi S, Gelain ME, Fonda D, Bronzo V, Zonca A. 2012. Evaluation of a ketamine-propofol drug combination with or without dexmedetomidine for intravenous anesthesia in cats undergoing ovariectomy. J Am Vet Med Assoc 241: 1307–1313. doi: 10.2460/javma.241.10.1307 [DOI] [PubMed] [Google Scholar]
- 31.Reid J, Scott EM, Calvo G, Nolan AM. 2017. Definitive Glasgow acute pain scale for cats: validation and intervention level. Vet Rec 180: 449. doi: 10.1136/vr.104208 [DOI] [PubMed] [Google Scholar]
- 32.Robertson SA, Gogolski SM, Pascoe P, Shafford HL, Sager J, Griffenhagen GM. 2018. AAFP feline anesthesia guidelines. J Feline Med Surg 20: 602–634. doi: 10.1177/1098612X18781391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson SA, Taylor PM. 2004. Pain management in cats--past, present and future. Part 2. Treatment of pain--clinical pharmacology. J Feline Med Surg 6: 321–333. doi: 10.1016/j.jfms.2003.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sibanda S, Hughes JM, Pawson PE, Kelly G, Bellenger CR. 2006. The effects of preoperative extradural bupivacaine and morphine on the stress response in dogs undergoing femoro-tibial joint surgery. Vet Anaesth Analg 33: 246–257. doi: 10.1111/j.1467-2995.2005.00261.x [DOI] [PubMed] [Google Scholar]
- 35.Simon BT, Scallan EM, Coursey CD, Kiehl WM, Moore EJ. 2018. The clinical effects of a low dose dexmedetomidine constant rate infusion in isoflurane anesthetized cats. Vet J 234: 55–60. doi: 10.1016/j.tvjl.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 36.Souza SS, Intelisano TR, De Biaggi CP, Moura CA, Selmi AL, Dias RA, Cortopassi SR. 2010. Cardiopulmonary and isoflurane-sparing effects of epidural or intravenous infusion of dexmedetomidine in cats undergoing surgery with epidural lidocaine. Vet Anaesth Analg 37: 106–115. doi: 10.1111/j.1467-2995.2009.00512.x [DOI] [PubMed] [Google Scholar]
- 37.Steagall PVM, Simon BT, Teixeira Neto FJ, Luna SPL. 2017. An update on drugs used for lumbosacral epidural anesthesia and analgesia in dogs. Front Vet Sci 4: 68. doi: 10.3389/fvets.2017.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steagall PV, Aucoin M, Monteiro BP, Moreau M, Simon BT, Burns PM. 2015. Clinical effects of a constant rate infusion of remifentanil, alone or in combination with ketamine, in cats anesthetized with isoflurane. J Am Vet Med Assoc 246: 976–981. doi: 10.2460/javma.246.9.976 [DOI] [PubMed] [Google Scholar]
- 39.Torruella X, Potter J, Huuskonen V. 2023. Sacrococcygeal epidural administration of 0.5% bupivacaine in seven cats undergoing pelvic or hind limb orthopaedic procedures. Ir Vet J 76: 1. doi: 10.1186/s13620-023-00231-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valverde A. 2008. Epidural analgesia and anesthesia in dogs and cats. Vet Clin North Am Small Anim Pract 38: 1205–1230, v. doi: 10.1016/j.cvsm.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 41.Valverde A, Skelding A. 2019. Comparison of calculated lumbosacral epidural volumes of injectate using a dose regimen based on body weight versus length of the vertebral column in dogs. Vet Anaesth Analg 46: 135–140. doi: 10.1016/j.vaa.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 42.Vesovski S, Makara M, Martinez-Taboada F. 2019. Computer tomographic comparison of cranial spread of contrast in lumbosacral and sacrococcygeal epidural injections in dog cadavers. Vet Anaesth Analg 46: 510–515. doi: 10.1016/j.vaa.2019.02.007 [DOI] [PubMed] [Google Scholar]