Abstract
Hepatocellular carcinoma (HCC) is the leading fatal malignancy worldwide. The tumor microenvironment (TME) can affect the survival, proliferation, migration, and even dormancy of cancer cells. Hypoxia is an important component of the TME, and hypoxia-inducible factor-1α (HIF-1α) is the most important transcriptional regulator. Noncoding RNAs (ncRNAs), including microRNAs (miRNAs), long noncoding RNAs (lncRNAs), and circular RNAs (circRNAs), comprise a large part of the human transcriptome and play an important role in regulating the tumorigenesis of HCC. This review discusses the role of ncRNAs in hepatocarcinogenesis, epithelial-mesenchymal transition (EMT), and angiogenesis in a hypoxic microenvironment, as well as the interactions between ncRNAs and key components of the TME. It further discusses their use as biomarkers and the potential clinical value of drugs, as well as the challenges faced in the future.
Keywords: noncoding RNAs, HIF-1α, hepatocellular carcinoma, tumor microenvironment
Introduction
Liver cancer is a fatal malignant tumor. According to CA: A Cancer Journal for Clinicians, the incidence of liver cancer ranks fifth among men, seventh among women, and second among malignant tumors in China [1]. Therefore, finding a treatment for liver cancer has become increasingly important. Liver cancer can be divided into primary liver cancer and metastatic liver cancer, with the most common being hepatocellular carcinoma (HCC), accounting for 75%–85% of primary liver cancers.
Noncoding RNAs (ncRNAs) are a class of RNA molecules that do not encode proteins but have important regulatory functions. According to their length and source, ncRNAs include microRNAs (miRNAs), long chain noncoding RNAs (lncRNAs), circular RNAs (circRNAs), and tRNA-derived fragments (tRFs). Yong et al. [2] reviewed the use of endogenous expression of miRNAs to silence target genes to achieve therapeutic approaches for HCC in 2007. With advances in bioinformatics and next-generation sequencing technologies, many ncRNAs were found to play important roles in the crosstalk between HCC cells and tumor microenvironment (TME) [3]. TME is a small microenvironment around tumor cells, including fibroblasts, immune cells, blood vessels, inflammatory cells, various signaling molecules and extracellular matrix (ECM). The interaction between tumor and TME plays a key role in tumorigenesis and tumor progression [4]. The oxygen concentration in the tissue is blocked, resulting in a low oxygen concentration in the TME, which is called hypoxia [5]. Hypoxia is a typical feature of the TME and a sign of cancer. To adapt to the hypoxic environment, cancer cells acquire invasiveness, metastasis and resistance to chemotherapy and radiotherapy, which together constitute a lethal cancer phenotype [6].
Recent studies also suggested possible therapeutic strategies to target HCC metabolism by regulating the expression of specific ncRNAs [ 7, 8] . ncRNAs are considered biomarkers and therapeutic targets for the treatment of cancers, including HCC. This review focuses on the regulatory role of ncRNAs in the TME of HCC.
TME and Tumorigenesis
Hypoxia inducible factors (HIFs) and TME
HIF-1 transcription factor activation is one of the widely studied pathways in the TME. HIF-1 has two subunits, an oxygen-sensitive α-subunit (HIF-1α) and a constitutively expressed β-subunit (HIF-1β) [9]. HIF-1α is a major regulator of hypoxia signal transduction and is widely expressed [10]. The α subunits degrade rapidly under normal oxygen conditions but remain stable during hypoxia [11]. The β subunit is constitutively expressed, not regulated by intracellular oxygen concentration, and has no transcriptional activity alone. Only the heterodimer of HIF-1α and HIF-1β subunits is active [12]. Under normal oxygen conditions, HIF-1α undergoes hydroxylation of conservative proline residues 402 and 564 by the prolyl hydroxylase domain (PHD) [13]. Subsequently, von protein (pVHL) mediates HIF-1α ubiquitination, followed by degradation by the proteasome [14]. Under anoxic conditions, the hydroxylation and acetylation of HIF-1α are inhibited, thereby stabilizing HIF-1α and allowing it to form a dimer with HIF-1β. The dimer then combines with CREB-binding protein (CBP)/p300, forming a transcription initiation complex and activating target genes [15]. In recognition of their contributions to the study of hypoxia, William G. Kaelin, Peter J. Ratcliffe, and Gregg L. Semenza were awarded the 2019 Nobel Prize in Physiology or Medicine.
Saurabh et al. [9] reviewed the role of HIF in tumor progression, specifically as a fuel for cancer progression. Chen et al. [16] reviewed the major impact of intratumoral hypoxia on the development and progression of breast cancer (BC). Instead of exerting a limited regional impact, hypoxia creates a positive environment for BC. HIF-1 is broadly induced under BC hypoxia, activating the transcription of multiple oncogenes. Zhang et al. [17] showed that HIF mediates a cascade of molecular events that enable cancer cells to adapt and multiply. In summary, hypoxia is beneficial to cancer cell growth in the TME. In most tumors within the TME, the hypoxic environment leads to the production of HIF-1α [18]. In an anoxic environment, activated HIF-1α increases the activity of Snail and Twist, which reduces the expression of E-cadherin and promotes EMT [19]. Goyette and his colleagues [20] found that interference with AXL (a member of the TAM receptor tyrosine kinase family) reduced HIF-1α levels during hypoxia, thereby altering the hypoxia response. This led to a reduction in the production of key cytokines involved in hypoxia-induced EMT, invasion, and macrophage behavior, ultimately enhancing the antitumor microenvironment and immunotherapy response. Mut homologue 6 (MSH6) is an overexpressed oncogene in glioblastoma (GBM). The expression of β-lactamase forms an anoxic TME in GBM, thus promoting EMT, proliferation, migration, invasion and angiogenesis and ultimately promoting the development of GBM [21]. Collectively, the pivotal role of HIF-1α in driving tumorigenesis and promoting tumor progression under hypoxic conditions has been extensively elucidated. Consequently, HIF-1α has emerged as a promising candidate for targeted cancer therapy, holding immense potential in the realm of oncological interventions.
HIF in HCC
Tumor hypoxia is a unique and critical environmental factor for tumor cells to survive under insufficient oxygen supply by modifying tumor cell metabolism through hypoxia, but normal cells cannot survive in the hypoxic TME. However, hypoxia could induce genomic changes, enabling tumor cells to adapt to the adverse microenvironment characterized by hypoxia and malnutrition so that living cell subsets with the genetic mechanism required for malignant tumor progression could survive [ 22, 23] . HCC is a highly metabolized tumor that consumes more oxygen than surrounding normal tissues [12]. In recent years, the in-depth study of the hypoxic microenvironment has provided new ideas for the treatment and possible prevention strategies of liver cancer [ 24, 25] . Zheng and his colleagues [26] found that HIF-1α overexpression is associated with poor overall survival (OS) and disease-free survival (DFS). HIF-1α is mainly involved in promoting tumor proliferation [27], migration, invasion and angiogenesis [28], as well as EMT [29], glycolysis regulation [30] and lipid metabolism [31], involving various signaling pathways ( Table 1).
Table 1 Role and potential mechanism of HIF-1α in HCC
|
Function |
Related genes and pathways |
Ref. |
|
Migration |
Increase TM4SF1-AS1 and TM4SF1 |
|
|
Invasion |
HIF-1α/IL-8/NF-κB axis |
|
|
Proliferation |
Activate KDM4A-AS1/KPNA2/AKT pathway |
|
|
Autophagy |
YTHDF1/ATG2A/ATG14 axis |
|
|
Angiogenesis |
Upregulation of Bclaf1 expression |
|
|
EMT |
TGM2/VHL/HIF-1α axis |
|
|
Glycolysis |
TFBM2/SIRT3/HIF-1α signal pathway |
|
|
Lipid metabolism |
FABP5/HIF-1α axis |
|
|
Drug resistance |
PFKFB3/HIF-1α feedback loop |
HIF-1α, through the IL-8/nuclear factor kappa B (NF-κB) axis, promotes the migration and invasion of hepatoma cells. In addition, HIF-1α-activated TM4SF1-AS1 plays an important role in promoting the proliferation, migration and invasion of liver cancer cells by enhancing the expression of TM4SF1 [32]. HIF-1α-induced EMT is a key process related to metastasis. Ma et al. [29] found that activated hepatic stellate cells promoted the upregulation of transglutaminase 2 (TGM2) in HCC cells through inflammatory signals, leading to HIF-1α accumulation. This results in a pseudo-hypoxic state and promotes EMT in HCC cells. Reprogramming of lipid metabolism has become a sign of cancer. Recently, it was reported that HIF-1α is related to this process. Fatty acid binding protein 5 (FABP5) promotes HIF-1α synthesis and disrupts the FIH/HIF-1α interaction, enhancing HIF-1α’s ability to promote lipid accumulation and cell proliferation in HCC cells [31].
ncRNAs and TME
Additional studies have shown that ncRNAs participate in intercellular communication [39] and regulate the activation, proliferation and cytokine secretion of tumor immune cells [40], thus affecting tumor invasion, metastasis and immune escape. Many ncRNAs have been found to play an important role between HCC cells and the TME. HIF-1α is involved in the regulation of the hypoxia response and could be used as the central hub for regulating multiple cancer markers. Therefore, exploring the interaction between ncRNAs and HIF-1α has become a promising target for anticancer therapy.
It was reported that more than 1000 target genes are affected by HIF-1α regulation to mediate the hypoxia-induced phenotype [6]. ncRNAs regulated by hypoxia signals are called hypoxia-responsive ncRNAs (HRNs). According to their interaction with the HIF-1α complex, HRNs can be divided into those participating in HIF-1α-mediated direct regulation and those participating in HIF-1α-mediated indirect regulation [14]. miRNA is the most studied subgroup of ncRNAs. Hypoxia-responsive miRNAs (HRMs) show promising carcinogenic or tumor suppressive functions in the occurrence and development of cancer [41]. lncRNA expression could be altered by hypoxia, which in turn regulates HIF-1α activity through a variety of mechanisms, such as chromatin modification, RNA stability and protein stability, and regulation of the transcriptional activity of HIF-1α [42]. It has also been shown that lncRNAs can act as competing endogenous RNAs (ceRNAs) for miRNAs to regulate the expression of related mRNAs at the posttranscriptional level [43], including HIF-1α mRNA. The mechanisms of ncRNA regulation of HIF-1α expression are summarized in Table 2.
Table 2 Major research advances in ncRNA and HIF-1α in cancer
|
ncRNAs |
Cancer |
Regulatory mechanisms |
Functions |
Ref. |
|
miRNA |
||||
|
miR-671-5p |
Oophoroma |
Inhibiting HIF-1α expression via activating HDAC5 |
Proliferation and apoptosis |
|
|
miR-29a |
HCC |
Supressing HIF-1α expression |
Proliferation |
|
|
miR-142-3p |
HCC |
Inhibiting PI3K/AKT/HIF-1α signaling |
Invasion and apoptosis |
|
|
miR-138-5p |
HCC |
Targeting HIF-1α and regulating its expression |
Vascular mimicry |
|
|
miR-322/424 |
Cirrhosis |
Inducing HIF-1α protein expression |
Angiogenesis and migration |
|
|
miR-200c |
HCC |
Downregulating the transcriptional activity of HIF-1α |
||
|
lncRNA |
||||
|
LINC00649 |
Breast cancer |
Maintaining the stability of HIF-1α |
Metastasis |
|
|
NORAD |
Colorectal cancer |
Acting as miR-495-3p sponge to adjust HIF-1α |
Angiogenesis and chemotherapy resistance |
|
|
FAM83A-AS1 |
Lung adenocarcinoma |
Suppressed combination of HIF-1α and VHL leads to HIF-1α accumulation |
Proliferation and migration |
|
|
LINC00525 |
Colorectal cancer |
Activating HIF-1α via miR-338-3p/UBE2Q1/β-catenin axis |
Proliferation |
|
|
PAARH |
HCC |
Promoting HIF-1α/VEGF signaling |
Progression and angiogenesis |
|
|
HIFAL |
Breast cancer |
Enhancing HIF-1α transactivation |
Proliferation |
|
|
MAPKAPK5-AS1 |
HCC |
Adjusting HIF-1α expression via PLAGL2 |
Progression |
|
|
circRNA |
||||
|
circ_03955 |
Pancreatic cancer |
Promoting HIF-1α through sponging miR-3662 expression |
Tumorigenesis and Warburg effect |
NF-κB Signaling Regulates the TME of HCC
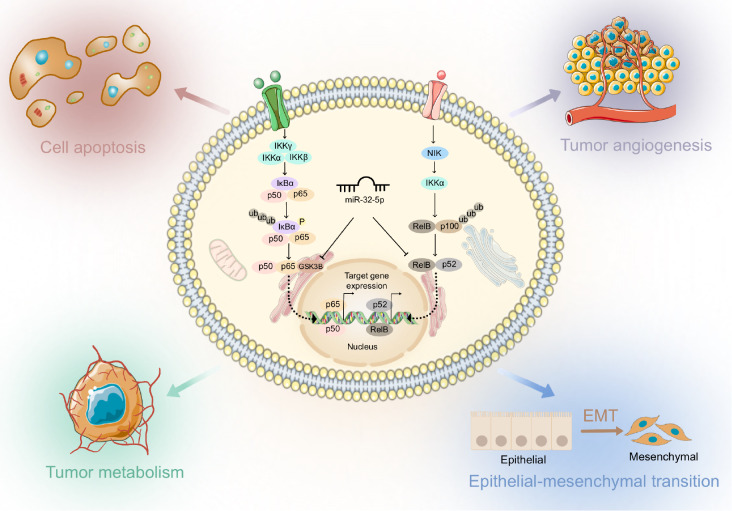
The NF-κB family is involved in a large number of biological processes, including inflammation, the immune response, and the regulation of cellular homeostasis [ 58, 59] . These biological phenomena are closely related to the occurrence, progression, and metastasis of various malignant tumors. Additionally, NF-κB plays multiple roles in the TME, including promoting tumor cell growth and invasion, suppressing immune surveillance, and promoting angiogenesis [ 60, 61] . Therefore, NF-κB signaling is not only an important regulatory pathway of inflammation and immunity but also a key factor in malignancy ( Figure 1). The NF-κB transcription factor family consists of five major subunits, including Rel (c-Rel), RelB, p65 (RelA, NF-κB3), p50 (NF-κB1) and p52 (NF-κB2), all of which have an N-terminal fragment containing approximately 300 amino acid residues, known as the Rel homeodomain (RHD). Among them, p65, c-Rel and RelB have C-terminal transactivation domains (TADs), which confers the ability to activate NF-κB and actively regulate its expression. Although p50 and p52 lack transcriptional activation domains, their homodimers inhibit transcription. The correlation between activation of the NF-κB pathway and hypoxic conditions, particularly in relation to HIF, has been shown to be a major mediator of the hypoxic response that promotes cancer progression [ 62, 63] . Additionally, the NF-κB signaling pathway is one of the most important signaling pathways involved in physiological and pathological conditions. It is always quiescent in normal tissues and activated in a variety of inflammatory diseases and tumors [64]. Growing evidence suggests that dysregulated NF-κB signaling enhances cancer cell proliferation and metastasis and mediates radio- and chemoresistance [ 65– 67] .
Figure 1 .
Regulation of NF-κB signaling by ncRNA in the HCC TME
Sustained activation of NF-κB is responsible for tumorigenesis, metastasis, tumor evasion, resistance to apoptosis, angiogenesis and proliferation in HCC [68]. ncRNAs have been found to regulate the NF-κB signaling pathway in different settings, and our laboratory has also reviewed the regulatory role and clinical significance of ncRNAs in NF-κB signaling in cancer [69].
Proliferation and apoptosis
Xie et al. [70] found that lncRNA-PDIA3P1 is upregulated in HCC, and its higher level is associated with recurrence and survival rates in human HCC. Additionally, upregulation of PDIA3P1 is significantly associated with elevated tumor necrosis factor receptor-associated factor 6 (TRAF6), p-p65, and NF-κB downstream anti-apoptotic genes in human HCC tissues. Mechanistically, PDIA3P1 binds to miR-125a/b and miR-124 and thereby deregulates their inhibitory effects on TRAF6, activating the NF-κB signaling pathway to confer chemoresistance [70]. Yang et al. [71] investigated the regulatory role of miR-20a on NF-κB in Huh7 HCC cells and its effect on the sensitivity of Huh7 cells to chemotherapeutic drugs. It was found that miR-20a activates the NF-κB signaling pathway and decreases the expressions of apoptosis-related proteins by upregulating the expressions of the proteins Livin and Survivin, which attenuates the sensitivity of cells to chemotherapeutic drugs and reduces the level of apoptosis [71]. The expression of miR-26b is significantly downregulated in human liver cancer tissues compared to paraneoplastic tissues. By forcibly upregulating miR-26b, it was found to inhibit cell proliferation and induce apoptosis, exerting an anticancer effect. Next, upregulation of miR-26b could significantly inhibit the NF-кB pathway and thus suppress tumor growth in human HCC [72]. Wen et al. [73] found that the expression of miR-27b in HCC is lower than that in adjacent nontumor tissue (ANT). The decreased expression of hsa-miR-27b is associated with poor survival among HCC patients. They demonstrated that miR-27b acts as an inhibitor of the NF-κB pathway in HCC by targeting transforming growth factor-activated kinase-binding protein 3 (TAB3). Furthermore, miR-27b significantly inhibits HCC cell proliferation.
Epithelial-mesenchymal transition (EMT), migration and invasion
The expression of miR-605-3p is downregulated in HCC tissues compared to paraneoplastic tissues, and the OS and DFS rates are lower in HCC patients with low miR-605-3p expression than in those with high miR-605-3p expression. Additionally, immunofluorescence and western blot analysis revealed that miR-605-3p inhibited EMT and attenuated the activation of NF-κB signaling in HCC cells, thus exerting its oncogenic function [74]. It was demonstrated that lncRNA fragment cancer susceptibility candidate 2 (CASC2) is downregulated in human HCC tissues and HCC cell lines compared to paraneoplastic tissues and the normal hepatocyte line LO 2. By downregulating its expression, it was found to significantly promote migration and invasion of HCC cells. Mechanistically, CASC2 was found to regulate hepatocellular carcinogenesis by targeting miR-362-5p and thereby inhibiting the NF-κB pathway [75]. To investigate the role of lncRNA and NF-κB in the regulation of cancer metastasis, Chen and colleagues [76] identified the lncRNA that interacts with NF-κB, NKILA, which was found to be downregulated in HCC tissues and cell lines, and its reduced level is associated with poor prognosis in HCC patients. In addition, NKILA inhibits the migration and invasion of HCC cells in vitro and in vivo. Mechanistically, NKILA blocks the Slug/EMT pathway by inhibiting the phosphorylation of IκBα, p65 nuclear translocation and NF-κB activation [76].
Regulation of the TME by ncRNAs in HCC
Role of ncRNAs in TME homeostasis
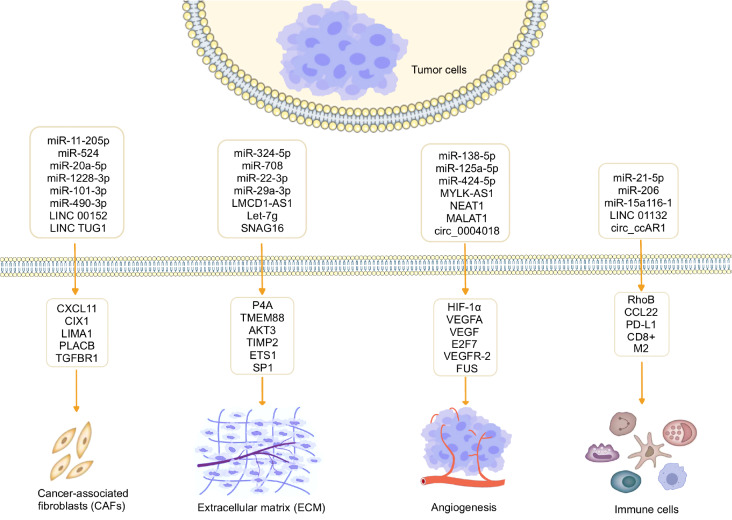
ncRNAs regulate cancer-associated fibroblasts (CAFs) in the TME of HCC
Fibroblasts were identified as the predominant cell population in solid tumors and are stimulated by various factors secreted by tumor cells or immune cells, leading to their transformation into CAFs. CAFs, recognized as a distinct subset of activated fibroblasts within the TME [77], play a crucial role in tumor growth, proliferation, and metastasis as one of the most abundant and critical components of the tumor mesenchyme. Studies have demonstrated the impact of CAFs on the malignant progression, metastasis, drug resistance, and recurrence of HCC [78]. Through a comparison between primary cultured CAFs and noncancerous fibroblasts (NFs) obtained from resected HCC specimens of the same patient, it was observed that CAFs significantly enhance HCC cell proliferation, migration, and invasion. The upregulation of CXCL11 expression in HCC tissues and CAFs has been reported, and CXCL11 secreted by CAFs was found to promote HCC cell proliferation and migration. To explore the specific mechanism, Liu et al. [79] identified that LINC00152 exerts a positive regulatory effect on CXCL11 expression in CAFs through direct binding to miR-11-205p. This regulatory axis influences the proliferation and migration abilities of HCC cells in vitro and the growth of HCC tumors in vivo. A growing body of evidence suggests that cellular interactions between cancer cells and surrounding stromal cells within the TME play a crucial role in modulating cancer progression and treatment response [ 80, 81] . CAFs were identified as key contributors to the promotion of human cancer growth, invasion, metastasis, and therapy resistance through exosome-mediated cellular communication [80]. It was found that exosomal lncRNA TUG1 derived from CAFs promotes the migration, invasion, and glycolysis of HepG2 cells. However, these effects could be attenuated by miR-524. Mechanistically, SIX1 was identified as a target gene of miR-524, and the inhibition of SIX1 abolishes the promoting effects of miR-524-5p inhibitors on migration, invasion, and glycolysis [82]. Qi et al. [83] reported that CAFs exert oncogenic effects on HCC cells through the transfer of exosomes carrying miR-20a-5p. Mechanistically, LIM domain and actin-binding 1 (LIMA1) was identified as a tumor suppressor in HCC, and miR-20a-5p was found to act as an oncogene in HCC. Furthermore, it was observed that miR-20a-5p is present in CAF-derived exosomes that are transferred from CAFs to HCC cells, leading to the suppression of LIMA1 expression [83]. CAF-derived extracellular vesicles (EVs) were shown to promote tumor progression through the delivery of miRNA. Zhang et al. [84] found that CAF-derived EVs could promote the proliferation, migration, invasion potential, and resistance to sorafenib in HCC cells. Specifically, miR-1228-3p carried by CAF-EVs was found to enhance the chemoresistance of HCC by activating the placenta-associated 8 (PLAC8)-mediated PI3K/AKT pathway [84]. CAFs have been recognized for their contribution to tumor progression, with miRNAs playing a crucial role in regulating the tumor-promoting properties of CAFs. The dysregulated expression of miRNAs in HCC-CAFs and their oncogenic characteristics were examined. The study revealed that miR-101-3p and miR-490-3p were downregulated in HCC-CAFs, and their common target gene was identified as TGFBR1 [85]. The downregulations of miR-101-3p and miR-490-3p, along with the upregulation of TGFBR1, were found to be associated with a poor clinical prognosis in HCC patients. Furthermore, increased expression of TGFBR1 is correlated with the infiltration of immunosuppressive immune cells such as MDSCs, M2 macrophages, and Treg cells [85].
ncRNAs affect extracellular matrix (ECM) remodeling
The ECM is recognized as one of the most crucial components of the TME and consists of protein components, including collagen, fibronectin, glycosaminoglycans, and proteoglycans. It serves as a significant tissue barrier against tumor invasion and metastasis [60]. The ECM exhibits a highly dynamic network structure, and matrix metalloproteinases (MMPs) play a vital role in the remodeling and turnover of the ECM. MMPs act as key regulators in multiple tumor pathological processes [ 86, 87] . ncRNAs were reported to participate in ECM remodeling by regulating the expressions of MMPs [ 88, 89] . Wang et al. [90] discovered that aspirin decreases the level of the tumor suppressor miRNA let-7g by inhibiting the lncRNA LMCD1-AS1, which acts as a sponge. Consequently, this inhibition enhances the targeting of let-7g on its target gene, prolyl 4-hydroxylase (P4H), thereby exerting inhibitory effects on tumor growth in HCC and collagen deposition. These findings revealed a novel role and regulatory mechanism of aspirin in inhibiting HCC through the disruption of abnormal collagen deposition. Excessive accumulation of ECM can lead to hepatic fibrosis (HF), where hepatic stellate cells (HSCs) are the main cells involved. In a study by Xu et al. [91], miR-708 was found to regulate HSC activation and enhance ECM accumulation by directly targeting transmembrane protein 88 (TMEM88). These findings provide a potential target for future research on the process of liver fibrosis. In a study conducted by Wang et al. [92], miR-22-3p and miR-29a-3p were found to act as fibrosis inhibitors and synergistically inhibit HF. Serine/threonine kinase 3 ( AKT3) was identified as the common target gene of these two miRNAs. This study provided new insights into the regulation of AKT3 expression in HF and opened up new possibilities for miRNA-based therapeutic regimens for HF. Similarly, Zhang et al. [93] found that the expression of the lncRNA SNHG16 is significantly increased in HCC tissues and cell lines and is associated with poor prognosis in HCC patients. Mechanistically, SNHG16 promotes the malignant behavior of HCC cells by activating the ECM-receptor interaction pathway [93]. ECM remodeling requires the concerted action of multiple proteolytic enzymes and their endogenous inhibitors, among which tissue inhibitor of metalloproteinases 2 (TIMP2) plays an important role. Kai and colleagues [94] found that TIMP2 is frequently and significantly downregulated in human HCC, and this downregulation is associated with aggressive tumor behavior and poorer patient prognosis. Mechanistically, TIMP2 suppression in a hypoxic environment is induced through a regulatory feedback circuit consisting of HIF-1α, miR-210 and HIF-3α [94]. Cao et al. [95] found that abnormal expression of miR-324-5p in HCC cells is involved in cell migration and invasion. Overexpression of miR-324-5p reduces the expressions of E26 transformation-specific 1 (ETS1) and specificity protein 1 (SP1) and potentially inhibits ECM degradation by suppressing MMP2 and MMP9 in HCC. Therefore, miR-324-5p could be considered a potential new target for the treatment of invasive HCC.
ncRNAs and angiogenesis in HCC
Angiogenesis plays a pivotal role in the pathophysiology of cancer and is intricately regulated by diverse components within the TME [96]. A large amount of vascularization was observed in rapidly growing tumors at an early stage, which was the significance of tumors in tumor treatment proposed by Judah Folkman [97]. Excessive proliferation of tumor cells leads to an increase in oxygen consumption, and when the tumor mass exceeds the blood supply, the tumor becomes hypoxic. Hypoxia induces the production of angiogenic factors, leading to enhanced angiogenesis [98]. Hypoxia-induced HIF-1α is stable and promotes the upregulation of several angiogenic factors, including vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF-2), and platelet-derived growth factor (PDGF) [99]. Liu et al. [47] identified that miR-138-5p targets HIF-1α and regulates the expressions of HIF-1α and vascular endothelial growth factor A (VEGFA), thereby inhibiting angiogenesis in HCC. Overall, miRNAs were identified to be involved in different stages of tumor progression, and some of them even play an important role in regulating multiple cancer features, making them promising targets for cancer therapy, which deserves further exploration.
Angiogenesis is essential for the occurrence, progression and metastasis of HCC. To investigate the biological function of the lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1) in HCC, Guo et al. [100] detected elevated levels of NEAT1 and reduced levels of miR-125a-5p in HCC tissues and cells. The dual luciferase reporter gene assay showed that NEAT1 binds to miR-125a-5p, which in turn binds to VEGF. NEAT1 enhances VEGF angiogenesis in HCC by regulating competitive endogenous RNA (ceRNA) for miR-125a-5p in the AKT/mTOR and ERK pathways. Fei and coworkers [101] demonstrated that upregulation of MYLK-AS1 is associated with enhanced angiogenesis and tumor progression in HCC tumor tissues and cell lines. MYLK-AS1 acts as a ceRNA that regulates angiogenesis in HCC, and miR-424-5p was identified as a direct target of MYLK-AS1. Mechanistically, MYLK-AS1 promotes tumor progression and angiogenesis by targeting the miR-424-5p/E2F7 axis and activating the VEGFR-2 signaling pathway in HCC. Oncogenic MALAT1 exerts an antiangiogenic effect in HCC by sponging miR-3064-5p as a competitive ceRNA with its attenuated inhibitory effect on the FOXA1/CD24/Src pathway [102]. These findings suggest that angiogenesis plays an important role in rapid tumor growth and metastasis [97]. Wu et al. [103] identified a downregulated circRNA, circ_0004018, in HCC by RT-qPCR. Through a series of functional assays, it was found that overexpression of circ_0004018 significantly inhibited angiogenesis in HCC. Mechanistically, circ_0004018, activated by estrogen receptor 1 (ESR1), inhibits angiogenesis in HCC by binding to FUS and stabilizing TIMP2 expression.
ncRNAs and immune modulation in the TME
In the TME, immune cells are the most abundant cellular component and have been the target of interest due to their potent cytotoxicity [ 104– 106] . Macrophages are one of the major components of the innate immune system and are responsible for pathogen clearance and antigen presentation. Tumor-associated macrophages (TAMs), the most abundant immune cells in the TME, are critical for cancer initiation and progression [105]. Based on various stimuli, macrophages were acknowledged to undergo polarization into either the M1 phenotype characterized by antitumor activity or the M2 phenotype characterized by protumor activity [60]. M2-like polarized TAMs represent a predominant subset of infiltrating immune cells in HCC, demonstrating substantiated evidence of profound immunosuppressive properties and protumor effects [107]. Yu et al. [108] found that exosomal miR-21-5p derived from HCC cells directly targeted the ras homolog family member B (RhoB) 3′-untranslated region (UTR), downregulating RhoB levels, which weakened mitogen-activated protein kinase (MAPK) axis signaling pathways and induced macrophage M2 polarization. Kupffer cells (KCs) have been recognized for their crucial role in HCC through intricate communication with various immune cell populations, thereby exerting a protective effect against HCC development. Liu et al. [109] found that the polarization of KCs towards the M2 phenotype is a pivotal factor contributing to the pathogenesis of HCC in AKT/Ras mice. Notably, the dysregulation of miR-206 was observed to promote the M1 polarization of KCs, thereby facilitating the augmented infiltration of CD8 +T cells and exerting a protective effect against HCC progression [109]. These significant findings underscore the potential of miR-206 as a promising immunotherapeutic intervention for HCC. A previous study demonstrated that miR-15a/16-1 exhibited the capacity to mitigate immune suppression by interfering with C-C motif chemokine 22 (CCL22)-mediated intercellular communication between KCs and regulatory T cells (Tregs) [110]. This modulation of the KC-Treg interaction highlights miR-15a/16-1 as a prospective immunotherapeutic approach for HCC. CD8 + T cell dysfunction is a critical factor in HCC immune escape. Hu et al. [111] discovered upregulated expression of circCCAR1 in HCC samples and cell lines, promoting HCC growth and development in vitro and in vivo. The circCCAR1/miR-127-5p/Wilms tumor 1-associated protein (WTAP) feedback loop enhances HCC proliferation and metastasis. Exosomal circCCAR1 from HCC cells impair activated CD8 + T cells by stabilizing PD8 protein, suggesting therapeutic potential in targeting exosomal circCCAR1 or cell division cycle and apoptosis regulator 1 (CCAR1) for improving HCC immunotherapy [111]. Zhang et al. [112] identified a significant upregulation of LINC01132 expression in HCC with a concurrent association with poorer OS in HCC patients. Functionally, LINC01132 overexpression was found to exert promotive effects on HCC cell growth, proliferation, invasion, and metastasis. Mechanistically, silencing of LINC01132 results in CD8 + T cell infiltration, implicating its role in modulating the tumor immune microenvironment [112]. Moreover, the combined approach of LINC01132 knockdown and anti-PDL1 treatment demonstrated enhanced antitumor immunity, highlighting the potential of this novel therapeutic combination for HCC.
In summary, ncRNAs play multiple roles in the TME, which could promote or inhibit the immune system and angiogenesis, increase the permeability of endothelial cells, promote cancer metastasis, and cause ECM remodeling, which together support tumor progression. The communication between cells and the TME mediated by ncRNAs is shown in Figure 2.
Figure 2 .
The communication mediated by ncRNAs between tumor cells and the TME
Conclusions and Prospects
Hepatocarcinogenesis is a multifactorial process in which ongoing liver injury and concurrent regeneration might produce an environment that ultimately leads to hypoxia and inflammation, which are key features of the liver TME [ 113, 114] . Under hypoxic conditions, HIF-1α is an important transcription factor that mediates the effects of hypoxia on the adaptive regulation of tumor cells and the TME [115]. The feasibility of using HIF-1α as a therapeutic target has been demonstrated in a number of studies, suggesting that interventions that alter HIF-1α activity by direct or indirect means could be effective in the treatment of HCC. In a review, Shant et al. [23] provided a comprehensive analysis of diverse potential novel therapeutic agents for HCC treatment. These agents include hypoxia-activated prodrugs, HIF inhibitors, nanomaterials, antisense oligonucleotides, and natural compounds, all of which specifically target the HIF/hypoxia signaling pathway in HCC. Their findings underscore the promising potential of HIFs as effective therapeutic targets in the management of HCC.
ncRNAs have emerged as key regulators of posttranscriptional activation in cancer. miRNAs, among the most extensively studied, were found to be significantly dysregulated in HCC, thereby promoting tumor progression [116]. Furthermore, alongside miRNAs, other ncRNAs, such as lncRNAs that predominantly function as miRNA sponges, are implicated in modulating sorafenib resistance by regulating EMT and stemness in HCC [117]. No studies have reported the role for circRNAs in sorafenib resistance; however, circRNAs are involved in regulating the stemness characteristics of HCC cells [118]. Several ncRNA biomarkers or therapeutic targets could be highly specific for a single liver disease, considering the tissue-specific expression of ncRNAs, thus enabling rapid diagnosis and improved management of HCC. These findings provide new insights into ncRNA-mediated interactions between the HCC microenvironment, metabolism and tumor cell state, thereby further enhancing our understanding of ncRNA-mediated cell state transitions in sorafenib resistance.
Due to the important role of the HIF pathway in conferring survival and resistance to cancer cells, the search for HIF inhibitors is critical to overcome the chemotherapy resistance that is observed in many cancers. Over the years, direct and indirect HIF inhibitors have been identified and evaluated in clinical trials at various stages [119]. Equally promising are miRNA mimics (agomiRs) that supplement tumor suppressor miRNAs and/or miRNA inhibitors (antagomiRs) targeting oncomiR-dependent tumor sites. Some of these inhibitors show promising response rates in patients, although many are still in early clinical trials. However, challenges persist concerning the specificity, stability, and short half-life of the target molecules. Hence, it is imperative that we amplify our endeavors to surmount the challenges hindering the translation of our present knowledge into clinical applications. By doing so, we can broaden our horizons and develop new therapeutic strategies against HCC.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported in part by the grants from the Shandong Province Medicine and Health Science and Technology Development Project (No. 2019WS596) and the Shanghai Science and Technology Committee (No. 20S11901300).
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022, 72: 7–33 . [DOI] [PubMed]
- 2.Chen Y, Cheng G, Mahato RI. RNAi for treating hepatitis B viral infection. Pharm Res. . 2008;25:72–86. doi: 10.1007/s11095-007-9504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xue C, Gu X, Bao Z, Su Y, Lu J, Li L. The mechanism underlying the ncRNA dysregulation pattern in hepatocellular carcinoma and its tumor microenvironment. Front Immunol. . 2022;13:847728. doi: 10.3389/fimmu.2022.847728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Meng T, Cui S, Liu D, Pang Q, Wang P. Roles of ubiquitination in the crosstalk between tumors and the tumor microenvironment (Review). Int J Oncol. 2022, 61: 84 . [DOI] [PMC free article] [PubMed]
- 5.Roy S, Kumaravel S, Sharma A, Duran CL, Bayless KJ, Chakraborty S. Hypoxic tumor microenvironment: implications for cancer therapy. Exp Biol Med (Maywood) . 2020;245:1073–1086. doi: 10.1177/1535370220934038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. . 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao W, Du J, Wang Z, Feng Q, Liao M, Liu H, Yuan K, et al. The role and mechanism of noncoding RNAs in regulation of metabolic reprogramming in hepatocellular carcinoma. Intl J Cancer. . 2022;151:337–347. doi: 10.1002/ijc.34040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng H, Niu R, Huang C, Li J. Circular RNA as a novel biomarker and therapeutic target for HCC. Cells. . 2022;11:1948. doi: 10.3390/cells11121948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satija S, Kaur H, Tambuwala MM, Sharma P, Vyas M, Khurana N, Sharma N, et al. Hypoxia-inducible factor (HIF): fuel for cancer progression. Curr Mol Pharmacol. . 2021;14:321–332. doi: 10.2174/1874467214666210120154929. [DOI] [PubMed] [Google Scholar]
- 10.Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. . 2004;5:437–448. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- 11.Ju C, Colgan SP, Eltzschig HK. Hypoxia-inducible factors as molecular targets for liver diseases. J Mol Med. . 2016;94:613–627. doi: 10.1007/s00109-016-1408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y, Xiao Z, Yang L, Gao Y, Zhu Q, Hu L, Huang D, et al. Hypoxiainducible factors in hepatocellular carcinoma (Review). Oncol Rep. 2020, 43: 3–15 . [DOI] [PMC free article] [PubMed]
- 13.Chua YL, Dufour E, Dassa EP, Rustin P, Jacobs HT, Taylor CT, Hagen T. Stabilization of hypoxia-inducible factor-1α protein in hypoxia occurs independently of mitochondrial reactive oxygen species production. J Biol Chem. . 2010;285:31277–31284. doi: 10.1074/jbc.M110.158485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng X, Gao H, Xu R, Wang H, Mei J, Liu C. The interplay between HIF-1α and noncoding RNAs in cancer. J Exp Clin Cancer Res. . 2020;39:27. doi: 10.1186/s13046-020-1535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nath B, Szabo G. Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology. . 2012;55:622–633. doi: 10.1002/hep.25497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Wu C, Zhong J, Shen Y, Zu X. Tumorigenesis and progression as a consequence of hypoxic TME: a prospective view upon breast cancer therapeutic targets. Exp Cell Res. . 2020;395:112192. doi: 10.1016/j.yexcr.2020.112192. [DOI] [PubMed] [Google Scholar]
- 17.Zhang MS, Cui JD, Lee D, Yuen VWH, Chiu DKC, Goh CC, Cheu JWS, et al. Hypoxia-induced macropinocytosis represents a metabolic route for liver cancer. Nat Commun. . 2022;13:954. doi: 10.1038/s41467-022-28618-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fathi M, Bahmanpour S, Barshidi A, Rasouli H, Karoon Kiani F, Mahmoud Salehi Khesht A, Izadi S, et al. Simultaneous blockade of TIGIT and HIF-1α induces synergistic anti-tumor effect and decreases the growth and development of cancer cells. Int Immunopharmacol. . 2021;101:108288. doi: 10.1016/j.intimp.2021.108288. [DOI] [PubMed] [Google Scholar]
- 19.Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, Shu Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. . 2019;18:157. doi: 10.1186/s12943-019-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goyette MA, Elkholi IE, Apcher C, Kuasne H, Rothlin CV, Muller WJ, Richard DE, et al. Targeting Axl favors an antitumorigenic microenvironment that enhances immunotherapy responses by decreasing Hif-1α levels. Proc Natl Acad Sci USA. . 2021;118:e2023868118. doi: 10.1073/pnas.2023868118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y, Jiang J, Cui Y, Chen Y, Dong T, An H, Liu P. MSH6 aggravates the hypoxic microenvironment via regulating HIF1A to promote the metastasis of glioblastoma multiforme . DNA Cell Biol. . 2021;40:93–100. doi: 10.1089/dna.2020.5442. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Wu K, Li H, Xia D, He T. Role of hypoxia in the tumor microenvironment and targeted therapy. Front Oncol. . 2022;12:961637. doi: 10.3389/fonc.2022.961637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sin SQ, Mohan CD, Goh RMW, You M, Nayak SC, Chen L, Sethi G, et al. Hypoxia signaling in hepatocellular carcinoma: challenges and therapeutic opportunities. Cancer Metastasis Rev. 2022. doi: 10.1007/s10555-022-10071-1 . [DOI] [PubMed]
- 24.Chen Y, Liu X, Yuan H, Yang Z, von Roemeling CA, Qie Y, Zhao H, et al. Therapeutic remodeling of the tumor microenvironment enhances nanoparticle delivery. Adv Sci. . 2019;6:1802070. doi: 10.1002/advs.201802070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ocaña MC, Martínez‐Poveda B, Quesada AR, Medina MÁ. Metabolism within the tumor microenvironment and its implication on cancer progression: an ongoing therapeutic target. Medicinal Res Rev. . 2019;39:70–113. doi: 10.1002/med.21511. [DOI] [PubMed] [Google Scholar]
- 26.Zheng SS, Chen XH, Yin X, and Zhang BH, Prognostic significance of HIF-1alpha expression in hepatocellular carcinoma: a meta-analysis. PLoS One. 2013, 8: e65753 . [DOI] [PMC free article] [PubMed]
- 27.Chen Y, Huang F, Deng L, Yuan X, Tao Q, Wang T, Li D, et al. HIF-1-miR-219-SMC4 regulatory pathway promoting proliferation and migration of HCC under hypoxic condition. Biomed Res Int. 2019, 2019: 8983704 . [DOI] [PMC free article] [PubMed]
- 28.Han L, Lin X, Yan Q, Gu C, Li M, Pan L, Meng Y, et al. PBLD inhibits angiogenesis via impeding VEGF/VEGFR2-mediated microenvironmental cross-talk between HCC cells and endothelial cells. Oncogene. . 2022;41:1851–1865. doi: 10.1038/s41388-022-02197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma H, Xie L, Zhang L, Yin X, Jiang H, Xie X, Chen R, et al. Activated hepatic stellate cells promote epithelial-to-mesenchymal transition in hepatocellular carcinoma through transglutaminase 2-induced pseudohypoxia. Commun Biol. . 2018;1:168. doi: 10.1038/s42003-018-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng J, Dai W, Mao Y, Wu L, Li J, Chen K, Yu Q, et al. Simvastatin re-sensitizes hepatocellular carcinoma cells to sorafenib by inhibiting HIF-1α/PPAR-γ/PKM2-mediated glycolysis. J Exp Clin Cancer Res. . 2020;39:24. doi: 10.1186/s13046-020-1528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo J, Jeong DW, Park JW, Lee KW, Fukuda J, Chun YS. Fatty-acid-induced FABP5/HIF-1 reprograms lipid metabolism and enhances the proliferation of liver cancer cells. Commun Biol. . 2020;3:638. doi: 10.1038/s42003-020-01367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng Z, Shi Z, Liu Y, Zhao J, Lu Q, Guo J, Liu X, et al. HIF-1α-activated TM4SF1-AS1 promotes the proliferation, migration, and invasion of hepatocellular carcinoma cells by enhancing TM4SF1 expression. Biochem Biophys Res Commun. . 2021;566:80–86. doi: 10.1016/j.bbrc.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Feng W, Xue T, Huang S, Shi Q, Tang C, Cui G, Yang G, et al. HIF-1α promotes the migration and invasion of hepatocellular carcinoma cells via the IL-8–NF-κB axis. Cell Mol Biol Lett. . 2018;23:26. doi: 10.1186/s11658-018-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen T, Liu R, Niu Y, Mo H, Wang H, Lu Y, Wang L, et al. HIF-1α-activated long non-coding RNA KDM4A-AS1 promotes hepatocellular carcinoma progression via the miR-411-5p/KPNA2/AKT pathway. Cell Death Dis. . 2021;12:1152. doi: 10.1038/s41419-021-04449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Q, Ni Y, Zhang L, Jiang R, Xu J, Yang H, Hu Y, et al. HIF-1α-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Sig Transduct Target Ther. . 2021;6:76. doi: 10.1038/s41392-020-00453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen Y, Zhou X, Lu M, He M, Tian Y, Liu L, Wang M, et al. Bclaf1 promotes angiogenesis by regulating HIF-1α transcription in hepatocellular carcinoma. Oncogene. . 2019;38:1845–1859. doi: 10.1038/s41388-018-0552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang H, Li J, Luo Y, Wu B, Yuan C, Geng X. TFB2M activates aerobic glycolysis in hepatocellular carcinoma cells through the NAD + /SIRT3/HIF‐1α signaling . J Gastro Hepatol. . 2021;36:2978–2988. doi: 10.1111/jgh.15548. [DOI] [PubMed] [Google Scholar]
- 38.Long Q, Zou X, Song Y, Duan Z, Liu L. PFKFB3/HIF-1α feedback loop modulates sorafenib resistance in hepatocellular carcinoma cells. Biochem Biophys Res Commun. . 2019;513:642–650. doi: 10.1016/j.bbrc.2019.03.109. [DOI] [PubMed] [Google Scholar]
- 39.Lin YH, Wu MH, Yeh CT, Lin KH. Long Non-coding RNAs as mediators of tumor microenvironment and liver cancer cell communication. Int J Mol Sci. . 2018;19:3742. doi: 10.3390/ijms19123742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z, Ma M, Yan L, Chen S, Li S, Yang D, Wang X, et al. miR-370 regulates ISG15 expression and influences IFN-α sensitivity in hepatocellular carcinoma cells. Cancer Biomark. . 2018;22:453–466. doi: 10.3233/CBM-171075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen G, Li X, Jia Y, Piazza GA, Xi Y. Hypoxia-regulated microRNAs in human cancer. Acta Pharmacol Sin. . 2013;34:336–341. doi: 10.1038/aps.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Zhao D, Xie H, Hu Y. Interplay of long non-coding RNAs and HIF-1α: a new dimension to understanding hypoxia-regulated tumor growth and metastasis. Cancer Lett. . 2021;499:49–59. doi: 10.1016/j.canlet.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Piao H, Liu Y, Kang Y, Wang Y, Meng X, Yang D, Zhang J. Hypoxia associated lncRNA HYPAL promotes proliferation of gastric cancer as ceRNA by sponging miR-431-5p to upregulate CDK14. Gastric Cancer. . 2022;25:44–63. doi: 10.1007/s10120-021-01213-5. [DOI] [PubMed] [Google Scholar]
- 44.Peng D, Wu T, Wang J, Huang J, Zheng L, Wang P, Li J, et al. microRNA-671-5p reduces tumorigenicity of ovarian cancer via suppressing HDAC5 and HIF-1α expression. Chemico-Biol Interactions. . 2022;355:109780. doi: 10.1016/j.cbi.2021.109780. [DOI] [PubMed] [Google Scholar]
- 45.Huang YH, Lian WS, Wang FS, Wang PW, Lin HY, Tsai MC, Yang YL. MiR-29a Curbs hepatocellular carcinoma incidence via targeting of HIF-1α and ANGPT2. Int J Mol Sci. . 2022;23:1636. doi: 10.3390/ijms23031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng C, Yuan G, Hu Y, Wang D, Shi X, Zhu D, Hu A, et al. Repressing phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma by microRNA-142-3p restrains the progression of hepatocellular carcinoma. Bioengineered. . 2022;13:1491–1506. doi: 10.1080/21655979.2021.2020549. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Liu H, Tang T, Hu X, Tan W, Zhou P, Zhang H, Liu Y, et al. miR-138-5p inhibits vascular mimicry by targeting the HIF-1alpha/VEGFA pathway in hepatocellular carcinoma. J Immunol Res. 2022, 2022: 7318950 . [DOI] [PMC free article] [PubMed]
- 48.Wang Q, Zhang F, Lei Y, Liu P, Liu C, Tao Y. microRNA-322/424 promotes liver fibrosis by regulating angiogenesis through targeting CUL2/HIF-1α pathway. Life Sci. . 2021;266:118819. doi: 10.1016/j.lfs.2020.118819. [DOI] [PubMed] [Google Scholar]
- 49.Byun Y, Choi YC, Jeong Y, Lee G, Yoon S, Jeong Y, Yoon J, et al. MiR-200c downregulates HIF-1α and inhibits migration of lung cancer cells. Cell Mol Biol Lett. . 2019;24:28. doi: 10.1186/s11658-019-0152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J, Du C, Zhang L, Wang Y, Zhang Y, Li J. LncRNA LINC00649 promotes the growth and metastasis of triple-negative breast cancer by maintaining the stability of HIF-1α through the NF90/NF45 complex. Cell Cycle. . 2022;21:1034–1047. doi: 10.1080/15384101.2022.2040283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L, Wu H, Zhang Y, Xiao X, Chu F, Zhang L. Induction of lncRNA NORAD accounts for hypoxia-induced chemoresistance and vasculogenic mimicry in colorectal cancer by sponging the miR-495-3p/ hypoxia-inducible factor-1α (HIF-1α) Bioengineered. . 2022;13:950–962. doi: 10.1080/21655979.2021.2015530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Z, Hu Z, Sui Q, Huang Y, Zhao M, Li M, Liang J, et al. LncRNA FAM83A-AS1 facilitates tumor proliferation and the migration via the HIF-1α/ glycolysis axis in lung adenocarcinoma. Int J Biol Sci. . 2022;18:522–535. doi: 10.7150/ijbs.67556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meng F, Luo X, Li C, and Wang G, LncRNA LINC00525 activates HIF-1alpha through miR-338-3p/UBE2Q1/beta-catenin axis to regulate the warburg effect in colorectal cancer. Bioengineered. 2022, 13: 2554–2567 . [DOI] [PMC free article] [PubMed]
- 54.Wei H, Xu Z, Chen L, Wei Q, Huang Z, Liu G, Li W, et al. Long non-coding RNA PAARH promotes hepatocellular carcinoma progression and angiogenesis via upregulating HOTTIP and activating HIF-1α/VEGF signaling. Cell Death Dis. . 2022;13:102. doi: 10.1038/s41419-022-04505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng F, Chen J, Zhang X, Wang Z, Chen J, Lin X, Huang H, et al. The HIF-1α antisense long non-coding RNA drives a positive feedback loop of HIF-1α mediated transactivation and glycolysis. Nat Commun. . 2021;12:1341. doi: 10.1038/s41467-021-21535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L, Sun L, Liu R, Mo H, Niu Y, Chen T, Wang Y, et al. Long non-coding RNA MAPKAPK5-AS1/PLAGL2/HIF-1α signaling loop promotes hepatocellular carcinoma progression. J Exp Clin Cancer Res. . 2021;40:72. doi: 10.1186/s13046-021-01868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu A, Xu J. Circ_03955 promotes pancreatic cancer tumorigenesis and Warburg effect by targeting the miR-3662/HIF-1α axis. Clin Transl Oncol. . 2021;23:1905–1914. doi: 10.1007/s12094-021-02599-5. [DOI] [PubMed] [Google Scholar]
- 58.Martin M, Sun M, Motolani A, Lu T. The pivotal player: components of NF-κB pathway as promising biomarkers in colorectal cancer. Int J Mol Sci. . 2021;22:7429. doi: 10.3390/ijms22147429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fang Z, Meng Q, Xu J, Wang W, Zhang B, Liu J, Liang C, et al. Signaling pathways in cancer‐associated fibroblasts: recent advances and future perspectives. Cancer Commun. . 2023;43:3–41. doi: 10.1002/cac2.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma Z, Shuai Y, Gao X, Wen X, Ji J. Circular RNAs in the tumour microenvironment. Mol Cancer. . 2020;19:8. doi: 10.1186/s12943-019-1113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malla RR, Kiran P. Tumor microenvironment pathways: cross regulation in breast cancer metastasis. Genes Dis. . 2022;9:310–324. doi: 10.1016/j.gendis.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Royds JA, Dower SK, Qwarnstrom EE, Lewis CE. Response of tumour cells to hypoxia: role of p53 and NFkB. Mol Pathol. . 1998;51:55–61. doi: 10.1136/mp.51.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature. . 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng Q, Zhang H, Nie X, Li Y, Chen WD, Wang YD. miR-149* suppresses liver cancer progression by down-regulating tumor necrosis factor receptor 1—associated death domain protein expression. Am J Pathol. . 2020;190:469–483. doi: 10.1016/j.ajpath.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 65.Yang C, Shi S, Su Y, Tong J, Li L. P2X7R promotes angiogenesis and tumour‐associated macrophage recruitment by regulating the NF‐κB signalling pathway in colorectal cancer cells. J Cell Mol Medi. . 2020;24:10830–10841. doi: 10.1111/jcmm.15708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang T, Zhang X, Wang H. Punicalagin inhibited proliferation, invasion and angiogenesis of osteosarcoma through suppression of NF‑κB signaling. Mol Med Rep. . 2020;22:2386–2394. doi: 10.3892/mmr.2020.11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mirzaei S, Zarrabi A, Hashemi F, Zabolian A, Saleki H, Ranjbar A, Seyed Saleh SH, et al. Regulation of nuclear factor-kappaB (NF-kappaB) signaling pathway by noncoding RNAs in cancer: inhibiting or promoting carcinogenesis? Cancer Lett. 2021, 509: 63-80 . [DOI] [PubMed]
- 68.Mohan CD, Bharathkumar H, Dukanya H, Rangappa S, Shanmugam MK, Chinnathambi A, Alharbi SA, et al. N-Substituted pyrido-1,4-oxazin-3-ones induce apoptosis of hepatocellular carcinoma cells by targeting NF-κB signaling pathway. Front Pharmacol. . 2018;9:1125. doi: 10.3389/fphar.2018.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu X, Shao Y, Zhou J, Qian G, Ma Z. Nuclear factor κB signaling and its related non-coding RNAs in cancer therapy. Mol Ther Nucleic Acids. . 2020;19:208–217. doi: 10.1016/j.omtn.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie C, Zhang L, Chen Z, Zhong W, Fang J, Zhu Y, Xiao M, et al. A hMTR4‐PDIA3P1‐miR‐125/124‐TRAF6 regulatory axis and its function in NF kappa B signaling and chemoresistance. Hepatology. . 2020;71:1660–1677. doi: 10.1002/hep.30931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang BM, Zhao JR, Huo TT, Zhang ML, Wu XH. MiR-20a lowers chemosensitivity of liver cancer Huh-7 cells by regulating NF-small ka, CyrillicB expression. Eur Rev Med Pharmacol Sci. 2020, 24: 11569–11577 . [DOI] [PubMed]
- 72.Feng Y, Zu LL, Zhang L. MicroRNA-26b inhibits the tumor growth of human liver cancer through the PI3K/Akt and NF-kappaB/MMP-9/VEGF pathways. Oncol Rep. 2018, 39: 2288–2296 . [DOI] [PubMed]
- 73.Wen J, Huang Z, Wei Y, Xue L, Wang Y, Liao J, Liang J, et al. Hsa-microRNA-27b-3p inhibits hepatocellular carcinoma progression by inactivating transforming growth factor-activated kinase-binding protein 3/nuclear factor kappa B signalling. Cell Mol Biol Lett. . 2022;27:79. doi: 10.1186/s11658-022-00370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu Y, Feng Y, Chen Y, Liu J, Su Y, Li P, Huang H, et al. SNHG16/miR‐605‐3p/TRAF6/NF‐κB feedback loop regulates hepatocellular carcinoma metastasis. J Cell Mol Medi. . 2020;24:7637–7651. doi: 10.1111/jcmm.15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao L, Zhang Y, Zhang Y. Long noncoding RNA CASC2 regulates hepatocellular carcinoma cell oncogenesis through miR‐362‐5p/Nf‐κB axis. J Cell Physiol. . 2018;233:6661–6670. doi: 10.1002/jcp.26446. [DOI] [PubMed] [Google Scholar]
- 76.Chen R, Cheng Q, Owusu-Ansah KG, Song G, Jiang D, Zhou L, Xu X, et al. NKILA, a prognostic indicator, inhibits tumor metastasis by suppressing NF-κB/Slug mediated epithelial-mesenchymal transition in hepatocellular carcinoma. Int J Biol Sci. . 2020;16:495–503. doi: 10.7150/ijbs.39582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kashima H, Noma K, Ohara T, Kato T, Katsura Y, Komoto S, Sato H, et al. Cancer‐associated fibroblasts (CAFs) promote the lymph node metastasis of esophageal squamous cell carcinoma. Intl J Cancer. . 2019;144:828–840. doi: 10.1002/ijc.31953. [DOI] [PubMed] [Google Scholar]
- 78.Kato K, Fukai M, Hatanaka KC, Takasawa A, Aoyama T, Hayasaka T, Matsuno Y, et al. Versican secreted by cancer-associated fibroblasts is a poor prognostic factor in hepatocellular carcinoma. Ann Surg Oncol. . 2022;29:7135–7146. doi: 10.1245/s10434-022-11862-0. [DOI] [PubMed] [Google Scholar]
- 79.Liu G, Yang ZF, Sun J, Sun BY, Zhou PY, Zhou C, Guan RY, et al. The LINC00152/miR-205-5p/CXCL11 axis in hepatocellular carcinoma cancer-associated fibroblasts affects cancer cell phenotypes and tumor growth. Cell Oncol. . 2022;45:1435–1449. doi: 10.1007/s13402-022-00730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS, Yan YR, Song FY, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer. . 2019;18:91. doi: 10.1186/s12943-019-1019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu ZH, Yang DL, Wang L, Liu J. Epigenetic and immune-cell infiltration changes in the tumor microenvironment in hepatocellular carcinoma. Front Immunol. . 2021;12:793343. doi: 10.3389/fimmu.2021.793343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu L, Huang J, Mo J, Da X, Li Q, Fan M, Lu H. Exosomal lncRNA TUG1 from cancer-associated fibroblasts promotes liver cancer cell migration, invasion, and glycolysis by regulating the miR-524-5p/SIX1 axis. Cell Mol Biol Lett. . 2022;27:17. doi: 10.1186/s11658-022-00309-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qi Y, Wang H, Zhang Q, Liu Z, Wang T, Wu Z, Wu W. CAF-released exosomal miR-20a-5p facilitates HCC progression via the LIMA1-mediated β-catenin pathway. Cells. . 2022;11:3857. doi: 10.3390/cells11233857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y, Pan Q, Shao Z. Extracellular vesicles derived from cancer-associated fibroblasts carry tumor-promotive microRNA-1228-3p to enhance the resistance of hepatocellular carcinoma cells to sorafenib. Hum Cell. . 2023;36:296–311. doi: 10.1007/s13577-022-00800-7. [DOI] [PubMed] [Google Scholar]
- 85.Eun JW, Ahn HR, Baek GO, Yoon MG, Son JA, Weon JH, Yoon JH, et al. Aberrantly expressed microRNAs in cancer-associated fibroblasts and their target oncogenic signatures in hepatocellular carcinoma. Int J Mol Sci. . 2023;24:4272. doi: 10.3390/ijms24054272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lachowski D, Cortes E, Rice A, Pinato D, Rombouts K, del Rio Hernandez A. Matrix stiffness modulates the activity of MMP-9 and TIMP-1 in hepatic stellate cells to perpetuate fibrosis. Sci Rep. . 2019;9:7299. doi: 10.1038/s41598-019-43759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jurado Acosta A, Rysä J, Szabo Z, Moilanen AM, Komati H, Nemer M, Ruskoaho H. Transcription factor PEX1 modulates extracellular matrix turnover through regulation of MMP-9 expression. Cell Tissue Res. . 2017;367:369–385. doi: 10.1007/s00441-016-2527-2. [DOI] [PubMed] [Google Scholar]
- 88.Ren S, Liu J, Feng Y, Li Z, He L, Li L, Cao X, et al. Knockdown of circDENND4C inhibits glycolysis, migration and invasion by up-regulating miR-200b/c in breast cancer under hypoxia. J Exp Clin Cancer Res. . 2019;38:388. doi: 10.1186/s13046-019-1398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang J, Liu X, Sun Y, Zhang X, Zhao Y, Zhang H, Mei Q, et al. ING5 overexpression upregulates miR-34c-5p/Snail1 to inhibit EMT and invasion of lung cancer cells. Acta Biochim Biophys Sin. . 2023;55:809–817. doi: 10.1002/ijc.31977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang T, Fu X, Jin T, Zhang L, Liu B, Wu Y, Xu F, et al. Aspirin targets P4HA2 through inhibiting NF-κB and LMCD1-AS1/let-7g to inhibit tumour growth and collagen deposition in hepatocellular carcinoma. EBioMedicine. . 2019;45:168–180. doi: 10.1016/j.ebiom.2019.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu T, Pan L, Li L, Hu S, Zhou H, Yang C, Yang J, et al. MicroRNA‐708 modulates hepatic stellate cells activation and enhances extracellular matrix accumulation via direct targeting TMEM88. J Cell Mol Medi. . 2020;24:7127–7140. doi: 10.1111/jcmm.15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y, Zhang R, Li J, Han X, Lu H, Su J, Liu Y, et al. MiR-22-3p and miR-29a-3p synergistically inhibit hepatic stellate cell activation by targeting AKT3. Exp Biol Med (Maywood) . 2022;247:1712–1731. doi: 10.1177/15353702221108379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang QJ, Li DZ, Lin BY, Geng L, Yang Z, Zheng SS. SNHG16 promotes hepatocellular carcinoma development via activating ECM receptor interaction pathway. Hepatobiliary Pancreatic Dis Int. . 2022;21:41–49. doi: 10.1016/j.hbpd.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 94.Kai AK, Chan LK, Lo RC, Lee JM, Wong CC, Wong JC, Ng IO. Down‐regulation of TIMP2 by HIF‐1α/miR‐210/HIF‐3α regulatory feedback circuit enhances cancer metastasis in hepatocellular carcinoma. Hepatology. . 2016;64:473–487. doi: 10.1002/hep.28577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cao L, Xie B, Yang X, Liang H, Jiang X, Zhang D, Xue P, et al. MiR-324-5p suppresses hepatocellular carcinoma cell invasion by counteracting ECM degradation through post transcriptionally downregulating ETS1 and SP1. PLoS One. 2015, 10: e0133074 . [DOI] [PMC free article] [PubMed]
- 96.Patil N, Allgayer H, Leupold JH. MicroRNAs in the tumor microenvironment. Adv Exp Med Biol. 2020, 1277: 1–31 . [DOI] [PubMed]
- 97.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971, 285: 1182–1186 . [DOI] [PubMed]
- 98.Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. . 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 99.Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. . 2020;77:1745–1770. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo J, Yuan Q, Fang Y, Liao J, Zhang Z. Long non-coding RNA NEAT1 promotes angiogenesis in hepatoma carcinoma via the miR-125a-5p/VEGF pathway. Open Life Sci. . 2022;17:1229–1239. doi: 10.1515/biol-2022-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Teng F, Zhang JX, Chang QM, Wu XB, Tang WG, Wang JF, Feng JF, et al. LncRNA MYLK-AS1 facilitates tumor progression and angiogenesis by targeting miR-424-5p/E2F7 axis and activating VEGFR-2 signaling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. . 2020;39:235. doi: 10.1186/s13046-020-01739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang P, Ha M, Li L, Huang X, Liu C. MicroRNA‐3064‐5p sponged by MALAT1 suppresses angiogenesis in human hepatocellular carcinoma by targeting the FOXA1/CD24/Src pathway. FASEB J. . 2020;34:66–81. doi: 10.1096/fj.201901834R. [DOI] [PubMed] [Google Scholar]
- 103.Wu Y, Zhang M, Bi X, Hao L, Liu R, Zhang H. ESR1 mediated circ_0004018 suppresses angiogenesis in hepatocellular carcinoma via recruiting FUS and stabilizing TIMP2 expression. Exp Cell Res. . 2021;408:112804. doi: 10.1016/j.yexcr.2021.112804. [DOI] [PubMed] [Google Scholar]
- 104.Chen Q, Li Y, Liu Y, Xu W, Zhu X. Exosomal non-coding RNAs-mediated crosstalk in the tumor microenvironment. Front Cell Dev Biol. . 2021;9:646864. doi: 10.3389/fcell.2021.646864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen C, Liu J, Luo Y. MicroRNAs in tumor immunity: functional regulation in tumor-associated macrophages. J Zhejiang Univ Sci B. . 2020;21:12–28. doi: 10.1631/jzus.B1900452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dutta A, Roy A, Chatterjee S. Long noncoding RNAs in cancer immunity: a new avenue in drug discovery. Drug Discov Today. . 2021;26:264–272. doi: 10.1016/j.drudis.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 107.Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol. . 2019;40:310–327. doi: 10.1016/j.it.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 108.Yu H, Pan J, Zheng S, Cai D, Luo A, Xia Z, Huang J. Hepatocellular carcinoma cell-derived exosomal miR-21-5p induces macrophage M2 polarization by targeting RhoB. Int J Mol Sci. . 2023;24:4593. doi: 10.3390/ijms24054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu N, Wang X, Steer CJ, and Song G, MicroRNA-206 promotes the recruitment of CD8(+) T cells by driving M1 polarization of Kupffer cells. Gut. 2022, 71: 1642–1655 . [DOI] [PMC free article] [PubMed]
- 110.Liu N, Chang CW, Steer CJ, Wang XW, Song G. MicroRNA-15a/16-1 prevents hepatocellular carcinoma by disrupting the communication between kupffer cells and regulatory T cells. Gastroenterology. . 2022;162:575–589. doi: 10.1053/j.gastro.2021.10.015. [DOI] [PubMed] [Google Scholar]
- 111.Hu Z, Chen G, Zhao Y, Gao H, Li L, Yin Y, Jiang J, et al. Exosome-derived circCCAR1 promotes CD8+ T-cell dysfunction and anti-PD1 resistance in hepatocellular carcinoma. Mol Cancer. . 2023;22:55. doi: 10.1186/s12943-023-01759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang J, Pan T, Zhou W, Zhang Y, Xu G, Xu Q, Li S, et al. Long noncoding RNA LINC01132 enhances immunosuppression and therapy resistance via NRF1/DPP4 axis in hepatocellular carcinoma. J Exp Clin Cancer Res. . 2022;41:270. doi: 10.1186/s13046-022-02478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang K, Sun D. Cancer stem cells of hepatocellular carcinoma. Oncotarget. . 2018;9:23306–23314. doi: 10.18632/oncotarget.24623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nishida N, Kudo M. Oncogenic signal and tumor microenvironment in hepatocellular carcinoma. Oncology. . 2017;93:160–164. doi: 10.1159/000481246. [DOI] [PubMed] [Google Scholar]
- 115.Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. . 2018;7:10. doi: 10.1038/s41389-017-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dietrich P, Koch A, Fritz V, Hartmann A, Bosserhoff AK, Hellerbrand C. Wild type kirsten rat sarcoma is a novel microRNA-622-regulated therapeutic target for hepatocellular carcinoma and contributes to sorafenib resistance. Gut. . 2018;67:1328–1341. doi: 10.1136/gutjnl-2017-315402. [DOI] [PubMed] [Google Scholar]
- 117.Zhang P, Wang F, Wu J, Wu Y, Huang W, Liu D, Huang X, et al. LncRNA SNHG3 induces EMT and sorafenib resistance by modulating the miR‐128/CD151 pathway in hepatocellular carcinoma. J Cell Physiol. . 2019;234:2788–2794. doi: 10.1002/jcp.27095. [DOI] [PubMed] [Google Scholar]
- 118.Wei Y, Chen X, Liang C, Ling Y, Yang X, Ye X, Zhang H, et al. A noncoding regulatory RNAs network driven by circ‐CDYL acts specifically in the early stages hepatocellular carcinoma. Hepatology. . 2020;71:130–147. doi: 10.1002/hep.30795. [DOI] [PubMed] [Google Scholar]
- 119.Fallah J, Rini BI. HIF inhibitors: status of current clinical development. Curr Oncol Rep. . 2019;21:6. doi: 10.1007/s11912-019-0752-z. [DOI] [PubMed] [Google Scholar]