Abstract
Stem cells are the foundational cells for every organ and tissue in our body. Cell-based therapeutics using stem cells in regenerative medicine have received attracting attention as a possible treatment for various diseases caused by congenital defects. Stem cells such as induced pluripotent stem cells (iPSCs) as well as embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), and neuroprogenitors stem cells (NSCs) have recently been studied in various ways as a cell-based therapeutic agent. When various stem cells are transplanted into a living body, they can differentiate and perform complex functions. For stem cell transplantation, it is essential to determine the suitability of the stem cell-based treatment by evaluating the origin of stem, the route of administration, in vivo bio-distribution, transplanted cell survival, function, and mobility. Currently, these various stem cells are being imaged in vivo through various molecular imaging methods. Various imaging modalities such as optical imaging, magnetic resonance imaging (MRI), ultrasound (US), positron emission tomography (PET), and single-photon emission computed tomography (SPECT) have been introduced for the application of various stem cell imaging. In this review, we discuss the principles and recent advances of in vivo molecular imaging for application of stem cell research.
Keywords: Stem cells, In vivo molecular imaging, Optical imaging, Magnetic resonance imaging, Positron emission tomography, Single-photon emission computed tomography
Introduction
In order to alleviate various chronic diseases, recent research are being conducted with transplanting sophisticated biomaterial scaffolds or transplanting organs. How-ever, these various ways could induce an immune response and require immunosuppressive drugs after transplanta-tion (1). In contrast, the regeneration of specific tissues and organs using stem cells could reduce the risk of side effects due to non-immune responses after transplantation. Stem cells have recently been studied in various ways as a cell-based therapeutic agent. Attention is focused on the treatment of diseases and body regeneration using induced pluripotent stem cells (iPSCs) as well as embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), and neuroprogenitors stem cells (NSCs). Stem cell is being studied as a strategy to regenerate tissues and organs damaged by congenital defects and diseases (2). An advantage of stem cell regenerative medicine is that stem cell-based therapies do not require systemic immunosuppression unlike other regenerative approaches (3-5). The degree of symptom alleviation in diseases varies according to the amount of transplanted stem cells in vivo (6). For stem cell transplantation, it is essential to determine the suitability of the stem cell-based treatment by evaluating the origin of stem, the route of administration, in vivo bio-distribution, transplanted cell survival, function, and mobility. Their effects and safety of stem cell would be enhanced by monitoring the accumulation of transplanted cells and long-term viability in target tissue. Moreover, assessing their function and toxicity in vivo could be achieved using various imaging modalities for applying cell-based therapeutics with promising preclinical results to clinical practices (7).
In vivo cell and molecular imaging is the visualization, characterization, and quantification of biological processes in humans and other living systems. In vivo molecular imaging could be used to determine the presence or absence of a disease and monitor the treatment of a disease (8-10). Various molecular images such as optical imaging, magnetic resonance imaging (MRI), positron emission tomography (PET), single-photon emission computed tomography (SPECT), and computed tomography (CT) allow us to visualize cellular and molecular processes with various genetic, metabolic, proteomic, and cellular biologic information. Comparing in vitro or ex vivo imaging, in vivo molecular imaging has many advantages to monitor the transplanted cells non-invasively in real time on an animal model. Especially, the unique characteristics and differentiation of the transplanted stem cells could be imaged through molecular imaging in the body. Currently, various stem cells are being imaged in vivo through various cell imaging methods (11-13). In this review, direct cell labeling methods using probes incorporated into cells or probes bound to cell membranes and indirect cell labeling methods that require genetic modification and image specific cells in vivo through an expressed reporter protein are described (Fig. 1). In addition, the basic principles of in vivo stem cell imaging are covered, the advantages and disadvantages of in vivo imaging methods, the latest research through in vivo stem cell imaging, and future research prospects are discussed.
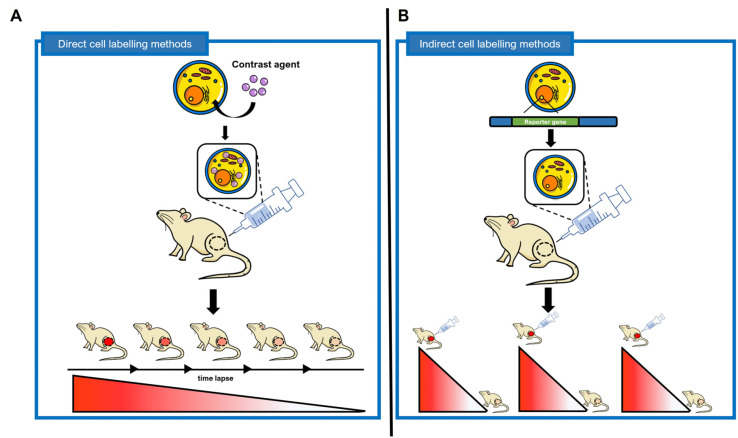
Fig. 1.
Difference between direct cell labeling and indirect cell labeling. (A) Direct cell labeling methods are non-permanent imaging methods that identify direct labeling agents such as fluorescent dyes, superparamagnetic iron oxide nanoparticles, and isotopes labeled on cells in vivo. Direct labeling agent after labeled stem cells can be detected for several hours to several days depending on the characteristics of the labeled material. (B) Indirect cell labeling methods are strategies for imaging stem cells by inserting a reporter gene into cells through genetic manipulation. The protein expressed by the reporter gene inserted into the stem cell functions as a cell receptor, transporter, enzyme, etc., and has the advantage of being able to image the stem cell permanently and repeatedly in vivo. In addition, since the reporter gene is also replicated during the cell division process, the degree of proliferation after stem cell transplantation in vivo can be analyzed.
Direct Cell Labeling Methods
Direct cell labeling methods are simple cell tracking methods that label specific target cells ex vivo/in vitro with a direct labeling agent and then inject and image in vivo (Fig. 2). In vivo cell imaging shows contrast agent-labeled cells transplanted into living organisms in various ways. In this method, labeled cells are explicitly detected in vivo and the degree of distribution in target organs can be monitored (14). Images can be taken repeatedly from several hours to several days depending on the half-life of the direct labeling agent or whether it is present in cells. However, since it does not allow imaging of cell proliferation, the imaging signal decreases due to the outflow of the direct labeling agent according to the time lapse (Fig. 1A). Direct cell markers are limited in that they cannot visualize cell activation or cell division. Also, labeled cells can be asymmetrically distributed in progeny cells or lose labeled material during cell division (15).
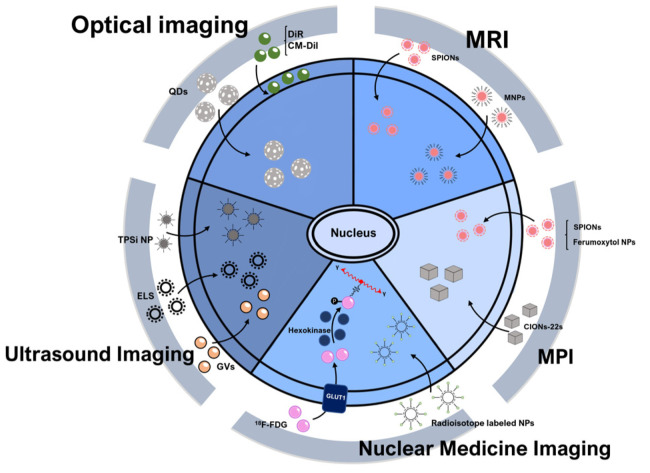
Fig. 2.
Principle of direct cell labeling. Principle of direct cell labeling methods is a strategy for imaging stem cells in vivo by introducing or labeling direct labeling agents into the body without genetic manipulation. Principle of direct cell labeling methods are relatively simple imaging methods that label stem cells with an agent in vitro and then implant them in vivo. QDs: quantum dots, CM-Dil: chloromethyl-benzamide dialkyl carbocyanine-Dil, MRI: magnetic resonance imaging, SPIONs: superparamagnetic iron-oxide nanoparticles, MNPs: magnetic nanoparticles, Ferumoxytol NPs: Feru-moxytol nanoparticles, CIONs-22s: cubic iron oxide nanoparticle, MPI: magnetic particle imaging, Radioisotope labeled NPs: Radio-isotope labeled nanoparticles, 18F-FDG: 2-deoxy-2-[18F]fluoro-D-glucose, GLUT1: glucose transporter 1, GVs: gas vesicles, ELS: exosome-like silica nanoparticles, TPSi NP: cell-penetrating peptide (virus-1 transactivator of transcription) conjugated porous silicon nanoparticle.
Optical imaging
Optical imaging is a imaging method acquiring optical signals from contrast agents (16). For optical imaging, a cooled Charge-Coupled Device (CCD) camera is used to reduce thermal noise and improve the sensitivity during imaging (17). Stem cells for fluorescence imaging could be labeled with fluorescent semiconductor nanocrystals such as quantum dots (QDs) or fluorophores (Fig. 2) (18, 19). QDs are semiconductor nanocrystals capable of emitting light at various wavelengths ranging from ultraviolet to near-infrared (NIR) (20). Because QDs have the advantages of high resolution, long duration, high sensitivity, and less toxicity, in vivo stem cells were labeled with QDs and imaged. This was demonstrated in vivo by labeling stem cells with 6 types of QDs representing various wavelengths (21). Murine embryonic stem (ES) cells were labeled with QD 525, QD 565, QD605, QD 655, QD 705, and QD 800, and 1×106 cells were injected subcutaneously into the backs of athymic nude mice. ES cells labeled with QD succeeded in multiplex imaging of ES cells in vivo using a single excitation wavelength (465 nm). Such in vivo multi-images have great potential for identifying the mobility of stem cells in vivo, which is difficult to reproduce in vitro, and at the same time identifying the movement of various stem cells. Another study on stem cell imaging using fluorophores showed that the cytoplasmic membrane of mES cells was stained with a DiR dye, a lipophilic NIR fluorescent cyanine dye, and then the stem cells were imaged in vivo (22). After labeling mES cells with DiR, 5×106 cells were intravenously injected into gastric tumor-bearing mice. The biodistribution of DiR-labeled mES cells was monitored by IVIS imaging within 24 hours. The migration rate of DiR-labeled mES cells to gastric cancer tissue after in vivo injection was as fast as 10 minutes and peaked at 2 hours. Ex vivo can confirm cell migration to specific organs, but it is not suitable for real-time monitoring. However, monitoring after implan-tation of stem cell images in vivo can identify the accessibility to gastric cancer tissue over time. This shows that DiR-labeled mES cells are applicable to gastric cancer target imaging. In addition, the NIR properties of the DiR dye are suitable for in vivo imaging since the autofluore-scence of the body itself is reduced at high wavelengths (23). Another study showed that a bone marrow mesenchymal stem cells (BMSCs) was labeled with chloromethyl-benzamide dialkyl carbocyanine-Dil and in vivo imaging was acquired to determine whether the stem cells were transplanted to the liver with portal hypertension (24). Other study showed that MSCs were labeled with conjugated polymer based water-dispersible nanoparticles (CPN) and applied to determine the positional infor-mation and viability of MSCs in vitro and in vivo (25). CPN exhibits higher brightness, improved photostability, higher fluorescence quantum yield, and lower cytotoxicity than conventional fluorescent dyes. The resulting fluore-scence signal was maintained for 3 weeks after in vitro differentiation into osteocytes, adipocytes, and chondrocytes. To analyze the in vivo effects of CPN-labeled MSCs on injured site mobility and liver regeneration, an animal model of liver injury was generated by partial hepate-ctomy in 6-month-old Sprague Dawley rats. CPN-labeled MSCs were injected through the tail vein at 106 cells each, and after 3 days, livers were removed and wavelengths for CPN were analyzed. Although this study is not real-time monitoring of MSCs in live animals, it suggests the possibility of safely tracking CPN-labeled MSCs in vivo. Further research is needed to determine whether CPN-MSCs migrated to the damaged liver differentiate and affect liver regeneration or function.
Magnetic imaging
The principle of operation of MRIs is that hydrogen nuclei are converted from a rotational motion by a magnetic field. When hydrogen nuclei in the procession state are exposed to electromagnetic waves, only electromagnetic waves that resonate with the precession are emitted. MRI contrast agents contain paramagnetic or superparama-gnetic metal ions that affect the MRI signal characteristics of the surrounding tissue and enhance the sensitivity of the MRI. In vivo imaging using MRI could be achieved by dosing contrast agents such as superparamagnetic iron-oxide nanoparticles (SPIONs) (Fig. 2). SPIONs treatment of human umbilical cord mesenchymal stem cells (hUC-MSCs) has been reported for non-invasive MRI tracking of stem cells in vivo (26). After spinal cord injury (SCI) was induced in rats using a weight drop device, SPIONs-labeled hUC-MSCs were injected into the spinal cord at a concentration of 4×104 cells/μl at 2.5 μl. SPIONs-labeled hUC-MSCs injected in vivo showed a significant decrease in MRI signal at 1 week and 3 weeks after transplantation. In vivo, SPIONs-labeled hUC-MSCs were transplanted into the spinal cord and survival was monitored for at least 8 weeks. This demonstrated that MSCs could survive and migrate in the spinal cord through MRI, and the effect of hUC-MSCs on functional recovery after SCI could be confirmed. It was also demonstrated that dental pulp stem cells (DPSCs) labeled with dextran-coa-ted SPIONs could be successfully monitored by MRI (27). In vivo MRI tracking has been reported with SPIONs-labeled BMSCs (28). BMSC transplanted into experimental animals displayed sensitive signals in T2/T2*-weighted images, enabling effective MRI tracking for up to 14 days after transplantation. Fluorescent magnetic nanoparticles (MNPs) has been reported that MSCs were successfully imaged in vivo using MNP (29). In a liver cirrhosis mouse model induced by intraperitoneal injection of dimethylnitrosamine (DMN), fluorescent MNP-labeled MSCs (3.0× 106 cells) were intrasplenicly injected. Then, the viability and mobility of MSCs in vivo were monitored using 3-T magnetic resonance equipment. MNP-labeled MSCs demonstrated lower liver-to-muscle noise ratios than those of the pre-injection and non-labeled groups at 3 and 5 hours after transplantation in vivo. Therefore, MNP-labeled MSC transplanted by intrasplenic injection in a mouse model of liver lesions by 3-T MRI were successfully mornitered. Through this, it was proved that MNP is suitable for stem cell monitoring through MRI and has mobility and viability in vivo. Magneto electroporation (MEP) is a technique based on the mechanism of low voltage pulses, and MEP-labeled MSCs proliferated normally after transplantation, and MRI was successful (30).
Recently, magnetic particle imaging (MPI) have introduced as a new imaging modality with high sensitivity and contrast (31). A study was reported on the dynamic trafficking of SPIONs-labeled MSCs after in vivo transplantation through MPI (32). It was demonstrated that the mobility and quantification of labeled MSCs to specific organs could be monitored with MPI. MPI could produce millimeter-scale resolution, high sensitivity, and high contrast angiographic images (33). SPIONs-labeled MSCs (5×106 to 8×106 cells) were intravenously administered to immunocompetent Fischer 344 rats, and the distribution of MSCs in vivo was confirmed by MPI. In this result, it was mornitered that the labeled MSCs were captured in the lung tissue and then removed to the liver within 1 day. MPI-CT imaging revealed that the elimination half-life of MSC iron oxide labels in the liver was 4.6 days, and the ex vivo MPI biodistribution of iron was measured in liver, spleen, heart, and lung after injection of labeled MSC. After confirming the distribution of MSCs in several specific organs ex vivo through MPI, in vivo noninvasive imaging of the labeled MSCs and real-time analysis of quantification provide usefulness. When Ferumoxytol na-noparticles (Ferumoxytol NPs) are labeled on MSC, MPI signals are significantly increased and quantitative information that cannot be obtained by MRI has been collected (34). The cubic iron oxide nanoparticle (CIONs-22s) is a cubic nanoparticle with a 22 nm edge tailored to MPI. It has a much larger saturation magnetization than the existing spherical nanoparticles, so it shows superior performance compared to the existing MPI nanopa-rticles. BMSCs labeled with CIONs-22 were monitored in real time by MPI regardless of tissue depth and cell location and distribution patterns (35). BMSCs (cell number of ∼100,000) labeled with CIONs-22 were administered intravenously to BALB/c mice. MPI over time was then able to accurately track cells over a long period of time, up to 7 days. This allowed long-term mornitering to examine the overall dynamics of stem cells administered into the body. In addition, when CIONs-22-labeled BMSCs were monitored in vivo through MPI, they accumulated in the lungs and migrated to the liver within 3 hours after injection. In this study, MPI succeeded in investigating cell dynamics over time, which could not be confirmed in vitro/ex vivo. It suggests the possibility of applying MPI to the accessibility of specific organs for studying stem cell therapy.
Nuclear medicine imaging
PET is a molecular imaging method that monitors positrons induced by radioactive isotopes. Positive charges emitted from radioactive isotopes interact with electrons in the body, generating two 511 KeV photons that are emitted at approximately 180° (36). PET has the advantage of being able to detect even picomolar concentrations, showing very sensitive and quantitative characteristics. For in vivo stem cell imaging, it is possible to non-invasively monitor the efficacy of cell-based therapeutics, including stem cell movement, metastasis, survival, and function. However, the half-life and in vivo toxicity of the radioactive substances should be considered, as well as the effects of the isotopes on stem cell viability, function, and differentiation (37). Recently, in vivo stem cell imaging study has reported with PET after 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG)-labeled stem cells were transplanted (38-41). In the study, 18F-FDG-labeled MSCs were implanted in vivo by various administration methods, and the in vivo distribution of the MSCs were analyzed. In this study, MSCs labeled with 18F-FDG were injected into the tail vein of mice (8.5×104 cells, 0.1 ml) and rats (4.6∼19.0×105 cells, 1.0 ml). In addition, 18F-FDG-labeled MSCs were injected through rat carotid artery (4.9∼16.3×105 cells, 1.0 ml) and intramyocardial (1.3∼1.6×106 cells, 0.25 ml) injections, and MSC localization was confirmed. It was confirmed that MSCs labeled with 18F-FDG injected through the peripheral vein were entrapped in lung tissue. In addition, 18F-FDG-labeled MSC administration through the carotid artery showed the highest activity in the head, and intramyocardial injection increased the signal from the heart. This study revealed that PET imaging of stem cells using radioactive isotopes varied in signal distribution depending on the stem cell injection route. In vitro, pre-labeling of 18F-FDG-labeled MSC is possible, and the uptake of radioactive isotopes can be determined. Furthermore, non-invasive visualization of the distribu-tion of 18F-FDG-labeled MSCs according to various administration modes in vivo has the potential to improve the understanding and accessibility of stem cell therapy. Injecting MSCs through the carotid artery may promote recovery of the function of the central nervous system after a brain injury by potentially replacing damaged pericytes associated with MSCs (42). After MSCs were injected into the myocardium, an accumulation of radiotracers near the heart was observed, suggesting that MSCs can potentially be used as a cell-based treatment for diseases such as myocardial infarction. The study not only identified the route of movement of the stem cells in vivo but also analyzed their accessibility in specific organs.
The SPECT imaging method is similar to the PET imaging method in that it uses radioactive isotopes, but SPECT uses relatively heavy radioactive isotopes such as 99mTC, 123I, and indium-111 (111In). Protons of SPECT radioactive isotopes combine with electrons in the inner shell to form neutrons and emit electron neutrinos. In this process, electrons from the outer shell are moved to the inner shell for stabilization, and auger electrons and gamma-ray photons are simultaneously generated (43). SPECT have the advantage of being used for relatively long-term in vivo imaging. Some stem cells have the ability to aggregate and integrate into tumors when injected into the body. Because of these capabilities, various studies on cell-based anticancer drugs using stem cells are being conducted. One study for in vivo stem cell imaging using SPECT showed that luciferase-expressing human adipocyte-derived stem cells (ADSCs) were co-cultured with 111In radiolabelled iron oxide nanoparticles (44). These ADSCs were administered intravenously and intracar-diacly to mice with orthotopic breast tumors, and the survival rate of intratumoral ADSCs was measured. As a result, it was demonstrated that more ADSCs implanted in vivo by intracardiac administration were present in tumors than by intravenous administration. This is a study that can reveal the accessibility of stem cells to tumors by presenting a simultaneous multiple mornitering method for various imaging means including SPECT. The multimodal imaging approach offers the possibility of analyzing in detail the degree of function, differentiation, and distribution of cells as well as the degree of survival (42). BMSCs labeled with 125I-conjugated nanoparticles were injected into ischemic mouse brains. transplanted BMSCs were imaged through SPECT into the brain, resulting that brain atrophy was reduced, and angiogenesis and neurogenesis were increased to promote nerve recovery. Through this, BMSC has the potential as a treatment for various brain diseases along with the treatment of brain ischemia, which can be continuously mornitered through SPECT.
Ultrasound imaging
Ultrasound imaging is a imaging tool for long-term, non-invasive cell tracking in stem cell-based therapies due to its features of deep penetration and excellent temporal and spatial resolution (45-47). Ultrasound uses Ultrasound Contrast Agents (UCA) to enhance contrast and enhance the echo signal upon detection. In the past, UCAs were micro-sized microbubbles composed of bioinert heavy gases such as lipids, proteins, and biocompatible polymers, but they were limited to large microsizes, poor structural stability, and short half-lives in stem cell tracking (48). Exosome-like silica nanoparticles (ELS) are novel cup-shaped silica nanoparticles and improves biocompatibility in MSCs (49). In addition, since ELS enhances echo generation and ultrasound sensitivity of human mesenchymal stem cell (hMSC) in vivo, when ELS-labelled hMSC (1 million) was injected into nude mice, stem cell sensitivity was increased through ultrasound. ELS increases echogenicity in vitro and in vivo and enables real-time cell tracking/imaging via relatively inexpensive ultrasound. In addition, the ability of ELS to load and release specific drugs has the prospect of improving the viability of stem cells in vivo and improving the efficiency of stem cell-based therapy. Cell-penetrating peptide conjugated porous silicon nanoparticle (TPSi NP) improved the viability of labeled cells and the accuracy of stem cell transplantation through a combinational theranostic strategy (50). Intrace-llular aggregation of TPSi NPs can amplify the coherent scattering of MSCs and thus amplify the ultrasonic signal. The function of TPSi NPs in vivo was studied by labeling MSCs and subcutaneously injecting them into nude mice. The ultrasound signal of TPSi NP-labeled MSCs was immediately observed when the number of injected cells in vivo was greater than 5×104 cells. Clear ultrasound signal amplification of TPSi NP-labeled MSCs can improve the precision of mornitering after stem cell transplantation in vivo. Gas vesicles (GVs) are biosynthetic nano-sized particles and perform excellent functions in ultrasound imaging. Recently, GVs were loaded into mouse MSCs and could be imaged in vivo in real time using ultrasound imaging (51). In vivo ultrasound imaging capability was demonstrated by taking ultrasound images by subcutaneously injecting 1×107 cells of GV@MSCs into their lateral malleolus of arthritic rats. GV@MSCs were able to apply real-time ultrasound imaging in vivo for 5 days. Bone/cartilage regeneration was induced when GV@MSCs and drugs were co-treated, and there is a possibility of analyzing the distribution and function of MSCs in vivo in the future.
Indirect Cell Labeling Methods
Indirect cell labeling methods require genetic manipulation to transplant the reporter gene. Through this, reporter proteins such as cell receptors, transporters, and enzymes are expressed in cells. These proteins promote absorption of radioactive tracers into cells or binding of radioactive tracers to cells, so that specific cells are imaged in vivo (Fig. 3). The characteristic of indirect cell labeling methods is that the reporter gene is transmitted during cell division in that genetically engineered cells are used. Through this, long-term imaging is possible by periodically administering the contrast agent. Over time, if the contrast agent is excreted in vivo or its function is lost, the imaging signal decreases, but imaging is possible again by re-injecting the contrast agent (Fig. 1B). Reporter gene imaging has the advantage of allowing repetitive “hotspot” imaging at locations within the body following stem cell administration (52). However, indirect cell labeling methods require complex genetic manipulations and additional stability evaluations.
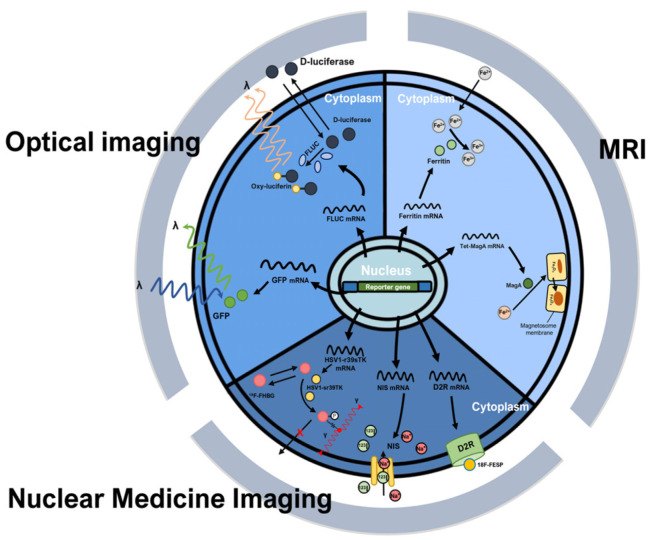
Fig. 3.
Principle of indirect cell labeling. Indirect cell labeling methods are methods that permanently detect the survival and proliferation of stem cells in vivo through genetic manipulation. There is a method in which the protein expressed by the reporter gene functions as an enzyme and emits a specific wavelength (GFP) and a method in which a contrast agent is additionally administered to the experimental animal (Fluc, D2R, NIS, HSV1-r39sTK). GFP: green fluorescent protein, Fluc: Photinus pyralis (firefly) luciferase, Tet: tetracycline, MRI: magnetic resonance imaging, D2R: dopa-mine 2 receptor, 18F-FESP: 3-(2’-[18F]fluoroethyl) spiperone, NIS: sodium iodide symporter, HSV1-r39sTK: herpes simplex virus 1 thymidine kinase, 18F-FHBG: 9-(4-(18)F-Fluoro-3-[hydroxymethyl]butyl) guanin.
Optical imaging
Cells expressing fluorescence proteins have been used to confirm protein expression at specific cellular sites and to track specific cell (Fig. 3). Using reporter gene imaging with fluorescent proteins, it is possible to observe the migration and movement of stem cells (53-57). However, fluorescence imaging is limited due to low signal-to-background ratios and autofluorescence (53, 58, 59). Various studies for in vivo stem cell imaging are being conducted using fluorescent proteins. One study showed that dynamic tracking of stem cells in an acute liver failure model was successfully achieved using fluorescent dyes in cells (60). In this study, green fluorescent protein (GFP)-ex-pressing ESCs were labeled with a DiR fluorescent dye. Acetaminophen 300 mg/kg was intraperitoneally adminis-tered to C57/BL6 male mice to form a liver injury model. Then, 5×106 cells of GFP-expressing ESCs labeled with DiR were transplanted into the spleen. ESCs implanted in the spleen in vivo were monitored via IVIS. In vivo ESCs were trapped inside the spleen 30 minutes after injection into the spleen and gradually moved to the splenic vein over time, and some were detected in the liver 3 hours later. This study presented a method that can evaluate the biodistribution and survival of transplanted cells through a relatively inexpensive method, and can be applied to cell therapy monitoring in that it is an easy-to-use method for experimenters. The cell mobility was analyzed according to the distribution of the fluorescence (61). However, in vivo cell imaging using GFP is difficult longer than two weeks after cell transplantation. Luciferases are used as bioluminescent reporters by catalyzing chemical reactions that produce light. Although various types of luciferases exist in nature, of which Photinus pyralis (firefly) luciferase (Fluc), Renilla reniformis (sea pansy) luciferase (Rluc), and Gaussia princeps (a marine copepod) luciferase (Gluc) have been studied in detail (62). Fluc emits a blue to yellow-green visible light with a wavelength of 490 to 620 nm (63). Since Fluc uses a different substrate than Rluc and Gluc, the production of light is distinct even in the same animal (61). However, the blue bioluminescence of Rluc and Gluc, which has a peak at 480 nm, is strongly absorbed by pigment molecules such as hemoglobin and melanin and has relatively more scatter by tissues, making it less suitable for in vivo imaging than Fluc (62). Bioluminescence imaging (BLI) can be also used to measure the expression of a specific protein in a cell and to monitor the transplanted cell in vivo (64). BLI is suitable for monitoring cell migration after stem cell transplantation. In one study, after the transplantation of stem cells in a myocardial infarction-induced mouse model using BLI, the cell location and cell survival patterns were analyzed and monitored over time (65). Recently, cell lines expressing luciferase were labeled with a fluorescent dye to analyze the optimal migration route and injection method for MSC migration to the target organ in vivo (66). The study evaluated the survival of syngeneic Luc-positive MSCs administered by different routes in non-obese diabetic (NOD) mouse model. The study injected MSCs through various routes, such as intravenous, intrapancreatic, intrasplenic, and subcutaneous. This method of labeling cells for monitoring can confirm the in vivo viability of specific cells after transplantation, and the expression of specific genes can be confirmed ex vivo. A study on the treatment effect of anaplastic thyroid cancer (ATC) by applying the Tet-On system to MSCs has been reported (67). A doxycycline (DOX)-controlled tetracycline (Tet) inducible system was developed using a retroviral vector expressing herpes simplex virus thymidine kinase (HSV1-sr39TK) with dual reporters (eGFP-Fluc2) in MSCs to develop the Tet-On system. The researchers constructed the MSC-Tet-TK/Fluc2 cell line with the Tet-On system and the MSC-TK/Fluc cell line without the Tet-On system. In an in vitro study, ATC (CAL62/Rluc) and engineered MSCs were cultured together, stimulated with DOX, and cell viability was measured according to the presence or absence of the prodrug ganciclovir (GCV). Fluc activity in vitro increased in a dose-dependent manner after DOX treatment in MSC-Tet-TK/Fluc cells, and no signal was confirmed in untreated cells. In vivo, we investigated the effect of GCV on the survival of MSC-Tet-TK/Fluc and CAK62 induced by DOX. In the left back of a nude mouse, 1.5×106 cells of MSC were separately injected in a 1:1 ratio with CAL62/Rluc cells. To confirm the decrease in ATC according to the presence or absence of the Tet-On system, 1.5×106 cells of MSC-Tet-TK and CAL62/Rluc cells were injected at a ratio of 1:1 into the right back of the same mouse. In an in vivo experiment, IVIS proved that GCV treatment reduced Rluc activity expressed in CAL62/Rluc when MSC was co-injected with ATC cells (CAL62/Rluc). The results of this study indicated that the MSC’s Tet-On/HSV-1-TK/GCV system induced the bystander effect. The suicide gene-based therapy using MSC identified in this study can be suggested as a treatment method for ATC, an aggressive malignant tumor.
MRI
MRI reporter genes contain a specific cellular receptor, an enzyme coding gene, and an endogenous reporter gene (68). The advantages of this reporter gene are that the signal does not weaken with cell division and the reporter gene is only expressed in viable cells, making it possible to track target cells in vivo indefinitely. With stem cell transplantation, it might be possible to alleviate many diseases, such as cardiovascular disorders, brain injuries, multiple sclerosis, urinary system diseases, cartilage lesions, and diabetes. Additionally, the reporter gene can be inserted under a specific promoter that is activated only when the stem cells differentiate into a specific phenotype, enabling specialized stem cell imaging to be implemented on a micro-scale (69). MRI reporter genes can be classified into three classes based on the types of encoded genes: reporter genes encoding an enzyme, such as tyrosinase and β-galactosidase; reporter genes encoding a receptor on the cell, such as transferrin receptor; and endogenous reporter genes, such as the ferritin reporter gene (70). Transferrin receptor and ferritin reporter genes are iron-based reporter genes. Various studies in MRI have demonstrated that the co-expression of ferritin and transferrin receptors of neural stem cells show signal loss in iron-rich environments (71-77). Tyrosinase and β-galactosidase are commonly used enzyme-based reporter genes for MRIs (71). The overexpression of human tyrosinase induces higher metal binding, which may result in enhanced MRI signal intensity. Additionally, MRIs based on the ferritin reporter gene can be used to trace the tendency of MSCs to accumulate in gliomas in vivo (78, 79). MSCs into which the reporter gene ferritin heavy chain (FTH1) was introduced were subcutaneously inoculated into nude mice, and signal changes in xenografts were observed through MRI in vivo. This study is a successful case of stem cell imaging in vivo through the change in T2 value after transduction of an MRI reporter gene into MSCs. And by developing a new MRI model based on FTH1 reporter gene expression, it is possible to more sensitively detect the occurrence of malignant transformation of MSCs. Stem cell mornitering in vivo has been studied by regulating MagA, a gene involved in iron transport and formation of forming magnetite (Fe3O4) crystals, through the Tet-On system (80). An mESC-MagA cell line was established through lentivirus transduction of Tet-MagA into mESC. Severe combined immune-deficient (SCID) mice were implanted by stereotactic injection with 1×105 cells of mESC-MagA and mESC-wild type, respectively, treated with Dox (1 μg/ml) and ferric citrate (25 μM) for 3 days. Through this experiment, we tried to monitor the suitability of mESC-MagA and mESC-wild type for 7T MRI in induced “ON” and non-induced “OFF” conditions in vivo. As a result, significant changes were shown in the transverse relaxation rate (R2 or 1/T2) and susceptibility weighted MRI contrast in the mESC-MagA cell line. Intracranial mESC-MagA grafts produced sufficient T2 and susceptibility weighted contrast at 7T. When DOX was injected into mice transplanted with mESC-MagA through diet, the presence of cells in vivo could be monitored through MRI. Based on these results, cells expressing MagA that can be controlled by the Tet-On system can be monitored non-invasively in vivo, and the status of repeated cell transplantation can be evaluated over a long period of time through repeated intake of ferric citrate and DOX. It was analyzed that the expression of MagA does not affect the function of mESCs at the in vitro level. In vivo, it was determined whether MagA expression in actual experimental animals was suitable for MRI and controllable imaging through the Tet-On system. The Tet-On induction system has the advantage of reducing the continuous accumulation of MagA and iron in vivo because it can inhibit the necessary expression of MagA. In view of these advantages, in vivo cell transplantation for stem cell-based therapy can be monitored only at a specific time point, which shows the possibility of reducing the burden of the body due to the accumulation of contrast agents in vivo.
Nuclear medicine imaging
For nuclear medicine imaging, there are various in vivo reporter gene in PET and SPECT imaging (81). Among the PET reporter genes, herpes simplex virus 1 thymidine kinase (HSV1-tk) is used for in vivo imaging using a substrate with a radioactive isotope such as 9-(4-(18)F-Fluoro-3-[hydroxymethyl]butyl)guanin (18F-FHBG) or 124I-FIAU (82, 83). Dopamine 2 receptor (D2R) is a protein expressed in the human striatum and pituitary and is a PET reporter gene that specifically pairs with radiolabeled compounds, such as 3-(2’-[18F]fluoroethyl)spiperone (18F-FESP) (84, 85). One study was conducted on whether D2R was suitable for non-invasive real-time imaging of stem cells in vivo by transplanting hMSC (86). The study succeeded in using D2R-overexpressing hMSC in vivo for imaging by utilizing the high sensitivity and high spatial resolution of the PET reporter gene system. In vitro, it was confirmed that the stem cell characteristics of hMSC were not changed by D2R, and in vivo, D2R-overexpressing hMSC (2.4×107 cells) was injected into the muscle of the hind limb of athymic nude rats. After transplantation, 20 MBq of 18F-fallypride was intravenously injected and mornitering was performed through PET. Specific signals in vivo were detected at the transplant site up to 7 days. Through this, the application period of D2R for periodic radioactive isotope injection and the location of hMSC relative to the injection site after cell labeling were confirmed in vivo. The sodium iodide symporter (NIS) reporter gene is a glycoprotein located in the basolateral membrane, which actively transports iodide (87, 88). The NIS introduces various radionuclides such as 131I, 123I, 125I, 124I, 99mTc, and 188Re into cells according to the purpose (89). The expression of NIS in stem cells could evaluate the survival rate and mobility of stem cells after transplantation in vivo, such as the transplantation of cardiac stem cells and the migration of MSCs to the breast cancer tumor stroma (90, 91). Since NIS is a non-immunogenic protein, it is an optimal reporter gene and gene therapy candidate. In addition, NIS can symport the radiotracer 99mTc-pertechnetate (99mTcO4-) for SPECT. Various NIS-expressing cells were imaged and monitored using 99mTcO4- in vivo (92, 93). NIS is also used as a therapeutic gene in research for stem cell therapy. MSCs that expressed NIS were used to image tumors through the recruitment of the MSCs to the tumor (94, 95).
Conclusion
This review described various methods for in vivo stem cell imaging (Table 1). Through direct and indirect labeling methods, the transplanted stem cell could be labeled and monitored in optical imaging, MRI, ultrasound, PET, and SPECT. Research on stem cells as a cell-based treatment and their clinical applications is ongoing. Future imaging technologies have the potential to monitor specific stem cells with high sensitivity and high resolution. In the future, more precise and complex in vivo cell imaging methods could be developed. Through this, new research directions for incurable diseases or previously unexplored fields may be presented.
Table 1.
Imaging techniques for stem cell labeling
| Goal | Strategy | Imaging modality | Labeling strategy | Cell type | Cells volume | Animals | Reference |
|---|---|---|---|---|---|---|---|
| Early cell localization and homing | Direct cell labeling methods | Optical imaging | QDs | Murine ESCs | 1×106 | Athymic nude mice | (20, 21) |
| Fluorophores | Murine ESCs | 5×106 | Gastric tumor-bearing mice | (22-24) | |||
| CPN | MSCs | 1×106 | Sprague Dawley rats | (25) | |||
| Magnetic imaging | SPIONs | Human umbilical cord MSCs | 1×106 | SCI induced rats | (26-28) | ||
| Fluorescent magnetic nanoparticles | MSCs | 3×106 | Liver cirrhosis mouse | (29) | |||
| Magneto electroporation | BMSCs | ∼1×106 | BALB/cmice | (30) | |||
| Magnetic particle imaging | SPIONs | MSCs | 5×106 ∼8×106 | Fischer 344 rats | (32) | ||
| Ferumoxytol nanoparticles | MSCs | 1×106 | C57B1/6 mice | (34) | |||
| CIONs-22s | BMSCs | ∼1×106 | BALB/cmice | (35) | |||
| Nuclear medicine imaging | 18F-FDG | MSCs | 8.5×104 | C57BL/6 mouse | (38-41) | ||
| Wistar rats | |||||||
| Indium-111 radiolabellediron oxide nanoparticles | Adipocyte-derived stem cells | 1×105 | NSG mice | (44) | |||
| Ultrasound imaging | ELS | hMSCs | 1×107 | Nude mice | (49) | ||
| TPSi NP | MSCs | 1×106 | BALB/c nude mice | (50) | |||
| GVs | Mouse MSCs | 1×107 | Arthritic rats | (51) | |||
| Long term monitering of cell viability | Indirect cell labeling methods | Optical imaging | GFP | ESCs | 5×106 | C57BL/6 mouse | (61) |
| Fluc | MSCs | 5×105 | FVB mice | (65) | |||
| MRI | Ferritin | MSCs | 2×106 | Wistar rats | (77) | ||
| Nuclear medicine imaging | D2R | hMSCs | 2.4×107 | Arthritic nude rats | (84) | ||
| NIS | MSCs | 1×106 | Athymic nude mice | (88, 89, 90-93) |
Labeling strategy for in vivo imaging methods is various depending on cell type, cell volume, animals and so on.
QDs: quantum dots, ESCs: embryonic stem cells, CPN: conjugated polymer based water-dispersible nanoparticles, MSCs: mesenchymal stem cells, SPIONs: superparamagnetic iron-oxide nanoparticles, SCI: spinal cord injury, BMSCs: bone marrow mesenchymal stem cells, CIONs-22s: cubic iron oxide nanoparticle, 18F-FDG: 2-deoxy-2-[18F]fluoro-D-glucose, NSG: non-obese diabetic/severe combined immunodeficiency/gamma, ELS: exosome-like silica nanoparticles, hMSCs: human mesenchymal stem cells, TPSi NP: cell-penetrating peptide (virus-1 transactivator of transcription) conjugated porous silicon nanoparticle, GVs: gas vesicles, GFP: green fluorescent protein, Fluc: Photinus pyralis (firefly) luciferase, MRI: magnetic resonance imaging, D2R: dopamine 2 receptor, NIS: sodium iodide symporter.
Funding Statement
Funding This research was supported by the Chung-Ang University Research Grants in 2021 to KOJ. This research was also supported by 2022 Advanced Facility Fund of the University of Seoul to DSL.
Footnotes
Potential Conflict of Interest
There is no potential conflict of interest to declare.
Authors’ Contribution
Conceptualization: SH, KOJ. Data curation: SH, KOJ. Formal analysis: SH, GWB, JJ, SR. Funding acquisition: DSL, KOJ. Investigation: SH, GWB, JJ. Methodology: SH, GWB, JJ. Project administration: DSL, SR, HKK, KOJ. Resources: SH, GWB, JJ. Software: SH, GWB, JJ. Supervi-sion: DSL, KOJ. Validation: DSL, SR, HKK, KOJ. Visualiza-tion: SH, KOJ. Writing – original draft: SH, GWB, JJ, KOJ. Writing – review and editing: SH, DSL, SR, HKK, KOJ.
References
- 1.Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med. 2008;3:1–5. doi: 10.2217/17460751.3.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol. 2016;2016:6940283. doi: 10.1155/2016/6940283.299fd671a7944b77afff0653031efe6f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leferink AM, Chng YC, van Blitterswijk CA, Moroni L. Distribution and viability of fetal and adult human bone marrow stromal cells in a biaxial rotating vessel bioreactor after seeding on polymeric 3D additive manufactured scaffolds. Front Bioeng Biotechnol. 2015;3:169. doi: 10.3389/fbioe.2015.00169.084e7c5cf3844c6fba70e5b7514f6d31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gubareva EA, Sjöqvist S, Gilevich IV, et al. Orthotopic transplantation of a tissue engineered diaphragm in rats. Biomaterials. 2016;77:320–335. doi: 10.1016/j.biomaterials.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Garzón I, Pérez-Köhler B, Garrido-Gómez J, et al. Evalua-tion of the cell viability of human Wharton's jelly stem cells for use in cell therapy. Tissue Eng Part C Methods. 2012;18:408–419. doi: 10.1089/ten.tec.2011.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson PA, Perera T, Marin D, et al. Double umbilical cord blood transplant is effective therapy for relapsed or refractory Hodgkin lymphoma. Leuk Lymphoma. 2016;57:1607–1615. doi: 10.3109/10428194.2015.1105370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boehm-Sturm P, Mengler L, Wecker S, Hoehn M, Kallur T. In vivo tracking of human neural stem cells with 19F magnetic resonance imaging. PLoS One. 2011;6:e29040. doi: 10.1371/journal.pone.0029040.6bd578a4ce194aa4adf19dde78d8d910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong H, Yang Y, Zhang Y, Cai W. Non-invasive cell tracking in cancer and cancer therapy. Curr Top Med Chem. 2010;10:1237–1248. doi: 10.2174/156802610791384234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Maslov K, Wang LV. Single-cell label-free photoacoustic flowoxigraphy in vivo. Proc Natl Acad Sci U S A. 2013;110:5759–5764. doi: 10.1073/pnas.1215578110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murakami T, Chun N. Bioluminescent imaging and organ-specific metastasis of human cancer cells [Internet] The International Society for Optics and Pho-tonics (SPIE); Bellingham: 2009. Dec 17, [cited 2023 Jun 1]. Available from: https://spie.org/news/2501-bioluminescent-imaging-and-organ-specific-metastasis-of-human-cancer-cells?SSO=1 . [DOI] [Google Scholar]
- 11.Iwano S, Sugiyama M, Hama H, et al. Single-cell biolu-minescence imaging of deep tissue in freely moving ani-mals. Science. 2018;359:935–939. doi: 10.1126/science.aaq1067. [DOI] [PubMed] [Google Scholar]
- 12.Herynek V, Turnovcová K, Gálisová A, et al. Manganese-zinc ferrites: safe and efficient nanolabels for cell imaging and tracking in vivo. ChemistryOpen. 2019;8:155–165. doi: 10.1002/open.201800261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Man F, Lim L, Volpe A, et al. In vivo PET tracking of 89Zr-Labeled Vγ9Vδ2 T cells to mouse xenograft breast tumors activated with liposomal alendronate. Mol Ther. 2019;27:219–229. doi: 10.1016/j.ymthe.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kircher MF, Gambhir SS, Grimm J. Noninvasive cell-tracking methods. Nat Rev Clin Oncol. 2011;8:677–688. doi: 10.1038/nrclinonc.2011.141. [DOI] [PubMed] [Google Scholar]
- 15.Youn H, Hong KJ. In vivo non invasive molecular imaging for immune cell tracking in small animals. Immune Netw. 2012;12:223–229. doi: 10.4110/in.2012.12.6.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Concilio SC, Russell SJ, Peng KW. A brief review of reporter gene imaging in oncolytic virotherapy and gene therapy. Mol Ther Oncolytics. 2021;21:98–109. doi: 10.1016/j.omto.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martelli C, Lo Dico A, Diceglie C, Lucignani G, Ottobrini L. Optical imaging probes in oncology. Oncotarget. 2016;7:48753–48787. doi: 10.18632/oncotarget.9066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michalet X, Pinaud FF, Bentolila LA, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalchenko V, Shivtiel S, Malina V, et al. Use of lipophilic near-infrared dye in whole-body optical imaging of hematopoietic cell homing. J Biomed Opt. 2006;11:050507. doi: 10.1117/1.2364903. [DOI] [PubMed] [Google Scholar]
- 20.Seleverstov O, Zabirnyk O, Zscharnack M, et al. Quantum dots for human mesenchymal stem cells labeling. A size-dependent autophagy activation. Nano Lett. 2006;6:2826–2832. doi: 10.1021/nl0619711. [DOI] [PubMed] [Google Scholar]
- 21.Lin S, Xie X, Patel MR, et al. Quantum dot imaging for embryonic stem cells. BMC Biotechnol. 2007;7:67. doi: 10.1186/1472-6750-7-67.a60bdb30a4f34ab690151668ec777612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan J, Song H, Li C, et al. DiR-labeled embryonic stem cells for targeted imaging of in vivo gastric cancer cells. Theranostics. 2012;2:618–628. doi: 10.7150/thno.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lassailly F, Griessinger E, Bonnet D. "Microenvironmental contaminations" induced by fluorescent lipophilic dyes used for noninvasive in vitro and in vivo cell tracking. Blood. 2010;115:5347–5354. doi: 10.1182/blood-2009-05-224030. [DOI] [PubMed] [Google Scholar]
- 24.Sun S, Chen G, Xu M, Qiao Y, Zheng S. Differentiation and migration of bone marrow mesenchymal stem cells transplanted through the spleen in rats with portal hyper-tension. PLoS One. 2013;8:e83523. doi: 10.1371/journal.pone.0083523.b515344b2b3748278a21284a018659eb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akhan E, Tuncel D, Akcali KC. Nanoparticle labeling of bone marrow-derived rat mesenchymal stem cells: their use in differentiation and tracking. Biomed Res Int. 2015;2015:298430. doi: 10.1155/2015/298430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu SL, Lu PG, Zhang LJ, et al. In vivo magnetic resonance imaging tracking of SPIO-labeled human umbilical cord mesenchymal stem cells. J Cell Biochem. 2012;113:1005–1012. doi: 10.1002/jcb.23432. [DOI] [PubMed] [Google Scholar]
- 27.Zare S, Mehrabani D, Jalli R, et al. MRI-tracking of dental pulp stem cells in vitro and in vivo using dextran-coated superparamagnetic iron oxide nanoparticles. J Clin Med. 2019;8:1418. doi: 10.3390/jcm8091418.45f4511c93a54217b7f18018df138c99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pang P, Wu C, Gong F, et al. Nanovector for gene transfection and MR imaging of mesenchymal stem cells. J Biomed Nanotechnol. 2015;11:644–656. doi: 10.1166/jbn.2015.1967. [DOI] [PubMed] [Google Scholar]
- 29.Kim TH, Kim JK, Shim W, Kim SY, Park TJ, Jung JY. Tracking of transplanted mesenchymal stem cells labeled with fluorescent magnetic nanoparticle in liver cirrhosis rat model with 3-T MRI. Magn Reson Imaging. 2010;28:1004–1013. doi: 10.1016/j.mri.2010.03.047. [DOI] [PubMed] [Google Scholar]
- 30.Walczak P, Kedziorek DA, Gilad AA, Lin S, Bulte JW. Instant MR labeling of stem cells using magnetoelectro-poration. Magn Reson Med. 2005;54:769–774. doi: 10.1002/mrm.20701. [DOI] [PubMed] [Google Scholar]
- 31.Zhou XY, Tay ZW, Chandrasekharan P, et al. Magnetic particle imaging for radiation-free, sensitive and high-contrast vascular imaging and cell tracking. Curr Opin Chem Biol. 2018;45:131–138. doi: 10.1016/j.cbpa.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du Y, Lai PT, Leung CH, Pong PW. Design of superparamagnetic nanoparticles for magnetic particle imaging (MPI) Int J Mol Sci. 2013;14:18682–18710. doi: 10.3390/ijms140918682.282299672aba4f159b667712a34d33bb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng B, von See MP, Yu E, et al. Quantitative magnetic particle imaging monitors the transplantation, biodistri-bution, and clearance of stem cells in vivo. Theranostics. 2016;6:291–301. doi: 10.7150/thno.13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sehl OC, Makela AV, Hamilton AM, Foster PJ. Trimodal cell tracking in vivo: combining iron- and fluorine-based magnetic resonance imaging with magnetic particle imaging to monitor the delivery of mesenchymal stem cells and the ensuing inflammation. Tomography. 2019;5:367–376. doi: 10.18383/j.tom.2019.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q, Ma X, Liao H, et al. Artificially engineered cubic iron oxide nanoparticle as a high-performance magnetic particle imaging tracer for stem cell tracking. ACS Nano. 2020;14:2053–2062. doi: 10.1021/acsnano.9b08660. [DOI] [PubMed] [Google Scholar]
- 36.Uenomachi M, Takahashi M, Shimazoe K, et al. Simulta-neous in vivo imaging with PET and SPECT tracers using a Compton-PET hybrid camera. Sci Rep. 2021;11:17933. doi: 10.1038/s41598-021-97302-7.5c1dd89e6a52433cb78a04570ae4efa4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong L, Zhao H, He Z, Li Z. In: Medical Imaging in Clinical Practice. Erondu OF, editor. InTech; 2013. Current perspectives on molecular imaging for tracking stem cell therapy; pp. 63–79. [DOI] [Google Scholar]
- 38.Jiang W, Chalich Y, Deen MJ. Sensors for positron emission tomography applications. Sensors (Basel) 2019;19:5019. doi: 10.3390/s19225019.0908d5f1944248c7a52b4b240891263d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapoor V, McCook BM, Torok FS. An introduction to PET-CT imaging. Radiographics. 2004;24:523–543. doi: 10.1148/rg.242025724. [DOI] [PubMed] [Google Scholar]
- 40.Cook GJ, Wegner EA, Fogelman I. Pitfalls and artifacts in 18FDG PET and PET/CT oncologic imaging. Semin Nucl Med. 2004;34:122–133. doi: 10.1053/j.semnuclmed.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Nose N, Nogami S, Koshino K, et al. [18F]FDG-labelled stem cell PET imaging in different route of administrations and multiple animal species. Sci Rep. 2021;11:10896. doi: 10.1038/s41598-021-90383-4.9212afd2f67542e299355bc54cce9b67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao M, Shi X, Zuo C, et al. Engineering of SPECT/photoacoustic imaging/antioxidative stress triple-function nanoprobe for advanced mesenchymal stem cell therapy of cerebral ischemia. ACS Appl Mater Interfaces. 2020;12:37885–37895. doi: 10.1021/acsami.0c10500. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Jokerst JV. Stem cell imaging: tools to improve cell delivery and viability. Stem Cells Int. 2016;2016:9240652. doi: 10.1155/2016/9240652.8f1af55894fb4a65970b22ba5e52b753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaw Thin M, Allan H, Bofinger R, et al. Multi-modal imaging probe for assessing the efficiency of stem cell delivery to orthotopic breast tumours. Nanoscale. 2020;12:16570–16585. doi: 10.1039/D0NR03237A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen F, Jokerst JV. Stem cell tracking with nanoparticle-based ultrasound contrast agents. Methods Mol Biol. 2020;2126:141–153. doi: 10.1007/978-1-0716-0364-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abou-Elkacem L, Bachawal SV, Willmann JK. Ultrasound molecular imaging: moving toward clinical translation. Eur J Radiol. 2015;84:1685–1693. doi: 10.1016/j.ejrad.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92:897–965. doi: 10.1152/physrev.00049.2010. [DOI] [PubMed] [Google Scholar]
- 48.Xu C, Feng Q, Ning P, Li Z, Qin Y, Cheng Y. Recent advances on nanoparticle-based imaging contrast agents for in vivo stem cell tracking. Mat Matters. 2021;16:2. [Google Scholar]
- 49.Chen F, Ma M, Wang J, et al. Exosome-like silica nanoparticles: a novel ultrasound contrast agent for stem cell imaging. Nanoscale. 2017;9:402–411. doi: 10.1039/C6NR08177K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi S, Zhang P, Ma M, et al. Cellular internalization-in-duced aggregation of porous silicon nanoparticles for ultrasound imaging and protein-mediated protection of stem cells. Small. 2019;15:e1804332. doi: 10.1002/smll.201804332. [DOI] [PubMed] [Google Scholar]
- 51.Gong Z, He Y, Zhou M, et al. Ultrasound imaging tracking of mesenchymal stem cells intracellularly labeled with biosynthetic gas vesicles for treatment of rheumatoid arthritis. Theranostics. 2022;12:2370–2382. doi: 10.7150/thno.66905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gawne PJ, Man F, Blower PJ T M de Rosales R, author. Direct cell radiolabeling for in vivo cell tracking with PET and SPECT imaging. Chem Rev. 2022;122:10266–10318. doi: 10.1021/acs.chemrev.1c00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang JH, Chung JK. Molecular-genetic imaging based on reporter gene expression. J Nucl Med. 2008;49 Suppl 2:164S–179S. doi: 10.2967/jnumed.107.045955. [DOI] [PubMed] [Google Scholar]
- 54.Müller-Taubenberger A. Application of fluorescent protein tags as reporters in live-cell imaging studies. Methods Mol Biol. 2006;346:229–246. doi: 10.1385/1-59745-144-4:229. [DOI] [PubMed] [Google Scholar]
- 55.Chudakov DM, Lukyanov S, Lukyanov KA. Fluorescent proteins as a toolkit for in vivo imaging. Trends Biotechnol. 2005;23:605–613. doi: 10.1016/j.tibtech.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Ke CC, Liu RS, Suetsugu A, et al. In vivo fluorescence imaging reveals the promotion of mammary tumorigenesis by mesenchymal stromal cells. PLoS One. 2013;8:e69658. doi: 10.1371/journal.pone.0069658.183006890e43408bb9c248320eb37121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Q, Wang X, Wu H, et al. Establishment of a dual-color fluorescence tracing orthotopic transplantation model of hepatocellular carcinoma. Mol Med Rep. 2016;13:762–768. doi: 10.3892/mmr.2015.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Troy T, Jekic-McMullen D, Sambucetti L, Rice B. Quan-titative comparison of the sensitivity of detection of fluorescent and bioluminescent reporters in animal models. Mol Imaging. 2004;3:9–23. doi: 10.1162/153535004773861688. [DOI] [PubMed] [Google Scholar]
- 59.Hoffman RM. Application of GFP imaging in cancer. Lab Invest. 2015;95:432–452. doi: 10.1038/labinvest.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ezzat T, Dhar DK, Malago M, Olde Damink SW. Dynamic tracking of stem cells in an acute liver failure model. World J Gastroenterol. 2012;18:507–516. doi: 10.3748/wjg.v18.i6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhaumik S, Lewis XZ, Gambhir SS. Optical imaging of Renilla luciferase, synthetic Renilla luciferase, and firefly luciferase reporter gene expression in living mice. J Biomed Opt. 2004;9:578–586. doi: 10.1117/1.1647546. [DOI] [PubMed] [Google Scholar]
- 62.Badr CE, Tannous BA. Bioluminescence imaging: progress and applications. Trends Biotechnol. 2011;29:624–633. doi: 10.1016/j.tibtech.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iyer M, Sato M, Johnson M, Gambhir SS, Wu L. Applications of molecular imaging in cancer gene therapy. Curr Gene Ther. 2005;5:607–618. doi: 10.2174/156652305774964695. [DOI] [PubMed] [Google Scholar]
- 64.Subramaniam D, Natarajan G, Ramalingam S, et al. Trans-lation inhibition during cell cycle arrest and apoptosis: Mcl-1 is a novel target for RNA binding protein CUGBP2. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1025–G1032. doi: 10.1152/ajpgi.00602.2007. [DOI] [PubMed] [Google Scholar]
- 65.van der Bogt KE, Sheikh AY, Schrepfer S, et al. Compa-rison of different adult stem cell types for treatment of myocardial ischemia. Circulation. 2008;118(14 Suppl):S121–S129. doi: 10.1161/CIRCULATIONAHA.107.759480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Preda MB, Neculachi CA, Fenyo IM, et al. Short lifespan of syngeneic transplanted MSC is a consequence of in vivo apoptosis and immune cell recruitment in mice. Cell Death Dis. 2021;12:566. doi: 10.1038/s41419-021-03839-w.c870d193b4064f9b8e3320f0af18e8c8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalimuthu S, Oh JM, Gangadaran P, et al. Genetically engineered suicide gene in mesenchymal stem cells using a Tet-On system for anaplastic thyroid cancer. PLoS One. 2017;12:e0181318. doi: 10.1371/journal.pone.0181318.9ca441cfcdb04debb51044b18b5f4cb3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao T, Wang P, Gong T, et al. Reporter genes for brain imaging using MRI, SPECT and PET. Int J Mol Sci. 2022;23:8443. doi: 10.3390/ijms23158443.7bab98650d014c0ca77d012a6e08ee5b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kraitchman DL, Bulte JW. Imaging of stem cells using MRI. Basic Res Cardiol. 2008;103:105–113. doi: 10.1007/s00395-008-0704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang C, Tian R, Liu T, Liu G. MRI reporter genes for noninvasive molecular imaging. Molecules. 2016;21:580. doi: 10.3390/molecules21050580.022ed8056b1f4a7fb5ef845aea873180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gilad AA, Ziv K, McMahon MT, van Zijl PC, Neeman M, Bulte JW. MRI reporter genes. J Nucl Med. 2008;49:1905–1908. doi: 10.2967/jnumed.108.053520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weissleder R, Moore A, Mahmood U, et al. In vivo magnetic resonance imaging of transgene expression. Nat Med. 2000;6:351–354. doi: 10.1038/73219. [DOI] [PubMed] [Google Scholar]
- 73.Ichikawa T, Högemann D, Saeki Y, et al. MRI of transgene expression: correlation to therapeutic gene expression. Neoplasia. 2002;4:523–530. doi: 10.1038/sj.neo.7900266.c62c2c31b86b4f0b85a5e26f2452e144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohen B, Dafni H, Meir G, Harmelin A, Neeman M. Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia. 2005;7:109–117. doi: 10.1593/neo.04436.75f7fd3e1feb45a68985f716d470fa01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cohen B, Ziv K, Plaks V, et al. MRI detection of transcriptional regulation of gene expression in transgenic mice. Nat Med. 2007;13:498–503. doi: 10.1038/nm1497. [DOI] [PubMed] [Google Scholar]
- 76.Genove G, DeMarco U, Xu H, Goins WF, Ahrens ET. A new transgene reporter for in vivo magnetic resonance ima-ging. Nat Med. 2005;11:450–454. doi: 10.1038/nm1208. [DOI] [PubMed] [Google Scholar]
- 77.Deans AE, Wadghiri YZ, Bernas LM, Yu X, Rutt BK, Turnbull DH. Cellular MRI contrast via coexpression of transferrin receptor and ferritin. Magn Reson Med. 2006;56:51–59. doi: 10.1002/mrm.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cao M, Mao J, Duan X, et al. In vivo tracking of the tropism of mesenchymal stem cells to malignant gliomas using reporter gene-based MR imaging. Int J Cancer. 2018;142:1033–1046. doi: 10.1002/ijc.31113. [DOI] [PubMed] [Google Scholar]
- 79.Sun J, Huang J, Bao G, et al. MRI detection of the malignant transformation of stem cells through reporter gene expression driven by a tumor-specific promoter. Stem Cell Res Ther. 2021;12:284. doi: 10.1186/s13287-021-02359-w.da18664f679441d69bfb4fc85c4dfd77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cho IK, Moran SP, Paudyal R, et al. Longitudinal monitoring of stem cell grafts in vivo using magnetic resonance imaging with inducible maga as a genetic reporter. Thera-nostics. 2014;4:972–989. doi: 10.7150/thno.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 82.Youn H, Hong KJ. In vivo noninvasive small animal molecular imaging. Osong Public Health Res Perspect. 2012;3:48–59. doi: 10.1016/j.phrp.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tjuvajev JG, Doubrovin M, Akhurst T, et al. Comparison of radiolabeled nucleoside probes (FIAU, FHBG, and FHPG) for PET imaging of HSV1-tk gene expression. J Nucl Med. 2002;43:1072–1083. [PubMed] [Google Scholar]
- 84.Peñuelas I, Haberkorn U, Yaghoubi S, Gambhir SS. Gene therapy imaging in patients for oncological applications. Eur J Nucl Med Mol Imaging. 2005;32 Suppl 2:S384–S403. doi: 10.1007/s00259-005-1928-3. [DOI] [PubMed] [Google Scholar]
- 85.Liang Q, Satyamurthy N, Barrio JR, et al. Noninvasive, quantitative imaging in living animals of a mutant dopamine D2 receptor reporter gene in which ligand binding is uncoupled from signal transduction. Gene Ther. 2001;8:1490–1498. doi: 10.1038/sj.gt.3301542. [DOI] [PubMed] [Google Scholar]
- 86.Schönitzer V, Haasters F, Käsbauer S, et al. In vivo mesenchymal stem cell tracking with PET using the dopamine type 2 receptor and 18F-fallypride. J Nucl Med. 2014;55:1342–1347. doi: 10.2967/jnumed.113.134775. [DOI] [PubMed] [Google Scholar]
- 87.Kitzberger C, Spellerberg R, Morath V, et al. The sodium iodide symporter (NIS) as theranostic gene: its emerging role in new imaging modalities and non-viral gene therapy. EJNMMI Res. 2022;12:25. doi: 10.1186/s13550-022-00888-w.7399ed72a2d84c20be101a3d6215c0fb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spitzweg C, Bible KC, Hofbauer LC, Morris JC. Advanced radioiodine-refractory differentiated thyroid cancer: the sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol. 2014;2:830–842. doi: 10.1016/S2213-8587(14)70051-8. [DOI] [PubMed] [Google Scholar]
- 89.Gu E, Chen WY, Gu J, Burridge P, Wu JC. Molecular imaging of stem cells: tracking survival, biodistribution, tumorigenicity, and immunogenicity. Theranostics. 2012;2:335–345. doi: 10.7150/thno.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Terrovitis J, Kwok KF, Lautamäki R, et al. Ectopic expression of the sodium-iodide symporter enables imaging of transplanted cardiac stem cells in vivo by single-photon emission computed tomography or positron emission tomo-graphy. J Am Coll Cardiol. 2008;52:1652–1660. doi: 10.1016/j.jacc.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dwyer RM, Ryan J, Havelin RJ, et al. Mesenchymal Stem Cell-mediated delivery of the sodium iodide symporter supports radionuclide imaging and treatment of breast cancer. Stem Cells. 2011;29:1149–1157. doi: 10.1002/stem.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharif-Paghaleh E, Sunassee K, Tavaré R, et al. In vivo SPECT reporter gene imaging of regulatory T cells. PLoS One. 2011;6:e25857. doi: 10.1371/journal.pone.0025857.0ea4ae1396b74ba491e13c829f5520f6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Price DN, McBride AA, Anton M, et al. Longitudinal assessment of lung cancer progression in mice using the sodium iodide symporter reporter gene and SPECT/CT ima-ging. PLoS One. 2016;11:e0169107. doi: 10.1371/journal.pone.0169107.d6246baf7cc9492692dea8a85eb76f80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Knoop K, Kolokythas M, Klutz K, et al. Image-guided, tumor stroma-targeted 131I therapy of hepatocellular cancer after systemic mesenchymal stem cell-mediated NIS gene delivery. Mol Ther. 2011;19:1704–1713. doi: 10.1038/mt.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Conrad C, Hüsemann Y, Niess H, et al. Linking transgene expression of engineered mesenchymal stem cells and angiopoietin-1-induced differentiation to target cancer angio-genesis. Ann Surg. 2011;253:566–571. doi: 10.1097/SLA.0b013e3181fcb5d8. [DOI] [PubMed] [Google Scholar]