Abstract
The application of robotic surgery technologies in neurological surgeries resulted in some advantages compared to traditional surgeries, including higher accuracy and dexterity enhancement. Its success in various surgical fields, especially in urology, cardiology, and gynecology surgeries was reported in previous studies, and similar advantages in neurological surgeries are expected. Surgeries in the central nervous system with the pathology of millimeters through small working channels around vital tissue need especially high precision. Applying robotic surgery is therefore an interesting dilemma for these situations. This article reviews various studies published on the application of brain and spine robotic surgery and discusses the current application of robotic technology in neurological cases.
Keywords: Robot surgery, Brain, Spine, Neurological surgeries
1. Introduction
Robotic surgery is developing for a broad range of surgical procedures. Robotic advanced technologies have been cooperated in the operating room, especially for microsurgical procedures [1]. It is obvious that robotic surgery can lead to important advantages against conventional surgeries; however, there are some concerns about it, such as higher costs [2,3], and having no higher clinical accuracy or effectiveness compared to freehand conventional methods in some reports [4,5]. Furthermore, there are limited evidence and theoretical/clinical benefits for using robotic surgery in some areas [6]. Recently, a warning was issued by the Food and Drug Administration (FDA) for robotic surgery applications in some surgeries such as breast and cervical cancer surgeries due to the lack of epidemiologic data [7]. Most of the previous studies are manufacturers’ claims, single-center reports [[8], [9], [10]], and technical statements or data [11], which may not be sufficient for a reliable conclusion about the use of robotic surgeries [12,13].
Because of its small structures and accurate procedures in neurosurgery, it is suitable for using advanced technologies such as robotic surgery technologies. Historically, Kwoh et al. in the late 1980s attempted to perform a brain biopsy with the aid of robotics for the first time. With the invention of the Gamma knife, as a simple and applicable supervisory-controlled robot, applications of robotic neurosurgery increased significantly. In 1992, an image-guided robotic surgery system named Robodoc® was developed for prosthetic hip replacement applications [14]. The ZEUS surgical robotic system was presented by Computer Motion in 1998. This system had surgical arms and instruments controlled by the surgeon. The ZEUS robotic system was used in 1998 (for the first time) at the Cleveland Clinic for uterine tube anastomosis surgery [15]. In 1998, the da Vinci system, the most successful robotic surgery platform until now, was introduced by Intuitive Company and obtained FDA approval for general laparoscopic procedures in 2000. The possible surgical procedures and applications are very wide, including mitral valve repair, thoracoscopic internal mammary harvesting, cholecystectomies, and brain surgeries [16]. The Computer Motion Company merged with Intuitive Surgical into a single company In 2003, and this resulted to discontinuing the development of the ZEUS system. Despite these two leadership big companies, there are several companies that developed surgical systems for special applications such as Mazor robotics (Medtronix, Caesarea, Israel) for spinal surgeries and neuroArm robotic systems by the University of Calgary for brain surgeries.
One of the first modern applications of robotic surgery is CyberKnife, developed by John Adler [17]. Although this system is a therapeutic X-ray generator and is limited to radiotherapy applications, CyberKnife is the first platform where the entire procedure can be executed with full remote control and without direct surgeon-patient contact. Due to the potential of this field and its clinical benefits, it has been increased and led to the invention and development of multiple systems [18].
Development and clinical applications of robotic surgery systems were increased rapidly in the past three decades. The minimally invasive robotic surgery platforms such as the da Vinci, NeuroArm, and SpinAssist surgical systems have experienced great increases in clinical applications and commercialization [19]. Neurosurgery is not a field with high use of robotic-aided surgery techniques, probably due to the complexity of neurosurgical operations compared to other anatomical regions; however, it seems that neurological surgery is appropriate for robotic surgery applications. Robotic surgery provides several benefits, such as remote control of the surgical procedure, higher quality, more accurate vision of the surgical site, motion scaling, higher accuracy of the surgical incision, and a higher degree of freedom. Applying robotic surgery is, therefore, an interesting technique for these situations.
There are many studies evaluating robotic surgery in brain and spinal surgeries, and it will be informative and applicable to present their important findings and outcomes in a systematic review. This article reviewed published studies and summarized the important findings of robotic surgical procedures in the brain and spine.
2. Methods
2.1. Searched databases and eligibility criteria
We performed a systematic search in electronic databases, including Science Direct, PubMed, Scopus, MEDLINE, POPLINE MEDLINE (Ovid), ProQuest, AIM, ELDIS, and CINAHIL to identify the current knowledge about the applications of robotic surgery in neurological cases.
Several terms and words were searched in combination and/or singly, including robotic surgery [*], neurological cancers [*], neurological surgeries [*], brain [*], spine [*], central nervous system [*], cancer surgery [*], and robotic [*]. These terms were searched in keywords, abstract, title, and the main text of the databases indexed articles. Furthermore, the founded articles were screened to identify original publications. Published manuscripts during the past 25 years, from 1997 to 2022 were considered. The founded article references were also assessed to identify additional studies that may have been older than the time frame. It must be noted that just the articles with available full texts were considered in our search.
2.2. Review criteria
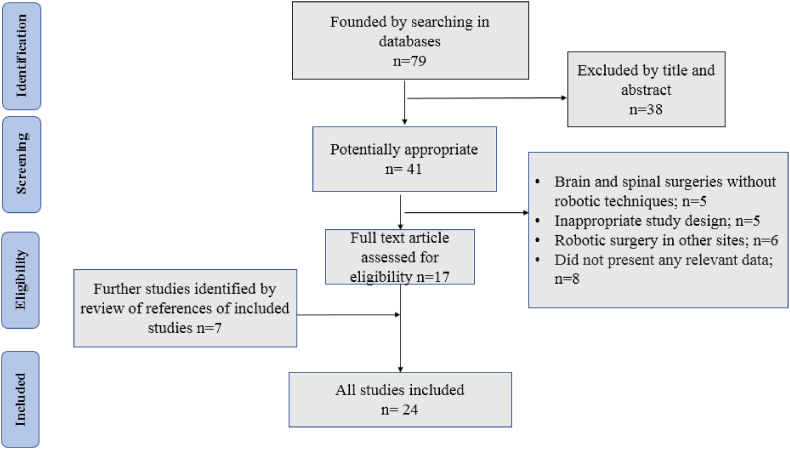
In this systematic review, PRISMA guidelines were followed for study selection and data extraction [20]. Screening the titles and abstracts of the relevant databases (eligibility criteria) were carried out by two independent reviewers, and a third reviewer resolved any probable disagreement. A manual search and a gray literature search were performed in order not to ignore eligible articles. The search and selection criteria were restricted to the English language of the article and original/review/case report/technical report studies. Fig. 1 illustrates the search and selection procedures.
Fig. 1.
The search and selection procedure of the reviewed studies.
For data extraction, we considered several variables, including performed data extraction and outcome measures for the following variables: type of robotic surgery system, type and number of patients/samples, grouping, patient outcomes, surgery-followed complications, and the most important findings/outcomes pointed out by the authors.
During the data extraction, the risk of bias was assessed for each study. Notably, there was a risk of publication bias in the current study. Patients’ outcomes were the primary outcome and surgery complications and the type of robotic system were considered the secondary outcomes of the study.
3. Results
3.1. Study selection
The search and selection process and the number of founded articles are presented in Fig. 1. In summary, after screening abstracts of eligible studies, and removing duplicates and unrelated records, 79 relevant articles were found. The animal, experimental, review, case report, and technical studies were excluded, and after reviewing the full texts, 24 studies met the inclusion criteria, used for further analysis.
3.2. History of robotic surgery
Minimal-invasive surgical techniques came into clinical practice in the late 1980s. There are several limitations for surgeons in these surgeries, such as loss of wrist articulation, fulcrum effect, and limited two-dimensional vision. Robotic surgery techniques have the potential to remove/decrease many of these limitations [21].
National Aeronautics and Space Administration (NASA) offered to develop a technique for providing surgical procedures for astronauts in 1972. In the late 1980s, Stanford Research Institute researchers tried to develop a method for increasing surgeons' skills. The scientists of Stanford Institute developed a system, and subsequently, their success motivated Defence Advanced Research Projects Administration (DARPA). They planned to improve this system to a telesurgical clinical system for performing essential life-saving surgeries on soldiers from experienced surgeons from far distances. Commercial telerobotic systems were introduced in 1995 by the Intuitive Surgical Corporation in a master-slave model. Since then, the applications of robotic surgeries have been successfully expanded to surgical disciples like gynecology, urology, and cardiology [22]. The enthusiasm for robotic surgery applications in neurology is a response to the need for minimally invasive surgical procedures with similar or better benefits compared to primary surgery and ongoing technological advances in imaging and robotic techniques. However, robotic surgical procedures have not obtained wide applications in neurological surgeries, probably due to their limitations in brain and spine regions.
Three classifications of robotic surgery systems were described by Nathoo et al. according to the robot–surgeon interaction [23]. Supervisory-controlled robotic system is the first classification. In this system, the robotic surgery procedure is programmed and then supervised by the surgeon during the intervention as the robot performs its programmed movements. Robotic telesurgery is the second system which is controlled and programmed by the surgeon in real-time through remote access. Shared control robotic surgery is the third one. In this system, a surgeon controls the movements and interventions of the robot as the robot enhances the surgeon's skills through dexterity enhancement, with some mechanical solutions for human limitations.
3.3. Robotic systems in brain surgeries
Based on the “Report of Brain Tumor Registry of Japan”, the survival rate (5-year) for malignant glioma for complete removal was 40 %; 95 % or more removal was 22 %; and under 95 % removal was 10–15 % [24], indicating that survival rates are more than halved with under 95 % resection in comparison with complete resection. Similar results were reported by Hentschel [25], Lacroix [26], and Claus [27]. Surgery is an important procedure, which indicates the first step for glioma treatment, with the aim of complete removal alongside preserving neurological function.
Several robotic ways have been expanded for the specific challenges associated with brain surgeries [[25], [26], [27], [28], [29]]. Performing pathology procedures for deep-sited tissues and open brain surgeries may lead to severe trauma to the parenchyma. This issue has resulted in the development of novel techniques and systems, such as robotic brain surgeries to minimize brain tissue damage [26,29].
Coupling robotic surgery systems with different imaging devices can increase and develop the precision of the surgery procedures and have some advances due to better feedback to the surgeon.
One of the simplest and most applicable supervisory-controlled robots is a particular kind of the Leksell Gamma Knife radiosurgical system. An automated system accurately adjusts the patient's head position based on geometrical definitions in a calculated radiotherapy plan. Although the Gamma knife is a radiotherapy system and not a real surgical system, it can replace the surgical procedures in the brain. Several studies reported the benefits and advantages of the robotic Gamma knife automatic system such as shorter adjustment times, reduction in patients and personnel radiation doses, and higher accuracy in radiation delivery to smaller targets [30,31].
NeuroMate (Integrated Surgical Systems, Sacramento, CA, USA) system was the first FDA-approved robotic system for neurosurgery [27]. This robotic device uses a robotic arm that can move in different pre-programmed directions guided by a navigation system for neurosurgical applications and stereotactic biopsy procedures [32].
Five prototyped systems for robotic microsurgery have existed, and only three of them can provide force feedback or haptic sensation, including NeuRobot [33], ROBOCAST [34], and neuroArm [35]. NeuRobot and neuroArm are the two systems applied to human surgeries, in which only the neuroArm remains in clinical use.
The Minerva project (University of Lausanne, Lausanne, Switzerland) used a CT scanner to provide 3-dimensional images and real-time cross-sectional as well as a robotic arm for brain surgery procedures. However, this project was discontinued due to radiation safety issues [1].
Recent studies have reported that NeuroMate localization and targeting performances and accuracies are similar to standard localizing systems [36]. Varma et al. reported a good accuracy for microelectrode placement in patients having Parkinson's disease with the use of NeuroMate system [37].
Another robotic surgery device was the Evolution 1 robotic system (Universal Robot Systems, Schwerin, Germany) was successfully assessed for various neurological surgeries such as endoscope-assisted pituitary adenoma resections and pedicle screw placements. It was reported that these applications were too cumbersome and time-consuming [38].
Another robotic surgical system named Neurobot telerobotic has been evaluated and applied successfully in neurosurgical complex procedures [33]. Goto et al. described the assistance of this system in craniotomy for the resection of superficial portions of a tumor. They reported enhancement in surgical dexterity as one of the advantages of using this system [31]. Goto et al. presented the NeuRobot, a tele-controlled microscopic system planned for neurosurgical procedures [31], consisting of a 3-dimensional endoscope and three robot arms that the surgeon operates without direct contact with the patient. Their system was successful in clinical neurosurgery.
Da Vinci surgical system is the most common and FDA-approved robotic surgical system for general and gynecologic surgeries. Furthermore, it has become a standard device for prostatectomy surgical procedures [39]. It can also be used for neurosurgical applications due to its capabilities, including an image-guided system, high degree of freedom in motion, minimally invasive surgery, and full range motion mimicking the human wrist. It was reported that using Da Vinci surgical system can have advantages over conventional surgical procedures such as higher accuracy, lower bleeding and trauma in brain tissues, and shorter patient recovery times [40]. However, it was also reported that this system may increase the surgery procedure time and has no higher accuracy in brain surgeries compared to the freehand technique [40]. Our review showed that there are just two studies reporting the accuracy of the Da Vinci system in brain surgeries and it seems that there is a need for more research evaluating the accuracy and usefulness of the Da Vinci robotic device in brain surgeries.
Identifying the lesion location is critical for brain tumor removal, which is improved by introducing navigation systems using medical imaging technology. Additionally, connecting tumor-detecting sensor systems like fluorescence sensors with a precise robotic arm would allow further development in tumor removal.
Sutherland et al. introduced a sensory robotic surgery workstation in which the surgeon can review and use the imaging data without interrupting the procedure of surgery [41]. The workstation composed of hand controllers, and can present a 3-dimensional view of MR images, a virtual image of the manipulators and a stereoscopic view of the operative field. This system was utilized initially for central nervous system neoplasia and cavernous angioma, indicating high accuracy.
Stummer reported that survival rates of brain tumor removal would improve by introducing new technologies like 5-ALA [28]. Although image-guided surgery improves the tumor removal rate, high levels of surgical skill or accurate techniques are still required in malignant tumors and the tumor is located around neural structures. Morita et al. reported the application of a micro-neurosurgery robotic system for deep brain surgery procedures [32]. It was reported that their system enhanced dexterity and maneuverability in deep surgical areas. NeuroArm was presented by Sutherland et al. as a surgical system equipped with two robotic arms [42]. It was reported that this system can be used during MR imaging. Image guiding during surgical procedures can improve tumor removal due to better identification of lesion location, size, and extension.
An important point of using robotic systems in neurosurgery is that their accuracy and precision are combined with the executive capacity of the human brain due to controlling the robotic system by a surgeon [[42], [43], [44]]. A robot with sub-millimeter accuracy and precision provides a viable solution for the need for high accuracy in minimally invasive surgeries on the brain. Furthermore, computer technology of the robotic surgery system with an imaging device can provide safer surgery directions and forbidden surgical zones; therefore, it can increase the safety and efficiency of the surgery procedure.
We tried to summarize and provide important findings of the past studies assisting the robotic surgery systems for the brain in Table 1.
Table 1.
Main findings of the previous studies evaluating the brain robotic surgery systems.
| Study | Robotic surgery system | Sample | Main findings |
|---|---|---|---|
| Varma et al. (2003) [37] | The frameless NeuroMate robot (Integrated Surgical Systems, Sacramento, CA, USA) | 50 patients underwent movement disorder surgery (MDS) | A significant improvement in motor scores at 6 (43 %) and 18 months (51.7 %). This robot can be used for MDS procedures based on only MRI data. |
| Nimsky et al. (2003) [38] | Evolution 1 (Universal Robot Systems, Schwerin, Germany) | Two patients with large invasive pituitary for transsphenoidal surgery. | This robotic system can be used for endoscopic skull base surgeries, and simultaneous application of two instruments under endoscopic view is possible in this system. |
| Goto et al. (2003) [31] | The NeuRobot telecontrolled microscopic micromanipulator system | Case report: a 54-year-old man who had a recurrent atypical meningioma | A part of the tumor was eliminated with use of NeuRobot by the aid of microscopic observation. No related complication was observed and reported after the operation and during the post-operative care course. |
| Arata et al. (2011) [45] | Novel intelligent Neurosurgical instruments consisted of a surgical robot, a master device and operating software. |
A phantom study | Mechanical tests on the components and a preliminary system evaluation were carried out. The test was performed in a phantom model, and a tumor-removal procedure was successfully carried out with the use of prototype intelligent neurosurgical instruments. |
| Hong et al. (2013) [46] | da Vinci Surgical System | Two fresh cadaver heads | The supraorbital trans-eyebrow keyhole was used for entering the robotic surgical arms. The carotid artery, third cranial nerve, optic nerve, and optic chiasm were visualized using the standard microdissection techniques. This system provides a broader vision compared to standard microsurgical systems for neurosurgeons. |
| Marcus et al. (2015) [40] | da Vinci Surgical System | One cadaver head | This system has great potentials to enhance the performance of transcranial minimally invasive surgeries. However, It has several limitations such as higher procedure time, loss of some sensational feedbacks, and lower degree of freedom. There is a need for researches and improvements in next-generation robots, better suited to keyhole neurosurgery procedures. |
| Sutherland et al. (2015) [41] | NeuroArm image-guided robot | Surgical treatment of glioma in 18 patients | This system can increase the safety and accuracy of brain surgeries with the aid of several novel features like augmented force feedback, haptic high-force warning, and virtual fixtures. |
| Morita et al. (2005) [32] | prototype MM-1 micro-neurosurgery robotic system | 20 Wistar rats, and several cadavers. | This robotic system improved the accuracy of pointing in the deep surgical field. The authors reported that they successfully closed the partial arteriotomy and anastomosed the rat CAs. The robotic instruments can move satisfactorily in cadavers, but the manipulators need to be smaller to fit into the narrow intracranial space. Furthermore, enhanced dexterity and maneuverability in deep surgical areas were reported. |
| Sutherland et al. (2008) [35,42] | MR-compatible image-guided neuroArm robot | 781 patients with glioma, meningioma, and pituitary adenoma | The robotic surgery improved surgical careers by decreasing tremors and increasing accuracy, and precision. The neuroArm system includes a haptic interface, and it is able to deform forces in both normal and pathological states. |
| Sutherland et al. (2013) [44] | Clinical integration of neuroArm, an MR-compatible image-guided robot, into surgical procedure | 35 cases with varying pathology | For the first 35 neuroArm cases, only 1 adverse event was encountered. Applying of neuroArm for routine dissection illustrates that robotic microsurgery procedures can be successfully performed with minimum adverse effects. Karnofsky's performance status scores were significantly improved postoperatively and at the 12-week follow-up. |
3.4. Robotic systems in spine surgeries
There are several robotic systems developed for the challenges in spinal surgery. These devices are enhanced by improvements and advances in intraoperative image guiding systems. Previous studies have focused on the accurate placement of spinal instrumentation [[47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]]. Furthermore, intra-operative radiotherapy (radiosurgery) can be assumed as one of the common spine robotic surgery methods; however, it's not a real surgery technique and can be used as an adjuvant or alternative method for surgery. In radiosurgery, robotics can track the spine movements due to respiration, and irradiate with high accuracy to the target tissue [58].
Like the cranial radiosurgical applications, the most common robotic subtype in spinal stereotactic radiosurgery is a supervisory-controlled system Cyberknife (Accuracy, Sunnyvale, CA, USA) relies on a calculated radiotherapy plan for focused beam radiotherapy. This system can adjust the trajectory of radiation fields using feedback mechanisms to correct patient respiration movements.
Furthermore, a RoboCouch Patient Positioning System (Accuracy) uses a similar technology of Cyberknife system to reposition the patient during the course of treatment.
For conventional spinal surgeries, there are several types of developed robotic systems [59,60]. Most of the previous studies used three robotic systems including the ROSA® robot (Medtech, Montpellier, France), SpineAssist (MAZOR Surgical Technologies, Caesarea), and Renaissance Surgical Guidance Robot (Mazor Robotics Ltd., Caesarea). These systems were used mainly for spinal screw and pedicle placement procedures. These devices, coupled with image-guided navigation systems, were tested for accurate pedicle screw placement.
Most of the studies reported higher or similar accuracy of robotic systems compared to conventional freehand surgeries [48,51,53,54,57,61]. However, the reported overall procedure times were lower in freehand surgeries [48,50,51,56,62,63].
The SpineAssist was evaluated more than other robotic systems. Lieberman et al. evaluated the SpineAssist (MAZOR Surgical Technologies, Caesarea) miniature robot for the two pedicle and translaminar facet screw placement [59]. The system consists of a passive arm mounted on a fixed part of the axial skeleton. The SpineAssist device is currently FDA-approved for spinal instrumentation. Most of the previous studies reported higher accuracy in instrument placement for the SpineAssist system compared to freehand surgeries. However, there are several studies reporting lower accuracy of this system. It seems that other robotic surgery systems, such as ROSA® robot needs more research to conclude about their accuracy compared with the freehand technique.
There are several studies determining improved screw placement accuracy using robotics in spinal surgery in comparison with conventional fluoroscopy-guided and navigation-guided screw placement [48,49,63]. Positional and force information during robotic surgery can be recorded for future analysis. This information can also be used for quality assurance of the robotic system and whole surgical procedure and case rehearsal. New surgeons unfamiliar with robotic systems can use the case rehearsal data in a virtual reality simulator to obtain initial experience in a more comfortable, less stressful, and safer manner. The recorded positional and force data can also be used for developing surgical simulators [64].
In Table 2, important findings of the previous studies evaluating the spine robotic surgery systems were summarized.
Table 2.
Main findings of the past studies evaluating the spine robotic surgery systems.
| Study | Robotic surgery system | Sample | Main findings |
|---|---|---|---|
| Lieberman et al. (2006) [60] | SpineAssist (MAZOR Surgical Technologies, Caesarea) and Hover-T frame in conjunction with the PathFinder system (Spinal Concept Inc., Austin, TX). | A cadaver lumbar spine | The average measured discrepancy between the planned and actual screw trajectories by a CT scan was 1.02 ± 0.56 mm. The authors reported that their results support the system's use in minimally invasive spine surgeries. |
| Choi et al. (2000) [47] | Fluorotactic guidance system (Z-Kat, Inc., Miami, FL) | Pedicle screw placement was carried out in six cadavers from T1 to S1 levels | The first fluoroscopy-based system for targeting the location of pedicle screws. The accuracy was similar to a conventional method, especially in the region of T9-L5. |
| Kantelhardt et al. (2011) [48] | SpineAssist (MAZOR Surgical Technologies, Caesarea) | Thirty-five patients underwent percutaneous, 20 open robotic-guided, and 57 open conventional pedicle screw placement. | The accuracy of screw positioning was increased with the use of this robotic system and X-ray exposure reduced. Patients seem to have a better perioperative course following percutaneous procedures. Accuracy of screw placement: Freehand ‐ 91.4 %; Robotic ‐ 94.5 % |
| Schizas et al. (2012) [49] | SpineAssist (MAZOR Surgical Technologies, Caesarea) | 11 patients, (robotic group), and 23 other patients (conventional fluoroscopic group) were instrumented with 64 pedicle screws. | No complications were reported after robotic surgery. Pedicle screw accuracy was 79 % in the robotically assisted group and 83 % in the fluoroscopic group. |
| Ringel et al. (2012) [50] | SpineAssist (MAZOR Surgical Technologies, Caesarea) | 60 patients (Freehand, 152; Robot-assisted, 146). | 93 % of screws had good positions (A or B) in freehand, and 85 % in robot-assisted. Surgical procedure time was lower in freehand (84 min) compared to robot-assisted (95 min) surgery. Most of the malpositioned screws in the robotic surgery group demonstrated a lateral deviation. |
| Schatlo et al. (2014) [51] | SpineAssist (MAZOR Surgical Technologies, Caesarea) | The robot cohort (Group 1; 55 patients, 244 screws) included an initial open robot-assisted subgroup (Subgroup 1 A; 17 patients) and a percutaneous cohort (Subgroup 1 B, 38 patients); fluoroscopy-guided cohort (Group 2; 40 patients, 163 screws) | Accuracy of screw placement: Freehand ‐ 87.1 %; Robotic‐guided Open surgery ‐ 90.4 %; Robotic‐guided Percutaneous surgery‐ 91.9 %. Robot-guided pedicle screw placement is a useful and safe tool for assisting spine surgeons in spine surgeries. However, It was proposed to consider fluoroscopy backup due to technical difficulties. |
| Dreval et al. (2014) [52] | SpineAssist (MAZOR Surgical Technologies, Caesarea) | 77 patients | This system enables minimal-invasive spine interventions with high accuracy (97.2 %) and safety for screw placement. A new technology, Guided Oblique Lumbar Interbody Fusion (GO-LIF), the procedure was performed for microdiscectomy and decompression of the spinal canal. It was reported that the fusion of spinal segments using the GO-LIF was not possible without the SpineAssist robotic system. |
| Molliqaj et al. (2017) [53] | SpineAssist (MAZOR Surgical Technologies, Caesarea) | Robot-assisted cohort (98 patients, 439 screws) freehand fluoroscopy-guided cohort (71 patients, 441 screws) | In the robot-assisted cohort, Grade A perfect (trajectories) were observed for 83.4 % of screws. In the fluoroscopy-guided group, grade A screws were found in 76 % (n = 335). Freehand ‐ 88.9; Robotic ‐ 93.4 |
| Lefranc and Peltier (2016) [65] | The ROSA® robot (Medtech, Montpellier, France) | 38 percutaneous transpedicular screws (between D8 and S1) implanted in two separate cadaver | Thirty-seven screws (97.4 %) were fully contained within the pedicle. The ROSA® Spine robot coupled with intraoperative flat-panel CT can performed highly accurate pedicle screw placement. High accuracy and safety in the treatment of degenerative lumbar disc diseases were reported with this system. |
| Lonjon et al. (2016) [54] | The ROSA® robot (Medtech, Montpellier, France) | ROSA group (10 patients, n = 40 screws); Freehand group (10 patients, n = 50 screws) | Accurate placement of the implant (score A and B) was reported in 97.3 % in ROSA robotic-assisted group and in 92 % of freehand group patients. |
| Solomiichuk et al. (2017) [55] |
SpineAssist (MAZOR Surgical Technologies, Caesarea) | 70 patients with the metastatic spinal disease who required instrumentation (35 patients with robotic surgery) | Accuracy of screw placement (Grade A or B): Freehand ‐ 83.6 %; Robotic ‐ 84.4 % |
| Hyun et al. (2017) [61] | Renaissance Surgical Guidance Robot (Mazor Robotics Ltd., Caesarea) |
robotic-guided and fluoroscopic-guided open surgery (30 patients in each group) | Robotic-guidance surgical procedures reduced radiation exposure and surgical overall time remarkably. Surgical technique did not affect the patient outcomes. Accuracy of screw placement: Freehand ‐ 98.6 %; Robotic ‐ 100 % |
| Kim et al. (2017) [56] | Renaissance Surgical Guidance Robot (Mazor Robotics Ltd., Caesarea) |
Robot: 37 patients; Freehand: 41 patients | Robotic-assisted pedicle screw placement contributed to fewer proximal facet joint violations and better convergence orientations. Accuracy (%): Freehand ‐ 99.4; Robotic ‐ 99.4 |
| Keric et al. (2017) [57] | Renaissance Surgical Guidance Robot (Mazor Robotics Ltd., Caesarea) |
Freehand fluoroscopy-guided surgery: 24 patients percutaneous robot-assisted: 66 patients | Robot-guided pedicle screw placement was effective and safe procedure in thoracic and lumbar spondylodiscitis with lower radiation dose, and decreased complication rates. Accuracy (%): Freehand ‐ 73.5; Robotic ‐ 90 |
3.5. The accuracy of robotic surgery in brain and spine
Robotic surgeries have been performed successfully in many anatomical regions related to different medical specialties. Previous studies applying robotic surgery reported higher accuracy in some procedures in brain and spinal surgeries, such as improved screw accuracy [48,49,54,63,66]. However, robotic surgery has several limitations, such as registration failure, soft-tissue hindrance, higher procedure time, higher costs, and lateral drill guide skiving [66]. These limitations hindered robotic surgery's widespread application [50,54,62]. It must be mentioned that there are not enough studies to conclude about the accuracy of all robotic surgical systems. We can just judge the accuracy of some surgical systems such as SpineAssist and Renaissance Surgical Guidance Robot for spine surgeries and neuroArm for brain surgeries.
There are also some studies that reported lower accuracy of robotic surgery in comparison with freehand operation. For instance, Ringel et al. [50] reported reduced screw accuracy in the robotic-assisted lumbosacral screws. These lower accuracies may be related to the robotic technology used in the study. They used the SpineAssist (MAZOR Robotics Inc®, Orlando, Florida) system in their study, and the authors related the robotic inaccuracy to K-wire placement instability, drill skiving on bony surfaces, movements of the bone mount, and drill sleeve lateral docking.
Higher screw placement accuracy in comparison with the freehand method was reported by Lonjon et al. [54] using the ROSA robotic system (Medtech S.A., Montpellier, France). Although the ROSA system requires K-wires to be placed before the pedicle screws, it is registered to the patient frame and can move independently.
Imaging is an important parameter in robotic surgery systems that can directly affect surgery accuracy. Intraoperative imaging or real-time imaging can show the cutting and placement of instruments and therefore is an appropriate tool to enhance the accuracy of surgical procedures. However, its application, along with a guide arm, can reduce surgeon physical fatigue. Intraoperative imaging can be used to detect the optimal surgical trajectory and therefore leads to a surgical operation with less invasions and side effects [48,49,63,67,68]. Challenging screws placement situations such as C1–C2 trans articular screws, L5-S1 trans-discal screws, and iliac screws, or in anatomic regions with anomalous structures such as surgical tumor resections near sensitive organs or operations needing a steep surgical trajectory can be obtained special higher benefits from intraoperative real-time imaging [48,62,[68], [69], [70], [71]].
4. Discussion
4.1. Spine robotic surgery
In spine surgery, appropriate instrumentation to supplement bony fusion remains critically important. Surgical robots have been developed for their ability to improve spinal instrumentation techniques. There are several robotic systems for spine surgeries; however, three robotic systems, including the ROSA® robot, SpineAssist, and Renaissance Surgical Guidance Robot, have been evaluated higher than other systems in previous studies. These systems were coupled with image-guided systems and showed high accuracy in spinal screw and pedicle placement procedures. The Mazor robot (SpineAssist or Renaissance) has been studied more compared to other systems. This system is a miniature robot with 6 degrees of freedom [60,72,73]. The robot uses intraoperative fluoroscopic imaging registered on pre-surgery CT simulation images and guides the surgeon to the appropriate trajectory. The ROSA robot consists of a mobile floor-fixed base attached to a robotic arm (with 6 degrees of freedom). A second mobile base has a mounted navigation imaging system. Furthermore, intraoperative fluoroscopy or intraoperative CT can be used for ROSA surgical planning [65,74].
Most studies reported higher or similar accuracy of robotic systems compared to conventional freehand surgeries [48,51,53,54,57,61]. However, the reported overall procedure times were lower in freehand surgeries [48,50,51,56,62,63].
Some of the previous studies explained that using robotic surgical systems in spine pedicle screws allows for avoiding the proximal facet joint violation and therefore preventing the development of adjacent-segment disease [75]. There are no large-scale investigations directly comparing robotic guidance of pedicle screws with image guidance. It was shown that Image guidance provided using intraoperative cone-beam CT is superior to freehand placement [76]. The ROSA robot does incorporate image guidance into its system, as mentioned previously.
Ionizing radiation exposure to the surgeon and operating room staff is a concern in spine surgeries [[77], [78], [79]]. Therefore, one of the robotic surgery potential advantage is minimizing the reliance on intraoperative fluoroscopy and radiation exposure. However, past robotic systems have several disadvantages, consisting of the requirement for the placement of Kirschner wires (K-wires), the potential for miss-registration due to patient or interspinous clamp movement, or skiving of screw hole preparation tools [50,68]. Other disadvantages are observed in systems that attach to the patient or operative table, which can be cumbersome, particularly in obese patients.
4.2. Brain robotic surgery
There are different applications related to various pathologies, such as brain tumors and movement control disorders. In most of the previous studies, it was reported that robotic surgery can improve the precision and accuracy of the surgical procedure as well as decrease the tremors and inverse side effects.
There are several robotic surgical systems, including NeuroMate robot, Evolution 1, NeuRobot, NeuroArm (with or without MR-compatible image guidance), da Vinci surgical system, and several prototype surgical systems. These systems were used mainly for tumor resection; however, other pathologies or even analyzing their performance on cadavers were considered in previous studies. The NeuroArm surgical system was the most used system in previous reports. NeuroArm surgical system is equipped with two robotic arms and can be used during MR imaging [42]. Its main advantage is MR-image guiding during surgical procedures, which can improve the accuracy due to better identification of lesion location, size, and extension. Other surgical systems, such as da Vinci may use optical imaging during the procedure; however, optical imaging can not assess the size and extension of the lesion in underlying or peripheral tissues. MRI can evaluate the lesion more carefully due to its tomographical imaging with high contrast between various soft tissues.
The lack of feedback to the operator is a clear drawback of all surgical robotic systems. Visual or imaging feedback can be improved remarkably with advances in optics and image-guidance systems; however, other sensory feedbacks such as sensing the lesion and position, acceleration or velocity of the instruments are lagging. This feedback might be identified through a combination of visual cues and other senses during conventional surgeries. For telesurgical or shared-surgical models, proprioceptive and visual cues can be used together for providing better feedback to the surgeon.
5. Conclusion
Although most of the previous studies reported higher accuracy in robotic-assisted surgeries in the brain and spine, several studies also reported higher accuracy of freehand surgeries compared to robotic procedures. This discrepancy may relate to differences in applied robotic systems and surgery techniques. Furthermore, the overall surgical time was higher in robotic procedures reported in previous studies, due to more time needed for preparing the robotic instruments. Generally, it seems that robotic surgery can be an accurate, safe, and suitable technique for minimally invasive surgeries on the brain and spine. However, the greatest disadvantage of robotic surgeries is the lack of sensory feedback such as sensing the lesion, and the position, velocity, or acceleration of the instruments. This disadvantage can be more important in complicated surgical regions like the brain, and this may be the reason for the low use of robotic-aided surgery techniques in the brain. However, all the sensory feedbacks, not only limited to visual cues, are under development, and robotic surgery will take a high portion of brain and spine surgeries in the future. In this regard, studies evaluating the accuracy, precision, and safety of developed novel systems will be necessary.
Ethical approval
Not applicable.
Funding
None.
Additional information
No additional information is available for this paper.
Data availability statement
Data included in article/supplementary material/referenced in article.
CRediT authorship contribution statement
Tong Lin: Data curation, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. Qinghai Xie: Conceptualization, Data curation, Investigation, Writing – original draft. Tao Peng: Conceptualization, Resources, Validation, Visualization. Xianxiao Zhao: Formal analysis, Investigation, Visualization, Writing – review & editing. Dongliang Chen: Investigation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Acknowledgment
Not applicable.
References
- 1.Nathoo N., Çavuşoğlu M.C., Vogelbaum M.A., Barnett G.H. In touch with robotics: neurosurgery for the future. Neurosurgery. 2005;56(3):421–433. doi: 10.1227/01.neu.0000153929.68024.cf. [DOI] [PubMed] [Google Scholar]
- 2.Wright J.D., Ananth C.V., Lewin S.N., Burke W.M., Lu Y.S., Neugut A.I., et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698. doi: 10.1001/jama.2013.186. [DOI] [PubMed] [Google Scholar]
- 3.Jeong I.G., Khandwala Y.S., Kim J.H., Han D.H., Li S., Wang Y., et al. Association of robotic-assisted vs laparoscopic radical nephrectomy with perioperative outcomes and health care costs, 2003 to 2015. JAMA. 2017;318(16):1561–1568. doi: 10.1001/jama.2017.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramirez P.T., Frumovitz M., Pareja R., Lopez A., Vieira M., Ribeiro R., et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N. Engl. J. Med. 2018;379(20):1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 5.Melamed A., Margul D.J., Chen L., Keating N.L., Del Carmen M.G., Yang J., et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N. Engl. J. Med. 2018;379(20):1905–1914. doi: 10.1056/NEJMoa1804923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karas C.S., Chiocca E.A. Neurosurgical robotics: a review of brain and spine applications. Journal of robotic surgery. 2007;1:39–43. doi: 10.1007/s11701-006-0006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Us Food and Drug Administrion . US Food and Drug Administration; 2019. Caution when Using Robotically-Assisted Surgical Devices in Women's Health Including Mastectomy and Other Cancer-Related Surgeries: FDA Safety Communication.https://www.fda.gov/medical-devices/safety-communications/update-caution-robotically-assisted-surgical-devices-mastectomy-fda-safety-communication [press release]. [Internet] Available from: [Google Scholar]
- 8.Kim H.J., Choi G.S., Park J.S., Park S.Y., Yang C.S., Lee H.J. The impact of robotic surgery on quality of life, urinary and sexual function following total mesorectal excision for rectal cancer: a propensity score-matched analysis with laparoscopic surgery. Colorectal Dis. 2018;20(5):O103–O113. doi: 10.1111/codi.14051. [DOI] [PubMed] [Google Scholar]
- 9.Lee S.R., Kim H.O., Shin J.H. Clinical outcomes of single-incision robotic cholecystectomy versus conventional 3-port laparoscopic cholecystectomy. Can. J. Surg. 2019;62(1):52. doi: 10.1503/cjs.000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tam V., Rogers D.E., Al-Abbas A., Borrebach J., Dunn S.A., Zureikat A.H., et al. Robotic inguinal hernia repair: a large health system's experience with the first 300 cases and review of the literature. J. Surg. Res. 2019;235:98–104. doi: 10.1016/j.jss.2018.09.070. [DOI] [PubMed] [Google Scholar]
- 11.Childers C.P., Maggard-Gibbons M. Estimation of the acquisition and operating costs for robotic surgery. JAMA. 2018;320(8):835–836. doi: 10.1001/jama.2018.9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsons J.K., Messer K., Palazzi K., Stroup S.P., Chang D. Diffusion of surgical innovations, patient safety, and minimally invasive radical prostatectomy. JAMA surgery. 2014;149(8):845–851. doi: 10.1001/jamasurg.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juo Y.Y., Mantha A., Abiri A., Lin A., Dutson E. Diffusion of robotic-assisted laparoscopic technology across specialties: a national study from 2008 to 2013. Surg. Endosc. 2018;32:1405–1413. doi: 10.1007/s00464-017-5822-4. [DOI] [PubMed] [Google Scholar]
- 14.Pransky J. ROBODOC-surgical robot success story. Ind. Robot: Int. J. 1997;24(3):231–233. [Google Scholar]
- 15.Marescaux J., Rubino F. The ZEUS robotic system: experimental and clinical applications. Surgical Clinics. 2003;83(6):1305–1315. doi: 10.1016/S0039-6109(03)00169-5. [DOI] [PubMed] [Google Scholar]
- 16.Freschi C., Ferrari V., Melfi F., Ferrari M., Mosca F., Cuschieri A. Technical review of the da Vinci surgical telemanipulator. Int. J. Med. Robot. Comput. Assist. Surg. 2013;9(4):396–406. doi: 10.1002/rcs.1468. [DOI] [PubMed] [Google Scholar]
- 17.Adler J.R., Jr. Surgical guidance now and in the future: the next generation of instrumentation. Clin. Neurosurg. 2002;49:105–114. [PubMed] [Google Scholar]
- 18.Kwoh Y.S., Hou J., Jonckheere E.A., Hayati S. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans. Biomed. Eng. 1988;35(2):153–160. doi: 10.1109/10.1354. [DOI] [PubMed] [Google Scholar]
- 19.Wang M.Y., Goto T., Tessitore E., Veeravagu A. Introduction. Robotics in neurosurgery. Neurosurg. Focus. 2017;42(5):E1. doi: 10.3171/2017.2.FOCUS1783. [DOI] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group* P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 21.Garg A., Dwivedi R.C., Sayed S., Katna R., Komorowski A., Pathak K.A., et al. Robotic surgery in head and neck cancer: a review. Oral Oncol. 2010;46(8):571–576. doi: 10.1016/j.oraloncology.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Camarillo D.B., Krummel T.M., Salisbury J.K., Jr. Robotic technology in surgery: past, present, and future. Am. J. Surg. 2004;188(4):2–15. doi: 10.1016/j.amjsurg.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Nathoo N., Çavuşoğlu M.C., Vogelbaum M.A., Barnett G.H. In touch with robotics: neurosurgery for the future. Neurosurgery. 2005;56(3):421–433. doi: 10.1227/01.neu.0000153929.68024.cf. [DOI] [PubMed] [Google Scholar]
- 24.Japan C of BTR of . Neurol Med Chir; Tokyo: 2009. Report of Brain Tumor Registry of Japan (1984-2000) p. 40. [PubMed] [Google Scholar]
- 25.Hentschel S.J., Sawaya R. Optimizing outcomes with maximal surgical resection of malignant gliomas. Cancer Control. 2003;10(2):109–114. doi: 10.1177/107327480301000202. [DOI] [PubMed] [Google Scholar]
- 26.Lacroix M., Abi-Said D., Fourney D.R., Gokaslan Z.L., Shi W., DeMonte F., et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J. Neurosurg. 2001;95(2):190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 27.Claus E.B., Horlacher A., Hsu L., Schwartz R.B., Dello-Iacono D., Talos F., et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer: Interdisciplinary Int. J. Am. Cancer Soc. 2005;103(6):1227–1233. doi: 10.1002/cncr.20867. [DOI] [PubMed] [Google Scholar]
- 28.Stummer W., Pichlmeier U., Meinel T., Wiestler O.D., Zanella F., Reulen H.J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 29.Guthart G.S., Salisbury J.K. Proceedings 2000 ICRA Millennium Conference IEEE International Conference on Robotics and Automation Symposia Proceedings (Cat No 00CH37065) IEEE; 2000. The Intuitive/sup TM/telesurgery system: overview and application; pp. 618–621. [Google Scholar]
- 30.Pott P.P., Scharf H.P., Schwarz M.L. Today's state of the art in surgical robotics. Comput. Aided Surg. 2005;10(2):101–132. doi: 10.3109/10929080500228753. [DOI] [PubMed] [Google Scholar]
- 31.Goto T., Hongo K., Kakizawa Y., Muraoka H., Miyairi Y., Tanaka Y., et al. Clinical application of robotic telemanipulation system in neurosurgery: case report. J. Neurosurg. 2003;99(6):1082–1084. doi: 10.3171/jns.2003.99.6.1082. [DOI] [PubMed] [Google Scholar]
- 32.Morita A., Sora S., Mitsuishi M., Warisawa S., Suruman K., Asai D., et al. Microsurgical robotic system for the deep surgical field: development of a prototype and feasibility studies in animal and cadaveric models. J. Neurosurg. 2005;103(2):320–327. doi: 10.3171/jns.2005.103.2.0320. [DOI] [PubMed] [Google Scholar]
- 33.Hongo K., Goto T., Miyahara T., Kakizawa Y., Koyama J., Tanaka Y. Telecontrolled micromanipulator system (NeuRobot) for minimally invasive neurosurgery. Med. Tchnol. Neurosurg. 2006:63–66. doi: 10.1007/978-3-211-33303-7_9. [DOI] [PubMed] [Google Scholar]
- 34.De Momi E., Ferrigno G. Robotic and artificial intelligence for keyhole neurosurgery: the ROBOCAST project, a multi-modal autonomous path planner. Proc. IME H J. Eng. Med. 2010;224(5):715–727. doi: 10.1243/09544119JEIM585. [DOI] [PubMed] [Google Scholar]
- 35.Sutherland G.R., Latour I., Greer A.D., Fielding T., Feil G., Newhook P. An image-guided magnetic resonance-compatible surgical robot. Neurosurgery. 2008;62:286–293. doi: 10.1227/01.neu.0000315996.73269.18. [DOI] [PubMed] [Google Scholar]
- 36.Li Q.H., Zamorano L., Pandya A., Perez R., Gong J., Diaz F. The application accuracy of the NeuroMate robot—a quantitative comparison with frameless and frame-based surgical localization systems. Comput. Aided Surg. 2002;7(2):90–98. doi: 10.1002/igs.10035. [DOI] [PubMed] [Google Scholar]
- 37.Varma T.R.K., Eldridge P.R., Forster A., Fox S., Fletcher N., Steiger M., et al. Use of the NeuroMate stereotactic robot in a frameless mode for movement disorder surgery. Stereotact. Funct. Neurosurg. 2003;80(1–4):132–135. doi: 10.1159/000075173. [DOI] [PubMed] [Google Scholar]
- 38.Nimsky C., Rachinger J., Iro H., Fahlbusch R. Adaptation of a hexapod-based robotic system for extended endoscope-assisted transsphenoidal skull base surgery. min-Minimally Invasive Neurosurgery. 2004;47(1):41–46. doi: 10.1055/s-2003-812465. [DOI] [PubMed] [Google Scholar]
- 39.Garisto J., Bertolo R., Reese S.W., Bove P., Kaouk J. Minimizing minimally invasive surgery: current status of the single-port robotic surgery in Urology. Actas Urol. Esp. 2021;45(5):345–352. doi: 10.1016/j.acuroe.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Marcus H.J., Hughes-Hallett A., Cundy T.P., Yang G.Z., Darzi A., Nandi D. Da Vinci robot-assisted keyhole neurosurgery: a cadaver study on feasibility and safety. Neurosurg. Rev. 2015;38:367–371. doi: 10.1007/s10143-014-0602-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutherland G.R., Maddahi Y., Gan L.S., Lama S., Zareinia K. Robotics in the neurosurgical treatment of glioma. Surg. Neurol. Int. 2015;6(Suppl 1):S1. doi: 10.4103/2152-7806.151321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutherland G.R., Latour I., Greer A.D. Integrating an image-guided robot with intraoperative MRI. IEEE Eng. Med. Biol. Mag. 2008;27(3):59–65. doi: 10.1109/EMB.2007.910272. [DOI] [PubMed] [Google Scholar]
- 43.Sutherland G.R. Surgeon at a workstation: information age surgery. Cureus. 2012;4(4) [Google Scholar]
- 44.Sutherland G.R., Lama S., Gan L.S., Wolfsberger S., Zareinia K. Merging machines with microsurgery: clinical experience with neuroArm. J. Neurosurg. 2013;118(3):521–529. doi: 10.3171/2012.11.JNS12877. [DOI] [PubMed] [Google Scholar]
- 45.Arata J., Tada Y., Kozuka H., Wada T., Saito Y., Ikedo N., et al. Neurosurgical robotic system for brain tumor removal. Int. J. Comput. Assist. Radiol. Surg. 2011;6:375–385. doi: 10.1007/s11548-010-0514-8. [DOI] [PubMed] [Google Scholar]
- 46.Hong W.C., Tsai J.C., Chang S.D., Sorger J.M. Robotic skull base surgery via supraorbital keyhole approach: a cadaveric study. Neurosurgery. 2013;72(suppl_1):A33–A38. doi: 10.1227/NEU.0b013e318270d9de. [DOI] [PubMed] [Google Scholar]
- 47.Choi W.W., Green B.A., Levi A.D. Computer-assisted fluoroscopic targeting system for pedicle screw insertion. Neurosurgery. 2000;47(4):872–878. doi: 10.1097/00006123-200010000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Kantelhardt S.R., Martinez R., Baerwinkel S., Burger R., Giese A., Rohde V. Perioperative course and accuracy of screw positioning in conventional, open robotic-guided and percutaneous robotic-guided, pedicle screw placement. Eur. Spine J. 2011;20:860–868. doi: 10.1007/s00586-011-1729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.SchizAS C., Thein E., KwiATKOwSKi B., KuliK G. Pedicle screw insertion: robotic assistance versus conventional C-arm fluoroscopy. Acta Orthop. Belg. 2012;78(2):240–245. [PubMed] [Google Scholar]
- 50.Ringel F., Stüer C., Reinke A., Preuss A., Behr M., Auer F., et al. Accuracy of robot-assisted placement of lumbar and sacral pedicle screws: a prospective randomized comparison to conventional freehand screw implantation. Spine. 2012;37(8):E496–E501. doi: 10.1097/BRS.0b013e31824b7767. [DOI] [PubMed] [Google Scholar]
- 51.Schatlo B., Molliqaj G., Cuvinciuc V., Kotowski M., Schaller K., Tessitore E. Safety and accuracy of robot-assisted versus fluoroscopy-guided pedicle screw insertion for degenerative diseases of the lumbar spine: a matched cohort comparison. J. Neurosurg. Spine. 2014;20(6):636–643. doi: 10.3171/2014.3.SPINE13714. [DOI] [PubMed] [Google Scholar]
- 52.Dreval O., Rynkov I., Kasparova K.A., Bruskin A., Aleksandrovskii V., Zil'Bernstein V. Results of using Spine Assist Mazor in surgical treatment of spine disorders. Interventions (transpedicular fixations) 2014;5(6):9–22. [PubMed] [Google Scholar]
- 53.Molliqaj G., Schatlo B., Alaid A., Solomiichuk V., Rohde V., Schaller K., et al. Accuracy of robot-guided versus freehand fluoroscopy-assisted pedicle screw insertion in thoracolumbar spinal surgery. Neurosurg. Focus. 2017;42(5):E14. doi: 10.3171/2017.3.FOCUS179. [DOI] [PubMed] [Google Scholar]
- 54.Lonjon N., Chan-Seng E., Costalat V., Bonnafoux B., Vassal M., Boetto J. Robot-assisted spine surgery: feasibility study through a prospective case-matched analysis. Eur. Spine J. 2016;25:947–955. doi: 10.1007/s00586-015-3758-8. [DOI] [PubMed] [Google Scholar]
- 55.Solomiichuk V., Fleischhammer J., Molliqaj G., Warda J., Alaid A., von Eckardstein K., et al. Robotic versus fluoroscopy-guided pedicle screw insertion for metastatic spinal disease: a matched-cohort comparison. Neurosurg. Focus. 2017;42(5):E13. doi: 10.3171/2017.3.FOCUS1710. [DOI] [PubMed] [Google Scholar]
- 56.Kim H.J., Jung W.I., Chang B.S., Lee C.K., Kang K.T., Yeom J.S. A prospective, randomized, controlled trial of robot-assisted vs freehand pedicle screw fixation in spine surgery. Int. J. Med. Robot. Comput. Assist. Surg. 2017;13(3):e1779. doi: 10.1002/rcs.1779. [DOI] [PubMed] [Google Scholar]
- 57.Keric N., Doenitz C., Haj A., Rachwal-Czyzewicz I., Renovanz M., Wesp D.M., et al. Evaluation of robot-guided minimally invasive implantation of 2067 pedicle screws. Neurosurg. Focus. 2017;42(5):E11. doi: 10.3171/2017.2.FOCUS16552. [DOI] [PubMed] [Google Scholar]
- 58.Adler J.R., Jr., Murphy M.J., Chang S.D., Hancock S.L. Image-guided robotic radiosurgery. Neurosurgery. 1999;44(6):1299–1306. [PubMed] [Google Scholar]
- 59.Chop W.W., Green B., Levi A. Fluoroscopic guided targeting system with a robotic arm for pedicle screw insertion. Neurosurgery. 2000;47(872–878) doi: 10.1097/00006123-200010000-00017. [DOI] [PubMed] [Google Scholar]
- 60.Lieberman I.H., Togawa D., Kayanja M.M., Reinhardt M.K., Friedlander A., Knoller N., et al. Bone-mounted miniature robotic guidance for pedicle screw and translaminar facet screw placement: Part I—technical development and a test case result. Neurosurgery. 2006;59(3):641–650. doi: 10.1227/01.NEU.0000229055.00829.5B. [DOI] [PubMed] [Google Scholar]
- 61.Hyun S.J., Kim K.J., Jahng T.A., Kim H.J. LWW; 2017. Minimally Invasive Robotic versus Open Fluoroscopic-Guided Spinal Instrumented Fusions: a Randomized Controlled Trial. [DOI] [PubMed] [Google Scholar]
- 62.Urakov T.M., Chang KH kan, Burks S.S., Wang M.Y. Initial academic experience and learning curve with robotic spine instrumentation. Neurosurg. Focus. 2017;42(5):E4. doi: 10.3171/2017.2.FOCUS175. [DOI] [PubMed] [Google Scholar]
- 63.Roser F., Tatagiba M., Maier G. Spinal robotics: current applications and future perspectives. Neurosurgery. 2013;72 doi: 10.1227/NEU.0b013e318270d02c. A12–8. [DOI] [PubMed] [Google Scholar]
- 64.Ottensmeyer M.P., Ben-Ur E. Medicine Meets Virtual Reality 2000. IOS Press; 2000. Input and output for surgical simulation: devices to measure tissue properties in vivo and a haptic interface for laparoscopy simulators; pp. 236–242. [PubMed] [Google Scholar]
- 65.Lefranc M., Peltier J. Evaluation of the ROSATM Spine robot for minimally invasive surgical procedures. Expet Rev. Med. Dev. 2016;13(10):899–906. doi: 10.1080/17434440.2016.1236680. [DOI] [PubMed] [Google Scholar]
- 66.Joseph J.R., Smith B.W., Liu X., Park P. Current applications of robotics in spine surgery: a systematic review of the literature. Neurosurg. Focus. 2017;42(5):E2. doi: 10.3171/2017.2.FOCUS16544. [DOI] [PubMed] [Google Scholar]
- 67.Amiot L.P., Lang K., Putzier M., Zippel H., Labelle H. Comparative results between conventional and computer-assisted pedicle screw installation in the thoracic, lumbar, and sacral spine. Spine. 2000;25(5):606–614. doi: 10.1097/00007632-200003010-00012. [DOI] [PubMed] [Google Scholar]
- 68.Overley S.C., Cho S.K., Mehta A.I., Arnold P.M. Navigation and robotics in spinal surgery: where are we now? Neurosurgery. 2017;80(3S):S86–S99. doi: 10.1093/neuros/nyw077. [DOI] [PubMed] [Google Scholar]
- 69.Collados-Maestre I., Lizaur-Utrilla A., Bas-Hermida T., Pastor-Fernandez E., Gil-Guillen V. Transdiscal screw versus pedicle screw fixation for high-grade L5-S1 isthmic spondylolisthesis in patients younger than 60 years: a case–control study. Eur. Spine J. 2016;25:1806–1812. doi: 10.1007/s00586-016-4550-0. [DOI] [PubMed] [Google Scholar]
- 70.Stokes I.A. Axial rotation component of thoracic scoliosis. J. Orthop. Res. 1989;7(5):702–708. doi: 10.1002/jor.1100070511. [DOI] [PubMed] [Google Scholar]
- 71.Wray S., Mimran R., Vadapalli S., Shetye S.S., McGilvray K.C., Puttlitz C.M. Pedicle screw placement in the lumbar spine: effect of trajectory and screw design on acute biomechanical purchase. J. Neurosurg. Spine. 2015;22(5):503–510. doi: 10.3171/2014.10.SPINE14205. [DOI] [PubMed] [Google Scholar]
- 72.Barzilay Y., Liebergall M., Fridlander A., Knoller N. Miniature robotic guidance for spine surgery—introduction of a novel system and analysis of challenges encountered during the clinical development phase at two spine centres. Int. J. Med. Robot. Comput. Assist. Surg. 2006;2(2):146–153. doi: 10.1002/rcs.90. [DOI] [PubMed] [Google Scholar]
- 73.Sukovich W., Brink-Danan S., Hardenbrook M. Miniature robotic guidance for pedicle screw placement in posterior spinal fusion: early clinical experience with the SpineAssist. Int. J. Med. Robot. Comput. Assist. Surg. 2006;2(2):114–122. doi: 10.1002/rcs.86. [DOI] [PubMed] [Google Scholar]
- 74.Chenin L., Peltier J., Lefranc M. Minimally invasive transforaminal lumbar interbody fusion with the ROSA TM Spine robot and intraoperative flat-panel CT guidance. Acta Neurochir. 2016;158:1125–1128. doi: 10.1007/s00701-016-2799-z. [DOI] [PubMed] [Google Scholar]
- 75.Kim H.J., Kang K.T., Park S.C., Kwon O.H., Son J., Chang B.S., et al. Biomechanical advantages of robot-assisted pedicle screw fixation in posterior lumbar interbody fusion compared with freehand technique in a prospective randomized controlled trial—perspective for patient-specific finite element analysis. Spine J. 2017;17(5):671–680. doi: 10.1016/j.spinee.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 76.Shin M.H., Hur J.W., Ryu K.S., Park C.K. Prospective comparison study between the fluoroscopy-guided and navigation coupled with O-arm–guided pedicle screw placement in the thoracic and lumbosacral spines. Clinical Spine Surgery. 2015;28(6):E347–E351. doi: 10.1097/BSD.0b013e31829047a7. [DOI] [PubMed] [Google Scholar]
- 77.Bindal R.K., Glaze S., Ognoskie M., Tunner V., Malone R., Ghosh S. Surgeon and patient radiation exposure in minimally invasive transforaminal lumbar interbody fusion. J. Neurosurg. Spine. 2008;9(6):570–573. doi: 10.3171/SPI.2008.4.08182. [DOI] [PubMed] [Google Scholar]
- 78.Grelat M., Zairi F., Quidet M., Marinho P., Allaoui M., Assaker R. Assessment of the surgeon radiation exposure during a minimally invasive TLIF: comparison between fluoroscopy and O-arm system. Neurochirurgie. 2015;61(4):255–259. doi: 10.1016/j.neuchi.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 79.Yu E., Khan S.N. Does less invasive spine surgery result in increased radiation exposure? A systematic review. Clin. Orthop. Relat. Res. 2014;472:1738–1748. doi: 10.1007/s11999-014-3503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.