Abstract
Ready-to-Use Therapeutic Food (RUTF) or Ready-to-Use Supplementary Food (RUSF) has been widely used in home-based treatment for severely and moderately acute malnourished children. These programs showed positive results in short term nutritional recovery in children, which were reported in some research settings. Nowadays, the RUTF/RUSF formulation has been improved using a variety of RUTF/RUSF from locally available food ingredients. This paper aims to review the essential aspects of the development and provision of RUTF/RUSF made from local food resources and monitor program effectiveness that warrants the program’s sustainability. The modified recipes of RUTF/RUSF were developed following the international dietary guidelines for the rehabilitation of severely and moderately acute malnourished children. The local production of RUTF/RUSF provided some benefits that include empowering the local community, consideration of the common eating pattern, promoting the diversification of food consumption, strengthening food security, as well as supporting the sustainability of RUTF/RUSF production. Results of the PRISMA-based systematic literature review revealed various ingredient developments and processing techniques which could improve the product characteristics and sensory evaluation. RUTF/RUSF in local food production provided different food carriers (e.g., biscuits, wafers) and seemed to be more readily accepted by the children. Furthermore, the program sustainability of RUTF/RUSF depends on a continuous ingredients supply and support from the local government. The findings presented the importance of development of such food supplements based on the local food resources and with improved technology for prevention and rehabilitation of malnourished children.
Keywords: RUTF, RUSF, Malnourished children, Food supplement, Food technology, Local foods
1. Introduction
In 1999, the World Health Organization (WHO) introduced a guideline for the treatment of severe acute malnutrition (SAM) in children in the clinical setting. High-energy dense milk fortified with a vitamin and mineral mix (therapeutic milk), also called F75 and F100, became a large portion of the hospitalized SAM children’s diet [1]. Furthermore, to facilitate the rehabilitation of the SAM children with fewer medical complications in the community and increase the rehabilitation coverage, a large portion of the milk was replaced with peanut butter/spread, to ease the community storage capacity, increase the shelf life of the foods, and reduce the bacterial contamination [2,3]. The food was subsequently called ready-to-use food (RUF) [4,5]. According to the World Food Programme (WFP) 2012 [6], RUF is defined as foods that require no preparation and cooking, hence, the food can be eaten directly after opening the packaging. The sealed package ensures that the food will have a low risk of bacterial contamination. The terminology has been developed beyond its original meaning, due to successful home-based therapy utilizing the RUFs for rehabilitation of SAM children [7,8]. Thereafter, the RUF terminology has evolved to ready-to-use therapeutic food (RUTF) for the treatment of SAM children. As stated jointly by WHO, WFP, the United Nations System Standing Committee on Nutrition (UNSCN) and the United Nations Children’s Fund (UNICEF) in 2007 [9], RUTF is an energy-dense food consisting of a homogenous mixture of powdered or dry ingredients, rich in protein and carbohydrates, embedded within the lipid matrix.
The current RUF developments involved the term, ready-to-use supplementary food (RUSF), for rehabilitation and prevention of moderate acute malnutrition (MAM) in children and/or deficiencies of micronutrients, respectively [[10], [11], [12]]. The use of RUTF and RUSF promotes the application of community-based programs, such as community management of acute malnutrition (CMAM). CMAM can reduce the risk of mortality in children with severe acute malnutrition in emergency and development settings. This is because of the decentralization of community outreach, and is expected to cover more children than other institutional approaches. The energy and nutrient density is lower in RUSF used in MAM cases compared to the RUTF due to the higher nutrient requirements in SAM children [13]. Thus, RUSF is expected to be given in combination with local diets to fulfill the dietary recommendation of MAM children [14]. In CMAM, the necessity of RUTF or RUSF depends on the local needs and context. Furthermore, a study among Malawian children found that locally produced RUTF was relatively inexpensive compared to the imported ones and achieved the same effect, an increase in the children’s weight gain [15]. Therefore, making some new formulations of RUTF/RUSF based on locally available foods is projected to be efficient and more cost-effective for treating the MAM and SAM children. It is important to note that some of the ingredients probably are still imported (especially the vitamin-mineral mix); however, some of the basic ingredients, e.g., nuts, beans, and cereals, can be obtained locally, which would decrease the total cost. Over the years, several RUTF/RUSF have been developed in countries where a high prevalence of malnutrition existed, namely in Africa and Asia. Most of the RUTF/RUSF provided for the undernourished children were formulated based on the first RUTF, by utilizing peanuts to replace milk with their local food resources. Although the inclusion of milk in the RUTF is still important for the growth of the SAM children, the MAM or SAM children with fewer medical complications, were treated at the community feeding center, where the presence of milk might be less essential than in a clinical setting. The SAM children hospitalized at the clinic usually had a very low body weight in relation to their height due to some medical complications. Based on this, they needed a high-energy milk and vitamin-mineral mix that could rehabilitate and promote their growth, indicative of a high weight gain rate (above 10 g/kg body weight/day). Hospitals are usually equipped with refrigerators to store the milk [7], while community centers usually have simple appliances and infrastructure. Hence, the provision of high-energy milk that needs a refrigerator for storing them is replaced by peanuts [2]. The RUTF/RUSF that replaced milk with other protein-based foods was given to the SAM and/or MAM children produced a lower weight gain, but was comparable to the therapeutic milk F-100 distributed at the community nutrition centers [10,12]. In addition, milk-free RUSF costs less, provides higher shelf life, and supports the local agriculture sector [16]. In recent years, more studies of developing countries have replaced increasing portions of milk with other protein sources, e.g., nuts, beans, or eggs. Furthermore, the nutrient balance in the RUTF/RUSF has been reviewed during its development to fulfill the dietary requirements of the SAM and MAM children, followed by sensory evaluation and acceptability trials [17,18]. In addition, program effectiveness of the RUTF/RUSF provision has been investigated according to the Sphere Project that usually includes weight gain (weight or rate of weight gain until recovery), length of stay (days the children stayed in the program), percentage of recovered children, and percentage of children who died [19]. Some intervention programs reported nutritional status changes, health conditions, and acceptance (percentage of compliance in consuming the RUF).
Currently, the utilization of local food resources is strongly encouraged to decrease the dependency on foreign resources, reducing the cost, and promoting the local bioeconomy and agriculture sector. The most important aspect of the RUTF/RUSF application is the sustainability of the program. Numerous successful programs using RUTF/RUSF for nutrition intervention should be adopted for large use. In order to be sustainable, the technology of RUTF/RUSF production should be accessible in terms of raw materials or ingredients availability, handling, processing technology, packaging and storing, and product quality [9,20].
When providing RUTF/RUSF for treatment and prevention of undernourishment in children, the food supplement should adhere to the WHO recommendation and criteria. The efficacy of RUTF/RUSF related to the protein source used (plant vs. milk) for the treatment of SAM and MAM children has become a longstanding debate, as milk-based RUTFs are considered to be more efficacious [21]. The challenges encountered during the development of RUTF/RUSF encompass not only the choice of raw materials but also the identification of an appropriate food carrier, determination of suitable food technology, and establishment of a sustainable supply chain to uphold the program's continuity. This review points to the utilization of the local food resources for the development of RUTF/RUSF products, and the application in the program for treating SAM and MAM children. Hence, the general research objective of this paper is to review critical aspects in the development and provisioning of RUTF/RUSF made from local food resources, along with assessing the program's effectiveness in ensuring sustainability of its execution.
2. Materials and methods
This research was conducted using a review of the literature following the modified Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines [22]. In July–December 2021 and updated on March 2023, a literature search was done in the Scopus database, continuing with the PubMed database to collect other papers that were not published in Scopus. Additional relevant databases from other source (i.e., WoS) was also used to ensure a more comprehensive retrieval of all related articles. The following keywords were used: “ready-to-use”, OR “ready-to-use therapeutic” OR “ready-to-use supplementary”. The articles were then selected based on the following criteria [1]: the relevancy of the paper’s content to answer the research objectives; thus, whether the literature described the development process and/or ingredients of a locally produced ready-to-use food (non-commercial) [2], the studies recruited SAM or MAM children above six months and below five years old for comparison [3], papers written in English [4], papers published within 1994–2023. The selected literatures were grouped based on the affiliated authors and the basic ingredients (milk-based and milk-free). Based on different terminologies used during the earlier development of RUSF/RUTF (Ready to Use Supplementary Food/Ready to Use Therapeutic Food), for common understanding, the definition of RUTF in this paper is referred to the WHO/WFP/UNSSCN/UNICEF guidelines for the treatment of the SAM [9], while RUSF was denoted for the RUF following the dietary guidelines for the MAM children [14]. The results from the bibliographic analysis included improvement of nutritional profiles, a wide range of local food ingredients, preferred properties by the consumers, and the adapted food processing that meets the needs in the target area. Some relevant program outcomes of the RUTF/RUSF were also presented, especially weight gain (rate of weight gain until recovery) and recovery (percentage of recovered children).
3. Results and discussion
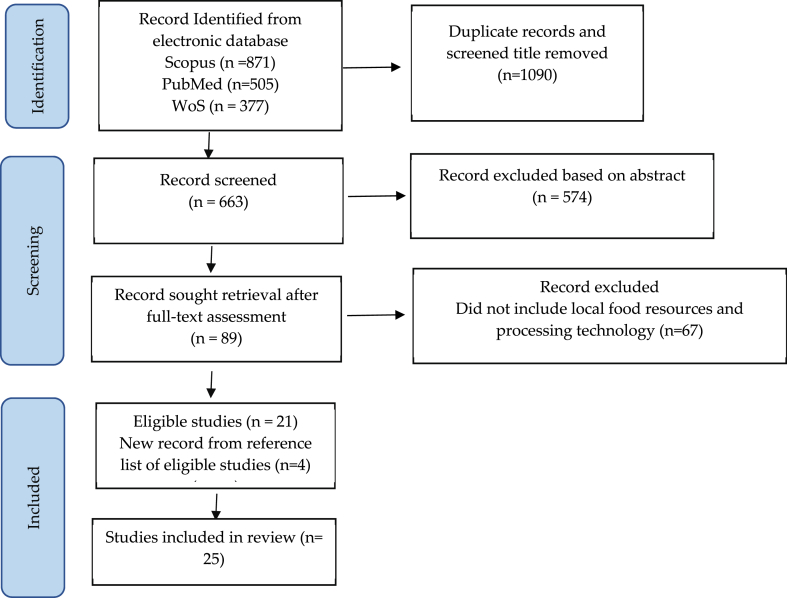
The Scopus search of the identified keywords: “ready-to-use”, OR “ready-to-use therapeutic” OR “ready-to-use supplementary”, yielded 871 documents. The set of specified terms, “ready-to-use therapeutic” (found in 279 articles) and “ready-to-use supplementary” (found in 87 articles), are part of the 871 documents. The PubMed literature search yielded 505 documents. After duplication and title screening, 591 articles were selected for abstract assessment. 89 articles were selected for full text assessment (Fig. 1). After applying the entry criteria, especially for articles reporting RUTF/RUSF with local food resources, the total number of papers selected for further analysis was 25 (Table 1).
Fig. 1.
PRISMA flow diagram of selection of eligible studies.
Table 1.
General information about the articles and descriptions of the study sites.
| RUTF/RUSF (country) | Descriptions during the research period | Authors (year) |
|---|---|---|
| Locally produced peanut-milk butter RUTF (Malawi) |
|
Sandige et al. (2004) [15] |
|
Linneman et al. (2007) [23] | |
| Fortified cereal/nut/legume-based RUF biscuits (Indonesia) |
|
Purwestri et al. (2012) [24], Scherbaum et al. (2015) [20] |
|
Fetriyuna et al. (2021) [25] | |
| Novel milk-free soybean-maize-sorghum RUTF (Zambia) |
|
Owino et al. (2012) [26] |
| Cereal and pulse-based RUTF (Democratic Republic of Congo) |
|
Bahwere et al. (2016) [27] |
| RUSF from local food resources based on linear programming, rice-lentil, chickpea-based (Bangladesh) |
|
Ahmed et al. (2014) [28], Choudhury et al. (2018) [29] |
| High-oleic RUTF (Malawi) |
|
Hsieh et al. (2015) [30] |
| RUSF with whey protein and whey permeate (Malawi and Mozambique) |
|
Stobaugh et al. (2016) [31] |
| RUTF from local ingredients from linear programming, Ethiopia, Ghana, Pakistan, and India |
Cereal/grain: maize (Ghana and Pakistan), oat (Ethiopia, India) Oil: canola rapeseed (all), coconut (Ghana and India), palm (Ethiopia, Ghana), sunflower (Ghana and Pakistan) Milk: different forms. |
Weber et al. (2017) [32] |
| Vietnamese RUTF (Vietnam) |
|
Nga et al. (2013) [33] |
| Locally produced lipid-based nutrient supplement (LNS) (RUTF/RUSF) with fish, in snack form (NumTrey wafer/paste RUSF and RUTF) (Cambodia) |
|
Borg et al. (2017) [34] Borg et al. (2018) [35] Borg et al. (2019) [36] |
|
Sigh et al. (2108) [37] Sigh et al. (2018) [38] |
|
| Alternative RUTF formulations using soy, sorghum and spirulina (Italy) |
|
Armini et al. (2018) [39], Miele et al. (2020) [40] |
| Newly developed RUTF (Iran) |
|
Azimi et al. [41] |
| Soy-protein isolate vs. milk-based (Bangladesh) |
|
Hossain et al. (2020) [42] |
| Chickpea, mungbean, and peanut-based (Pakistan) |
|
Javed et al. (2021) [43] |
Note: RUTF: Ready to Therapeutic Food; RUSF: Ready to Use Supplementary Food.
3.1. Food sources of RUTF
In the early development of RUTF/RUSF, production using local food resources in the recipes mostly consisted of peanut-milk spread/butter, sugar, plant-based oil, and the micronutrient premix to improve the recommended nutritional composition [10,15,44]. Afterward, the improvisation of the recipes took place using the various available food resources in the respective areas to adopt the local familiarity and eating patterns of the target group (Table 1). RUTF/RUSF development was mostly done on two continents: Africa (Malawi, Zambia, Democratic Republic of Congo, Mozambique, Ethiopia, Ghana), and Asia (Indonesia, Bangladesh, Pakistan, India, Vietnam, Cambodia, Iran); hence the food sources used in the recipes were based on the study sites. The study sites in Africa and Asia were characterized by a deprived population, lack of health services and improper hygienic sanitation practices, unfavorable climatic conditions that caused household food insecurity, and an elevated moderate to severe acute malnutrition in children (Table 1). In addition, two countries in Europe (France and Italy) were also involved in developing the RUTF products during its initial development [2], and with more advanced technology [30,39]. In Africa, the ingredients are mostly combined with sorghum, maize, and chickpea and were recently improved with the crystalline amino acid-enriched plant [45,46]. While in Asia, the combination of rice, cereals, legumes, and animal sources [38,37] were usually used, as well as omega 3 and omega six sources [17]. The different recipes were used in different regions, based on the locally available food resources in both Africa and Asia. The intervention using the RUTF/RUSF made from the local food resources is presented in Table 1. This table mentioned a brief description of the research period. Details of the program are presented in section 3.4 Program effectiveness of RUTF/RUSF provision in the community.
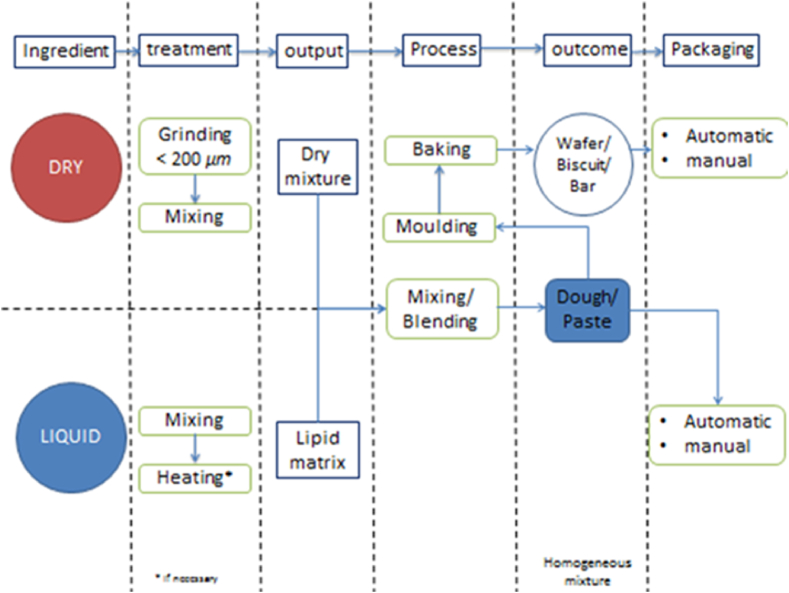
Typically, the initial RUTF recipes consist of 30% milk, 28% sugar, 15% vegetable oil, 25% peanut butter, and 2% vitamin and mineral premix. Nowadays, the composition of milk may be replaced by other protein sources from freshwater fish, legumes, and cereals, mainly due to the higher economic cost of milk and also other factors, such as lactose intolerance, milk protein allergy, and difficulties in handling fresh milk because of the susceptibility to microbial contamination [47]. The preparation of local commodities to be used in RUTF and RUSF can be seen in Fig. 2.
Fig. 2.
The common process of rutf/RUFS production.
The preparation of local food ingredients was mostly conducted by sorting and cleaning, roasting or drying; some included dehulling, milling, and sieving. Drying, milling, and sieving are standard protocols in producing flour. The benefits of processing ingredients into flour are its flexibility to be used in many products, a longer shelf life, and simple packaging and storage. For freshwater fish, the crucial step of drying needs to be taken into account to prevent the loss of protein in the product. According to Ref. [48], sun drying is the most desirable method used for fish due to protein retention.
Meanwhile, legumes are a rich source of protein, but on the other hand, they also contain a higher amount of antinutrient compounds, such as phytic acid. The presence of phytic acid inhibits the absorption of certain minerals, such as iron, zinc, calcium, and magnesium. Roasting, besides its aims in reducing moisture content, is also successfully reported to decrease the phytic acid of legumes [49].
3.1.1. Carbohydrates
Carbohydrates have various functions in living organisms. Besides the energy source in the form of glucose, carbohydrates can also be used as food reserves, for example, starch in plants and glycogen in animals. Moreover, they can also be utilized as a building material in plants (cellulose), animals, and fungi (chitin). Carbohydrates can be grouped into two categories simple carbohydrates, which, when ingested, are readily converted to energy, such as sugar, and complex carbohydrates, which need further steps to be used as an energy source, such as starch [50]. Due to their function, carbohydrates are used as an energy source in treating severe acute malnutrition children [51]. In the WHO F75, for the stabilization stage of SAM treatment, maltodextrin and lactose are used to provide energy for undernourished children. Simple and ready-to-be-converted carbohydrates are needed because of various complications that occur in the metabolic system of malnourished children [51]. The enzymatic hydrolysis of the ingested disaccharides into monosaccharides, such as glucose and galactose in the small intestinal villi, fulfills the energy required. Nevertheless, the malnourished children tended to exhibit a lactose intolerance condition, thus the use of lactose for nutrition intervention needs to be cautiously monitored [52].
Meanwhile, carbohydrates are also widely used for other forms of malnutrition and nutrition deficiency conditions. In the development of RUF, sugar or sucrose (made from glucose and fructose) is one of the carbohydrates present in almost all of the reports related to RUF [8,15,23,24,26,28,33]. Those can be effective energy sources that can be easily digested and absorbed in the form of glucose and delivered through the bloodstream. In addition, due to the sweet nature of sugar, it helps to increase the acceptability of the product [53]. Sweetness is mainly preferred by children, as they are the group most vulnerable to nutritional deficiency conditions. Therefore, the presence of sugar is essential. In addition to its function as a sweetener, sugar plays an important role in RUF product development. For example, in cookies or biscuit products, which are the most common product developed for RUF, sugar can also help to increase the spread ratio of cookies, increase the crunchiness of the cookies as well as the moistness within the center of the cookie due to its ability to entrap moisture [54]. Sugar can also act as a flavor enhancer to offset the flat or bland taste of products which is essential in the production of RUF due to the limited flavoring agents used [55].
In an extruded snack, sugar contributes in shaping the structural characteristics of the extruded product, particularly influencing the product's density and porosity of the composition. The presence of sugar contributes to the higher density of the product, and due to the synergistic effect with salt, it adds to the crunchy texture of the extruded product [56]. Meanwhile, for paste and liquid food, which is also a common form of RUF, sugar contributes to the pasting properties of the product, creating a firm and soft texture with an emphasis on the smoothness of the paste [57]. In addition, sugar increases the viscosity of liquid food due to the hydroxyl bond that binds water molecules to create a larger structure which affects the movement of molecules, thus increasing the viscosity of liquid foods [58].
Furthermore, carbohydrates in foods are also commonly present in their native form, for example, rice, maize, and legumes [26,29,33]. The traditional dietary pattern of a usually high-energy starch-based diet can be responsible for forming a habit or preference for a certain food. In addition to its function as an energy source, starchy staple foods are used to maintain the palatability of RUF development and can increase the level of acceptability of the new product because children are familiar with the taste of some ingredients used. Moreover, as staple foods, the commodity is commonly available in the area and accessible by the household.
3.1.2. Lipids
Lipids or fats are organic compounds found in tissue cells, insoluble in water but soluble in non-polar solvents. Lipids are essential part of the body’s homeostatic function and help with some of the most critical processes in the body. Lipids include fats and oils called triglycerides, phospholipids, waxes, and steroids. Triglycerides store energy, insulate cells and help absorb fat-soluble vitamins.
Even though the intake of lipids creates a critical discussion regarding weight gain and other health issues [59], the presence of lipids in the human diet is essential due to their various critical functions, including the treatment of malnourished children with lipid-based nutrient supplements which are divided into three categories and ready-to-use therapeutic foods that are usually prescribed for severe acute malnutrition treatment [60]. This category of supplementation is given in large doses because it is the sole source of energy for malnourished children. The second category is fat-based supplements or ready-to-use supplements in moderate amounts. This category can be used to treat severe acute malnutrition or to prevent wasting or growth retardation due to difficulties in obtaining food at certain times of the year. This category is designed to provide approximately 50% of the energy intake. The third category is low-level lipid-based supplements. This category has been used to prevent wasting and stunting in infants and children and accounts for less than 50% of their energy intake [60].
Lipids in the form of oil are the most common ingredient in nutrition supplementation. Providing high-energy-density products requires oil that contributes to the replenishment of energy. Together with a carbohydrate source, lipids will provide energy, and assist the transport of lipid-soluble micronutrients fortified in the product. Providing lipid-based dietary supplements improves recovery rates compared to specially formulated foods [61]. In addition, fatty acid as a significant component of triglyceride is limited in children with malnutrition, which can be observed in skin conditions, limited growth rate, an increase of infection, and the hampering of the child's development [62]. Therefore, numerous studies on nutrition intervention for different categories of malnourished children have included oil as an essential part of the formulation, such as cottonseed oil [15], vegetable oil [8,23,33], palm oil [20,30,24,27,31,32], soybean oil [[28], [29], [31], [32]], linseed oil [30,27], canola oil [37,32], rapeseed oil [27], coconut oil [32], and sunflower oil [39,32,40]. Different sources of oil provide specific configurations of fatty acids, which can be chosen according to the locally available commodities and the specific fatty acid needed.
Furthermore, oil is critical for producing ready-to-use therapeutic food (RUFT), which are lipid-based emulsions containing cooked, dried ingredients suspended in an oil matrix [63]. Meanwhile, oil within the RUF formulation is responsible for the moistness of cookies [64] and provides a cake-like crumbly texture preferred by children. The existence of oil can retain the gas produced during dough preparation and baking and, therefore, creates a crumbly and moist texture [65]. In addition, oil also provides viscous texture and smoothness to the paste product [66].
3.1.3. Plant and animal protein (specific amino acid)
The plant protein consumed is mainly derived from vegetable proteins, such as cereals, tubers, vegetables, beans, and fruits. Proteins obtained from plants can be separated from other components called protein concentrates and isolates. Concentrates have a protein content of at least 70%, whereas protein isolates must contain more than 90% of protein from dry ingredients. Bean protein concentrates, or isolates are obtained by depositing proteins at their isoelectric point. Some researchers set their isoelectric point at pH 3.7–5.5 to produce different yields and characteristics [67]. The use of temperature and extraction time variations [68] and acid type [69] in protein isolation techniques determine the functional characteristics and properties that protein isolates produce.
For the animal protein source, such as whey protein from cow milk, some researchers have found that whey protein substances are rich in amino acids and its composition milk is about 20%. Whey protein contains various essential amino acids, such as isoleucine, leucine, arginine, valine, lysine, and cysteine. In addition, whey is very easy to digest, so it can increase the concentration of amino acids in plasma and accelerate protein synthesis by body tissue. This good-quality protein can trigger the synthesis of hemoglobin [70]. Whey protein is also used to increase biological value, develop physical properties, texture or function, improve sensory properties, as a formulation of high-protein or low-lactose food products as well as the formation of structures in food products, including emergency food [71,72].
Protein in RUF formulation should be high in amino acids (AA), whether naturally synthesized by the body or not. The criteria for high protein biological value is generally determined by the digestibility and composition of amino acids content in a product [73]. Protein is categorized as the primary nutrient required for growth and development, especially in children and older people [70]. Protein food for children contains a standard protein of 7.5% of total energy, while high-protein food contains up to 15% of total energy [74].
Various protein-rich food products were developed, such as baked goods, snacks, sport drinks, and even as an additional component in ready-to-used food (RUF) for children [75,76]. Intermediate Moisture Food (IMF) is a product that is highly recommended as a high protein food because it has a soft texture and moisture content of 10–40% and a value of water activity (Aw) around 0.60–0.85, making microbes unable to thrive [77].
It is necessary to provide the particle size of the powder ingredient, which is less than 200 μm, to ensure the stability of the mixture and avoid segregation during the process and storage. According to Ref. [78], the homogenous particle size is essential to the mixture quality. Moreover, the particle size also affects the quality of the final product; for example, biscuits or cookies are one of the most commonly used products for RUTF. A study on the development of gluten-free cookies from maize flour indicates that the smaller particle size could improve the water-holding capacity and swelling power of the dough, as well as increase the cohesiveness of the dough, thus producing a more robust structure of cookies [79].
The efficacy of plant-based RUTF can be attained by improving the quality of protein and amino acid balance through the augmentation of crystallized amino acids. This notion is supported by the comparable results in recovery rates of most plasma essential amino acids (except methionine and tryptophan) after discharge. The efficacy of amino acid-enriched plant-based (soy, maize, and sorghum) RUTF treatment is not inferior to peanut-milk RUTF treatment in reestablishing plasma amino acid concentrations in SAM children with oedematous or non-oedematous malnutrition [45]. An efficacy study of the plant-based RUTFs (with or without milk) in Malawi showed both were as efficacious as PM-RUTF in the treatment of patients with SAM in terms of recovery rates and were more efficacious in alleviating anemia and restoring body iron stores in children aged 6–59 months suffering from oedematous and non-oedematous malnutrition [46,80,81].
Since RUTF/RUSF is for the treatment of children with severe acute malnutrition and moderate acute malnutrition, its toxicity [25] should be considered. Legumes and cereals are not only good sources of macronutrients and micronutrients but also have substantial anti-nutritional factors. Common anti-nutritional factors that can be found in edible plants are saponins, tannins, phytic acid, gossypol, lectins, protease inhibitors, amylase inhibitor, and goitrogens. Anti-nutritional factors might cause micronutrient malnutrition and mineral deficiencies due to reduced nutrient bioavailability. Several traditional methods and technologies can be applied to decrease the concentrations of these anti-nutritional factors. Techniques and methods such as fermentation, germination, debranning, autoclaving, soaking, etc., are commonly used to reduce the anti-nutritional levels in foods. However, human consumption is often varied in terms of composition and time and increasingly involves highly processed food. On the other hand, a number of antinutrients have been shown to possess beneficial properties, for example, anticancer and antimicrobial effects. Finding alternative food crops less prone to mycotoxin contamination, such as root and tuber crops, due to how they are used in culinary preparation, should be taken into consideration, especially for infants and young children [82].
Legumes, beans, and especially peanuts, can be contaminated by aflatoxins, which may increase the risk of liver cancer, impaired immune function, and malnutrition [82,83]. In application, the legumes and beans were milled/ground and roasted to avoid contamination [8,20]. However, if locally produced peanuts used in the RUTF/RUSF formulation are stored in non-safe conditions, it can increase the contamination risk connected to the presence of aflatoxins. Moreover, peanuts contain potent allergens, which may be enhanced further during cooking [84]. Consumption of aflatoxin can result in hepatic oxidative stress, and predispose the individual to hepatic cancers. RUTF should conform to international standards for maximum aflatoxin content, 10–20 ppb [8]. Very high doses of aflatoxin can produce acute intoxication [85]. Moderate doses may depress child growth [86].
Malnutrition can be associated with diarrhea and intestinal inflammation, leading to increase permeability and the release of bacterial endotoxins into the bloodstream [[87], [88], [89]]. Milk with whey protein as one of its components and soybean are common protein sources in RUTF/RUSF. A quality protein to some extent can contribute beneficial effects for intestinal inflammation. Glutamine and whey protein, have been shown to help restore gut integrity and reduce gut permeability, thus reducing the release of endotoxins and inflammation [90]. Furthermore, whey and soy protein can produce anti-inflammatory effects by decreasing the levels of IL-6 and TNF-α in the bloodstream [91]. However, to prevent excessive protein intake, which could harm health by fostering pathogens and protein-fermenting bacteria, it is recommended to have a diet with an appropriate ratio of protein to carbohydrates [92]. Furthermore, it has been proposed that lower consumption of essential amino acids (AAs) reduces linear growth because the AAs regulate the mechanistic target of rapamycin (mTOR) signaling pathway [93]. Activation of the mTOR pathway is essential for cellular growth and metabolic processes [94].
3.1.4. Micronutrients (vitamins and minerals)
Suboptimal nutritional status is caused by a lack of intake, absorption or use of one or more vitamins or minerals called micronutrient deficiencies. Excessive intake of some micronutrients may also result in adverse effects. The international community has focused on several micronutrients that remain issues globally, including iron, zinc, vitamin A, folate, vitamin B12, and iodine, as they are the most difficult to satisfy without diverse diets. One general indicator of micronutrient deficiencies is anemia, as this syndrome is caused by the deficiency of many of them, and its effects are exacerbated by several diseases.
Nutrient deficiencies have been recognized as playing an important role in limiting growth since children who are lacking of energy and protein are usually also deficient in other essential minerals (e.g., iron, zinc, iodine, potassium, magnesium, calcium), vitamins (A, C, E, D) and essential fatty acids, which are largely responsible for growth failure and increased proneness to infectious diseases [95].
The vitamins are divided into two groups, fat-soluble vitamins (A, D, E, and K) and water-soluble vitamins (B and C). Vitamin A is a necessary micronutrient in the diet for vision, growth, tissue differentiation, reproduction, and maintenance of the immune system. Carotenoids, present in fruits and vegetables, are widely believed to protect human health. Vitamin B1 (Thiamine) is found in nature in animal organisms, plants, and microorganisms. The most important sources of vitamin B1 are grain products (whole grain products, especially oatmeal), legumes, potatoes, and meat [96]. Grains and potatoes are frequently consumed as staple foods globally, often in larger quantities per meal. Various legumes and meat are classified as essential sources of dietary protein.
Dietary minerals are the chemical elements required by living organisms, other than the four elements carbon, hydrogen, nitrogen, and oxygen, present in common organic molecules. Appropriate intake levels of certain chemical elements are thus required to maintain optimal health. Essential macro minerals are minerals that the body needs in significant quantities, such as calcium, and this is usually measured in milligrams. In the other hand, essential trace minerals are minerals that are needed in minute quantities, and this usually measured in micrograms. These essential minerals are crucial to the growth and production of bones, teeth, hair, blood, nerves, skin, vitamins, enzymes, and hormones; and the healthy functioning of nerve transmission, blood circulation, fluid regulation, cellular integrity, energy production, and muscle contraction. Minerals are more resistant to manufacturing processes than vitamins. However, in the cooking process, mineral availability and absorption are also affected as foods are cooked, processed, and refined, and many naturally occurring minerals in food are lost. In contrary to vitamins, minerals are very stable under extreme processing conditions, including heating and storage [25,97].
3.2. The principle of RUTF/RUSF production
The formulation of the RUTF/RUSF was initiated with the preparation of ingredients to meet the standard for SAM and MAM children using nutritional composition prediction. The local production of RUSF/RUTF follows the principles of specific mixing procedures that combine different phases of liquid and dry/powder ingredients separately. Usually, liquid ingredients contain lipids and a small quantity of the protein, while powder ingredients consist of carbohydrates, protein, and vitamin and mineral premix. In principle, the liquid and dry ingredients are individually stirred. This initial step sometimes requires heating to achieve a homogenous mixture. During vigorous stirring, the powdered ingredients are added to the liquid ingredients and are continuously stirred for several minutes (Fig. 2).
Fig. 2 shows that the outcome of RUTF/RUSF production can be divided into two categories, namely, baked-based products, such as wafers, biscuits, and bars, and also mixed-only products, such as paste or dough. Baked-based products have been widely applied in RUTF/RUSF production because of the preparation simplicity, longer shelf life, easy packaging, and distribution, and they are also preferred by children, who are the most vulnerable group to micronutrient deficiency. Meanwhile, the paste is chosen especially for children who need assistance in consuming food due to their age or individual condition. The paste products are fresh and easily made for consumption.
Both outcome categories need proper packaging and storage to maintain the quality of the finished product. Baked products, due to their nature of low moisture content, should be kept in an air-tight packed container because of the hygroscopicity nature of the product [98]. Proper packaging and storage keep the product from direct contact with the environment which could lead to moisture absorption. The excess water absorption in the products contributes to a negative impact on the texture and shelf life of the product. The higher moisture content of the product is strongly correlated with the growth of unwanted microorganisms, which can shorten the shelf life of products [99]. Meanwhile, for paste and dough, the shelf life will not be as long as the baked products due to higher moisture content. In addition, the higher content of lipids in paste and dough will increase the susceptibility of the product to oxidation caused by overexposure to oxygen and the presence of light. The lipid oxidation was responsible for the unpleasant taste and rancid aroma of the product [100].
3.3. The accessibility of technology in RUTF/RUSF production
For the program sustainability in the treatment of SAM and AM children regarding the production of RUTF/RUSF, it is necessary to ensure the accessibility of technology in RUTF/RUSF production. Table 2 shows the development of RUTF/RUSF production in terms of technology used in the formulation and processing, local food utilization, specific nutrient premix, and measured quality parameters.
Table 2.
The development of RUTF/RUSF production method and technology.
| Year | Authors | Local ingredient | Additional ingredients | Formulation method | Mixing | Processing | Quality parameter |
|---|---|---|---|---|---|---|---|
| 2004 | Sandige et al. [15] | Full-fat milk powder, icing sugar, cottonseed oil, salt-free peanut butter |
Mineral and vitamin mixture | Manual | Mixing the Paste | – | Nutritional composition (macronutrient), aflatoxin, and microbial contamination |
| 2006 | Manary M.J [44]. | Milk powder, sugar, peanut butter, vegetable oil | Powdered vitamins and minerals | Manual | Mixing the Paste | – | Choice of ingredients, Nutritional composition (macronutrient), Aflatoxin contamination, Bacterial contamination, Prevention of oxidation, Composition of RUTF, Costs, and sustainability |
| 2007 | Linneman et al. [23] | Peanut butter, sugar, full-cream milk, vegetable oil | Vitamin and mineral supplement | Manual | Mixing the Paste | – | Nutritional composition (macronutrient), bacterial contamination, aflatoxins |
| 2012 | Owino et al. [26] | Soybean, maize and sorghum, oil and sugar | Vitamins and mineral premix | Linear Programming (LP) using Microsoft Excel software |
Extrusion continue with mixing | – | Storage stability, microbiological contamination, peroxide value, aflatoxin contamination, nutritional composition (macro and micronutrient) |
| 2012 | Purwestri et al. [24] | Wheat flour, peanut, refined sugar, palm oil, egg yolk and white, soybean or mungbean | Micronutrient powder | Manual- Nutrisurvey | Mixing the dough of the biscuit | Baking, t = 15 min T = 150–180 °C |
Nutritional composition |
| 2013 | Nga et al. [33] | Mung and soybeans, rice, sesame, sugar, whole milk powder, whey protein, vegetable fat, vegetable oil | Micronutrient premix | Manual | Mixing | Pressing (compressed bar) | Nutritional composition (macro and micronutrients), Omega-3 and Omega-6 fatty acids |
| 2014 | Ahmed et al. [28] | Rice, lentil, chickpea, dried skimmed milk powder, sugar, soybean oil | Micronutrient premix | Linear programming | Roasting rice, lentil/chickpea and continue mixing with ingredients | – | Microbial contamination (total viable count, yeasts, molds, Coliforms, Escherichia coli, Bacillus cereus, Staphylococci, Listeria monocytogenes, Cronobacter sakazaki), chemical properties (ph, water activity, moisture, peroxide value, total aflatoxin), nutritional composition (protein, fat, energy, carbohydrates) and micronutrient composition (vitamins, and minerals), storage stability |
| 2015 | Scherbaum et al. [20] | Peanut milk-based: Peanut, palm oil, refined sugar, skimmed milk powder/whole milk powder, Cereal/nut/legume-based: wheat flour, peanut, refined sugar, palm oil, eggs (yolk and white), and soybean/mungbean |
Vitamin mineral mix | Manual- Nutrisurvey | Mixing the dough of the biscuit | Baking, t = 15 min T = 150–180 °C |
Nutritional composition |
| 2015 | Hsieh et al. [30] | Skimmed milk, sweet whey, peanuts, linseed oil, palm oil, soy oil, sugar, and maltodextrin | Micronutrients and monoglyceride and diglyceride emulsifier | No information | Mixing | – | Aflatoxin, microbial contamination, fatty acid analysis, saturated fat, monounsaturated fat, ALA (α -linolenic acid), LA: linoleic acid, LA: ALA ratio |
| 2016 | Bahwere et al. [27] | Dehulled soybean, degerminated maize, sorghum (white, whole grain), dried skim milk, sugar, peanut paste, palm oil, linseed oil, palm stearin | Vitamins and mineral premix | Manual (USDA food composition Database) |
Mixing the paste | – | Nutritional composition (macro and micronutrients), amino acid profiles |
| 2016 | Stobaugh et al. [31] | Peanut paste, sugar, extruded soy flour, whey permeates, whey protein concentrate (WPC) 80, palm oil, soy oil, | Micronutrient mixture, Mono- and diglycerides | No information | Mixing | – | Aflatoxin and microbial contamination, nutritional composition (macro and micronutrients), antinutrient (Phytic acid), DIAAS, Digestible Indispensable Amino Acid Score, PDCAAS, Protein Digestibility–Corrected Amino Acid |
| 2017 | Weber et al. [32] | Peanuts, legumes (almond, groundnut, lentil, soybean), cereal/grain (maize, oat), milk powder (acid whey, non-fat dry, whey protein concentrate/WPC 34 and 80, refined vegetables oil (canola rapeseed, coconut, palm, soybean, sunflower), sugar | Vitamin mineral powder and emulsifier | Linear programming | Mixing | Extrusion | Nutritional composition (macro and micronutrients), water activity, and ph. |
| 2018 | Choudhury et al. [29] | Rice, lentil, chickpea, dried skimmed milk powder, sugar, soybean oil | Soy lecithin, vitamin mineral premix | Linear programming | Roasting, particle size reduction, homogeneous blending, and packaging |
– | Microbial contamination (total viable count, yeasts, molds, Coliforms, Escherichia coli, Bacillus cereus, Staphylococci, Listeria monocytogenes, Cronobacter sakazaki), chemical properties (ph, water activity, moisture, peroxide value, total aflatoxin), nutritional composition (protein, fat, energy, carbohydrates) and micronutrient composition (vitamins and minerals) |
| 2018 | Sigh et al. [37] | The indigenous small freshwater fish species, mungbean, rice, soybean, icing sugar, canola oil, maltodextrin, palm vegetable shortening, desiccated coconut, rice bran, vitamin and mineral mix, rice flour, duck egg, refined sugar, coconut, salt, vanilla or sesame seeds, cooking oil | Micronutrient premix | Manual | Mixing | Extrusion and baking (wafer) | Organoleptic assessment, nutritional composition, microbial contamination |
| 2018 | Armini et al. [39] | sunflower oil, dehulled and roasted sorghum and soy flour, icing sugar, dried microalgae Spirulina | Soy lecithin | Response surface method | Mixing | – | Particle size distribution, viscosity, water activity, and optimization formula |
| 2019 | Borg et al. [36] | Rice, small freshwater fish, soy, and mung beans, oil, sugar, coconut | Multiple micronutrients | Manual | Mixing | Baking (wafer) | Nutritional composition, microbial contamination |
| 2020 | Miele et al. [40] | Sunflower oil, dehulled and roasted sorghum and soy flour, icing sugar, dried microalgae Spirulina | Soy lecithin | Response surface method | Mixing | – | Particle size distribution, viscosity, oxidation indexes, water activity, sensory evaluation (nonoral evaluation and customer test), volatile compound |
| 2020 | Azimi et al. [41]. | Soy protein isolate, whey protein, egg white, dates, vegetable oils, sugar, starch | Micronutrient premix | Manual | Mixing | – | Quality control of the product, microbial and aflatoxin contamination |
| 2020 | Hossain et al. [42] | Skim milk, soy protein isolate, sugar, vegetable oil, peanut paste | Mineral vitamin mix | Manual | Mixing | – | Nutritional composition (macro and micronutrients) |
| 2021 | Javed et al. [43] | Mungbean (Vigna radiatus) chickpea (Cicer arietinum) and peanut (Pyrus communis), sugar, vegetable oil, and milk powder | Vitamin/mineral premix | Manual | Mixing | – | Microbiological analysis, water activity, peroxide value, thiobarbituric acid (TBA) value as well as color analysis |
| 2021 | Fetriyuna et al. [25] | Taro (Xanthosoma undipes K. Koch), red rice (Oryza sativa), maize (Zea mays), peanut (Arachis hypogaea), mungbean (Vigna radiata), banana (Nangka) (Musa textilia) |
Vitamin/mineral premix | Manual- Nutrisurvey | Mixing the biscuit dough | Baking, t = 15 min T = 150 °C |
Nutrient composition (macro and micronutrients), physical properties (color and dimension) sensory evaluation test (seven-point hedonic scale) |
| 2022 | Yazew. Y [101]7) | maize (Zea mays L.), soybeans (Glycine max), and banana (Musa spp) corn kernels, soybeans, plantain fruits, soybean oil, minerals, and sugar as sweeteners. | Vitamin/mineral premix | Manual | Mixing | mixtures of germinated and nongerminated raw | Proximate, minerals, antinutrition factors, sensory evaluation |
| 2022 | Hadi et al. [102]8) | Soybean, milk protein concentrate corn flour, sugar cacao butter, Nigella sativa, and sesame seeds | vitamin/mineral, beta-alanine, arginine | Manual | Mixing | ball mill refiner at the temperature of 45 °C, | Macro nutrient and micro nutrient (Vitamins such as ascorbic acid, thiamine, folic acid, and retinol), sensory evaluation |
| 2022 | Adewumi et al. [103]9) | Bambara groundnut, Moringa oleifera leaf protein isolate, millet, melon seed (egusi), oat, cocoa powder, icing sugar, canola oil, and honey | Bambara groundnut-Moringa oleifera leaf protein complex (BAMOLP) | SuperPro designer (Version 9) | Mixing | baked in a pre-heated oven at 160 °C for 30 min | Physical Properties (Morphology, Water Activity, color, texture), Nutritional(Amino acid, Minerals), Proximate Composition and sensory |
| 2023 | Akande et al. [104]0) | Dried migratory locust (Locusta migratoria), peanut, Skimmed milk, icing sugar, soybean oil | Vitamin/mineral premix | Manual | Mixing |
Proximate composition, energy and total sugars, minerals, vitamin, amino acid profiling, Phytochemical compositions Antioxidant activity |
Note: RUTF: Ready to Use Food; RUSF: Ready to Use Sulementary Food; USDA: United State Department of Agriculture.
Undernourishment is more prevalent in developing countries where the majority of people have low level of education and poor economic status, simple technology is more appropriate than sophisticated equipment or machinery. For a small production of RUTF/RUSF, manual mixing is possible, while in large production, a mechanical mixer is required. Manual mixing is not recommended for a large scale production [44] due to the inconsistent results and less homogenous mixture during the process and storage for liquid forms of RUTF/RUSF. Small-scale production of RUTF/RUSFs can be done in the community with simple technology, such as manual mixing and baking [44,24], and it has been developed recently for the separation of whey protein as the main ingredient of RUTF/RUSF [31,105]. Meanwhile, large-scale production needs a mechanized facility.
The RUTF/RUSF were developed based on the guidelines from the WHO for the treatment of Severe Acute Malnutrition (SAM) [9] and the recommended treatment for Moderate Acute Malnutrition (MAM) [14]. The fulfillment of the RUTF/RUSF formulation were first analyzed using a food software, e.g., NutriSurvey [24,106] using a linear programming model [28,105,107], or response surface methodology [39].
In the production of RUTF/RUTF, the processing techniques used vary from simple techniques (mixing and baking) to modern techniques using the extrusion method [90]. In general, the food supplement type is categorized as semi-liquid (paste and porridge) and solid form (biscuit, wafer, snack bar). Both of these forms have their strengths and weaknesses with regard to the appointed target group of undernourished children. The paste form of RUTF/RUSF is easy to squeeze from the package [108,109], while the biscuit and bar form are most favorable to children [33,110]. The studies in Indonesia presented that the rate of weight gain of the participating children was significantly correlated to the compliance of consuming the RUSF [20,110], indicating the importance of producing the RUSF that are palatable and favored by the children and accepted by the caretakers. The development of RUTF/RUSF is presented in Table 2.
In the early RUSF/RUTF development, the acceptance of the target group was usually considered, along with an intervention study [12,15,111]. Recently, the development of local production of RUTF/RUSF placed the product review (usually sensory test) as a part of product development and continued with the improvement of the product before the actual intervention studies [80,108].
3.4. Program effectiveness of RUTF/RUSF provision in the community
Not all the articles reported on the program effectiveness had the RUTF/RUSF provision in a community setting. The program effectiveness of the RUTF/RUSF provision is indicated by several health/nutrition indicators and the acceptability of the food supplement. It is usually compared to the Sphere emergency nutrition standard [19]. Among others, the health nutrition indicators are the rate of weight gain [19]. The child mortality rate was normally reported among the participants with SAM children [8,10,12,15] but less likely found among the MAM children. The reported nutritional status improvement, percentage of recovery, and length of stay depended on the entry and discharge criteria of the program.
The first community trial using peanut-milk spread RUTF was in Malawi (Table 2). The duration of the RUTF provision studies in Malawi was between four to eight weeks. Rates of weight gain of the SAM children after the treatment in the clinical setting that were given the standard therapy (therapeutic milk F100) and commercial peanut-milk spread RUTF were 10.1 and 15.6 g/kg/day [7], which were above the Sphere emergency nutrition standard (≥8 g/kg/day) [19]. However, in the community setting, the average rate of weight gain of the SAM children consuming peanut-milk spread RUTF was lower, between 3.5 and 5.2 g/kg/day [8,10,12,15]. MAM children in Malawi and those at risk of malnutrition participating in the community nutrition center who were assigned to consume peanut-milk RUTF had a comparatively lower weight gain (2.8 and 3.1 g/kg/day) [11,112]. Furthermore, by utilizing the local food resources in Indonesia, locally produced RUSF-Nias biscuits were developed. The study was implemented among mildly and moderately wasted children. Mean rates of weight gain among the mildly and moderately wasted children were between 2.0 and 3.9 g/kg/day [24,110]. In Zambia, the MAM children consuming a soy-maize-sorghum-based RUTF produced a comparable weight gain (approximately 3 g/kg/day) [113].
Different preparations of RUTF have been proposed to provide sustainable and affordable locally-produced RUTF formulation. Most of the peanut-milk spread, RUTF distributed and presented in the earlier studies in Malawi was commercially produced. A later study, also in Malawi, reported that the provision of the locally produced peanut-milk spread RUTF resulted in a similar mean range of weight gain of the treated SAM children consuming the commercial one [15]. Locally produced RUTF might be an option in particular areas with lower acceptability for the standard RUTF and could provide better sustainability in the future; hence, the use of local food resources is further encouraged.
In Indonesia, locally produced RUSF-Nias biscuits were developed by utilizing local food resources. The study was implemented among mildly and moderately wasted children, and the program effectiveness of the RUSF-Nias biscuits was compared to the locally-based peanut-milk spread RUTF. The results showed similar mean rates of weight gain among the mildly and moderately wasted children (between 2.0 and 3.9 g/kg/day) [24,110]. In Zambia, the MAM children consuming a soy-maize-sorghum-based RUTF, produced a comparable weight gain (approximately 3 g/kg/day) [113] to the Indonesian study (Table 3). A study in Cambodia described the average weight gain with the standard RUTF and the locally produced RUTF by 2.97 (±1.57) and 2.52 (±1.23) g/kg body weight/day, respectively. Both RUTF formulations increased height and HAZ-scores significantly, with an average height gain of 22.4 (±18.7) mm and 32.3 (±36.3) mm for respective standard and locally produced RUTF, whereas acceptability was higher for locally produced RUTF [114]. These results are in line with a broader metanalysis study that suggest the current evidence does not favor a particular formulation RUTF, except for a relapse, which is reduced with standard RUTF [115]. Table 3 presents the outcomes of tested RUTF/RUSF for some research setting.
Table 3.
Relevant program outcomes of the provision of RUTF/RUSF.
| Group assignment | Entry and discharge criteria | Weight gain | Recovery rate, Mortality rate, or Default rate | Compliance/acceptance and length of stay in the program (in days) | References |
|---|---|---|---|---|---|
| Locally produced RUTF: 135 children Commercially/imported RUTF: 125 children Only Targeted Children with HIV-Negative: 99 locally produced RUTF 83 Commercially/imported RUTF |
Entry: Children who were not hospitalized with malnutrition were eligible if they were referred from the outpatient department for the treatment of malnutrition with either edema or a weight-for-height Z score < −2 (WHZ) Discharge: Children completed the study when they reached a weight-for-height Z score > −0.5 (WHZ), relapsed, died, or failed to achieve WHZ > −0.5 after 16 weeks. |
Locally produced RUTF: 5.2 ± 4.6 g/kg/day Imported RUTF: 4.8 ± 4.0 g/kg/day Only Targeted Children with HIV-Negative: Locally produces: 5.6 ± 4.6 Imported RUTF: 5.5 ± 3.8 |
Recovery rate: 80% percent of those receiving locally produced RTUF and 75% of those receiving imported RTUF reached WHZ > −0.5. Mortality rate: HIV negative: Locally produced RUTF = 3% of death/failure Imported RUTF 2% of death/failure HIV positive: 30% of death/failure Default Rate: 91% of all children maintained a normal WHZ |
- Compliance rate: HIV negative: Locally produced RUTF = 95% Imported RUTF = 95% HIV positive: 59% - Length of stay: 180 days/six months |
Sandige et al. (2004) [15] |
| 51 Daily Program; 48 Weekly Program |
Entry: In semi-urban areas, 51 children aged ≥6 to <60 months old, with weight-for-height (WHZ) ≥ -2 to < -1.5 SD WHO 2006 standard reference data and no congenital disability or disease that could limit the ad libitum food intake, were continuously recruited from the existing community-based screening programs in the Church World Service project area, and assigned to the Daily Programs. Forty-eight children in remote rural regions were allocated to Weekly Programs. Discharge: The individual discharge criterion of the programs was WHZ ≥ -1.5 SD. |
Weight gain of the children in Daily Programs was higher (3.1 ± 3.6 g/kg/day) than in Weekly Programs (2.0 ± 2.1 g/kg/day), | Recovery Rate: Of the 76 children who reached the discharge criterion (WHZ ≥ -1.5 SD), 47 (27 children in Daily and 20 children in Weekly Program) were followed up on average after 4.9 months (data not shown). Mortality Rate: No information |
Compliance Rate: Of the index children admitted in Daily Programs (n = 51), 80.4% reached target WHZ, which was higher than in Weekly Programs (72.9%; n = 48) Amount consumption of the RUTF: Daily Programs enabled supervision and monitoring of onsite consumption of 30–40% of the daily portion of RUF-Nias biscuits, in contrast to only about 5–10% portion in the Weekly Programs. Length of stay: Similar length of stay of about 32 days, Each program: Daily Program = 42.7 ± 36.5 Weekly Program = 43.5 ± 31.3 |
Purwestri et al. (2012) [24] |
| 34 Daily Programs 20 Weekly Programs |
Entry: aged ≥6 to <60 months with weight-for-height z-score (WHZ) ≥ −3 to < −2 SD were recruited Discharge: The individual discharge criterion was WHZ ≥ −1.5 SD. |
Weight gain: Daily Programs: +3.9 ± 3.8 g/kg/day Weekly Programs: +2.0 ± 2.0 g/kg/day, |
|
RUTF Intake: Daily Program: 83.3 ± 32.6 Weekly Program: 78.4 ± 33.0 Length of stay: Daily Program: 51.2 ± 40.0 Weekly Program: 61.8 ± 35.1 |
Purwestri et al. (2013) [110] |
| 29 Peanut/Milk-based spreads (PM-S) 44 cereal/nut/legume-based biscuits (CNL-B) 38 cereal/nut/legume-based biscuits whose mothers received intensive nutrition education (CNL-B + INE) |
Entry: Moderately and mildly wasted children with a weight-for-height z-score (WHZ) ≥ -3 to < -1.5SD, aged ≥6–60 months and with no sign of congenital disabilities or disease which would limit the food intake Discharge: They were individually discharged after reaching WHZ ≥ − 1.5SD. |
Weight Gain PM-s: 1.01 (0.43, 2.36) CNL-B: 1.76 (1.12, 2.79) CNL-B + INE: 2.31 (1.64, 3.26) |
Recovery Rate: PM-S: 62 [18] CNL-B: 84 [31] CNL-B + INE: 79(30) |
Compliance >80%: PM-S: 45(13) CNL-B: 86(38) CNL-B + INE: 84 [25] Children in CNL-B programs showed a significantly higher frequency of high compliance (>80%) to the RUF (86% and 84% vs. 45%, p < 0.001) Length of Stay: PM-S: 25.3 (17.5, 36.5) CNL-B: 32.9 (25.4, 42.6) CNL-B + INE: 29.6 (20.8, 42.6) |
Scherbaum et al. (2015) [20] |
| 1103 P-RUTF 824 SMS-RUTF |
Entry: Children aged 6–59 mo; have been diagnosed suffering from SAM (MUAC <11 cm; pitting edema grade 1 (+) or grade 2 (++) without complications of the 24 HCs (lost appetite, dehydration) Excluded: SAM children w/complications; relapse; refused transfer |
Weight Gain P-RUTF (95%CI) in g/kg/day: All forms of SAM: 3.2 (2.9, 3.5) Non-oedamatous cases: .5 (4.0, 5.0) Oedamatus cases: 2.6 (2.2, 2.9) Weight Gain SMS-RUTF (95%CI) in g/kg/day: All forms of SAM: 2.2 (1.9, 2.5) Non-oedamatous cases: . 3.4 (2.8, 4.1) Oedamatus cases: 1.7 (1.4, 2.1) Children in the SMS-RUTF arm had a lower weight gain than those in P-RUTF arm (p = 0.007) in both oedematous (p = 0.018) and non-oedematous (p = 0.091) cases. There was no statistically significant interaction between weight gain and other baseline variables. |
Recovered: P-RUTF: 671/1103 (60.8%) SMS-RUTF: 439/824 (53.3%) Mortality Rate: P-RUTF: 138/1103 (12.5%) SMS-RUTF: 113/824 (13.7%) Default Rate: P-RUTF: 278/1103 (25.2%) SMS-RUTF: 233/824 (28.3%) |
The median length of stay for children recruited into the P-RUTF arm was 35 days (23–49 days), and for SMS-RUTF, it was 35 days (21–56 days), For those who were discharged as recovered, the median length of stay was 35 days [[23], [24], [25], [26], [27], [28], [29], [31], [32], [33], [34], [35], [36], [37], [38], [40], [41], [42], [43], [47], [48], [49], [50], [51], [52]] and 47 days [[23], [24], [25], [26], [27], [28], [29], [31], [32], [33], [34], [35], [36], [37], [40], [41], [42], [43], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70]] for those in the P-RUTF arm and those in the SMS-RUTF, respectively (p < 0.001). | Irena et al. (2015) [113] |
| 445 SMS-RUTF 441 P-RUTF |
Entry: Children w/MUAC <115 mm; good appetite; no medical complication: and those with bilateral pitting edema assessed as + or ++ who also had a good appetite and no medical complication. Excluded: bilateral pitting edema assessed as +++ degree or with any medical complication was referred to the participating inpatient |
Weight gain: margin 1.2 g/kg/day | Children aged 24–59 months SMA-RUTF: Recovery rate: 83.3% Mortality rate: 1.7% Default: 7.8% P-RUTF: Recovery rate: 90.3% Mortality rate: 0.4% Default: 7.6% Children aged 6–23 months SMA-RUTF: Recovery rate: 54.3% Mortality rate: 3.4% Default: 24.5% P-RUTF: Recovery rate: 75.1% Mortality rate: 1% Default: 15.7% |
|
Bahwere et al. (2016) [27] |
| 13 panels from Dhaka Hospital for the development of RUTF 90 Children aged 6–18 months for Acceptability Trial (30 for Rice-Lentil based RUSF; 30 for Chickpea RUSF; 30 for Pusthi Pucket) |
Entry for Development RUTF: Not directly involved with the present study Entry for Acceptability Trial: age 6–18 months, started semi-solid food Discharge Involved in other studies, not available, Age >18 months, refused, the child is sick, weight-for-age or weight-for-height Z-score was < −3 if they had any acute illness or features suggestive of any chronic disease such as tuberculosis, any congenital anomalies such as trisomy 21, cleft lip, or palate. |
Favorable rate: 18/30 children (60%) liked rice-lentil, and 20/30 (66%) preferred chickpea-based RUSF. In the Pushti packet group, only 12/30 (40%) caregivers reported that their children liked the supplement. |
Amount consumed g (mean ± SD): Rice Lentil-RUSF: 23.8 ± 14 Chickpea RUSF: 28.4 ± 15 Pusthi Pucket: 17.1 ± 14 (40.8 ± 35*) Percentage of food consumed from offered food: Rice Lentil-RUSF: 47.2 ± 28 Chickpea RUSF: 56.7 ± 31 Pusthi Pucket: 34.4 ± 28 Feeding time (minutes) Rice Lentil- RUSF: 23 ± 10 Chickpea RUSF: 20.7 ± 10 Pusthi Pucket: 20.4 ± 7 |
Ahmed et al. (2014) [28] |
|
| 1438 Control Group 1492 Plumpy doz group 825 Rice lentil group 843 Chickpea group 851 WSB++ group |
Entry: Infants were enrolled at six months Discharge: Not met/not into; refused; the child died; aged out at seven months, permanently moved. |
Weight gain kg/months, in mean (SE) Control: 0.24 (0.01) Plumpy doz: 0.28 (0.01) Rice Lentil: 0.26 (0.01) Chickpea: 0.28 (0.01) WSB++: 0.26 (0.01) Treatment effect b (95% CI) Control: Plumpy doz: 0.03 (0.02, 0.05) Rice Lentil: 0.02 (0.003, 0.04) Chickpea: 0.04 (0.02, 0.05) WSB++: 0.01 (−0.002, 0.03) |
Mortality: 39 children died after enrolment, mortality rate <1% |
Length of stay: 540 days/18 months | Christian et al. (2015) [116] |
| 70 RUTF 71 HO-RUTF |
Entry: Having a mid-upper arm circumference <11.5 cm and/or bilateral pitting edema, who qualify for community-based management of SAM. Appetite was assessed by giving the child 30 g of RUTF and requiring him/her to consume it within 20 min. Excluded: Exclusion criteria were treatment for SAM in the previous six months, the presence of a chronic, debilitating condition such as cerebral palsy, congenital heart disease, or peanut allergy. HIV infection was not an exclusion criterion. |
Weight gain after four weeks, in g/kg/day: RUTF = 2.0 ± 2.6 HO-RUTF = 2.8 ± 3.1 |
Recovered: RUTF: 50/70 (71%) HO-RUTF: 48/71 (68%) Mortality Rate: RUTF: 5/70 (7%) HO-RUTF: 1/71 (1%) Defaulted/remained malnourished: RUTF: 9/70 (13%) HO-RUTF: 19/71 (27%) |
Acceptance: Likeability on the first day for the first and second components showed a score of 5, the highest on the scale, for 64/74 participants receiving RUTF and 59/74 receiving HO-RUTF (p = 0.38). On the fourth day of the acceptability survey, in the second component of the trial, all participants reported a likeability score of 5. During the first activity of the acceptability trial, children consumed 30 g of standard RUTF in 9.3 min and 30 g of HO-RUTF in 12.3 min. In the second activity, standard RUTF and HO-RUTF consumption took 10.4 and 12.8 min, respectively. The amount of food remaining at the end of the taste test was greater among the HO-RUTF food taste testers than standard RUTF tasters in both components (3.7 ± 8.0 vs 1.3 ± 4.6 g, p = 0.03). Length of stays: 84 days/12 weeks |
Hsieh et al. (2015) [30] |
| 1144 for whey RUSF group; 1086 for soy RUSF group | Entry: Children aged 6–59 months with MAM, as defined by a mid-upper arm circumference (MUAC) of 11.5–12.4 cm without bipedal edema Discharge: Having continued MAM, developing SAM (MUAC, 11.5 cm and/or bipedal edema), dying, or defaulting (failing to return for three consecutive visits). |
Weight gain from enrollment to final visit (g/kg/day): Soy RUSF: 2.79 ± 2.16 Whey RUSF: 2.95 ± 2.04 |
Recovered: Soy RUTF: 874/1086 (80.5%) Whey RUTF: 960/1144 (83.9%) Mortality Rate: Soy RUTF: 4 (0.37%) Whey RUTF: 2 (0.17%) Default rate: Soy RUTF: 28/1086 (2.6%) Whey RUTF: 16/1144 (1.4%) |
Length of stays: 84 days/12 weeks Acceptability testing: 60 children aged 6–51 months enrolled in the acceptability trial; all but one returned for the follow-up questionnaire. The mean times for children to consume both RUSF foods were similar on the initial visit. Both foods were deemed highly acceptable based on the hedonic scale ratings and comments from the caregivers. One child in the soy RUSF group and two children in the whey RUSF group had a new onset of diarrhea after starting the RUSF, all lasting 1–2 d. |
Stobaugh et al. (2016) [31] |
| 61 BP-100 60 NumTrey |
Entry: Aged 6–59 months with SAM (WHZ ≤ −2.8 and/or MUAC ≤115 mm, and/or presence of nutritional edema); pass an appetite test Excluded: Uncontrolled or untreatable systemic opportunistic infection, severe cerebral palsy, apparent dysmorphic features, general mental health problems, or participation in other clinical trials. |
Weight gain: BP-100 = 1.06 g/kg/day 95% CI (0.72, 1.41) NumTrey = 1.08 g/kg/day 95% CI (0.75, 1.41). |
Recovery rate: Mortality rate: BP-100 = 2/61 |
The proportion of patients expressing that they liked the product increased after the eight-week intervention for NumTrey and remained ∼90% for BP-100TM during the trial. The steady increase in acceptability of NumTrey can be seen as an adaptation to an unfamiliar product, not uncommonly seen in the acceptability testing of novel products |
Sigh et al. (2018) [38] |
| 105 M-RUTF 108 Soy-RUTF |
Entry criteria Children (both boys and girls) with SAM defined by WHZ < −3 of WHO—2006 standard, without other medical illness, or clinically improved from a medical condition, no edema, regaining appetite, and aged 6–59 months were included. Additional enrollment criteria were: no signs of concurrent infection; mothers/caregivers agreed to stay in their current address for the next four months (for tracking the children); and informed written consent given by the parent or guardian. Discharge criteria: Discontinued after two skipped visits, Children without any fixed address; tuberculosis (according to WHO criteria) or any congenital/acquired disorder affecting growth, i.e., trisomy-21 or cerebral palsy; children on an exclusion diet for the treatment of persistent diarrhea, and having a history of soy, peanut or milk protein allergies. |
The absolute gain in body weight of the children was 0.698 ± 0.438 kg versus 0.741 ± 0.381 (p = 0.553); and the rate of weight gain was 3.9 ± 3.2 g/kg/day (median: 3.63; 95% confidence interval: 3.29–4.51) versus 5.2 ± 4.6 (median: 4.29; 95% CI: 4.35–6.07) g/kg/d (p = 0.078) in Soy-RUTF group and M-RUTF group. | The number of total follow-ups (attended): Soy-RUTF = 3.7 ± 2.4 M-RUTF = 3.4 ± 2.2 Recovery rate: Soy that is less processed has lower digestibility, and the amino acids are less bio-available for use by the body to support recovery. The anti-nutrients present in less processed soy might have contributed to the lower recovery rate. |
Total days enrollment: Soy-RUTF = 44 ± 34 M-RUTF = 39 ± 30 Acceptability of product: A total of 36 children participated in the pilot taste acceptability trial: Absolute amount (g) taken over 30 min (single mealtime): mean ± SD Soy-RUTF = 38.8 ± 16.1 M-RUTF = 40.7 ± 17.4 Amount (g) taken per kg: mean ± SD Soy-RUTF = 6.4 ± 2.8 M-RUTF = 6.8 ± 3.4 |
Hossain et al. (2020) [42] |
| 50 Control Group 50 RUSF |
Entry: Children aged 24–59 months with mild or moderate malnutrition, were enrolled from six urban health centers in Shahr-e-Rey, Tehran, Iran, between April and October 2017. Malnutrition was defined as mild-moderate wasting with a WHZ between 1 and 3 standard deviations (SDs) of the WHO reference population. Exclude: Children with a chronic or debilitating illness, such as severe anemia, liver and kidney diseases, malabsorption syndromes, or a history of allergy to egg white or soy protein, developed severe acute malnutrition (WHZ <3 and/or bipedal edema) or were absent for two consecutive visits. |
Weight gain after 8 weeks of supplementation RUSF: 1.44 ± 0.38 kg Control: 0.7 ± 0.32 kg |
Recovery Rate: About 92% of the children in the RUSF group, and 12% of those in the control group have reached a WHZ>1, as the recovery cutoff, at the end of the study, which was significantly higher in the intervention group (p < 0.001) RUSF has a statistically significant effect on the rate of recovery adjusted for the other potentially confounding variables (AHR: 16.3, 95% confidence interval: 6.1 to 43.3, P 1⁄4 0.001). |
Lenght of stay = 56 days/8 weeks Compliance Rate: RUSF = 100% Control Group = 98% |
Azimi et al. (2020) [41] |
Note: RUF: Ready to Use Food; RUTF: Ready to Use Food; RUSF: Ready to Use Sulementary Food; WHZ: Weight-For-Height Z score; HIV: Human Immunodeficiency Virus; WHO: World Health Organization; SD: Standard Deviation.
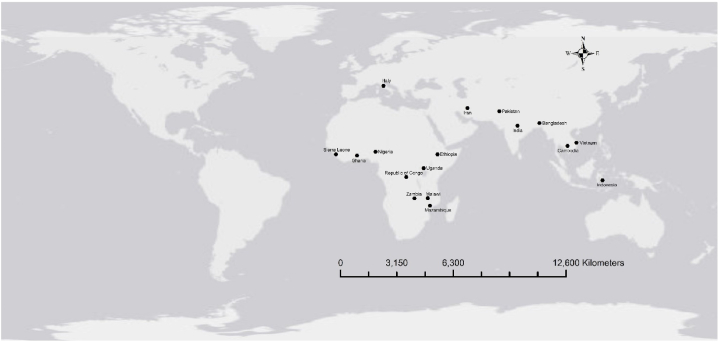
The implementation or RUF/RUTF from the local food ingredients has been tested in several countries especially in Africa and Asia. Fig. 3 shows the mapping of the implementation of RUF/RUTF or RUSF made from local food resources.
Fig. 3.
The location of the implementation of RUF, RUTF and RUSF from local food resources.
3.5. Sustainability aspects and future perspective of the provision of RUTF/RUSF from local food resources
Nutrition centers in the community-based setting aim to reduce the mortality of children at risk – to severe acute malnutrition in emergency and development settings due to the decentralization of community outreach. It is expected to provide for more children than other clinical institutional approaches [117]. The necessity of RUTF/RUSF in the community nutrition centers depends on the local need and context. A study among Malawian children found that locally produced peanut-milk spread RUTF was relatively inexpensive compared to the imported ones and achieved the same effect: an increased weight gain of the children [15]. Therefore, making new formulations of RUTF/RUSF based on locally available foods is expected to be efficient and cost-effective. Another critical issue concerning the production and distribution of RUTF/RUSF is the lack of sustainability and financial-logistic-distribution problems on the NGOs or government side. The ingredients of RUTF/RUSF, especially the premix (micronutrients), are still not accessible in terms of supply and are costly to the most vulnerable group. Therefore, in many low income countries with a high proportion of malnourished children, RUTF/RUSF will be sustainable only if its production is supported by the government or humanitarian organizations. Moreover, the duration of intervention is set to a limited period. After the health/nutritional status of the malnourished children is improved, the children should return to their regular diet.
The programs for rehabilitating MAM children need a more comprehensive and complex approach than for SAM children. It involves the improvement of families’ livelihoods and changing poor childcare behavior; therefore, intensive nutritional education is needed to accompany the provision of RUTF/RUTF. The leading solutions are to improve local production focused on a diversified group of nutritious crops and to increase public awareness regarding feasible and low-cost approaches to a healthy diet.
In terms of food supply, the utilization of locally available commodities needs to be promoted. Lessons learned from previous nutrition intervention programs is the assurance of its sustainability, for example, using homegrown items or low-cost commodities available in traditional neighborhood markets. Meanwhile, the technology used in producing RUTF/RUSF should be accessible. Most of the reported studies use simple technology that is commonly implemented at the household level. However, the factor usually neglected is the proper method of utilizing simple technology because it will greatly affect the quality of RUF products. For example, the sun drying method for animal-based commodities needs to be properly monitored regarding the time of drying, cleanliness, and possible cover to protect from dust and other unwanted material. Roasting can also be done at the household level, however, the time, temperature, and final products will determine the quality of RUTF/RUSF, especially in the nutritional content. In principle, solid manufacturing practices widely known in food production at the higher level should be adapted on the microscale or household scale because proper processing methods and technology affect the quality of products.
For the country level, the implementation of RUTF/RUSF for the nutrition intervention has been guaranteed with the national nutrition strategy like in Vietnam [118], as well in the international level by FAO [119]. In Vietnam, integrated management of acute malnutrition (IMAM) using local products was found to be highly acceptable and effective after product optimization and training of health workers. Children with SAM have been treated very successfully, with over 90% of children recovering. Its locally manufactured RUTF capacity is being increased to allow for the expansion of the IMAM program and the potential export of products to countries in the region [120].
4. Concluding remarks
Ready-to-Use Therapeutic Food (RUTF) or Ready-to-Use Supplementary Food (RUSF) have been widely used in home-based treatment for severely and moderately acute malnourished children. These products have been deemed very effective at short-term nutritional recovery in children and have shown substantial success. RUTF and RUSF are expected to be given in combination with local diets to fulfill the dietary recommendation of SAM and MAM children. The new formulation of RUTF/RUSF based on locally available foods is projected to be efficient and more cost-effective for treating MAM and SAM children. Taking into account regional preferences, closeness to their intended consumers, accessibility to raw materials, and production sustainability, locally generated RUF/RUTF can be used for MAM/SAM processing that is safe and sustainable. Our findings presented the importance of development of such food supplements based on the local food resources and with improved technology for prevention and rehabilitation of malnourished children, which encourage the development in another developing countries with similar nutritional problem. The results underscore the significance of developing food supplements using local resources and enhanced technology for the prevention and rehabilitation of malnourished children. Furthermore, the review findings encourage creation of such food supplements in other developing countries facing similar nutritional challenges.
Funding
This review research was funded by Hibah Artikel Review Universitas Padjadjaran, grant number No. 1959/UN6.3.1/PT.00/2021. F·F and R.C.P. were supported by the Federal Ministry of Education and Research within the project Humboldt reloaded (01PL11003) at the University of Hohenheim, Stuttgart. R.C.P. was also funded by the Operational Program Research, Development, and Education (OP-RDE), Ministry of Education of the Czech Republic, grant no. Z.02.1.01/0.0/0.0/16_019/0000803 (EVA 4.0).
Data availability statement
No. Data for Review is included in article/supp. material/referenced in article.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Fetriyuna Fetriyuna: Writing – review & editing, Writing – original draft, Project administration, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization. Ratna Chrismiari Purwestri: Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Ignasius R.A.P. Jati: Writing – review & editing, Visualization, Methodology, Formal analysis. Budhi Setiawan: Writing – review & editing, Writing – original draft, Formal analysis. Syamsul Huda: Writing – review & editing, Writing – original draft, Project administration, Funding acquisition, Formal analysis. Nia Novita Wirawan: Writing – review & editing, Writing – original draft, Methodology, Formal analysis. Robi Andoyo: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors appreciate the help of Theodore Tanudji and Regina Magaña Vázquez within the Humboldt reloaded project, and Rizky Fitriani Syahbana in the literature research assignment. The support of the project “Advanced research supporting the forestry and wood-processing sector's adaptation to global change and the 4th industrial revolution” (EVA 4.0) at the Czech University of Life Sciences Prague, Directorate of Research and Community Services Universitas Padjadjaran for the opportunity to conduct this review study is also acknowledged.
Contributor Information
Fetriyuna Fetriyuna, Email: fetriyuna@unpad.ac.id.
Ratna Chrismiari Purwestri, Email: purwestri@fld.czu.cz.
Ignasius R.A.P. Jati, Email: radix@ukwms.ac.id.
Budhi Setiawan, Email: budhisetiawan@uwks.ac.id.
Syamsul Huda, Email: syamsul.huda@unpad.ac.id.
Nia Novita Wirawan, Email: nia_wirawan.fk@ub.ac.id.
Robi Andoyo, Email: r.andoyo@unpad.ac.id.
References
- 1.World Health Organization . World Health Organization; 1999. Management of Severe Malnutrition: a Manual for Physicians and Other Senior Health Workers. [Google Scholar]
- 2.Briend A., Lacsala R., Prudhon C., Mounier B., Grellety Y., Golden M.H. Ready-to-use therapeutic food for treatment of marasmus. Lancet. 1999;353(9166):1767–1768. doi: 10.1016/S0140-6736(99)01078-8. [DOI] [PubMed] [Google Scholar]
- 3.Briend A. Highly nutrient-dense spreads: a new approach to delivering multiple micronutrients to high-risk groups. Br. J. Nutr. 2001;85(S2):S175–S179. [PubMed] [Google Scholar]
- 4.Collins S., Dent N., Binns P., Bahwere P., Sadler K., Hallam A. Management of severe acute malnutrition in children. Lancet. 2006;368(9551):1992–2000. doi: 10.1016/S0140-6736(06)69443-9. [DOI] [PubMed] [Google Scholar]
- 5.Wold Health Organization . World Health Organization; Geneva, Switzerland: 2013. Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children.https://www.who.int/publications/i/item/9789241506328 [cited 2023 Aug 15] p. 115. Available from: [PubMed] [Google Scholar]
- 6.World Food Programme (WFP) World Food Programme; Rome, Italy: 2012. Nutrition at the World Food Programme. Programming for Nutrition-specific Interventions.https://documents.wfp.org/stellent/groups/public/documents/communications/wfp258650.pdf [cited 2023 Aug 15] p. 38. Available from: [Google Scholar]
- 7.Diop E.H.I., Dossou N.I., Ndour M.M., Briend A., Wade S. Comparison of the efficacy of a solid ready-to-use food and a liquid, milk-based diet for the rehabilitation of severely malnourished children: a randomized trial. Am. J. Clin. Nutr. 2003;78(2):302–307. doi: 10.1093/ajcn/78.2.302. [DOI] [PubMed] [Google Scholar]
- 8.Manary M.J., Ndkeha M.J., Ashorn P., Maleta K., Briend A. Home based therapy for severe malnutrition with ready-to-use food. Arch. Dis. Child. 2004 Jun 1;89(6):557–561. doi: 10.1136/adc.2003.034306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO/WFP/UNSSCN/UNICEF . World Health Organization; 2007. Community-Based Management of Severe Acute Malnutrition : a Joint Statement by the World Health Organization, the World Food Programme, the United Nations System Standing Committee on Nutrition and the United Nations Children’s Fund [Internet]https://apps.who.int/iris/handle/10665/44295 [cited 2021 Sep 25] p. 7. Available from: [Google Scholar]
- 10.Ciliberto M.A., Sandige H., Ndekha M.J., Ashorn P., Briend A., Ciliberto H.M., et al. Comparison of home-based therapy with ready-to-use therapeutic food with standard therapy in the treatment of malnourished Malawian children: a controlled, clinical effectiveness trial. Am. J. Clin. Nutr. 2005;81(4):864–870. doi: 10.1093/ajcn/81.4.864. [DOI] [PubMed] [Google Scholar]
- 11.Patel M.P., Sandige H.L., Ndekha M.J., Briend A., Ashorn P., Manary M.J. Supplemental feeding with ready-to-use therapeutic food in Malawian children at risk of malnutrition. J. Health Popul. Nutr. 2005;23(4):351–357. [PubMed] [Google Scholar]
- 12.Ciliberto M.A., Manary M.J., Ndekha M.J., Briend A., Ashorn P. Home-based therapy for oedematous malnutrition with ready-to-use therapeutic food. Acta Paediatr. 2006;95(8):1012–1015. doi: 10.1080/08035250600606803. [DOI] [PubMed] [Google Scholar]
- 13.Isanaka S., Roederer T., Djibo A., Luquero F.J., Nombela N., Guerin P.J., et al. Reducing wasting in young children with preventive supplementation: a cohort study in Niger. Pediatrics. 2010 Aug 1;126(2):e442–e450. doi: 10.1542/peds.2009-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golden M.H. Proposed recommended nutrient densities for moderately malnourished children. Food Nutr. Bull. 2009 Sep 1;30(3_suppl3):S267–S342. doi: 10.1177/15648265090303S302. [DOI] [PubMed] [Google Scholar]
- 15.Sandige H., Ndekha M.J., Briend A., Ashorn P., Manary M.J. Home-based treatment of malnourished Malawian children with locally produced or imported ready-to-use food. J. Pediatr. Gastroenterol. Nutr. 2004 Aug;39(2):141–146. doi: 10.1097/00005176-200408000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Scherbaum V., Srour M.L. Milk products in the dietary management of childhood undernutrition – a historical review. Nutr. Res. Rev. 2018 Jun;31(1):71–84. doi: 10.1017/S0954422417000208. [DOI] [PubMed] [Google Scholar]
- 17.Brenna J.T., Akomo P., Bahwere P., Berkley J.A., Calder P.C., Jones K.D., et al. Balancing omega-6 and omega-3 fatty acids in ready-to-use therapeutic foods (RUTF) BMC Med. 2015 Dec;13(1):117. doi: 10.1186/s12916-015-0352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briend A., Akomo P., Bahwere P., de Pee S., Dibari F., Golden M.H., et al. Developing food supplements for moderately malnourished children: lessons learned from ready-to-use therapeutic foods. Food Nutr. Bull. 2015;36(1S):S53–S58. doi: 10.1177/15648265150361S109. [DOI] [PubMed] [Google Scholar]
- 19.The Sphere Project. Humanitarian Charter and Minimum Standards in Disaster Response [Internet] The Sphere Project; Geneva, Switzerland: 2004. https://www.ifrc.org/docs/idrl/I283EN.pdf [cited 2021 Sep 30] p. 344. Available from: [DOI] [PubMed] [Google Scholar]
- 20.Scherbaum V., Purwestri R.C., Stuetz W., MPhil D.A.I., Suryantan J., Bloem M.A., et al. Locally produced cereal/nut/legume-based biscuits versus peanut/milk-based spread for treatment of moderately to mildly wasted children in daily programmes on Nias Island, Indonesia: an issue of acceptance and compliance? Asia Pac. J. Clin. Nutr. 2015;24(1):152–161. doi: 10.6133/apjcn.2015.24.1.15. [DOI] [PubMed] [Google Scholar]
- 21.Manary M., Callaghan M., Singh L., Briend A. Protein quality and growth in malnourished children. Food Nutr. Bull. 2016;37(1_suppl):S29–S36. doi: 10.1177/0379572116629023. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009 Jul 21;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linneman Z., Matilsky D., Ndekha M., Manary M.J., Maleta K., Manary M.J. A large-scale operational study of home-based therapy with ready-to-use therapeutic food in childhood malnutrition in Malawi. Matern. Child Nutr. 2007 Jul;3(3):206–215. doi: 10.1111/j.1740-8709.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purwestri R.C., Scherbaum V., Wirawan N.N., Suryantan J., Bloem M.A., Pangaribuan R.V., et al. Supplementary feeding with locally-produced Ready-to- Use Food (RUF) for mildly wasted children on Nias Island, Indonesia: comparison of daily and weekly program outcomes. Asia Pac. J. Clin. Nutr. 2012;21(3):374–379. [PubMed] [Google Scholar]
- 25.Fetriyuna F., Purwestri R.C., Susandy M., Köhler R., Jati I.R.A.P., Wirawan N.N., et al. Composite flour from Indonesian local food resources to develop cereal/tuber nut/bean-based ready-to-use supplementary foods for prevention and rehabilitation of moderate acute malnutrition in children. Foods. 2021 Dec 5;10(12):3013. doi: 10.3390/foods10123013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owino V.O., Irena A.H., Dibari F., Collins S. Development and acceptability of a novel milk‐free soybean–maize–sorghum ready‐to‐use therapeutic food (SMS‐RUTF) based on industrial extrusion cooking process. Matern. Child Nutr. 2012;10(1):126–134. doi: 10.1111/j.1740-8709.2012.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahwere P., Balaluka B., Wells J.C., Mbiribindi C.N., Sadler K., Akomo P., et al. Cereals and pulse-based ready-to-use therapeutic food as an alternative to the standard milk- and peanut paste–based formulation for treating severe acute malnutrition: a noninferiority, individually randomized controlled efficacy clinical trial. Am. J. Clin. Nutr. 2016 Apr 1;103(4):1145–1161. doi: 10.3945/ajcn.115.119537. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed T., Choudhury N., Hossain M.I., Tangsuphoom N., Islam M.M., de Pee S., et al. Development and acceptability testing of ready-to-use supplementary food made from locally available food ingredients in Bangladesh. BMC Pediatr. 2014 Dec;14(1):164. doi: 10.1186/1471-2431-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhury N., Ahmed T., Hossain M.I., Islam M.M., Sarker S.A., Zeilani M., et al. Ready-to-Use therapeutic food made from locally available food ingredients is well accepted by children having severe acute malnutrition in Bangladesh. Food Nutr. Bull. 2018 Mar;39(1):116–126. doi: 10.1177/0379572117743929. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh J.C., Liu L., Zeilani M., Ickes S., Trehan I., Maleta K., et al. High-oleic ready-to-use therapeutic food maintains docosahexaenoic acid status in severe malnutrition. J. Pediatr. Gastroenterol. Nutr. 2015 Jul;61(1):138–143. doi: 10.1097/MPG.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stobaugh H.C., Ryan K.N., Kennedy J.A., Grise J.B., Crocker A.H., Thakwalakwa C., et al. Including whey protein and whey permeate in ready-to-use supplementary food improves recovery rates in children with moderate acute malnutrition: a randomized, double-blind clinical trial. Am. J. Clin. Nutr. 2016 Mar 1;103(3):926–933. doi: 10.3945/ajcn.115.124636. [DOI] [PubMed] [Google Scholar]
- 32.Weber J.M., Ryan K.N., Tandon R., Mathur M., Girma T., Steiner-Asiedu M., et al. Acceptability of locally produced ready-to-use therapeutic foods in Ethiopia, Ghana, Pakistan and India: acceptability of local RUTF. Matern. Child Nutr. 2017 Apr;13(2) doi: 10.1111/mcn.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nga T.T., Nguyen M., Mathisen R., Hoa D.T., Minh N.H., Berger J., et al. Acceptability and impact on anthropometry of a locally developed Ready-to-use therapeutic food in pre-school children in Vietnam. Nutr. J. 2013 Dec;12(1):120. doi: 10.1186/1475-2891-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borg B., Mihrshahi S., Griffin M., Chamnan C., Laillou A., Wieringa F.T. Crossover trial to test the acceptability of a locally produced lipid-based nutrient supplement (LNS) for children under 2 years in Cambodia: a study protocol. BMJ Open. 2017 Sep;7(9) doi: 10.1136/bmjopen-2017-015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borg B., Mihrshahi S., Griffin M., Sok D., Chhoun C., Laillou A., et al. Randomised controlled trial to test the effectiveness of a locally-produced ready-to-use supplementary food (RUSF) in preventing growth faltering and improving micronutrient status for children under two years in Cambodia: a study protocol. Nutr. J. 2018 Dec;17(1):39. doi: 10.1186/s12937-018-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borg B., Mihrshahi S., Griffin M., Sok D., Chhoun C., Laillou A., et al. Acceptability of locally‐produced Ready‐to‐Use Supplementary Food (RUSF) for children under two years in Cambodia: a cluster randomised trial. Matern. Child Nutr. 2019 Jul;15(3) doi: 10.1111/mcn.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sigh S., Roos N., Sok D., Borg B., Chamnan C., Laillou A., et al. Development and acceptability of locally made fish-based, ready-to-use products for the prevention and treatment of malnutrition in Cambodia. Food Nutr. Bull. 2018 Sep;39(3):420–434. doi: 10.1177/0379572118788266. [DOI] [PubMed] [Google Scholar]
- 38.Sigh S., Roos N., Chamnan C., Laillou A., Prak S., Wieringa F. Effectiveness of a locally produced, fish-based food product on weight gain among Cambodian children in the treatment of acute malnutrition: a randomized controlled trial. Nutrients. 2018 Jul 16;10(7):909. doi: 10.3390/nu10070909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armini V., Miele N.A., Albero M., Sacchi R., Cavella S. Formula optimization approach for an alternative ready-to-use therapeutic food. Food Sci. Technol. 2018;6 [Google Scholar]
- 40.Miele N.A., Armini V., Troccoli A.M., Puleo S., Paduano A., Sacchi R., et al. Sensory evaluation and volatile compounds of an alternative ready‐to‐use therapeutic food for malnourished children. J. Food Sci. 2020 Apr;85(4):1265–1273. doi: 10.1111/1750-3841.15110. [DOI] [PubMed] [Google Scholar]
- 41.Azimi F. Effect of a newly developed ready-to-use supplementary food on growth indicators in children with mild to moderate malnutrition. Publ. Health. 2020;8 doi: 10.1016/j.puhe.2020.06.025. [DOI] [PubMed] [Google Scholar]
- 42.Hossain MdI., Huq S., Islam M.M., Ahmed T. Acceptability and efficacy of ready-to-use therapeutic food using soy protein isolate in under-5 children suffering from severe acute malnutrition in Bangladesh: a double-blind randomized non-inferiority trial. Eur. J. Nutr. 2020 Apr;59(3):1149–1161. doi: 10.1007/s00394-019-01975-w. [DOI] [PubMed] [Google Scholar]
- 43.Javed F., Jabeen S., Sharif M.K., Riaz A., Manzoor M.F., Sahar A., et al. Development and storage stability of chickpea, mung bean, and peanut‐based ready‐to‐use therapeutic food to tackle protein‐energy malnutrition. Food Sci. Nutr. 2021 Jul 19 doi: 10.1002/fsn3.2479. fsn3.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manary M.J. Local production and provision of ready-to-use therapeutic food (rutf) spread for the treatment of severe childhood malnutrition. Food Nutr. Bull. 2006 Sep;27(3_suppl3):S83–S89. doi: 10.1177/15648265060273S305. [DOI] [PubMed] [Google Scholar]
- 45.Sato W., Furuta C., Matsunaga K., Bahwere P., Collins S., Sadler K., et al. Amino-acid-enriched cereals ready-to-use therapeutic foods (RUTF) are as effective as milk-based RUTF in recovering essential amino acid during the treatment of severe acute malnutrition in children: an individually randomized control trial in Malawi. van Wouwe JP. PLoS One. 2018 Aug 10;13(8) doi: 10.1371/journal.pone.0201686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato W., Furuta C., Akomo P., Bahwere P., Collins S., Sadler K., et al. Amino acid-enriched plant-based RUTF treatment was not inferior to peanut-milk RUTF treatment in restoring plasma amino acid levels among patients with oedematous or non-oedematous malnutrition. Sci. Rep. 2021 Dec;11(1) doi: 10.1038/s41598-021-91807-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chalupa-Krebzdak S., Long C.J., Bohrer B.M. Nutrient density and nutritional value of milk and plant-based milk alternatives. Int. Dairy J. 2018 Dec;87:84–92. [Google Scholar]
- 48.Tenyang N., Ponka R., Tiencheu B., Djikeng F.T., Womeni H.M. Effect of traditional drying methods on proximate composition, fatty acid profile, and oil oxidation of fish species consumed in the far‐north of Cameroon. Glob. Chall. 2020 Aug;4(8) doi: 10.1002/gch2.202000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agume A., Njintang N., Mbofung C. Effect of soaking and roasting on the physicochemical and pasting properties of soybean flour. Foods. 2017 Feb 10;6(2):12. doi: 10.3390/foods6020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cummings J.H., Stephen A.M. Carbohydrate terminology and classification. Eur. J. Clin. Nutr. 2007 Dec;61(S1):S5–S18. doi: 10.1038/sj.ejcn.1602936. [DOI] [PubMed] [Google Scholar]
- 51.Bandsma R.H.J., Voskuijl W., Chimwezi E., Fegan G., Briend A., Thitiri J., et al. A reduced-carbohydrate and lactose-free formulation for stabilization among hospitalized children with severe acute malnutrition: a double-blind, randomized controlled trial. Persson LÅ. PLoS Med. 2019 Feb 26;16(2) doi: 10.1371/journal.pmed.1002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iddrisu I., Monteagudo-Mera A., Poveda C., Pyle S., Shahzad M., Andrews S., et al. Malnutrition and gut microbiota in children. Nutrients. 2021 Aug 8;13(8):2727. doi: 10.3390/nu13082727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillison F., Grey E., Verplanken B., Barnett J., Baber F. Food Standards Agency; 2022. A Rapid Review of the Acceptability and Impact of Approaches to Reduce the Salt, Fat and Sugar Content of People’s Diets on Consumers and Industry.https://www.food.gov.uk/research/behaviour-and-perception/a-rapid-review-of-the-acceptability-and-impact-of-approaches-to-reduce-the-salt-fat-and-sugar-content-of-peoples-diets-on Mar [cited 2022 Jun 29]. Available from: [Google Scholar]
- 54.Ashwath Kumar K., Sudha M.L. Effect of fat and sugar replacement on rheological, textural and nutritional characteristics of multigrain cookies. J. Food Sci. Technol. 2021 Jul;58(7):2630–2640. doi: 10.1007/s13197-020-04769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shang Y., Cao H., Wei C., Thakur K., Liao A., Huang J., et al. Effect of sugar types on structural and flavor properties of peony seed derived Maillard reaction products. J. Food Process. Preserv. 2020 Mar;44(3) [Google Scholar]
- 56.Bobade H., Singh A., Sharma S., Gupta A., Singh B. Effect of extrusion conditions and honey on functionality and bioactive composition of whole wheat flour‐based expanded snacks. Food Process. Preserv. 2022 Jan;46(1) [Google Scholar]
- 57.Bousi C., Sismanidou O.X., Marinopoulou A., Raphaelides S. Effect of sugar addition on the elongational and shearing deformation behavior of sesame paste systems. LWT. 2022 Jan;153 [Google Scholar]
- 58.Bertelsen A.S., Mielby L.A., Byrne D.V., Kidmose U. Ternary cross-modal interactions between sweetness, aroma, and viscosity in different beverage matrices. Foods. 2020 Mar 30;9(4):395. doi: 10.3390/foods9040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forouhi N.G., Krauss R.M., Taubes G., Willett W. Dietary fat and cardiometabolic health: evidence, controversies, and consensus for guidance. BMJ. 2018 Jun 13 doi: 10.1136/bmj.k2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Das J.K., Hoodbhoy Z., Salam R.A., Bhutta A.Z., Valenzuela-Rubio N.G., Weise Prinzo Z., et al. Cochrane Developmental, Psychosocial and Learning Problems Group. Cochrane Database of Systematic Reviews; 2018 Aug 31. Lipid-based nutrient supplements for maternal, birth, and infant developmental outcomes. [cited 2022 Jun 29];2018(8). Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huybregts L., Le Port A., Becquey E., Zongrone A., Barba F.M., Rawat R., et al. Impact on child acute malnutrition of integrating small-quantity lipid-based nutrient supplements into community-level screening for acute malnutrition: a cluster-randomized controlled trial in Mali. Persson LÅ, editor. PLoS Med. 2019 Aug 27;16(8) doi: 10.1371/journal.pmed.1002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smit E.N., Muskiet F.A.J., Boersma E.R. The possible role of essential fatty acids in the pathophysiology of malnutrition: a review. Prostagl. Leukot. Essent. Fat. Acids. 2004 Oct;71(4):241–250. doi: 10.1016/j.plefa.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 63.Zuzarte A., Mui M., Ordiz M.I., Weber J., Ryan K., Manary M.J. Reducing oil separation in ready-to-use therapeutic food. Foods. 2020 Jun 1;9(6):706. doi: 10.3390/foods9060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tzompa-Sosa D.A., Dewettinck K., Gellynck X., Schouteten J.J. Replacing vegetable oil by insect oil in food products: effect of deodorization on the sensory evaluation. Food Res. Int. 2021 Mar;141 doi: 10.1016/j.foodres.2021.110140. [DOI] [PubMed] [Google Scholar]
- 65.Gutiérrez-Luna K., Astiasarán I., Ansorena D. Gels as fat replacers in bakery products: a review. Crit. Rev. Food Sci. Nutr. 2022 Aug 6;62(14):3768–3781. doi: 10.1080/10408398.2020.1869693. [DOI] [PubMed] [Google Scholar]
- 66.Evlogimenou A., Paraskevopoulou A., Kiosseoglou V. Exploitation of hazelnut, maize germ and sesame seed aqueous extraction residues in the stabilisation of sesame seed paste (tahini): physical stabilisation of sesame seed paste. J. Sci. Food Agric. 2017 Jan;97(1):215–221. doi: 10.1002/jsfa.7714. [DOI] [PubMed] [Google Scholar]
- 67.Kong X., Li X., Wang H., Hua Y., Huang Y. Effect of lipoxygenase activity in defatted soybean flour on the gelling properties of soybean protein isolate. Food Chem. 2008 Feb;106(3):1093–1099. [Google Scholar]
- 68.Speroni F., Milesi V., Añón M.C. Interactions between isoflavones and soybean proteins: applications in soybean-protein–isolate production. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2010 Oct;43(8):1265–1270. [Google Scholar]
- 69.Triyono A. Semarang, Indonesia. Universitas Diponegoro; 2010. Mempelajari pengaruh penambahan beberapa asam pada proses isolasi protein terhadap tepung protein isolat kacang Hijau (phaseolus radiatus L.) pp. 4–5.http://eprints.undip.ac.id/27996/1/C-10.pdf Available from: [Google Scholar]
- 70.Camargo L. da R., Doneda D., Oliveira V.R. Whey protein ingestion in elderly diet and the association with physical, performance and clinical outcomes. Exp. Gerontol. 2020 Aug;137 doi: 10.1016/j.exger.2020.110936. [DOI] [PubMed] [Google Scholar]
- 71.Alimoradi F., Hojaji E., Jooyandeh H., Moghadam S.A.H.Z., Moludi J. Whey protein : health benefit and food applications whey protein. J. Int. Res. Med. Pharmaceut. Sci. 2016;9(2):63–73. [Google Scholar]
- 72.Andoyo R., Dianti Lestari V., Mardawati E., Nurhadi B. Fractal dimension analysis of texture formation of whey protein-based foods. Int. J. Food Sci. 2018;2018:17. doi: 10.1155/2018/7673259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weindl I., Ost M., Wiedmer P., Schreiner M., Neugart S., Klopsch R., et al. Sustainable food protein supply reconciling human and ecosystem health: a Leibniz Position. Global Food Secur. 2020 Jun;25 [Google Scholar]
- 74.Kabir I., Rahman M.M., Haider R., Mazumder R.N., Khaled M.A., Mahalanabis D. Increased height gain of children fed a high-protein diet during convalescence from shigellosis: a six-month follow-up study. J. Nutr. 1998 Oct 1;128(10):1688–1691. doi: 10.1093/jn/128.10.1688. [DOI] [PubMed] [Google Scholar]
- 75.Khairunnisa S., Andoyo R., Marta H., Saripudin G.L.U. Process optimization of emergency food originated from denatured whey protein concentrate and dried sweet potato puree. IOP Conf. Ser. Earth Environ. Sci. 2018 May;157 [Google Scholar]
- 76.Andoyo R., Mahani M., Ain M.N., Wira D.W., Wilar G., Darwis R.S., et al. Pre-clinical study of the high protein food based on denaturized whey protein. Sys. Rev. Pharm. 2021;12(1):759–770. [Google Scholar]
- 77.Prabhakar K. Encyclopedia of Food Microbiology. Elsevier; 2014. Intermediate moisture foods.https://linkinghub.elsevier.com/retrieve/pii/B9780123847300001701 [cited 2022 Jun 29]. pp. 372–6. Available from: [Google Scholar]
- 78.Shenoy P., Viau M., Tammel K., Innings F., Fitzpatrick J., Ahrné L. Effect of powder densities, particle size and shape on mixture quality of binary food powder mixtures. Powder Technol. 2015 Mar;272:165–172. [Google Scholar]
- 79.Belorio M., Sahagún M., Gómez M. Influence of flour particle size distribution on the quality of maize gluten-free cookies. Foods. 2019 Feb 23;8(2):83. doi: 10.3390/foods8020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bahwere P., Akomo P., Mwale M., Murakami H., Banda C., Kathumba S., et al. Soya, maize, and sorghum–based ready-to-use therapeutic food with amino acid is as efficacious as the standard milk and peanut paste–based formulation for the treatment of severe acute malnutrition in children: a noninferiority individually randomized controlled efficacy clinical trial in Malawi. Am. J. Clin. Nutr. 2017 Oct;106(4):1100–1112. doi: 10.3945/ajcn.117.156653. [DOI] [PubMed] [Google Scholar]
- 81.Akomo P., Bahwere P., Murakami H., Banda C., Maganga E., Kathumba S., et al. Soya, maize and sorghum ready-to-use therapeutic foods are more effective in correcting anaemia and iron deficiency than the standard ready-to-use therapeutic food: randomized controlled trial. BMC Publ. Health. 2019 Dec;19(1):806. doi: 10.1186/s12889-019-7170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wagh V.D. Ready to use therapeutic food [RUTF] formulation and packaging for malnutrition. Nutr. Food Sci. Int. J. 2017;4(5) [Google Scholar]
- 83.Barrett J.R. National Institute of Environmental Health Sciences; 2005. Liver Cancer and Aflatoxin: New Information from the Kenyan Outbreak. [Google Scholar]
- 84.Wagh V.D., Deore B.R. Ready to use therapeutic food (RUTF): an overview. Adv. Life Sci. Health. 2015;2(1):15–30. [Google Scholar]
- 85.Centers for Disease Control and Prevention (CDC) Outbreak of aflatoxin poisoning--eastern and central provinces, Kenya, January-July 2004. MMWR Morb. Mortal. Weekly Rep. 2004 Sep 3;53(34):790–793. [PubMed] [Google Scholar]
- 86.Gong Y., Hounsa A., Egal S., Turner P.C., Sutcliffe A.E., Hall A.J., et al. Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environ. Health Perspect. 2004 Sep;112(13):1334–1338. doi: 10.1289/ehp.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patterson G.T., Osorio E.Y., Peniche A., Dann S.M., Cordova E., Preidis G.A., et al. Pathologic inflammation in malnutrition is driven by proinflammatory intestinal microbiota, large intestine barrier dysfunction, and translocation of bacterial lipopolysaccharide. Front. Immunol. 2022 May 26;13 doi: 10.3389/fimmu.2022.846155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M., et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012 Jul;487(7408):477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tidjani Alou M., Lagier J.C., Raoult D. Diet influence on the gut microbiota and dysbiosis related to nutritional disorders. Human Microb. J. 2016 Sep;1:3–11. [Google Scholar]
- 90.Benjamin J., Makharia G., Ahuja V., Anand Rajan K.D., Kalaivani M., Gupta S.D., et al. Glutamine and whey protein improve intestinal permeability and morphology in patients with crohn’s disease: a randomized controlled trial. Dig. Dis. Sci. 2012 Apr;57(4):1000–1012. doi: 10.1007/s10620-011-1947-9. [DOI] [PubMed] [Google Scholar]
- 91.Prokopidis K., Mazidi M., Sankaranarayanan R., Tajik B., McArdle A., Isanejad M. Effects of whey and soy protein supplementation on inflammatory cytokines in older adults: a systematic review and meta-analysis. Br. J. Nutr. 2022 Jun 16:1–12. doi: 10.1017/S0007114522001787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma N., Tian Y., Wu Y., Ma X. CPPS; 2017 Jun 2. Contributions of the Interaction between Dietary Protein and Gut Microbiota to Intestinal Health.http://www.eurekaselect.com/150179/article [cited 2023 Apr 10];18(8). Available from: [DOI] [PubMed] [Google Scholar]
- 93.Jewell J.L., Russell R.C., Guan K.L. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013 Mar;14(3):133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012 Apr;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Biesalski H.K. Springer Science & Business Media; Heidelberg, Germany: 2013 Jan. Hidden Hunger; p. 265. [Google Scholar]
- 96.Hrubša M., Siatka T., Nejmanová I., Vopršalová M., Kujovská Krčmová L., Matoušová K., et al. Biological properties of vitamins of the B-complex, part 1: vitamins B1, B2, B3, and B5. Nutrients. 2022;14(3):484. doi: 10.3390/nu14030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Watzke H.J. Impact of processing on bioavailability examples of minerals in foods. Trends Food Sci. Technol. 1998 Aug;9(8–9):320–327. [Google Scholar]
- 98.de Almeida M.M.C., Francisco C.R.L., de Oliveira A., de Campos S.S., Bilck A.P., Fuchs R.H.B., et al. Textural, color, hygroscopic, lipid oxidation, and sensory properties of cookies containing free and microencapsulated chia oil. Food Bioprocess Technol. 2018 May;11(5):926–939. [Google Scholar]
- 99.Qiu L., Zhang M., Tang J., Adhikari B., Cao P. Innovative technologies for producing and preserving intermediate moisture foods: a review. Food Res. Int. 2019 Feb;116:90–102. doi: 10.1016/j.foodres.2018.12.055. [DOI] [PubMed] [Google Scholar]
- 100.Gama T., Wallace H.M., Trueman S.J., Hosseini-Bai S. Quality and shelf life of tree nuts: a review. Sci. Hortic. 2018 Dec;242:116–126. [Google Scholar]
- 101.Yazew T. In: Therapeutic Food Development from Maize Grains, Pulses, and Cooking Banana Fruits for the Prevention of Severe Acute Malnutrition. Capurso C., editor. The Scientific World Journal; 2022 Jan 29. pp. 1–7. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hadi S., Amani R., Tehrani M.M., Hadi V., Hejri S., Askari G. Ready-to-Use therapeutic food (RUTF) formulations with functional food and nutrient density for the treatment of malnutrition in crisis. Int. J. Prev. Med. 2022;13:16. doi: 10.4103/ijpvm.IJPVM_304_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adewumi O.O., Felix-Minnaar J.V., Jideani V.A. Physiochemical and nutritional characteristics of ready-to-use therapeutic food prepared using Bambara groundnut-moringa oleifera leaf protein complex. Foods. 2022 Jun 8;11(12):1680. doi: 10.3390/foods11121680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Akande O.A., Oluwamukomi M., Osundahunsi O.F., Ijarotimi O.S., Mukisa I.M. Evaluating the potential for utilising migratory locust powder (Locusta migratoria) as an alternative protein source in peanut-based ready-to-use therapeutic foods. Food Sci. Technol. Int. 2023 Apr;29(3):204–216. doi: 10.1177/10820132211069773. [DOI] [PubMed] [Google Scholar]
- 105.Bahwere P., Banda T., Sadler K., Nyirenda G., Owino V., Shaba B., et al. Effectiveness of milk whey protein‐based ready‐to‐use therapeutic food in treatment of severe acute malnutrition in M alawian under‐5 children: a randomised, double‐blind, controlled non‐inferiority clinical trial. Matern. Child Nutr. 2014 Jul;10(3):436–451. doi: 10.1111/mcn.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fetriyuna F., Purwestri R.C., Susandy M., Köhler R., Jati I.R.A.P., Wirawan N.N., et al. Composite flour from Indonesian local food resources to develop cereal/tuber nut/bean-based ready-to-use supple-mentary foods for prevention and rehabilitation of mod-erate acute malnutrition in children. Foods. 2021 doi: 10.3390/foods10123013. (not yet published) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dibari F., Bahwere P., Huerga H., Irena A.H., Owino V., Collins S., et al. Development of a cross-over randomized trial method to determine the acceptability and safety of novel ready-to-use therapeutic foods. Nutrition. 2013 Jan;29(1):107–112. doi: 10.1016/j.nut.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 108.Borg B., Mihrshahi S., Laillou A., Sigh S., Sok D., Peters R., et al. Development and testing of locally-produced ready-to-use therapeutic and supplementary foods (RUTFs and RUSFs) in Cambodia: lessons learned. BMC Publ. Health. 2019;19(1):1–9. doi: 10.1186/s12889-019-7445-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kshirsagar R., Bach M., Forteza M.E. 2019. Expert Meeting on Ready-To-Use-Therapeutic Foods (RUTF) UNICEF, Supply Division | Report 1 | 2nd-3rd September.https://www.unicef.org/supply/media/3316/file/RUTF-technical-expert-meeting-report-02-03092019.pdf.pdf [Internet]. 2019 [cited 2021 Sep 10] p. 21. Available from: [Google Scholar]
- 110.Purwestri R.C., Scherbaum V., Inayati D.A., Wirawan N.N., Suryantan J., Bloem M.A., et al. Impact of daily versus weekly supply of locally produced ready-to-use food on growth of moderately wasted children on nias island, Indonesia. ISRN Nutr. 2013:1–10. doi: 10.5402/2013/412145. 2013 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maleta K., Kuittinen J., Duggan M.B., Briend A., Manary M., Wales J., et al. Supplementary feeding of underweight, stunted Malawian children with a ready-to-use food. J. Pediatr. Gastroenterol. Nutr. 2004 Feb;38(2):152–158. doi: 10.1097/00005176-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 112.Matilsky D.K., Maleta K., Castleman T., Manary M.J. Supplementary feeding with fortified spreads results in higher recovery rates than with a corn/soy blend in moderately wasted children. J. Nutr. 2009;139(4):773–778. doi: 10.3945/jn.108.104018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Irena A.H., Bahwere P., Owino V.O., Diop E.I., Bachmann M.O., Mbwili-Muleya C., et al. Comparison of the effectiveness of a milk-free soy-maize-sorghum-based ready-to-use therapeutic food to standard ready-to-use therapeutic food with 25% milk in nutrition management of severely acutely malnourished Zambian children: an equivalence non-blin: effectiveness of a soya-maize-sorghum RUTF. Matern. Child Nutr. 2015 Dec;11:105–119. doi: 10.1111/mcn.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nguyen M., Berger J., Wieringa F., Tran T.N., Do T.B.H., Nguyen H.M., et al. Effectiveness of a locally produced RUTF for the treatment of acute malnutrition in Vietnam. IAEA. 2014 Jul 1;2014 [Google Scholar]
- 115.Schoonees A., Lombard M.J., Musekiwa A., Nel E., Volmink J. Ready‐to‐use therapeutic food (RUTF) for home‐based nutritional rehabilitation of severe acute malnutrition in children from six months to five years of age. Cochrane Database Syst. Rev. 2019;5 doi: 10.1002/14651858.CD009000.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Christian P., Shaikh S., Shamim A.A., Mehra S., Wu L., Mitra M., et al. Effect of fortified complementary food supplementation on child growth in rural Bangladesh: a cluster-randomized trial. Int. J. Epidemiol. 2015 Dec;44(6):1862–1876. doi: 10.1093/ije/dyv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Food and Nutrition Technical Assistance III Project (FANTA). Training Guide for Community-Based Management of Acute Malnutrition (CMAM) FHI 360/FANTA; Washington DC: 2018. https://www.fantaproject.org/focus-areas/nutrition-emergencies-mam/cmam-training 2018 [cited 2021 Oct 10] p. 175. Available from: [Google Scholar]
- 118.Republik Vietnam . Medical Publishing House; 2011. National Nutrition Strategy for 2011-2020, with A Vision toward 2030.https://extranet.who.int/nutrition/gina/sites/default/filesstore/VNM%202011%202.%20National%20Nutrition%20%20Strategy%202011-2020.pdf [cited 2023 Mar 4]. Available from: [Google Scholar]
- 119.FAO . Food and Agriculture Organization; United Nation: 2022. New Codex Alimentarius Guideline Means Ready-To-Use Therapeutic Foods Can Be Manufactured and Incorporated into More Countries’ Health Plans.https://www.fao.org/fao-stories/article/en/c/1619824/ [cited 2022 Mar 4]. Available from: [Google Scholar]
- 120.Phuong H., Nga T.T., Mathisen R., Nguyen M., Hop L.T., Hoa D.T.B., et al. Development and implementation of a locally produced ready-to-use therapeutic food (RUTF) in Vietnam. Food Nutr. Bull. 2014 Jun;35(2_suppl1):S52–S56. doi: 10.1177/15648265140352S108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No. Data for Review is included in article/supp. material/referenced in article.