Abstract
The fast decline in the physiological quality of seeds during storage is a serious problem. It is known that the reduction of seed quality may be related to its biochemical constitution. However, the relationship between the composition and the mechanisms linked to the loss of vigor of soybean seeds during aging has not been elucidated yet. Thus, the aim of this work was to analyze the role of the biochemical composition of soybean seeds in the physiological quality and in the tolerance to deterioration due to natural and artificial aging. Seeds of six soybean genotypes were analyzed initially and after being submitted to natural aging, storage for eight months, and artificial aging, using the temperature of 41 °C and 100 % relative humidity for 96 h. Moisture content, germination and vigor tests were carried out. Also, there were measured the content of oil, total protein, soluble protein, malonaldehyde, and fatty acids palmitic, stearic, oleic, linoleic, and linolenic. It was verified that the physiological quality of soybean seeds decreased with both kinds of aging. However, the deterioration process occurs by distinct mechanisms. The biochemical composition of the seeds is associated with the physiological quality and their storage potential is changed by natural and artificial aging. The tolerance of the seed to deterioration is related to soluble protein and fatty acids content. Oleic fatty acid and soluble protein can be used as indicators of physiological quality in soybean seeds.
Keywords: Glycine max (L.) storage, Longevity, Vigor, Seed physiological quality
Highlights
-
•
Fast decline of the seeds quality during the storage is a serious problem.
-
•
It depends on the aging process, genotype and biochemical composition.
-
•
The relation between the biochemical composition and vigor seeds loss still unclear.
-
•
We investigated this relation in naturally and artificially aged soybean seeds.
-
•
We found valuable information about soybean seeds quality and their composition.
1. Introduction
Soybean [Glycine max (L.) Merrill] is a crop of major importance for worldwide agribusiness. Its grains are considered an important source of oil and protein [1], which makes them a diversified raw material. They are used for the food industry, with the production of bran for animal consumption, in natura soybean and its derivatives for human consumption, and, also, for the biofuel industry, cosmetics, dyes, biodegradable plastics, and tires, among others [[2], [3], [4]].
Because of its renowned importance, the obtainment of high yield has been the producers’ focus, and seed physiological quality is indispensable to achieve an appropriate plant stand and, consequently, high yield rates of the culture [5].
Among the traits related to seed physiological quality, germination and vigor, which are the maximums at the physiological maturity point, stand out [6]. From this point on, several metabolic changes occur, which usually incur decreases in quality parameters, caused by the deterioration process, which can be faster or slower according to the genetic constitution of the seeds and to the conditions they are exposed to Ref. [7].
There is evidence that seed performance of naturally aged genotypes can be simulated by artificial aging [8]. Both kinds of aging contribute to the reduction of the physiological quality of the seeds [9]. However, the speed and intensity of the aging process depend on several factors, among them environmental conditions, species, and genotype [9].
Oilseeds are the most affected by aging, and soybean is described as one of the cultures whose seeds suffer the most from deterioration [10,11]. With seed aging, there is an increase in the production of reactive oxygen species and in mitochondrial dysfunction, which lead to deterioration [12]. Aging also causes an increase of lipid peroxidation and of electrical conductivity, while promoting the reduction of germination, vigor, content of total sugars, soluble proteins, and enzymatic activity [13], which culminates in loss of quality, with a reduction of seed vigor and germination.
Soybean seeds present high levels of polyunsaturated fatty acids, which have a high propensity to non-enzymatic peroxidation (self-peroxidation) and enzymatic peroxidation (lipoxygenase), which result in a fast decline of seed quality [1]. For this reason, soybean seeds generally are considered as low longevity in storage.
Many factors contribute to the predisposition of seeds to deterioration, among them the genetic factor is of great importance [6,14]. Thus, some genotypes present greater seed longevity than others [14], regardless of the conditions they are submitted to.
Seed biochemical composition, which is strictly associated with the genetic component, also exerts influence on the deterioration process and affects seed quality and longevity [1]. The great effect of biochemical compounds on longevity is related to the sorption properties, the potential sites for attacks of free radicals, and to the presence of protective compounds in the seeds [1], which vary according to the type and amount of predominant reserve in the seeds [15], as well as to the fractions of compounds that form them [1].
It is known that the biochemical composition has a relationship with the physiological quality and longevity of the seeds [1]. However, the mechanisms linked to the rapid loss of vigor or tolerance to deterioration of soybean seeds in storage still need to be elucidated. Thereby, this work aimed at analyzing the role of the biochemical composition of the seeds of soybean cultivars on their physiological quality and in the tolerance to deterioration due to natural and artificial aging.
2. Material and methods
This experiment was carried out in the Laboratório de Sementes and in the Instituto de Biotecnologia Aplicado à Agropecuária (BIOAGRO) (“Seed laboratory” and “Institute of Biotechnology Applied to Farming”) of the Federal University of Viçosa- MG- Brazil.
The soybean seeds used were from pre commercial strains. They were used genetically modified seeds glyphosate resistant – roundup read (RR) technology or with resistance to the herbicide glyphosate and the main caterpillars of the crop (IPRO technology). In this research the genotypes were called GEN1 (maturity group 5.9, with RR technology); GEN2 (maturity group 5.6, with RR technology); GEN3 (maturity group 5.8, with IPRO technology); GEN4 (maturity group 5.5, with IPRO technology), GEN5 (maturity group 6.8, with IPRO technology) and GEN6 (maturity group 5.5, with IPRO technology). We have chosen these genotypes for this research because our previous studies indicated these genotypes showed genetic variability for tolerance to deterioration during storage.
The seeds of the six genotypes were produced under the same cultivation conditions in the town of Passo Fundo, RS, Brazil by GDM Seeds Company. The fields were conducted in the 2019/2020 harvest, sown under a population of 250,000 plants per hectare. Fertilization recommendations were followed and the cultural practices recommended for soybean cultivation were carried out.
The seeds were analyzed when freshly harvested and then submitted to storage (natural aging) and artificial aging.
For natural aging, the seeds were stored in Kraft Multifolha paper bags, appropriately sealed and identified, and submitted to storage under a non-refrigerated condition, in a seed shed, in the town of Passo Fundo, RS, Brazil, for eight months. During this period, from April to December, the minimum and maximum temperatures observed were 8.8 and 30.7 °C, respectively, and the variation of relative humidity (RH) of the environment was from 50 to 92 %.
For artificial aging, the seeds were distributed on metal mesh trays coupled to plastic boxes (11 x 11 × 3.5 cm), containing 40 mL of distilled water on the bottom and kept under relative humidity of 100 % at 41 °C, for a period of 96 h. After aging, the seeds were left on a countertop in a laboratory environment for natural drying until they reached their initial moisture content (approximately 12 %).
The seeds which were freshly harvested, artificially aged, and stored for eight months were submitted to physiological quality and biochemical composition analysis. All the measurements related to the seed quality were done according to the ISTA rules, as following tests, and determinations.
2.1. Moisture content of seeds (MC)
The moisture content of seeds was determined through the oven method, at 105 ± 3 °C, for 24 h, using four replications of 50 seeds each [16]. Water content was measured immediately after aging and before the performance of the tests.
2.2. Germination (G)
Four replications of 50 seeds each were used. The seeds were sown in moistened germitest paper with water volume equivalent to 2.5 times the weight of the dry substrate and kept in a germinator at 25 °C. Evaluations were carried out, with the record of the percentage of the normal seedlings on the 5th and 8th days after sowing [16].
2.3. Accelerated aging (AA)
Four replications of 50 seeds each were used. The seeds were distributed on a single layer on a metal mesh tray coupled to the plastic boxes (11 x 11 × 3.5 cm), which contained, on the bottom, 40 mL of distilled water. The boxes were covered to obtain 100 % RH inside them and kept in a Bio-Oxygen Demand (BOD incubator) at 41 °C for 48 h [17]. After this period, the seeds were submitted to the germination test and the percentage of normal seedlings was evaluated on the 5th day after sowing.
2.4. Seedling growth (SG)
Four replications of 20 seeds were used, evenly sown on two sheets of germitest paper, moistened with distilled water at the proportion of 2.5 times the weight of the dry paper [18]. The seeds were left to germinate, in moistened paper rolls, and kept in a germinator, at 25 °C, for three days. The seedlings were scanned and, using Software VigorS®, the measures of root length and of the aerial part were taken. The data of length and germination were used to calculate vigor index (VI) [19], obtained by means of package SeedCalc of Software R [20].
2.5. Seedling dry mass (SDM)
The same seedlings used for length determination were used for dry mass determination. The dry mass was obtained after the seedlings were dried in the air-forced circulation oven, at 70 °C for 72 h.
2.6. Emergence speed index (ESI)
Four replications of 50 seeds were sown on polystyrene trays containing 2 L of sand as substrate. The substrate was initially moistened until it reached 60 % of the water retention capacity and it was irrigated daily. Daily counts of the number of emerged seeds were carried out until the 12th day after sowing. The count data were used to calculate the emergence speed index, as proposed by Maguire [21].
2.7. Electrical conductivity (EC)
Four replications of 50 seeds were weighed and put in plastic cups containing 75 mL of distilled water, and kept in a BOD incubator at 25 °C, for 24 h [22]. After this period, the electrical conductivity of the solution was determined, using a conductivity meter of the DIGIMED-DM 32 model.
2.8. Content of oil and of total protein
Three replications of 50 seeds each were used. The seeds were freeze-dried and grinded in a cutting mill, and an aliquot of 10g of powder of the grinded seed was harvested per sample. The contents of oil and total protein were determined through near-infrared spectrometry (NIR), by means of Analyzer Equipment FT-NIR, Thermo Scientific, Antaris II model.
2.9. Content of soluble protein
Four replications of 10 seeds each were used. The seeds were soaked for 16 h. The teguments were removed, and the embryos of the seeds were freeze-dried and grinded in a ball mill for the obtainment of fine powder. A subsample of 100 mg of the grinded material was used for each one of the replications of each treatment. The determination of the content of soluble protein was carried out according to the methodology described by Bradford [23], using BSA as standard. The reading was carried out in a spectrophotometer at a wavelength of 595 nm.
2.10. Content of malonaldehyde (MDA)
Four replications of 10 seeds were used per treatment. For the preparation of the samples, the seeds were soaked for 16 h, and the teguments were removed. The embryos were freeze-dried, grinded in a ball mill for the obtainment of fine powder, and 200 mg of the grinded material was used per sample. An aliquot of 1.8 mL of trichloroacetic acid (TCA 0.1 %, w/v) was added to each sample, followed by homogenization. The homogenized solution was centrifuged at 19.000 g, for 15 min, at 4 °C. After this procedure, 500 μL of the supernatant was collected and added to 1.5 mL of thiobarbituric acid solution (TBA 0.5 % in TCA 20 %). 500 μL of TCA 0.1 % was added for the composition of the blank, instead of the sample. The samples and the blank were incubated for 30 min at 90 °C in bain-marie, under stirring [24]. After this period, an ice bath was carried out in order to stop the reaction. Aliquots of 250 μL were collected and readings were carried out in a spectrophotometer at the wavelengths of 532 nm and 600 nm. The calculations of the MDA concentration were done according to Heath and Packer [25], by using the molar extinction coefficient of 155 mM−1 cm−1.
2.11. Content of fatty acids
The content of fatty acids stearic, palmitic, oleic, linoleic, and linolenic in the fraction of soybean oil was determined through gas chromatography. Ten seeds were used per treatment, with three replications per treatment. The seeds were freeze-dried and grinded in a cutting mill, and 150 mg of the grinded material was used in each sample. The samples were placed in microtubes, and 1 mL of hexane was added to them, being kept at 4 °C for 16 h. After this period, the lipid fraction was poured into tubes and the solvent was evaporated by N2 bubbling. For the obtainment of methyl esters, the methodology described by Jham et al. [26] was employed. After the preparation of the samples, aliquots were injected into a CG-17a gas chromatographer, equipped with an automatic sampler (Shimadzu, model AOC-17) and integrator (Shimadzu, model C–R7A). The Carbowax capillary column (30 m x 0,32 mm) was kept at 225 °C, and the temperatures of the injector and of the detector were 245 °C and 280 °C, respectively. Nitrogen gas was used as a carrier, at a flow 1.1 mL min−1.
2.12. Statistical analysis
The experiment was carried out in a completely randomized design, in a 6 x 3 factorial scheme, with six genotypes and three kinds of aging: non-aged seeds (freshly harvested), naturally aged seeds (stored for eight months), and artificially aged seeds (in a BOD chamber, at 41 °C and 100 % RH, for 96 h). The data were submitted to the analysis of variance and the means were compared by using the Tukey test (p < 0,05). In order to verify the performance of the genotypes and the relationships between the physiological and biochemical variables, the data obtained from the physiological evaluation and from the biochemical composition of the seeds were submitted to the principal component analysis (PCA). The Pearson's correlation coefficients were also obtained, whose significance was evaluated by the t-test (p < 0,05). Software R 4.0.0 [27] was used to carry out these analyses.
3. Results
3.1. Physiological quality of soybean seeds after aging processes
The initial moisture content of the seeds was 11,8 ± 0,9 %. After storage, the seeds presented water content of 11.5 ± 0.8 %, and after artificial aging, they reached moisture of 30.2 ± 2.5 %. At the moment the tests were performed, seed moisture content was 11.7 ± 0.6 %.
The physiological quality of the seeds was reduced after natural and artificial aging (Fig. 1).
Fig. 1.
Germination (G), accelerated aging (AA), seedling dry mass (SDM), vigor index (VI), emergence speed index (ESI) and electrical conductivity (EC), in freshly harvested soybean seeds (initial) and submitted to natural aging, storage for eight months (8 months), and artificial aging for 96 h (96 h AA). GEN1, GEN2, GEN3, GEN4, GEN5 and GEN6 were the genotypes used in this research. % = percentage; mg sl−1 = milligrams per seedling; mS cm−1 g−1 = milliSiemens per centimeter per gram of seed. *** Means followed by the same capital letter, comparing the initial period and the different, kinds of aging, and lowercase, comparing the genotypes in each environment, do not differ from each other by the Tukey test at 5 % probability.
After storage for eight months, the seeds of GEN1 did not reduce their germination, in relation to the initial condition, unlike the other cultivars (Fig. 1). The seeds of GEN2 kept their high vigor after being stored, as it was observed by the AA test. ESI did not differ between the initial condition and after storage, for the seeds of genotypes GEN1, GEN2, GEN4 and GEN5. Also, the EC of the seeds in the initial condition was like those stored for all the genotypes. However, VI was sharply reduced in all genotypes, pointing out a reduction in seedling performance and, consequently, a decrease in seed vigor after storage.
There was a difference in performance among the genotypes after the natural aging of the seeds (Fig. 1). All genotypes presented high physiological quality of the seeds when freshly harvested. However, after being submitted to storage, the seeds of genotype GEN1 presented a lower decrease in physiological quality, while the seeds of GEN6 had a sharper decrease.
After the seeds were submitted to artificial aging, there was a decrease in germination and vigor, with a decrease in parameters G, AA, SDM, VI, ESI, and an increase in the EC of the seeds of all genotypes (Fig. 1).
The performance of the seeds of some genotypes was similar when they were submitted to artificial and natural aging. There was no difference in seed germination between natural and artificial aging for genotypes GEN3, GEN4, GEN5 and GEN6. Likewise, after the AA test, the seeds of genotypes GEN3 and GEN6 presented the same performance when submitted to the two kinds of aging. Also, there was a decrease of SDM in the same proportion for genotypes GEN1, GEN2, GEN4, and a decrease of VI in the same intensity for GEN2, GEN3 and GEN4. However, artificial aging caused a greater increase of EC and a more marked decrease in ESI in the seeds of all genotypes, if compared to the stored ones (Fig. 1).Thus, it is suggested that both aging processes occur by distinct mechanisms.
By means of the principal component analysis (PCA), a clear difference was noticed in the physiological quality of the seeds which were freshly harvested, artificially aged, and stored (Fig. 2A).
Fig. 2.
Biplot of the principal component analysis (A) and contribution of the physiological variables to the PCA (B) of freshly harvested soybean seeds (purple) and submitted to natural aging (green) and artificial aging (orange). VI = vigor index; SDM = seedling dry mass; G = germination; EC = electrical conductivity; ESI = emergence speed index; AA = accelerated aging. GEN1, GEN2, GEN3, GEN4, GEN5 and GEN6 refer to the genotypes used; initial means freshly harvest seeds; 8_months refers to natural aging and AA_96_hours to artificial aging.
More than 90 % of the total variation of the physiological data was explained by the two first components (Fig. 2A). VI, SDM, G and EC were the physiological variables that most contributed to the differentiation of the treatments (Fig. 2B).
The genotypes were grouped according to the kind of aging they were submitted to, except for genotype GEN6 after storage for eight months, which had an atypical behavior, being similar to the genotypes that were submitted to artificial aging for 96 h (Fig. 2A). The seeds aged for 96 h and genotype GEN6 after storage presented higher values of EC (Fig. 2A). For the grouping of the genotypes stored for eight months, ESI was the most important variable (Fig. 2A).
3.2. Chemical composition of seeds and aging tolerance of soybean genotypes
In order to investigate the effect of the biochemical composition of the seeds in physiological quality and tolerance to deterioration during natural and artificial aging, the following contents of the seeds of the different soybean genotypes were analyzed: total protein content, oil, soluble protein, malonaldehyde content (MDA) (Fig. 3) and the fractions of fatty acids: palmitic, stearic, oleic, linoleic and linoleic (Fig. 4). For total protein content, soluble protein and MDA, there was a significant interaction between the genotypes and the aging treatment. However, for oil content and the content of fatty acids, this interaction was not significant.
Fig. 3.
Contents of total protein (Total_Prot), soluble Protein (Soluble_Prot), oil and malonaldehyde (MDA), in freshly harvested soybean seeds (initial) and submitted to natural aging, storage for eight months (8 months), and artificial aging, aged for 96 h (96 h AA). GEN1, GEN2, GEN3, GEN4, GEN5 and GEN6 were the genotypes used in this research. % = percentage; mg g-1 = milligrams per gram; nmol g−1 = nanomole per gram. *** Means followed by the same capital letter, comparing the initial period and the different kinds of aging, and lowercase, comparing the genotypes in each environment, do not differ from each other by the Tukey test at 5 % probability. For the analysis of oil content there was no significant effect of the interaction between types of aging and genotypes, so the effects were analyzed independently.
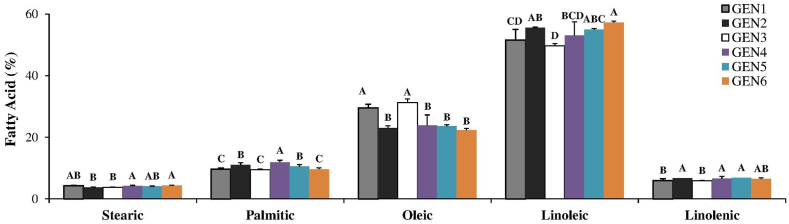
Fig. 4.
Contents of fatty acids stearic, palmitic, oleic, linoleic and linolenic in freshly harvested seeds of soybean cultivars and submitted to artificial and natural aging. GEN1, GEN2, GEN3, GEN4, GEN5 and GEN6 were the genotypes used in this research. *** There was no statistical difference among the seeds in the initial period, after storage (8 months) and after artificial aging (96 h of AA) for any of the cultivars. Means followed by the same letters do not differ from each other by the Tukey test at 5 % probability.
After artificial aging, there was a reduction in the content of total protein only for the seeds of genotypes GEN2 and GEN6. However, a sharp decrease in the content of soluble protein was observed for all genotypes after storage. The seeds of genotype GEN6 were the ones that presented the lowest content of total protein and the lowest content of soluble protein, in the initial condition and after artificial aging. The aging of the seeds promoted an increase in oil content for all the genotypes, being higher in the seeds of GEN6. There was a decrease in the MDA content in the seeds of genotypes GEN5 and GEN6, after artificial aging, and in the seeds of genotypes GEN1, GEN3, GEN5 and GEN6, after storage (Fig. 3).
The seeds of GEN1 and GEN3 presented the greatest proportions of oleic acid and the smallest ones of acids linoleic and linolenic (Fig. 4). On the other hand, the seeds of GEN6 presented the highest content of linoleic acid (Fig. 4).
3.3. Physiological quality and chemical composition of the soybean seeds: what is the relationship between them?
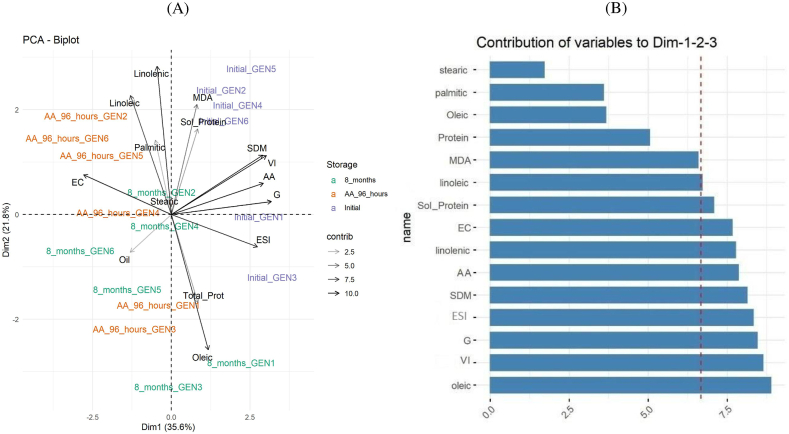
By means of the PCA analysis, using all the physiological and biochemical variables, it was noted that the content of fatty acid oleic was the most important variable to explain the differences among the treatments, followed by variables VI, G, ESI, SDM, AA, linolenic acid, EC, and soluble protein (Fig. 5). The distinction between the freshly harvested and the aged seeds was also evident, but the distinction between the naturally and artificially aged seeds was not clear (Fig. 5A).
Fig. 5.
Principal component analysis (A) and analyses of variable contribution (B) to verify the relationship between the physiological and biochemical variables and seed performance of freshly harvested soybean seeds (purple) and submitted to natural aging (green) and artificial aging (orange). GEN1, GEN2, GEN3, GEN4, GEN5 and GEN6 refer to the genotypes used; initial means freshly harvest seeds; 8_months refers to natural aging and AA_96_hours to artificial aging.
In order to verify the relationship between the physiological and biochemical variables, the data of the freshly harvested, naturally and artificially aged seeds (Fig. 6A) were analyzed jointly, and separately only, from the data of the freshly harvested (Fig. 6B), naturally (Fig. 6C) and artificially (Fig. 6D) aged seeds.
Fig. 6.
Correlation between the physiological variables (germination (G), accelerated aging (AA), seedling dry mass (SDM), vigor index (VI), emergence speed index (ESI) and electrical conductivity (EC)) and the biochemical composition (content of oil, total protein (Total_Prot), soluble Protein (Soluble_Prot), malonaldehyde (MDA) and fatty acids stearic, palmitic, oleic, linoleic and linolenic) of soybean seeds: freshly harvested and naturally and artificially aged (A); freshly harvested seeds (B); seeds naturally aged (storage) (C); seeds submitted to artificial aging (D).
Considering only the significant correlation of a high magnitude (>80 %), there was a positive correlation among variables G, SDM and VI; VI and SDM; MDA and soluble protein, and a negative correlation between ESI and EC; and oleic acid and linoleic and linolenic fatty acids, when the freshly harvested, naturally and artificially aged seeds were analyzed jointly (Fig. 6A).
For the freshly harvested seeds (Fig. 6B), it was observed that the greater the proportion of oleic acid and the smaller the proportion of oil and of acids linoleic and linolenic, the greater seed vigor and germination, according to the AA test.
Considering the seeds kept under storage for eight months, naturally aged, there was a negative correlation between the contents of oleic acid and acids linoleic and linolenic (Fig. 6C). Artificial aging for 96 h, however, pointed out a negative and significant correlation only between the contents of acids oleic and linoleic and, in addition, it showed a positive correlation between EC and linoleic acid; and a negative one between soluble protein and EC (Fig. 6D).
4. Discussion
The water content of the freshly harvested and naturally or artificially aged seeds, at moment of the performance of the tests, was around 12 %. The uniformity of this parameter is important so that there is no interference in the results obtained, since water content affects seed metabolism [28].
In this study, a difference in performance among the genotypes was observed after storage. The seeds of GEN1 presented the lowest reduction, while those of GEN6 had the highest reduction in physiological quality (Fig. 1). This distinct behavior among genotypes may be related to their genetic differences, since seed longevity during storage is genetically controlled [29]. However, the storage potential of the seeds is also influenced by their initial quality, history, water content and biochemical composition, as well as by biotic agents, humidity, temperature, and storage time [1].
In this work, the seeds were stored under a non-refrigerated condition, in which the temperature varied from 8.8 to 30.7 °C, and the relative humidity of the environment igfrom 50–92 %, with high means of temperature and humidity in November and December. When seeds are stored in an environment with high humidity, they absorb water, increase the level of moisture, leading to an increase in the activity of hydrolytic enzymes, respiration and of the proportion of free fatty acids [1]. High temperature also increases the metabolic rate and enzymatic reactions, accelerating the deterioration process [1]. Inadequate storage, under high temperature and/or high relative humidity, may cause temperature rise of the seed mass, acidity increase, respiration intensification, reserve degradation, change of the fractions of the fatty acids, membrane decomposition, lipid peroxidation, among other factors [[19], [30]], which culminate in loss of seed vigor and germination capacity (Fig. 1).
In addition to the non-ideal storage conditions, artificial aging also promotes a decrease in seed physiological quality. It was observed that, when submitted to artificial aging, the seeds also presented a reduction in vigor (Fig. 1). Artificial aging is based on the application of high temperatures and humidity to the seeds, causing quick deterioration [31].
In this research, the seeds were aged under the temperature of 41 °C and RH of 100 %, for 96 h. Preliminary tests showed that the time of 96 h of artificial aging presented a high correlation to the results obtained after eight months of storage. Freitas et al. [8] also found a high correlation between artificial aging at 41 °C for 96 h and the performance of cotton seeds in storage over 10 months.
We noticed that the seeds of some genotypes presented the same performance when submitted to natural and artificial aging (Fig. 1). However, by means of tests EC and ESI, it was possible to note differences between the two kinds of aging. After artificial aging, the seeds of all genotypes presented a sharp increase in electrical conductivity and a greater decrease in ESI, when compared to storage.
The PCA analysis carried out with data of the physiological variables also pointed to a clear distinction between natural and artificial aging (Fig. 2A). Through this analysis, it was pointed out that the genotypes were grouped according to the kind of aging to which they were submitted, and that genotype GEN6, after storage for eight months, presented an atypical behavior, being similar to those artificially aged. As noted by the tests of EC, ESI (Fig. 1) and the PCA analysis (Fig. 2A), it was pointed out that artificial aging was sharper than natural aging. Therefore, for the seeds of GEN6, the deterioration process was more marked during storage, if compared to the other genotypes.
The seeds that were aged for 96 h and those from GEN6 after storage presented higher EC values. The EC test points out the structure level of the cell membranes during the soaking process [9]. Thus, the higher the EC, that is, the higher the leaching of exudates of the seeds, the lower the integrity level of the membranes is, which may indicate the occurrence of deterioration and, consequently, lower seed vigor [32]. The great EC influence on genotype GEN6 after storage may be an indicative of greater sensitivity of this genotype to deterioration, with a decrease in the integrity of the cell membranes, which may justify the worst performance of the seeds in storage and after artificial aging. The EC increase is related to the peroxidation of lipids of membranes, pointed as the main mechanism of deterioration of oilseeds [33,34].
In addition, ESI was the most important variable for the grouping of the genotypes stored for eight months (Fig. 2A), demonstrating that, even with the decrease in the physiological quality of these seeds, the high speed of emergence of the seedlings indicates higher physiological quality if compared to the artificially aged seeds.
The results of the evaluation tests of seed physiological quality and the PCA analysis led to infer that, although there is a great similarity between the two aging treatments, artificial and natural aging probably are stimulated by distinct mechanisms. The seeds after storage presented moisture content of 11.5 ± 0.8 %, while those under artificial aging reached water content of 30.2 ± 2.5 %. This high moisture content of the seeds, in combination with the high temperature of the environment for subsequent hours (96 h), favors respiration intensification, causing the accumulation of reactive oxygen species, which results in deleterious reactions and promotes membrane destabilization and the increase of the level and speed of deterioration processes [9,12]. This way, it was observed that artificial aging promoted damage to the membranes of the seeds, which culminated with EC increase and ESI decrease, which were not observed in the seeds after storage (Fig. 1).
Although the artificial aging of the seeds is used for the prediction of performance during natural aging, it is still not clear how similar these two aging processes are [35]. Shaban [14] mentions that natural and artificial aging do not occur in the same order and magnitude. Machado Neto et al. [36] argue that standards of protein degradation in artificial and natural aging are probably stimulated by different physiological mechanisms. Freitas et al. [8] describe that it was not possible to establish a correlation between natural and artificial aging for the changes in the content of lipids and in the lipoxygenase activity in cotton seeds. In turn, Sung [37] states that both kinds of aging affect lipid peroxidation and the inhibition of enzyme activity. Rajjou and Debeaujon [35] also point out several similar molecular events between both kinds of aging. However, regardless of the intensity and the way of action, both artificial and natural aging promote deterioration, which culminates in decrease of seed viability and vigor [8,35,36], as it was highlighted in this work (Fig. 1).
The process of seed deterioration, stimulated either by natural or by artificial aging, is associated with the conditions to which the seeds are exposed and to their genetics, and biochemical composition is one of the factors which most affects seed quality and longevity [6,14,29].
It was noted that there is a variation in the biochemical composition among the soybean genotypes, and this variation can explain the differences of performance among the genotypes, either when freshly harvested, or naturally and artificially aged (Fig. 3, Fig. 4, Fig. 5, Fig. 6). The seeds of genotype GEN6, which presented the worst performance after storage (Fig. 1), contained the smallest content of total protein and of soluble protein before being aged.
In addition, it was observed that the soluble proteins are very prone to degradation during aging. While the content of total protein was reduced only in the seeds of GEN2 and GEN6, when under artificial aging, there was a sharp decrease in the levels of soluble protein for all the genotypes after storage, and for GEN6 after artificial aging (Fig. 4). The decrease in the content of soluble protein may be attributed to the Amadori and Maillard reactions, which have, as a consequence, the aggregation and loss of solubility of soluble proteins, according to results obtained by Castellión et al. [38]. These authors found a reduction in the content of soluble protein after the storage of quinoa seeds and attributed greater longevity of the seeds of some genotypes during natural aging to the higher content of soluble protein in the freshly harvested seeds. Thus, a correlation between protein stability and the useful life of the seeds was suggested. Mathias et al. [39] also pinpoint that the content of soluble protein can be used as an indicator of the physiological quality of soybean seeds.
It was noted that, while the content of soluble protein was sharply decreased in the seeds after storage, the high levels were maintained after artificial aging, for most genotypes (Fig. 3). This can be connected to a higher metabolic rate of the seeds during the artificial aging process. In this condition, the seeds were kept under high temperature and relative humidity (41 °C and 100 % RH for 96 h) and reached average moisture content of 30 %. Possibly, this water content favored the intensification of the metabolism, with protein synthesis, including enzymes of reserve mobilization and linked to the defense mechanisms against this oxidative stress, which did not occur during natural aging, in which the seeds had reduced water content (approximately 12 %). In this case, the metabolic rates are very reduced [9] and, unlike artificial aging, during storage, the deterioration processes linked to the oxidation of fatty acids [1] are predominant, together with the degradation of proteins by means of the Amadori and Maillard reactions [38].
It was found out that there was an increase in oil content with the aging, and that GEN6 seeds presented the greatest proportion of oil if compared to the others (Fig. 3). Although an increase in oil content is not expected with seed aging, Priestley and Leopold [32] also found similar results in soybean seeds. Oil content is inversely proportional to germination and to vigor (Fig. 6B), which can also justify the worst performance of the seeds of the GEN6 seeds.
After natural and artificial aging, it was pointed out that the MDA of the seeds either remained the same or decreased if compared to the seeds before aging (Fig. 3). MDA is a secondary product, generated with the oxidation of the poly-unsaturated fatty acids, whose determination, in biological samples, is an indicator of the level of oxidative stress [40]. As it is a product originating from lipid peroxidation, an increase in its production is expected with the aging of the seeds. However, in this work, no increment in MDA was observed with aging. Ataíde et al. [41], who also found a reduction of MDA in Pterogyne nitens seeds after 72 h of aging, justify this drop by two possible reasons: cell death and consequent compound degradation, and enzyme action of the anti-oxidative complex, whose activity is increased under oxidative stress, and which acts in opposition to peroxidation.
Even though conflicting results have been found for oil content and MDA content in this work, the analysis of the contribution of the variables for PCA with the data of the entire experiment shows that these traits were not very important for the differentiation of the treatments (Fig. 5B). Both variables were less important than the average importance, when considering all the variables.
The seeds of GEN1 and GEN3, which had a small reduction in their physiological quality after natural and artificial aging (Fig. 1), also presented the greatest proportions of oleic acid and the smallest ones of acids linoleic and linolenic (Fig. 4). On the other hand, the seeds of GEN6 presented the greatest content of linoleic acid (Fig. 4) and the greatest decrease in physiological quality after both aging treatments (Fig. 1).
Soybean seeds have on average about 20 % of oil in their constitution, and the oil is mainly composed of fatty acids palmitic (16:0), stearic (18:0), oleic (18:1), linoleic (18:2) and linolenic (18:3). The percentages of these five fatty acids in soybean oil are, in average, 10 %, 4 %, 18 %, 55 % and 13 %, respectively [42], but there is a variation of concentration among commercial varieties [43], as it was also observed in this work (Fig. 4). The composition of fatty acids is the most important factor to determine oil susceptibility to oxidation [44], and the unsaturation level of the fatty acids has a significant influence on their degradation [45]. Previous works pointed out that the viability of soybean seeds in storage is associated with the oxidation of unsaturated fatty acids, especially linoleic acid [46]. Our results also show that a high linoleic acid content in soybean seeds leads to deterioration and rapid loss of vigor, as evident in GEN6.The correlation analyses of this work point to an inverse relationship between fatty acid oleic and fatty acids linoleic and linolenic (Fig. 6). Acids linoleic and linolenic stand out as the most susceptible to enzymatic and non-enzymatic oxidative degradation [1] and, therefore, the seeds that presented the greatest proportions of these fatty acids also presented greater propensity to deterioration. Oliveira et al. [47] observed that the low content of linolenic acid present in the oil fraction of the soybean seed favors the production of seeds of better quality. Wang et al. [48], when working with peanut seeds, pointed out that the seeds with a higher content of oleic acid presented a smaller reduction in physiological quality during storage. Thus, it is known that linoleic and linolenic fatty acids are harmful to the longevity of seeds. But in this work, we suggest oleic acid as an indicator of seed quality, since it is inversely proportional to acids linoleic and linolenic (Fig. 6).
Oily seeds, in general, present low longevity and may suffer from intense deterioration with aging. Soybean is among the oilseeds whose seeds deteriorate the most during storage [10,11], which might be associated with their high content of fatty acid linoleic, which, according to the data obtained from this work, has an inverse relationship with the potential of germination and of vigor of the seeds.
While the high content of soluble protein can be an indicator of the maintenance of the physiological quality in soybean aged seeds [39], high EC points out a greater level of deterioration and lower vigor of the seeds [9]. The increase of EC may be a consequence of the peroxidation of the unsaturated fatty acids, such as linoleic acid, present in the membranes [1], which may justify the positive correlation between the content of linoleic acid and the EC values (Fig. 6).
5. Conclusions
The physiological quality of soybean seeds decreased with natural and artificial aging. However, the deterioration process occurs by distinct mechanisms.
The biochemical composition of the seeds is associated with physiological quality and their storage potential is changed by natural and artificial aging.
The tolerance of the seed to deterioration is associated with the soluble protein and the content of fatty acids. A high content of linoleic and linolenic acid favors deterioration and rapid loss of vigor, while oleic fatty acid and soluble protein can be used as indicators of physiological quality in soybean seeds.
Author contribution statement
Martha Freire Silva: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Julia Martins Soares: Performed the experiments; Analyzed and interpreted the data.
Wanderson Andrade Xavier: Performed the experiments.
Francisco Charles dos Santos Silva; Felipe Lopes da Silva: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Laercio Junio da Silva: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Additional information
Supplementary content related to this article has been published online at [URL].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) [grant numbers 001]; the Conselho Nacional de Desenvolvimento Científico e Tecnológico – Brasil (CNPq); and the Fundação de Amparo à Pesquisa do Estado de Minas Gerais - Brasil (FAPEMIG).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21628.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Singh J., Paroha S., Mishra R.P. Factors affecting oilseed quality during storage with special reference to soybean (Glycine max) and Niger (Guizotia abyssinica) seeds. International Journal of Current Microbiology and Applied Sciences. 2017;6(10):2215–2226. doi: 10.20546/ijcmas.2017.610.262. [DOI] [Google Scholar]
- 2.Ghosal K., Bhattacharjee U., Sarkar K. Facile green synthesis of bioresorbable polyester from soybean oil and recycled plastic waste for osteochondral tissue regeneration. Eur. Polym. J. 2020;122:1–16. doi: 10.1016/j.eurpolymj.2019.109338. [DOI] [Google Scholar]
- 3.Mu B., Liu L., Li W., Yang Y. High sorption of reactive dyes onto cotton controlled by chemical potential gradient for reduction of dyeing effluents. J. Environ. Manag. 2019;239:271–278. doi: 10.1016/j.jenvman.2019.03.062. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz N., Atmanli A., Vigil F.M. Quaternary blends of diesel, biodiesel, higher alcohols and vegetable oil in a compression ignition engine. Fuel. 2018;212:462–469. doi: 10.1016/j.fuel.2017.10.050. [DOI] [Google Scholar]
- 5.Ebone L.A., Caverzan A., Tagliari A., Chiomento L.T., Silveira D.C., Chavarria G. Soybean seed vigor: uniformity and growth as key factors to improve yield. Agronomy. 2020;10(545):1–15. doi: 10.3390/agronomy10040545. [DOI] [Google Scholar]
- 6.Tripathi N., Khare D. Molecular approaches for genetic improvement of seed quality and characterization of genetic diversity in soybean: a critical review. Biotechnol. Lett. 2016;38:1645–1654. doi: 10.1007/s10529-016-2154-8. [DOI] [PubMed] [Google Scholar]
- 7.Nagel M., Kranner I., Neumann K., Rolletscheck H., Seal C.E., Couville L., Fernandez-Marín B., Borner A. Genome-wide association mapping and biochemical markers reveal that seed ageing and longevity are intricately affected by genetic background and developmental and environmental conditions in barley. Plant Cell Environ. 2014;38:1011–1022. doi: 10.1111/pce.12474. [DOI] [PubMed] [Google Scholar]
- 8.Freitas R.A., Dias D.C.F.S., Oliveira M.G.A., Dias L.A.S., José I.C. Physiological and biochemical changes in naturally and artificially aged cotton seeds. Seed Sci. Technol. 2006;34:253–264. [Google Scholar]
- 9.Bewley J.D., Bradford K.J., Hilhorst H.W.M., Nonogaki H. third ed. Spring; New York: 2013. Seeds: Physiology of Development, Germination and Dormancy. [Google Scholar]
- 10.Naik S.M., Madhusudan K., Motagi B.M., Mugali S., Nadaf H.L. Molecular characterization of seed longevity and associated characters using SSR markers in soybean [Glycine max (L.) Merrill] J. Pharmacogn. Phytochem. 2019;8(1):2357–2360. [Google Scholar]
- 11.Shelar V.R., Shaikh R.S., Nikam A.S. Soybean seed quality during storage: a review. Agric. Rev. 2008;29(2):125–131. [Google Scholar]
- 12.Xin X., Tian Q., Yin G., Chen X., Zhang J., Ng S. Reduced mitochondrial and ascorbate–glutathione activity after artificial ageing in soybean seed. J. Plant Physiol. 2014;171:140–147. doi: 10.1016/j.jplph.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Chandel R.K., Khan Z., Gandotra S. Alterations in protein and isozymes profiles during accelerated ageing in soybean (Glycine max (l.) merrill) Journal of Functional and Environmental Botany. 2015;5(1):64–69. doi: 10.5958/2231-1750.2015.00010.4. [DOI] [Google Scholar]
- 14.Shaban M. Review on physiological aspects of seed deterioration. Intl. J. Agric. Crop Sci. 2013;6(11):627–631. [Google Scholar]
- 15.Nagel M., Borner A. The longevity of crop seeds stored under ambient conditions. Seed Sci. Res. 2010;20:1–12. doi: 10.1017/S0960258509990213. [DOI] [Google Scholar]
- 16.Brasil . 2009. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: Mapa/ACS. [Google Scholar]
- 17.Mcdonald M.B., Phaneendranath B.R. A modified accelerated aging seed vigor test for soybeans. J. Seed Technol. 1978;3:27–37. [Google Scholar]
- 18.Nakagawa J. In: Vigor de sementes: conceito e testes. Abrates, Londrina. Krzyzanowski F.C., Vieira R.D.E., França-Neto J.B., editors. 1999. Teste de vigor baseados no desempenho das plântulas. [Google Scholar]
- 19.Medeiros A.D., Pereira M.D. SAPL®: a free software for determining the physiological potential in soybean seeds. Pesqui. Agropecuária Trop. 2018;48(3):222–228. doi: 10.1590/1983-40632018v4852340. [DOI] [Google Scholar]
- 20.Silva L.J., Medeiros A.D., Oliveira A.M.S. SeedCalc, a new automated R software tool for germination and seedling length data processing. Journal of Seed Science. 2019;41:250–257. doi: 10.1590/2317-1545v42n2217267. [DOI] [Google Scholar]
- 21.Maguire J.D. Speed of germination-aid selection and evaluation for seedling emergence and vigor. Crop Sci. 1962;2:176–177. [Google Scholar]
- 22.Vieira R.D., Krzyzanowski F.C. In: Vigor de sementes: conceitos e testes. Londrina: Abrates. Krzyzanowski F.C., Vieira R.D., França-Neto J.B., editors. 1999. Teste de condutividade elétrica. [Google Scholar]
- 23.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Cakmak I., Horst W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max) Physiol. Plantarum. 1991;83(3):463–468. doi: 10.1111/j.1399-3054.1991.tb00121.x. [DOI] [Google Scholar]
- 25.Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 26.Jham G.N., Teles F.F.F., Campos L.G. Use of aqueous HCl/MeOH as esterification reagent for analysis of fatty acids derived from soybean lipids. J. Am. Oil Chem. Soc. 1982;59:132–133. doi: 10.1007/BF02662261. [DOI] [Google Scholar]
- 27.R CORE TEAM . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing. Disponível em. [Google Scholar]
- 28.Raveneau M., Benamar A., Macherel D. Water content, adenylate kinase, and mitochondria drive adenylate balance in dehydrating and imbibing seeds. J. Exp. Bot. 2017;68(13):3501–3512. doi: 10.1093/jxb/erx182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sano N., Rajjou L., North H.M., Debeaujon I., Marion-Poll A., Seo M. Staying alive: molecular aspects of seed longevity. Plant Cell Physiol. 2016;57:660–674. doi: 10.1093/pcp/pcv186. [DOI] [PubMed] [Google Scholar]
- 30.Šimic B., Sudaric A., Liovic I., Kalinovic I., Rozman V., Cosic J. Influence of storage condition on seed oil content of maize, soybean and sunflower. Agric. Conspectus Sci. 2007;72(3):211–213. [Google Scholar]
- 31.Delouche J.C., Baskin C.C. Accelerated aging techniques for predicting the relative storability of seed lots. Seed Sci. Technol. 1973:427–452. [Google Scholar]
- 32.Priestley D.A., Leopold A.C. Absence of lipid oxidation during accelerated aging of soybean seeds. Plant Physiology. 1979;63:726–729. doi: 10.1104/pp.63.4.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mcdonald M.B. Seed deterioration: physiology, repair and assessment. Seed Sci. Technol. 1999;27:177–237. [Google Scholar]
- 34.Vijayakumar H.P., Vijayakumar A., Srimathi P., Somasundaram G., Prasad S.R., Natarajan S., Raja K., Dhandapani R., Vishwanath K. Correlation among physiological and histological changes in soybean seeds during storage. J. Environ. Biol. 2019;40:217–225. doi: 10.22438/jeb/40/2/MRN-753. [DOI] [Google Scholar]
- 35.Rajjou L., Debeaujon I. Seed longevity: survival and maintenance of high germination ability of dry seeds. Comptes Rendus Biol. 2008;331(10):796–805. doi: 10.1016/j.crvi.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Machado Neto N.B., Custódio C.C., Takaki M. Evaluation of naturally and artificially aged seeds of Phaseolus vulgaris L. Seed Sci. Technol. 2001;29(1):137–149. [Google Scholar]
- 37.Sung J.M. Lipid peroxidation and peroxide-scavenging in soybean seeds during aging. Physiol. Plantarum. 1996;97:85–89. doi: 10.1111/j.1399-3054.1996.tb00482.x. [DOI] [Google Scholar]
- 38.Castellion M., Matiacevich S., Buerab P., Maldonado S. Protein deterioration and longevity of quinoa seeds during long-term storage. Food Chem. 2010;121:952–958. doi: 10.1016/j.foodchem.2010.01.025. [DOI] [Google Scholar]
- 39.Mathias V., Coelho C.M.M., Garcia J. Soluble protein as indicative of physiological quality of soybean seeds. Revista Caatinga, Mossoró. 2019;32(3):730–740. doi: 10.1590/1983-21252019v32n317rc. [DOI] [Google Scholar]
- 40.Lima E.S., Abdalla D.S.P. Peroxidação lipídica: mecanismos e avaliação em amostras biológicas. Rev. Bras. Ciencias Farm. 2001;37(3):293–303. [Google Scholar]
- 41.Ataíde G.M., Flores A.V., Borges E.E.L. Alterações fisiológicas e bioquímicas em sementes de Pterogyne nitens tull. durante o envelhecimento artificial. Pesqui. Agropecuária Trop. 2012;42(1):71–76. doi: 10.1590/S1983-40632012000100010. [DOI] [Google Scholar]
- 42.Clemente E.T., Cahoon E.B. Soybean oil: genetic approaches for modification of functionality and total content. Plant Physiology. 2009;151:1030–1040. doi: 10.1104/pp.109.146282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamagno S., Aznar-Moreno J.A., Durrett T.P., Prosad P.V.V., Rotundo J.L., Ciampitti I.A. Dynamics of oil and fatty acid accumulation during seed development in historical soybean varieties. Field Crops Res. 2020;248 doi: 10.1016/j.fcr.2020.107719. [DOI] [Google Scholar]
- 44.Morello J.R., Motilva M.J., Tovar M.J., Romero M.P. Changes in commercial virgin olive oil (cv Arbequina) during storage, with special emphasis on the phenolic fraction. Food Chem. 2004;85(3):357–364. doi: 10.1016/j.foodchem.2003.07.012. [DOI] [Google Scholar]
- 45.Priestley D.A. Comstock Publishing Associates; New York: 1986. Seed Aging Implications for Seed Storage and Persistence in the Soil. [Google Scholar]
- 46.Tatić M., Balešević-Tubić S., Ðorđević, Nikolić Z., Đukić V., Vujaković M., Cvijanovic G. Soybean seed viability and changes of fatty acids content as affected by seed aging. Afr. J. Biotechnol. 2012;11(45):10310–10316. doi: 10.5897/AJB11.3505. [DOI] [Google Scholar]
- 47.Oliveira O.D.A., Piovesan N.D., José I.C., Barros E.G.A., Dias D.C.F.S., Moreira M.A. Lipoxigenases e teor de ácido linolênico relacionados à qualidade de sementes de soja. Rev. Bras. Sementes. 2006;28(1):30–35. doi: 10.1590/S0101-31222006000100005. [DOI] [Google Scholar]
- 48.Wang H., Yu S.T., Wang C.T., Yu G.Q., Cui X.Y., You S.L., Gao Z.Y., Shi P.X., Yu H.B., Ren L. Effect of different aging treatments on the vigor of high-oleic acid peanut seeds. Earth and Environmental Science. 2019;346:1–9. doi: 10.1088/1755-1315/346/1/012061. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.