Abstract
Buruli ulcer (BU), a neglected tropical disease (NTD), is an infection of the skin and subcutaneous tissue caused by Mycobacterium ulcerans. The disease has been documented in many South American, Asian, and Western Pacific countries and is widespread throughout much of Africa, especially in West and Central Africa. In rural areas with scarce medical care, BU is a devastating disease that can leave patients permanently disabled and socially stigmatized. Mycobacterium ulcerans is thought to produce a mycolactone toxin, which results in necrosis of the afflicted tissue and may be involved in the etiology of BU. Initially, patients may notice a painless nodule or plaque on their skin; as the disease progresses, however, it may spread to other parts of the body, including the muscles and bones. Clinical signs, microbial culture, and histological analysis of afflicted tissue all contribute to a diagnosis of BU. Though antibiotic treatment and surgical removal of infected tissue are necessary for BU management, plant-derived medicine could be an alternative in areas with limited access to conventional medicine. Herein we reviewed the geographical distribution, socioeconomic, risk factors, diagnosis, biology and ecology of the pathogen. Complex environmental, socioeconomic, and genetic factors that influence BU are discussed. Further, our review highlights future research areas needed to develop strategies to manage the disease through the use of indigenous African plants.
Keywords: Neglected tropical diseases, NTDs, Herbal medicine, Skin and subcutaneous tissue infections, Putative vectors, Treatment and management options
1. Introduction
Buruli ulcer (BU) is a necrotizing cutaneous disease caused by Mycobacterium ulcerans, an acid-fast mycobacterium [[1], [2], [3]]. While the precise mechanism of transmission is not well understood and is likely to be varied, the causative agent appears to spread from aquatic habitats to people via penetrating the skin or indirect transmission mediated by a biting insect vector [4]. The disease is considered the third most prevalent mycobacterial disease worldwide, after leprosy and tuberculosis [5]. The World Health Organization (WHO) considers BU as one of the neglected tropical diseases (NTDs) that affect the skin [6]. Children account for more than 50 % of all cases, and populations at greatest risk are those with no access to improved sanitation and clean water [7]. Preulcerative lesions, such as nodules, plaques, or oedematous infiltrates, are the typical symptoms of BU [5,8,9]. About 31 % of patients have severe symptoms that can be debilitating and stigmatizing [10]. All the biological effects associated with BU are caused by mycolactone A/B, an exotoxin secreted by M. ulcerans [11,12].
This chronic ulcer was first reported in 1897 by the British physician Albert Cook in the Mengo Hospital Notes in Kampala, Uganda [13]. The disease is primarily prevalent in tropical and subtropical areas of the world, particularly in Africa which accounts for 99 % of the disease's worldwide burden, and in some places with moderate climates like Japan, Papua New Guinea, and southern Australia [14,15]. In the tropical, subtropical, and temperate zones, many countries have recorded occurrences of BU, with most cases being reported in western and central African sub-regions, where approximately 1750 new cases were reported in 2017 to the WHO [2,16]. However, considerable underreporting of BU has been shown by cross-sectional surveys in endemic areas partly due to the chronic nature of the disease, the stigma often associated with the condition, the prevalence in rural areas, patients' limited access to medical care, and a lack of resources within health systems and most importantly the preference for herbal medicine [13,17].
Because there is no effective vaccine to prevent BU, early case discovery, and thorough patient treatment are the main priorities in current disease control strategies [18]. Treatment options available for BU include surgery debridement and antibiotics, such as the combination of rifampicin/streptomycin, and rifampicin/clarithromycin alone or in conjunction with surgery to speed up the healing of wounds and avoid deformities [3,19,20]. Although the use of antibiotic medication has significantly improved BU management and produced superior results, streptomycin injections are associated with significant nephrotoxicity and ototoxicity [21]. Also, rifampicin has been implicated in drug-drug interactions [21]. Surgical treatments are only practicable in a small number of medical facilities with appropriate technology, and are neither economical nor available to a substantial portion of the population, particularly in Africa [6,22].
Traditional plant-based remedies remain the primary choice for BU management for many communities, particularly in Africa, due to cultural beliefs, inadequate healthcare facilities, stigmatization, and fear of amputation [23]. The use of traditional, complementary, and alternative medicine is receiving more and more attention on a global scale because of the threat that antibiotic-resistant microbes pose to human health and development. Because of the abundance and diversity of chemicals found in plants, there are countless opportunities for new therapeutic leads. In addition to providing alternative treatment options for medical professional and patients, the identification of novel metabolites isolated from plants can also hold considerable commercial potential [24]. In this review,we assess African medicinal plants that have historically been utilized to treat BU and for which ethnopharmacological accounts backed by pharmacological data have been collected. This information will enable further research toward developing plant-based management strategies for BU. In addition, we comprehensively assemble information on the distribution of BU in Africa, associated risk factors, clinical and laboratory diagnostic tools available, and vector-disease interactions.
2. Methodology
2.1. Protocol
A protocol was created outlining the research processes, including the databases to use and study eligibility requirements, before commencing the search. However, because this review was not a systematic review, we did not strictly follow the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines and thus our protocol was not registered.
2.2. Eligibility criteria
The review process contained a priori definitions of the studies' inclusion and exclusion criteria. Articles that identified BU in endemic and non-endemic areas from different parts in Africa, transmission studies, and biology of the causal organism were considered relevant to this review and included in the study. No restriction by geographical location was applied. Articles that developed from field surveys and laboratory conditions and validated them for risk assessment and decision analyses were also considered relevant to the review and included in the study. Review articles on health systems and implications on BU were considered relevant and included. Articles that developed and validated detection tools for early detection of BU were included. We restricted the literature search to studies published in English between 2012 and 2023, except for twelve articles published from 2006 to 2011. Information from Wikipedia were excluded from the study.
2.3. Information sources
Using the following keywords ‘Buruli ulcer’, ‘Buruli ulcer distribution’, ‘vectors of Buruli ulcer’, ‘Buruli ulcer cases in Africa’, ‘Herbal medicine for treatment and management of Buruli ulcer’, ‘Buruli ulcer risk factors’, ‘methods for Buruli ulcer detection’, Antibiotics for treating Buruli ulcer’, ‘causal agents of Buruli ulcer’, ‘drugs for Buruli ulcer’ and ‘compounds in indigenous plants for Buruli ulcer treatment’, we searched numerous academic databases, including Scopus, Web of Science, Science Direct, PubMed, BMC, Research Gate, and Google Scholar (Fig. 1.). In the case of literature from books, we considered only online versions and not printed versions.
Fig. 1.
A schematic diagram showing the methodology followed during the review.
2.4. Risk of bias assessment
Articles from different databases were downloaded and carefully reviewed by authors. All authors conducted title and abstract screening followed by full-text screening following predefined inclusion and exclusion criteria. Screening was done independently and by deliberation and consensus, all disagreements were resolved.
2.5. Data collection and analysis
Data from the WHO BU database (https://www.who.int/data/gho/data/indicators/indicator-details/GHO/number-of-new-reported-cases-of-buruli-ulcer) was used to describe the currently endemic and previously endemic African countries. The relationship between the year of record and number of BU cases from 2000 to 2020 was analysed using the Spearman Rank Correlation at 95 % confidence interval. All analyses were performed using R software (version 4.3.1) [25].
3. The geographical distribution of BU in Africa
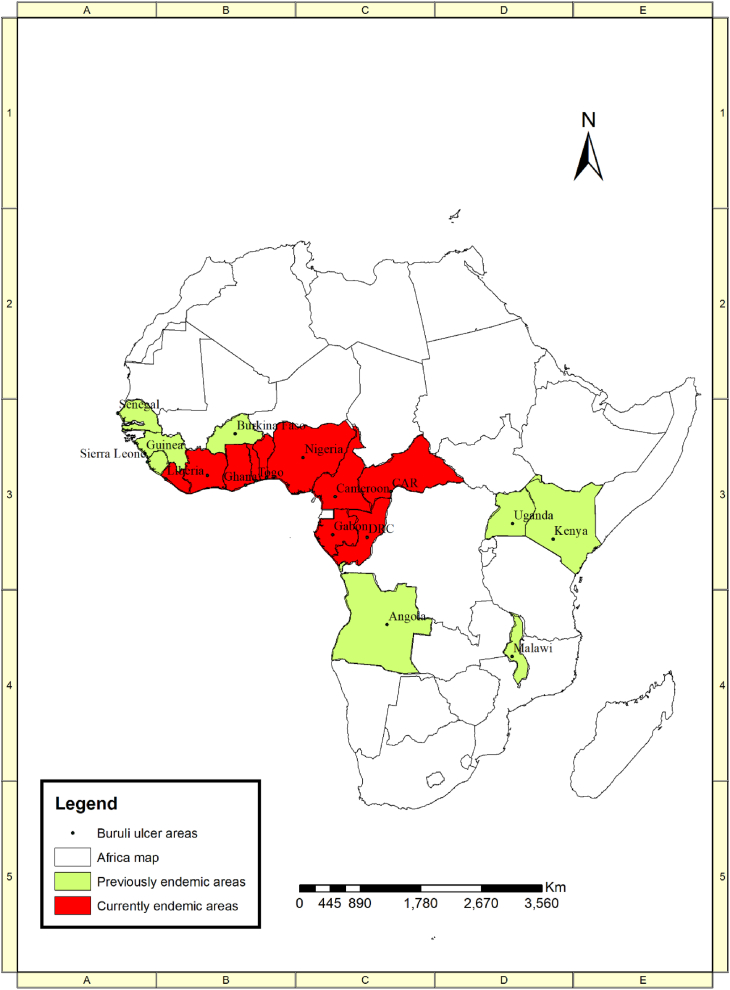
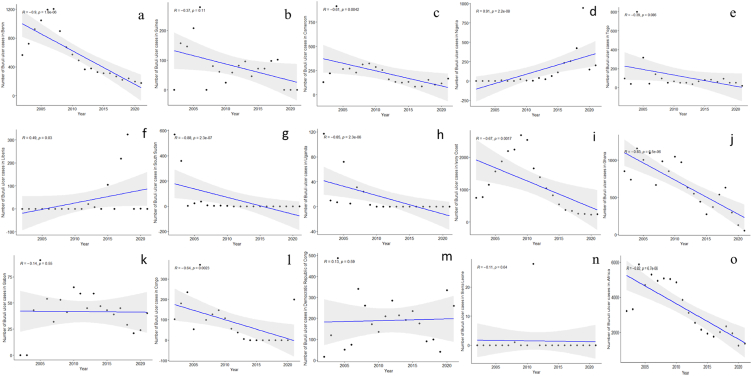
Buruli ulcer has been recorded in about 33 countries across the globe including Africa, Asia, Oceania, and South America [26]. Africa records most cases of BU worldwide. Although over 95 % of all cases are recorded in Africa, the West African sub-region bears the main brunt [27]. The disease is concentrated within rural communities which often have limited access to health facilities [28]. Data from the World Health Organization (WHO) were used to describe the currently endemic and previously endemic countries (Fig. 2) [29]. The correlation analysis revealed a significant decline in the number of BU cases in Benin, Cameroon, South Sudan, Uganda, Ghana, Ivory Coast and Congo but rising numbers in Nigeria and Liberia between 2000 and 2020 (Fig. 3). Yet overall, BU cases significant declined during the said period in Africa (Fig. 3; Supplemental Information Table SI).
Fig. 2.
A map depicting the distribution of Buruli ulcer across Africa. Source: Adapted from [29].
Fig. 3.
Spearman correlation scatter plots (linear regression [blue line] with its confidence interval [light gray area]) for the year vs. the number of Buruli ulcer cases in different countries: a) Benin, b) Guinea, c) Cameroon, d) Nigeria, e) Togo, f) Liberia, g) South Sudan, h) Uganda, i) Ivory Coast, j) Ghana, k) Gabon, l) Congo, m) Democratic Republic of Congo, n) Sierra Leon, and o) the overall cases in Africa from 2002 to 2021, as measured by the World Health Organization. Upper left corner with r, Spearman correlation coefficient and p, associated p-value. Data from Ref. [29]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. The biology of M. ulcerans
M. ulcerans belong to the family of Mycobacteriaceae, order Actinomyetales, and the phylum Actinobacteria [30]. These pathogens exist in diverse forms, and are mostly harmless saprophytes in soils or aquatic environments such as natural water reservoirs and engineered water systems [31,32]. Some mycobacterial species have evolved the ability to cause diseases in mammals, especially human diseases such as tuberculosis (M. tuberculosis), leprosy (M. leprae), and BU (M. ulcerans) [[33], [34], [35], [36]].
Mycobacterium ulcerans constitute a group of closely related and niche-adapted pathogenic bacteria species commonly detected in various aquatic environments such as mud, plant biofilms, and detritus [37,38]. Characteristically, this Mycobacterium species shows sensitivity to streptomycin and rifampicin but is resistant to para-aminosalicylic acid, isoniazid, and ethambutol [37]. It represents the etiologic agent of the NTD BU [39], and BU infections can occur in any human organ, where M. ulcerans multiply and spread in immunocompromised persons [30]. mycobacterial pathogens frequently infect the mammalian skin and soft tissues and represent the most common cause of mycobacteriosis, ranking only behind tuberculosis and leprosy [32,40].
Mycobacterial infections cause a broad range of dermatologic symptoms on the skin and soft tissues, including cellulitis, single or multiple abscesses, subacute or chronic nodular lesions, macules, superficial lymphadenitis, plaques, non-healing ulcers, necrotic plaques, verrucous lesions, and several other symptoms [30,41]. Clinically, following infection, the mycobacterium is challenging to treat due to the presence of its tough outer cell membrane. Mycobacterium ulcerans, in addition to related species like M. pseudoshottsii, M. shinshuense, M. liflandii and M. marinum have evolved a distinct ability to secrete an immunosuppressive polyketide toxin called mycolactone which acts as a necrotizing agent and a trigger for cellular death [[42], [43], [44], [45]]. The necrosis that occurs provides a suitable environment for the further proliferation of the pathogens. Mycolactone produced by M. ulcerans is known to be responsible for the ulcerative skin condition of BU. However, this hypothesis appears to be debatable as other mycolactone-producing mycobateria such as M. shinshuense, M. pseudoshottsii, M. marinum, and M.liflandii are not by default associated with BU [43,46].
In the 1930s, a group of Australian scientists led by Perter MacCallum for the first time successfully cultured M. ulceran from lesions of patients from the Bainsdale region in Australia [47]. Using molecular approaches, to date over 150 mycobacterial species have been identified [30].
5. Putative vectors of BU
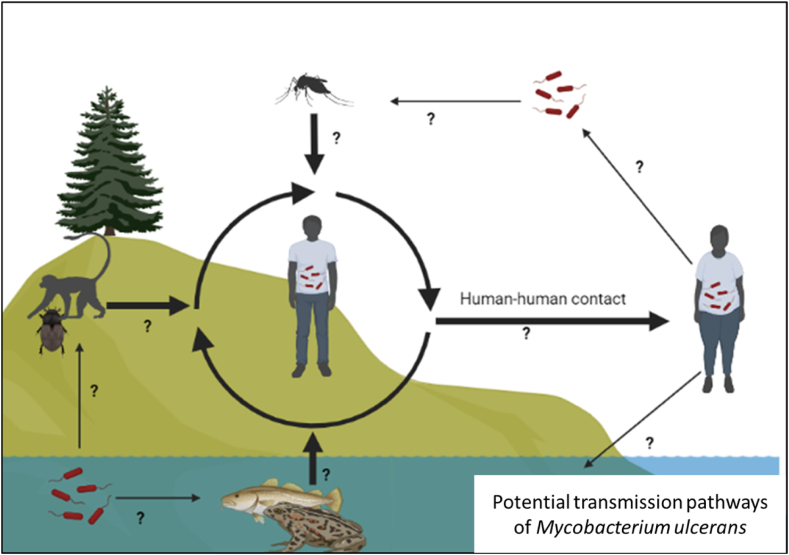
A BU infection manifests as a painful swelling (nodule), a sizeable painful area of induration (plaque), or a diffuse painful swelling of the legs, arms, or face (edema), eventually leading to damaging the skin and soft tissue [10,48]. According to WHO infections caused by M. ulcerans fall into one of three categories: Category I is characterized by a single lesion with a diameter of <5 cm (32 %); Category II represent non-ulcerative and ulcerative plaque and oedematous forms with a diameter of 5–15 cm (35 %); and Category III has lesions >15 cm (33 %), including disseminated and mixed forms such as osteomyelitis and joint involvement [49]. Most documented cases occur in regions with bordering sluggish or stagnant bodies of water (such as ponds, bogs, marshes, backwaters, dams, or men-made lakes lakes) [6,10]. A BU transmission from person to person has not been confirmed, but it can spread if someone comes into contact with the bacterium, either through drinking contaminated water or touching infected soil or wounds. Several studies have focused on identifying potential (arthropod) vectors associated with the disease using molecular techniques [50]. Many divergent views exist regarding the transmission mode of M. ulcerans (Fig. 4) [37,40,51].
Fig. 4.
Possibly transmission pathways of Mycobacterium ulcerans in humans. Generally, aquatic habitats harbour insects such as water bugs which are capable of transmitting M. ulcerans to humans and other animals including mice. Host-to-host transmission also occurs between humans and other animal reservoirs. Moreover, transmissions by other insect vectors such as mosquitoes have been reported.
Buruli ulcer has been observed in a variety of species, including opossums [52]. In Australia, they are thought to be reservoirs for the etiologic agent, M. ulcerans. Not much is known about the precise mechanisms and channels of transmission from possums to people or other hosts. More investigation on the role of possums in the transmission of BU is required, as is the development of efficient methods for containing and preventing the disease. Moreover, the specific mechanisms by which M. ulcerans spreads from possums to other hosts or vectors, as well as how possums themselves become infected, are not clear [10]. The study further noted that utilizing bacterial comparative genomics and phylogeographic studies could aid in uncovering the pathways through which M. ulcerans is disseminated among hosts, thereby identifying potential intervention measures for disease control. The rate at which M. ulcerans is shed from possum faeces is correlated with the incidence of BU in humans [53].
Several studies postulate that insects can serve as vectors of BU (e.g. Ref. [54]). [51], showed evidence of aquatic insect involvement as vectors of BU by demonstrating that more than 30 % of aquatic insects sampled from BU endemic populations harbour M. ulcerans. Another study showed that the prevalence of M. ulcerans was higher in water bugs from BU endemic regions than in water bugs from non-endemic regions [50]. The disease is assumed to be transmitted mainly through the bites of aquatic insects, both biting and non-biting, such as the water strider and water boatman. These insects are believed to operate as intermediary hosts for the bacterium that can transmit the disease from infected animals like possums and rats to people via polluted water [10].
Mosquitoes have been implicated as potential mechanical vectors for M. ulcerans in Australia, as molecular studies have detected the pathogen DNA in mosquito populations in endemic areas [10,55,56]. [10] found that M. ulcerans can persist and proliferate possibly via mechanical transmission by mosquitoes. Wild-type M. ulcerans, isogenic toxin-negative mutants, and M. marinum isolates were ingested by Aedes aegypti (Linnaeus in Hasselquist), Aedes albopictus (Skuse), Ochlerotatus triseriatus (Say) and Culex restuans (Theobald) larvae and survived in their intestines [51]. A study conducted between 2002 and 2008 revealed a correlation between BU rates and the rates of other vector-borne diseases in Victoria (Southern Australia) [57]. Moreover, an important geographical link exists between M. ulcerans in mosquito populations and human BU incidence in Australia, suggesting mosquitoes could be a primary vector for the disease [51,56]. Another study indicated that M. ulcerans was highest among mosquitoes of the genus Aedes, known to feed on humans and animals [58]. Moreover, the symptoms of BU look like those resulting from mosquito bites and may thus be confounded [57]. BU lesions tend to form on the ends of toes, fingers, and other exposed parts of the body, which further supports the idea that mosquitoes have a role in transmitting this disease [59]. Furthermore, data collection alongside human BU case data in Victoria, [56] observed a significant and direct relationship between the detection of M. ulcerans DNA in mosquitoes and the incidence of BU cases in each town, indicating a strong correlation.
Though several studies have detected M. ulcerans in mosquitoes suggesting that they may operate as carriers, this is still debatable [[60], [61], [62]]. For instance, M. ulcerans does not survive long enough until the mosquito vector accomplishes its pre-adult development [51,55], making vertical transmission of the pathogen from larvae to mosquito adults rather unlikely. This was later confirmed by a study in Benin which failed to detect M. ulcerans at either the pupae or adult stages [61]. Field studies conducted in endemic areas of Benin found that mosquitoes do not contribute to the ecology or transmission of M. ulcerans, and are less likely responsible for the spread of BU [62].
Buruli ulcer may spread among mammals through wild koalas and ringtail possums [63]. Common brushtail possums, a prevalent mammal in many BU-endemic regions, have been found carrying M. ulcerans DNA in their faeces [50]. In Ghana possums captured in BU endemic areas had a higher prevalence of M. ulcerans infection than those captured in non-endemic areas [50]. Possibly possums pick up M. ulcerans from contaminated water and then spread it to humans through their waste or by coming into direct contact with them, though more research is needed to ascertain this hypothesis and better understand the spread of BU.
In Ghana the incidence of M. ulcerans infections in rodents from BU endemic areas was higher than those from non-endemic areas [51]. DNA from M. ulcerans was found in the faeces of Australian native rodents such as the long-tailed mouse and the bush rat [59]. Likely, the pathogens spread from rats to humans via their faeces or through direct contact, with rodents becoming infected after coming into contact with contaminated water. Yet, to what extent rodents actually contribute to the epidemiology and spread of BU is presently not clear and would warrant more research. Bats and primates are two more potential animal hosts for BU. In Ghana in locations where BU was prevalent, the faeces of fruit bats tested positive for M. ulcerans DNA [50]. Possibly the latter mammals may (also) pick up M. ulcerans from contaminated water and subsequently spread it to humans through their droppings or by direct contacts with humans. Like for rats, the importance of bats and monkeys BU's epidemiology is all but clear and certainly will necessitate more research.
The possibility of M. ulcerans transmission by other organisms has also been proposed. In Ghana, frogs and fish tested positive for M. ulcerans [64], and [65] found M. ulcerans DNA in soil, water, and plant samples collected in and around the Haho and Zio rivers of Togo. Finally, grasscutters have recently been suggested as hosts for M. ulcerans in Africa [66].
6. Risk factors associated with the disease in Africa
Several factors can predispose people in BU endemic regions to the disease. Surveys and experimental studies have shown that the risk factors for BU can be environmental/ecological, behavioural, and socio-demographic factors (Table 1). Moreover, in perceptional studies mythical or superstitious risk factors like witchcraft and ancestral curse were mentioned in connection with BU infection [67,68], though those are beyond the scope of this review.
Table 1.
Summary of risk factors and their levels of association with Buruli ulcer in Africa.
| Risk factors | Classification | Level of association | References |
|---|---|---|---|
| Stagnant water/wetland | Ecological/environmental | High | [7,38,[67], [69], [70]] |
| Insect bite | Ecological/environmental | High | [69,70] |
| Secondary vectors (pets, infected persons) | Ecological/environmental | High | [7,67] |
| Hygiene | Personal/individual | High | [7,69,67] |
| No Bacillus Calmette–Guérin vaccination | Personal/individual | Low | [67,71] |
| Occupation | Socio-demographic | High | [7,69] |
| Education | Socio-demographic | High | [67] |
| Age | Socio-demographic | High | [71,72] |
| Gender | Socio-demographic | Medium | [[67], [71], [72]] |
| Superstition/beliefs | Misconception | Cannot be proven | [70,68] |
The most important ecological predisposing factor to BU is contact with stagnated water, e.g., ponds, dams, swamps, and lakes. Surveys across endemic communities in different African countries like Ghana, Côte d‘Ivoire, Nigeria, and Benin, confirmed the presence of wetland or water bodies in the vicinity of patients suffering from BU (Table 1; [7,[67], [69], [70],72]. People who swim, bathe, and drink water from slow-flowing sources are at risk to get infected with BU as these bodies can be contaminated with M. ulcerans. Insects like mosquitoes and water bugs constitute nnother ecological risk factor that can expose people to BU [70]. Using multivariate conditional logistic regression analysis [69], found bites from insects residing in mud or water to be highly associated with BU infections in Ghana. Poor personal hygiene, such as improper wound care, irregular bathing, and improper handwashing (i.e., without soap), can also promote BU infections [38].
Several socio-demographic factors have been associated with BU infection in Africa (Table 2). In Côte d’Ivoire, most of the BU patients had low levels of education and were less informed about the disease [67]. People with higher income levels can afford clean drinking water and often practice better personal hygiene. People who engage in agricultural activities are more predisposed to BU because of frequent insect bites and contact with contaminated water and soil [7]. Yet, [111] found no association between the risk to develop BU and the socioeconomic status and occupational hygiene in parts of the Ashanti Region of Ghana.
Table 2.
Advantages and disadvantages of laboratory diagnostic tools for BU.
| Laboratory investigation | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Culture | Useful for monitoring drug resistance, for confirmatory test and the ability to distinguish between viable and nonviable organisms. | Requires sophisticated laboratory, strict quality control, highly skilled personal; it takes a long time to obtain results and has a low sensitivity of between 20 and 60 %. | [8,73] |
| Histopathology and cultivation | Gives fairly rapid results, high sensitivity of about 90 %; advantageous in establishing differential diagnosis and monitoring response to treatment | Requires a sophisticated laboratory, is expensive, needs skilfully trained personnel and the procedure is invasive. | [8,73] |
| Microscopy | Very quick, relatively simple, and less expensive procedure | Limited sensitivity | [8,73] |
| PCR | Very sensitive with a specificity of >90 % and rapid results. | Expensive procedure, requires highly skilled personnel and does not differentiate between viable and nonviable organism | [8,73] |
Age and gender can be associated with BU infections. Children below 15 years and adults above 49 were more predisposed to BU in Benin [72], possibly because of comparatively lower immunity levels, men often more involved in agricultural activities which increases likelihood of insect bites. [67] found significantly higher BU infections rates among males than females, and among children, a meta-anaysis found that boys are more likely to be infected with BU than girls [71].
Evidence from the literature on the importance of ecological risk factors for the epidemiology of BU is rather strong. Yet, this is not so much the case for the socio-demographic ones. Thus, future studies should elucidate more the role and importance of various potential risk factors of BU and also their possible interactions, since this will be key for eradicating the disease.
7. Available clinical and laboratory diagnostic tools for BU detection
Buruli ulcer presents various clinical features, including relatively unspecific, painless nodules, plaques, and edema, which may eventually progress to chronic ulcerative lesions. This complicate the clinical diagnosis of BU within a wide range of differential diagnoses, especially in the tropics, where the prevalence of other skin conditions with similar features is high [74]. However, clinical diagnosis of BU can be done in endemic areas with some degree of accuracy by experienced health professionals. Given the characteristic clinical features of the disease, a reliable clinical diagnosis of BU is generally straightforward [29], particularly for patients who come from endemic areas or have a travel history and present with typical signs and symptoms including painless ulcers with undermined edges [39].
Rapid and precise diagnosis of M. ulcerans is vital for effective and successful management (Table 2). It is commonly diagnosed based on WHO clinical case definition and on clinical and epidemiological grounds and microbiological tests [75]. High-quality diagnosis is essential in all settings, as misdiagnosis can result in high morbidity and mortality. Mycobacterium ulcerans is clinically diagnosed based on the patient's clinical manifestations and physical findings at examination and confirmed by identifying the causative bacterium in the patient's blood via appropriate laboratory tests. Laboratory diagnosis of M. ulcerans is multifaceted and has evolved over the years. Globally, there are currently four main laboratory approaches employed for the clinical diagnosis of M. ulcerans, i.e., microscopy, culture, Polymerase Chain Reaction (PCR), and histopathological analysis of sections obtained from affected tissue and primary cultivation of the mycobacteria [73]. Microscopy and PCR are used routinely for diagnosis of the disease. PCR targeting IS2404 is presently considered the gold standard for laboratory confirmation because it provides the highest sensitivity and quickly available data [76,110].
8. Health systems in Africa and implications for BU management
WHO and most national health systems in Africa have procedures to monitor and treat BU and its consequences [6]. Most treatment plans combine conventional and complementary therapies. Antibiotics for immediate therapy, wound care, surgery, disability prevention, and social and mental support have all been used as treatment and management strategies [77,78]. Such an approach, yet, necessitates easy access to healthcare facilities for laboratory diagnosis, treatment, physiotherapy, and surgical interventions. For the treatment and management of both moderate and severe cases, rehabilitation infrastructures such as operating theatres, wards, and physiotherapy units are required in some municipal and district hospitals as well as some health centres [79]. Periodic training within various healthcare systems has helped to develop the human capacity for treating cases by exposing healthcare workers to new and cutting-edge techniques for treating BU and its debilitating sequelae [80]. However, inadequate medical facilities such as BU testing laboratories, physiotherapy, and well-equipped surgical theatres continues to hamper the progress of eradicating the disease in Africa.
8.1. Treatment and management options for buruli ulcer
8.1.1. Clinical drugs
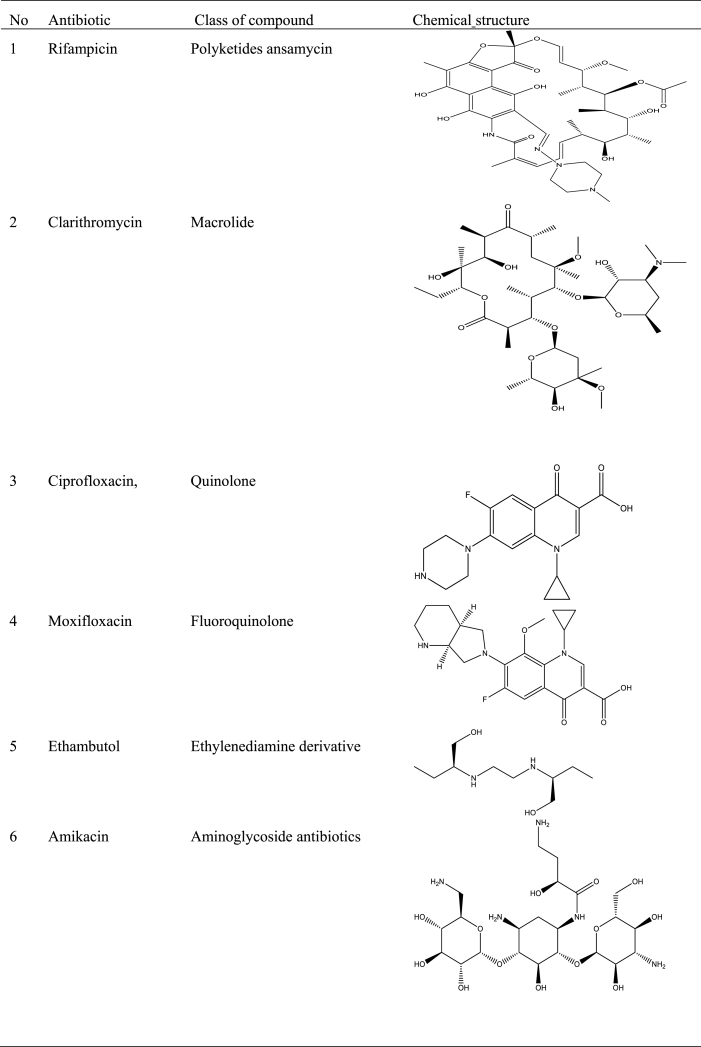
Most national health systems have embraced some WHO recommended antibiotics for treating and managing BU patients. Rifampicin and clarithromycin, ciprofloxacin, moxifloxacin, ethambutol, amikacin, azithromycin, streptomycin, and levofloxacin are currently the preferred medications for first-line treatment and infection control [18,81]. Moreover, many national health systems have approved using a combination of one of these antibiotics for wound care, infection control, and treatment [82] (Table 3).
Table 3.
Antibiotics used for treating BU.
8.1.2. Surgery/body grafting
Treatment with surgical and body grafting techniques is a more specialized approach for the most complex types of BU. However, this treatment option is expensive and typically out of reach for most patients Africa [3]. Excision and wound debridement are steps in the surgical procedures. Further seclusion and repair are performed on the afflicted body part.
8.1.3. Vaccines
A specific vaccine to prevent BU has not yet been authorized. However, the Bacillus Calmette-Guérin (BCG) vaccine created for tuberculosis immunity has been applied to BU, though offers minimal protection [83]. In addition, more specific M. ulcerans vaccines under development have so far not been able to pass the protocols for vaccine design approval [84].
8.1.4. Other treatment methods
The Negative Pressure Wound Treatment (NPTW) is an innovative treatment plan for patients with advanced BU. It implies the use of a portable NPWT equipment for outpatients to promote tissue granulation growth and wound blood flow, and which can reduce edema and bacterial growth [6,85]. The application of ozone for the treatment of BU has successfully been tested in Central Africa, involving placing a bag over the lesion and inhaling a combination of O2–O3 with a concentration of 30 g/ml [86].
8.2. African plants used for the treatment and management of BU
Worldwide, medicinal plants have been successfully used to treat various diseases, including BU [87]. Some African plants contain active ingredients that make them good candidates and choices for herbal preparations to treat and manage BU. Moreover, a recent survey in Nigeria showed that about one-fourth of the respondents preferred herbal medicine compared to conventual interventions to treat BU [88].
Herbal medicinal products have been utilized as treatment options for BU in many African countries [89]. A previous study by [92] reported that, the administration of an herbal formulation comprising of Erythropheleum suaveolens and Stemonocoleus micrantus completely healed BU wounds. A great reservoir of herbal medicinal resources for treating BU has also been discovered by an ethnobotanical survey in the West Africa, particularly in Ivory Coast, Ghana, and Benin [22].
Recently [90], provided a detailed compilation of traditional plant medicine, including botany and geographical distribution, used to treat BU in Ghana, Benin, and Ivory Coast. Additionally,[89] collected 27 native plants used to treat BU in Ghana and Cameroon and reported on their antimicrobial effects. The list included common plant species such as Mangifera indica L. [Anacardiaceae], Azadirachta indica A. Juss. [Meliaceae], Solanum torvum Sw. [Solanaceae], Carica papaya L. [Caricaceae], Chromolaena odorata (L.) R.M. King & H.Rob [Asteraceae], Jatropha curcas L. [Euphorbiaceae], etc.[89]. In Benin,[93] collected and studied 44 plant species used by herbalists to treat BU. They found high antimycobacterial activity of extracts from aerial organs of Holarrhena floribunda (G. Don) T. Durand and Schinz against M. ulcerans, explaining why local herbalists use this species to treat BU in Benin. In Cameroon, decoction and powder from the barks of the two tree species Erythrophleum suaveolens ([Guill. & Perr.], Brenan), and Stemonocoleus micranthus (Harms) were used to completely heal a patient with BU (Table 4, [91].
Table 4.
Some plant species used to treat BU in Africa.
| Plant species | Propagation method | Type/growth habit | Part used for preparation | Method of preparation | Mode of administration | Reference |
|---|---|---|---|---|---|---|
| Spathodea campanulata P. Beauv | seed, root sucker, cuttings | evergreen tree | stem bark, root | bitters | topical | [21,92] |
| Jathropha curcas L. [Euphorbiaceae] | seed, cuttings | semi-evergreen shrub | root, leaf | powder | poultice | [21,92] |
|
Ricinus communis L. |
seed, stem cuttings | evergreen shrub | root, leaf | bitters | topical | [21,92] |
| Khaya senegalensis (Desv.) A. Juss | seed, root, and stem cuttings | evergreen tree | stem | powder | poultice | [92] |
| Cryptolepis sanguinolenta (Lindl.) Schltr. | seeds | evergreen climbing shrub | root | decoction or powder | topical | [92] |
|
Picralima nitida (Stapf) T.Durand & H.Durand |
seeds, leafy stem cuttings |
evergreen tree | seed | decoction or powder | poultice | [92] |
| Bombax buonopozense P. Beauv | seeds, cuttings | deciduous tree | stem bark | decoction | oral | [92] |
|
Newbouldia laevis (P. Beauv) Seem. Ex Bureau |
cuttings | evergreen shrub | stem bark | decoction | oral | [92] |
| Erythrophleum suaveolens [(Guill. & Perr.), Brenan] | seeds | evergreen tree | stem barks | decoction and powder | topical | [91] |
| Stemonocoleus micranthus [Harms]) | seeds | evergreen tree | stem barks | powder | topical | [91] |
| Holarrhena floribunda (G. Don) T. Durand and Schinz | seeds | shrub or tree | stem bark, leaf | powder | topical | [21,93] |
| Annona senegalensis subsp. oulotricha Le Thomas [Annonaceae] | seeds, cuttings | evergreen shrub | leaf, stem | decoction or powder | topical, poultice |
[21,94] |
A more recent study showed that herbal medicine practitioners in Ghana used preparations from eight plant species to treat BU [92]. The preparations were mainly from roots and stem barks, with minimal use of seeds. The botanical preparations were administered to patients dermally or orally (Table 4). Traditional healers in the Democratic Republic of the Congo use plant species such as Aloe tenuifolia Lam., Annona senegalensis subsp. oulotricha Le Thomas, Brillantaisia owariensis P. Beauv, Vernonia amygdalina Delile and Strychnos icaja Baill. to treat BU [95].
Many BU patients in Africa prefer using traditional herbal medicine rather than the standard WHO recommended treatment of daily rifampicin (10 mg/kg orally) and streptomycin (15 mg/kg intramuscularly) for eight weeks [88,92,91]. Plant species used for bitters, powder, and decoction to treat BU grow naturally in forests or are cultivated by seeds or vegetative methods (Table 4). Strategies should be adopted to promote natural regeneration, conservation, and cultivation of reported potent plant species against BU in endemic regions. The different parts of plant species that can be used to treat BU and the availability of both evergreen and deciduous species make the use of herbal preparations independent of seasonal availability (Table 4). Most botanicals used to treat BU proved to be safe for patients [21] and should be promoted hitherto in BU endemic regions. However, there needs to be more evidence to support proper standardization to streamline and integrate them into the established healthcare system.
9. Compounds isolated from indigenous African plants for the treatment and management of BU
9.1. Isolation procedure for targeted compounds
Natural products are considered a rich reservoir of bioactive substances with therapeutic potential because of their chemical diversity [96,97]. Generally, drug discovery from plants for the treatment of BU, starts from an ethnobotanical survey to provide background information regarding the type of plant and plant part used, formulation (such as decoction, trituration, infusion, powders, maceration, pomade), and mode of administration (bandage, dermal) [98]. Survey data are generally derived from structured questionaries, personal observations, and/or focus group discussions [22]. Identification and scientific authentication of the plants used for BU treatment then follows the ethnobotanical survey. Once the plant's identity has been confirmed, extraction of the plant's active component(s) is carried out either using organic solvents such as hexane, ethylacetate dichloromethane, and water or essential oils through distillation. Antimycobacterial screening of crude plant extracts using cultured M. ulcerans strains is generally performed to assess the bioactivity of the plant species in question. Standard methods used for the antimycobacterial activity include the resazurin method, Alamar blue assay, radiorespirometry method, bioluminescent methods, and susceptibility assays to provide data on the minimal inhibitory concentration (MIC) of the plant extract ([89]. Extracts with a MIC of 10 mg/mL are often regarded as appropriately active [99]. Plant extracts that show bioactivity are then fractionated into active components following a bio-guided fractionation using column chromatography or preparative high-pressure liquid chromatography. The structure of the bioactive compound is determined using a physical, chromatographic, computational, and spectroscopic method of analysis. Antimycobacterial activity of the proposed structure is then confirmed through an in vitro assay using the compound obtained through synthesis or isolation from nature. Fig. 5 shows the various steps involve in the discovery of plant-derived compounds with antimycobacterial properties.
Fig. 5.
Flow chart summarizing the various steps involved in the drug discovery from plants.
9.2. Some isolated plant metabolites used for the treatment of BU
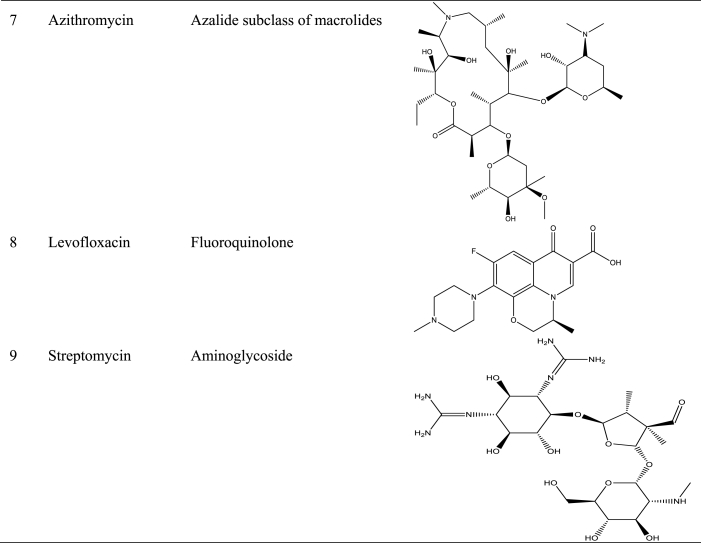
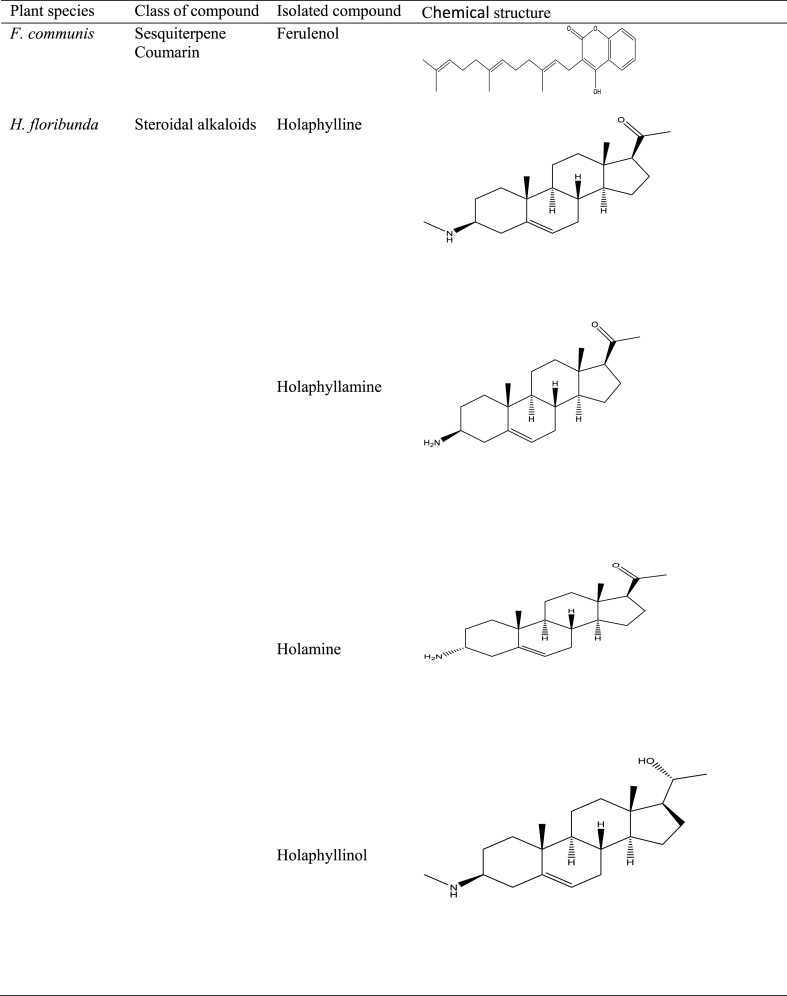
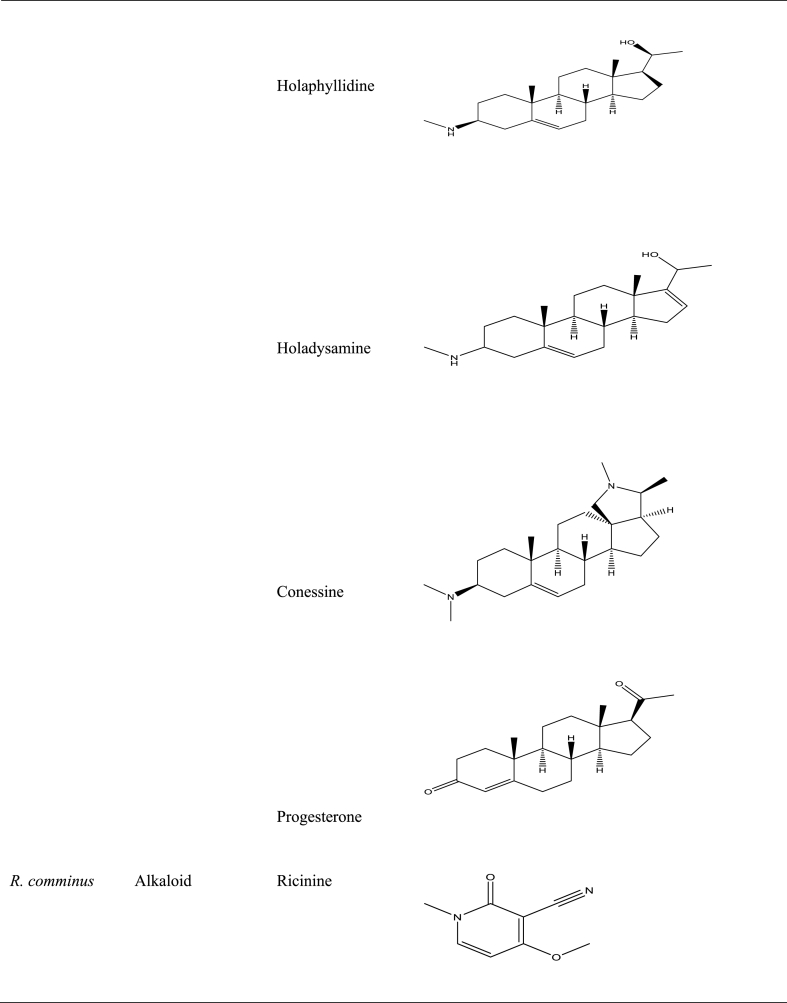
Numerous studies have shown the use of medicinal plants for the treatment of a variety of illnesses as well as the assessment of certain biological features with potential for application in pharmaceutical products [23,91,[100], [101], [102], [103]]. Several studies in Central and West Africa reported the use of traditional medicinal plants in the treatment of BU [98,104], with some going ahead to isolate, identify, validate, and evaluate the effectiveness of some secondary metabolites in vitro against M. ulcerans [89]. For example, holaphylline, holaphyllamine, holamine, holaphyllinol, holaphyllidine, holadysamine, holarrhesine, conessine, and progesterone were isolated from the leaves of Holarrhena floribunda (G.Don) T.Durand & Schinz [Apocynaceae] which have been reported to show bioactivity against a M. ulcerans strain [93]. When holadysamine was tested in vitro against M. ulcerans it showed an MCI of 0.05 mg/mL [93]. Also, ricinine, an alkaloid isolated from Ricinus comminus L., accounted for the plant's pharmacological activity against M. ulcerans [87]. Furthermore, Ferula communis [Apiaceae], which contains as active ingredient ferulenol, poses substantial antimycobacterial activity [99]. Table 5 summarises some isolated compounds from African plants with reported antimycobacterial activity.
Table 5.
Phytochemical constituents such as alkaloids, glucids, coumarins, flavonoids, triterpenes, anthocyanins, glucids, and many others have been linked to the antimycobacterial activity of plants used for BU treatment. For example, cryptolepine, an alkaloid derived from Cryptolepis sanguinolenta (Lindl.) Schltr. [Apocynaceae], demonstrated substantial antimycobacterial activity and a MIC comparable to those of the antibiotics ethambutol and isoniazid [99]. Also, Zea mays [Poaceae] known for its capacity to heal wounds caused by BU [89] produces silk that is abundant in phenolic chemicals, particularly flavonoids [98]. Table 6 lists the phytochemical constituents of some African medicinal plants used for the treatment of BU.
Table 6.
Phytochemical composition of some African medicinal plants used to treat Buruli ulcer.
| Plant | Plant part | Saponins | Tannins | Alkaloids | Flavonoids | Coumarins | Anthocyanins | Terpenoids | Phytosterols | Glycosides | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aloe tenuifolia Lam | Leaves | – | + | + | ND | – | + | + | + | ND | [94] |
| Alstonia boonei De Wild | Leaves | + | + | + | + | ND | ND | + | + | + | [22] |
| Annona senegalensis subsp. oulotricha Le Thomas | Leaves | + | + | – | ND | + | – | – | + | ND | [94] |
| Annona muricata L. [Annonaceae] | Root | + | + | + | + | ND | – | ND | ND | + | [89] |
| Annona reticulata L. [Annonaceae] | Fruit | – | + | + | + | ND | – | ND | ND | + | [22] |
|
Annickia chlorantha (Oliv.) Setten & Maas syn |
Stem bark | + | – | + | – | ND | – | ND | ND | + | [89] |
| Artabotrys rufus De Wild. | Stem | + | + | + | + | ND | – | ND | ND | + | [22] |
| Brillantaisia patula P. Beauv. | Leaves | – | + | + | ND | + | + | – | + | ND | [94] |
| Bridelia ferruginea Benth | Stem bark | + | + | – | + | ND | – | ND | ND | – | [89] |
| Capsicum annum L. [Solanaceae] | Fruit | + | ND | + | + | + | ND | – | + | + | [22] |
| Cleistopholis patens (Benth.) Engl. and Diels | Stem bark | – | – | + | + | – | ND | + | – | + | [104]) |
| Cryptolepis sanguinolenta (Lindl.) Schltr. | Leaves | – | + | + | – | – | ND | + | – | + | [104] |
| Carica papaya L. [Caricaceae] | Leaves | + | + | + | – | ND | ND | ND | ND | + | [89] |
| Erythrophleum suaveolens (Guill. & Perr.) Brenan [Fabaceae] | Leaves | ND | + | + | + | ND | ND | ND | + | ND | [104] |
| Eucalyptus globulus Labill. | Leaves | – | + | + | + | ND | ND | ND | ND | – | [22] |
| Holarrhena floribunda (G.Don) T.Durand & Schinz [Apocynaceae] | Leaves | + | + | + | + | ND | – | ND | ND | ND | [105] |
| Jatropha curcas L. [Euphorbiaceae] | Leaves | + | ND | ND | + | ND | ND | ND | + | ND | [89] |
| Pycnanthus angolensis (Welw) Warb | Stem bark | + | + | ND | ND | + | ND | + | + | + | [106] |
| Vernonia amygdalina Delile | Leaves | + | + | + | ND | + | + | + | + | ND | [94] |
| Sacoglottis gabonensis (Baill.) Urb. | Stem bark | + | + | + | + | – | – | – | + | – | [22] |
| Spathodea campanulata P. Beauv. | Leaves | + | + | ND | + | ND | ND | ND | ND | + | [89] |
| S. campanulate P. Beauv. | Root | + | + | – | + | ND | – | ND | ND | – | [22] |
| Strophanthus hispidus DC | ND | + | + | + | ND | ND | ND | + | ND | [21] | |
| Strychnos icaja Baill | Stem | + | + | + | ND | + | + | + | + | ND | [94] |
| Sorindeia juglandifolia (A.Rich.) Planch. ex Oliv | Fruit | + | + | – | + | ND | – | ND | ND | + | [21] |
| Zea mays L. [Poaceae] | Corn silk | + | ND | + | ND | ND | ND | ND | ND | ND | [89] |
| Zanthoxylum zanthoxyloides (Lam.) Zepern. & Timler | Root-bark | + | + | + | + | + | ND | ND | + | – | [22] |
ND: data not available.
9.3. Computer-aided botanical-drug design for BU
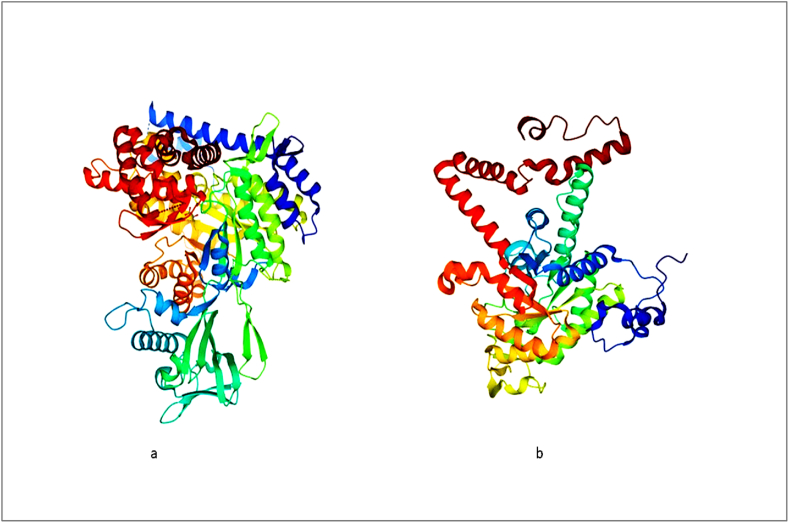
The traditional approaches to drug discovery are costly, labour- and time-intensive. Using computer-aided structure-based drug design (SBDD) is an alternate strategy to address these difficulties [102]. Moreover, a comprehensive BU database (BuDb) that provides information on drug targets, tested compounds, existing drugs, ethnopharmacological plants, and the genome of M. ulcerans has been established [107]. One noteworthy application of SBDD is a study that computationally predicted binders of isocitrate lyase, a key enzymes of the mycobacterium's glyoxylate shunt (Fig. 6) produced from natural products (ZINC38143792, ZINC95485880, and ZINC95486305) [107]. The inhibitors of M. tuberculosis have also been studied using SBDD approaches [102]. Numerous biomedical databases are nowadays available to study natural product interactions with M. ulcerans (Table 7). BuDb also contains cross-referenced links to comprehensive databases such as PubMed, PubChem, DrugBank, protein data bank (PDB) NCBI, Gene Ontology (GO), UniProt, Prota4u, String database, KEGG Pathway and KEGG genome database [107].
Fig. 6.
A 3D structures of malate synthase (a) and isocitrate lyase (b) a key enzymes of mycobacterium's glyoxylate shunt. Structures were obtained from Protein data bank.
Table 7.
Databases containing information on mycobacteria.
| Data Base | Information | Reference |
|---|---|---|
| Mycobrowser | Database for genomic and proteomic information | [107] |
| AfroDb database | 3D structures of natural products originating from different geographical regions in Africa. | [102] |
| GenoMycDB | Analysis of functional genomics | [107] |
| TDR target | A web-based resource with a variety of datasets to help with the pathogen drug research for neglected diseases | [107] |
| BioCyc | Contains data on the phenotypic characteristics of the organism, including its body place in the human microbiome, its aerobicity, and its temperature range. | [107] |
| BuDb | Database for BU Drug Development | [107] |
| DrugBank | A bioinformatics and cheminformatics database that integrates extensive drug target (i.e., sequence, structure, and route) information with detailed drug (i.e., chemical, pharmacological, and pharmaceutical) data | [107] |
| GeneOntology | Genome database | [107] |
| KEGG | Genome database | [107] |
10. Antimycobacterial activities of some African plants
The antimycobacterial activities and cytotoxicities of a selected group of 65 extracts obtained from 27 plant species from Ghana and Cameroon were tested against M. ulcerans, using the Resazurin Microtiter Assay (REMA) and MTT [(3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide] assay, respectively [21]. Most of these plant species originated from larger families such as Anacardiaceae, Annonaceae, Apocynaceae, Myrtaceae, Meliceae, Rutaceae, Solanaceae and Bignoniaceae [21]. Seventy (17) extracts displayed minimal inhibitory concentration (MIC) activities against M. ulcerans with MIC values between 125 μg/mL to 250 μg/mL [21]. Except for Carica papaya, Cleistopholis patens, and Polyalthia suaveolens, which had approximately 50 % cell cytotoxic concentrations (CC50), most of the other tested plant species showed no cytotoxic effects against normal human liver cells [21]. A follow-up study employed bioactivity-guided isolation to identify and characterize nine active compounds with significant in vitro anti-M ulcerans activities (MIC = 16–128 μg/mL) [21].
Indeed, several plants from sub-Saharan African may exhibit potential antimycobacterial effects against BU (Table 8) [89]. Using the resazurin microplate assay, Ghanaian herbal formulations containing the roots of Cryptolepis sanguinolenta and seeds of Picralima nitida proved to be significantly active against M. ulcerans (MIC = 32 μg/mL) and relatively non-cytotoxic against Chang liver cells [92]. Several other in vitro antimycobacterial assays such as agar diffusion, radiorespiratory method, and dilution methods, are also employed to characterize potential anti-M. ulcerans natural products [93]. Moreover, methanolic and aqueous crude extracts of Ficus binjamina, Ficus elastica, Ficus saussureana, and Terminalia superba from Cameroon were screened against M. ulcerans using the resazurin microtiter assay method [90]. Eleven (11) tested extracts displayed promising anti-M. ulcerans activities with MICs ranging from 62.5 μg/mL to 250 μg/mL, while phytochemical screening revealed also the presence of secondary metabolites such as phenols, flavonoids, tannins, triterpenes, glucosides, and saponins [90]. Thus, there is the possibility that most African plants may possess potent antimycobacterial properties.
Table 8.
Antimycobacterial activities of some African plants.
| Plants | MIC (μg/ml) | CC50 (μg/mL) Chang Liver Cell | Reference |
|---|---|---|---|
| Aglaonema commutatum Schott [Araceae] | 40 | N/D | [22] |
| Aloe vera (L.) Burm.f. [Asphodelaceae] | 40 | N/D | [89] |
| Alstonia boonei De Wild. [Apocynaceae] | >250 | >250 | [21] |
| Annona muricata L. [Annonaceae] | >250 | N/D | [21] |
| Annona reticulata L. [Annonaceae] | >250 | >250 | [21] |
| Annona senegalensis L. [Annonaceae] | 100–250 | N/D | [21] |
| Artabotrys rufus De Wild. | 125–250 | N/D | [21] |
| Annickia chlorantha (Oliv.) Setten & Maas syn | 250 | [21] | |
| Azadirachta indica A.Juss. [Meliaceae] | >250 | N/D | [21] |
| Capsicum annuum L. [Solanaceae] | 40 | N/D | [21] |
| Carica papaya L. [Caricaceae]. | >250 | 3.8–54 | [21] |
| Chromolaena odorata (L.) R.M.King & H.Rob. [Asteraceae] | >250 | N/D | [21] |
| Cleistopholis patens (Benth.) Engl. & Diels [Annonaceae] | 125–250 | 20.8 | [21] |
| Eucalyptus globulus Labill. [Myrtaceae] | 250 | >250 | [21] |
| Gratiola officinalis L. [Plantaginaceae] | 1.56–25 | N/D | [22] |
| Mangifera indica L. [Anacardiaceae] | >250 | N/D | [21] |
| Ricinus communis L. [Euphorbiaceae] | >250 | N/D | [21] |
| Greenwayodendron suaveolens (Engl. & Diels) Verdc. [Annonaceae] | >250 | 223–250 | [21] |
| Phyllanthus fraternus G.L.Webster [Phyllanthaceae] | >250 | N/D | [21] |
| Pycnanthus angolensis (Welw) Warb [Myristicaceae] | 256 | 383.9 | [106] |
| Holarrhena floribunda (G.Don) T.Durand et Schinz | >250 | N/D | [21] |
| Jatropha curcas L. [Euphorbiaceae] | 250 | N/D | [89] |
| Sacoglottis gabonensis (Baill.) Urb. [Humiriaceae] | 780 | N/D | [22] |
| Spathodea campanulata P.Beauv. [Bignoniaceae] | >250 | >250 | [21] |
| Spigelia anthelmia L. [Loganiaceae] | 6.25–25 | N/D | [22] |
| Syzygium aromaticum (L.) Merr.& L.M.Perry [Myrtaceae] | 25 | N/D | [89] |
| Sorindeia juglandifolia (A.Rich.) Planch. ex Oliv [Anacardiaceae] | 250 | >250 | [21] |
| Zea mays L. [Poaceae] | 6.25–25 | [22] | |
| Zanthoxylum zanthoxyloides (Lam.) Zepern.&Timler [Rutaceae] | >250 | >250 | [21] |
| Antibiotics | |||
| Streptomycin | 0.25 | N/D | [23] |
| Rifampicin | 0.125 | N/D | [21,106] |
Minimum Inhibitory Concentration (MIC), Sample's concentrations required to inhibit 50 % of cell proliferation (CC50). ND: data not available.
11. Social perceptions of the use of plants for BU treatment in Africa
Plant-based medicines are often used to treat different kinds of diseases in Africa. Some plants have been found to inhibit M. ulcerans [22]. In Africa, BU is mostly dominant in remote areas where traditional medicine remains highly preferred over conventional medicines and, in some cases, herbal medicine remains the only source of medication [89]. People's perception of using plant medicine for treating BU can enhance our understanding and control of the disease. Often traditional healers are the first point of call when people contract BU in Ghana, Benin, Cameroon and beyond, and they use different kinds of methods including the application of herbal medicine [108].The rural folks seeking herbal treatment share the perception that spirits and witchcraft cause BU, and the combination of treatments such as placating spirits, prayer, and the application of herbal medicine might effectively cure the disease [108]. Some also use plant medicine because they believe they will heal quickly [80].
Studies in Ghana and Benin have also shown that people resort to traditional healers because they fear they may have infections after surgical treatment, get scars, and become disabled [80]. Furthermore, some resort to plant treatment because of high transport costs, the high cost of food when hospitalized, and the socio-economic implications these may have on their relatives when they are on hospital admission. Besides, the herbalists are closer to them in their communities, who are sometimes their relatives, and they can easily access their services at a lower cost [80]. A study in the Ga West district of Ghana revealed that 32 out of 86 BU patients applied plant medicine to treat the disease [109].
People use plant medicine to treat BU because they perceive it to be effective in treating the disease and use it as a cheaper alternative. A study conducted in three health? centres in Côte d’Ivoire found that out of 273 people, 219 had their wounds healed when they applied plant medicine and 41 of them had their wounds stabilized [112]. A case was also reported of an 11-year-old patient who was taken to the hospital for diagnosis, and it was found to be a BU. However, the father refused the WHO-recommended treatment for BU for eight weeks and took the child to the house where he applied decoctions of Erythrophleum suaveolens. The wound was washed twice a day with a decoction derived from boiling the bark of E. suaveolens. And then, a mixture of salt and powdered bark of S. micranthus, and the E. suaveolens decoction was applied to the wound every day for three months. Two years later, the patient was diagnosed with no bone fractures or other defects [91]. Such cases can boost peoples' perceptions about the healing power of plant medicines and increase their desire to use them. In general, the treatment of BU requires multiple strategies [27], and the application of different plant-based approaches should be investigated further to reveal more healing properties of plants and help patients to have alternatives to the treatment of BU.
12. Conclusion and future perspective
BU vectors are only partially understood. However, human-to-human contact, mosquitoes, and aquatic insects have all been implicated in the spread of the disease. Possible mammalian BU vectors include possums, rodents, bats, and monkeys. These animals can contract M. ulcerans from water and subsequently spread the infection to humans through their waste or by coming into physical contact with humans. More studies are required to fully understand how these vectors possibly contribute to the spread of BU. The epidemiology of BU, especially the mechanisms that contribute to its transmission and dissemination, requires more research. This could aid in unravelling vulnerable regions and directing the development of specific management measures. Recent studies have provided insight into the significance of the bacterial toxin mycolactone in causing tissue damage, but the precise processes by which M. ulcerans produces toxins and these toxins transmitted in humans remain unknown.
Traditional African plant species have long been explored as potential sources of alternative chemotherapy for BU treatment. In vitro studies have played a crucial role in confirming or refuting the antimycobacterial properties of these plants, sometimes supplemented with rigorous in silico models. However, the majority of these plants have not undergone in vivo testing, which would provide data closer to natural biological conditions. Future research should prioritize investigating the in vivo efficacy of promising antimycobacterial plant compounds. Moreover, it is essential to follow up on in vivo studies with isolation, characterization, and possible structural modification of promising antimycobacterial compounds to facilitate the discovery and development of novel drugs. Additionally, high-quality clinical research is needed to demonstrate the safety and efficacy of herbal medicine for BU treatment. While herbal treatments may have a role in certain situations, they should be used cautiously and under the guidance of competent healthcare professionals to avoid delays in seeking conventional medical treatment, which could worsen the course of the disease and lead to complications. In conclusion, addressing these research gaps and advancing our understanding of BU transmission, toxin production, and potential treatments will be critical in improving the management and control of this debilitating disease.
CRediT authorship contribution statement
Jonathan Osei-Owusu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Owusu Fordjour Aidoo: Conceptualization, Formal analysis, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. Fatima Eshun: Writing – original draft, Writing – review & editing. David Sewordor Gaikpa: Writing – original draft, Writing – review & editing. Aboagye Kwarteng Dofuor: Writing – original draft, Writing – review & editing. Bright Yaw Vigbedor: Writing – original draft. Bernard Kofi Turkson: Writing – original draft. Kingsley Ochar: Writing – original draft. John Opata: Writing – original draft. Maxwell Jnr Opoku: Writing – original draft. Kodwo Dadzie Ninsin: Conceptualization, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Christian Borgemeister: Conceptualization, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22018.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Pluschke G., Röltgen K. Springer; 2019. Buruli Ulcer Mycobacterium Ulcerans Disease. [DOI] [PubMed] [Google Scholar]
- 2.Marion E., Hycenth N., Vedithi S.C., Robbe-Saule M., Donkeng V., Ganlonon L.M., Dissou A., Ngazoa S.K., Kabedi M.J., Mabika A.M., Phillips R., Frimpong M., Yeboah-Manu D., Walker V.Y., Akinwale O., Issaka M., Bretzel G., Asiedu K., Eyangoh S. A combined effort of 11 laboratories in the WHO African region to improve quality of Buruli ulcer PCR diagnosis: the “BU-LABNET.”. PLoS Neglected Trop. Dis. 2022;16(11):11. doi: 10.1371/journal.pntd.0010908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wadagni A.C., Steinhorst J., Barogui Y.T., Catraye P.M., Gnimavo R., Abass K.M., Amofa G., Frimpong M., Sarpong F.N., van der Werf T.S., Phillips R., Sopoh G.E., Johnson C.R., Stienstra Y. Buruli ulcer treatment: rate of surgical intervention differs highly between treatment centers in West Africa. PLoS Neglected Trop. Dis. 2019;13(10):10. doi: 10.1371/journal.pntd.0007866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson H., Tabah E.N., Phillips Ro F.M., Maman I., Ampadu E. Apping Suitability for Buruli Ulcer at Fine Spatial Scales across Africa: A Modelling Study. 2021;15:3. doi: 10.1371/journal.pntd.0009157. others. (n.d.) https://doi.org/10.1371/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent Q.B., Ardant M.-F., Adeye A., Goundote A., Saint-André J.-P., Cottin* J., Kempf M., Agossadou D., Johnson C., Abel L., Marsollier L., Chauty A., Alca"\is A. Clinical epidemiology of laboratory-confi rmed Buruli ulcer in Benin: a cohort study. Lancet Glob Health. 2014 doi: 10.1016/S2214-109X(14)70223-2. [DOI] [PubMed] [Google Scholar]
- 6.Yotsu R.R., Richardson M., Ishii N. Drugs for treating Buruli ulcer (Mycobacterium ulcerans disease) Cochrane Database Syst. Rev. 2018;2018(8):8. doi: 10.1002/14651858.CD012118.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leuenberger A., Koné B.V., N’krumah R.T.A.S., Koffi D.Y., Bonfoh B., Utzinger J., Pluschke G. Perceived water-related risk factors of Buruli ulcer in two villages of south-central Côte d'Ivoire. PLoS Neglected Trop. Dis. 2022;16(12):12. doi: 10.1371/journal.pntd.0010927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guarnera J. Buruli ulcer: review of a neglected skin mycobacterial disease. J. Clin. Microbiol. 2018;56(4):1507–1517. doi: 10.1128/JCM.01507-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manry J., Vincent Q.B., Johnson C., Chrabieh M., Lorenzo L., Theodorou I., Ardant M.F., Marion E., Chauty A., Marsollier L., Abel L., Alcaïs A. Genome-wide association study of Buruli ulcer in rural Benin highlights role of two LncRNAs and the autophagy pathway. Commun. Biol. 2020;3(1):3. doi: 10.1038/s42003-020-0920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muleta A.J., Lappan R., Stinear T.P., Greening C. Understanding the transmission of mycobacterium ulcerans: a step towards controlling buruli ulcer. PLoS Neglected Trop. Dis. 2021;15(8):8. doi: 10.1371/journal.pntd.0009678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chany A.C., Tresse C., Casarotto V., Blanchard N. History, biology and chemistry of Mycobacterium ulcerans infections (Buruli ulcer disease) Nat. Prod. Rep. 2013;30(12):1527–1567. doi: 10.1039/c3np70068b. [DOI] [PubMed] [Google Scholar]
- 12.Saint-Auret S., Chany A., Tresse C., Parmentier L., Hajer Abdelkafi H., Nicolas Blanchard N. Total syntheses of mycolactone A/B and its analogues for the exploration of the biology of buruli ulcer. Chimia. 2017;71:836–840. doi: 10.2533/chimia.2017.836. [DOI] [PubMed] [Google Scholar]
- 13.Pluschke G., Röltgen K. Epidemiology and disease burden of Buruli ulcer: a review. Res. Rep. Trop. Med. 2015;2015(6):59. doi: 10.2147/rrtm.s62026. [DOI] [Google Scholar]
- 14.Anagonou E.G., Johnson R.C., Barogui Y.T., Sopoh G.E., Ayelo G.A., Wadagni A.C., Boko M., Wardle A.J.G. Decrease in Mycobacterium Ulcerans Disease (Buruli Ulcer) in the Lalo District of Bénin (West Africa). BMC Infectious Diseases. 2019;19:247. doi: 10.1186/s12879-019-3845-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyamfi E, Narh Charles A., Quaye C., Abbass Adiza, Dzudzor B., Mosi Lydia. Microbiology of secondary infections in Buruli ulcer lesions; implications for therapeutic interventions. BMC Microbiol. 2021;21:4. doi: 10.1186/s12866-020-02070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandelannoote K., Phanzu D.M., Kibadi K., Eddyani M., Meehan C.J., Jordaens K., Leirs H., Portaels F., Stinear T.P., Harris S.R., de Jong B.C. No title. Mycobacterium Ulcerans Population Genomics to Inform on the Spread of Buruli Ulcer across Central Africa. 2019;4:418–472. doi: 10.1128/mSphere.00472-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson Hope, Deribe K., Tabah E.N., Peters A., Maman I., Frimpong M., Ampadu E., Phillips R., Saunderson P., Pullan R.L., Cano J. Mapping the global distribution of Buruli ulcer: a systematic review with evidence consensus. Lancet Global Health. 2019;7(7):e912–e922. doi: 10.1016/S2214-109X(19)30171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omansen T.F., van der Werf T.S., Phillips R.O. Buruli Ulcer: Mycobacterium Ulcerans Disease. 2019. Antimicrobial treatment of mycobacterium ulcerans infection; pp. 203–220. [DOI] [PubMed] [Google Scholar]
- 19.Douine M., Gozlan R., Nacher M., Dufour J., Reynaud Y., Elguero E., Combe M., Velvin C.J., Chevillon C., Berlioz-Arthaud A., Labbé S., Sainte-Marie D., Guégan J.-F., Pradinaud R., Couppié P. Mycobacterium ulcerans infection (Buruli ulcer) in French Guiana, South America, 1969--2013: an epidemiological study. Lancet Planet. Health. 2017;(1):65–73. doi: 10.1016/S2542-5196(17)30009-8. 2017. [DOI] [PubMed] [Google Scholar]
- 20.Phillips R.O., Robert J., Abass K.M., Thompson W., Sarfo F.S., Wilson T., Sarpong G., Gateau T., Chauty A., Omollo R., Ochieng Otieno M., Egondi T.W., Ampadu E.O., Agossadou D., Marion E., Ganlonon L., Wansbrough-Jones M., Grosset J., Macdonald J.M.…Faber W. Rifampicin and clarithromycin (extended release) versus rifampicin and streptomycin for limited Buruli ulcer lesions: a randomised, open-label, non-inferiority phase 3 trial. Lancet. 2020;395(10232):1259–1267. doi: 10.1016/S0140-6736(20)30047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsouh P.V.F., Addo P., Yeboah-Manu D., Boyom F.F. Methods used in preclinical assessment of anti-Buruli ulcer agents: a global perspective. J. Pharmacol. Toxicol. Methods. 2016;73:27–33. doi: 10.1016/j.vascn.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Tsouh P.V.F., Nyarko A.K., Appiah-Opong R., Tchokouaha Yamthe L.R., Addo P., Asante I.K., Boyom F.F. Ethnopharmacological reports on anti-Buruli ulcer medicinal plants in three West African countries. J. Ethnopharmacol. 2015;172(2015):297–311. doi: 10.1016/j.jep.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 23.Atchogloa P.K. Isaac kingsley amponsaha , patrick valere Tsouh Fokoub,c. Benjamin Kingsley Harley , Michael Kwesi Baahe , Francis Ackah Armahf , Silas Adjei. Anti-Mycobacterium Ulcerans Activity and Pharmacognostic Standardisation of Pycnanthus Angolensis (Welw) Warb. Scientific African. 2021;13 doi: 10.1016/j.sciaf.2021.e00935. [DOI] [Google Scholar]
- 24.Rubegeta E., Makolo F., Kamatou G., Enslin G., Chaudhary S., Sandasi M., Cunningham A.B., Viljoen A. The African cherry: a review of the botany, traditional uses, phytochemistry, and biological activities of Prunus africana (Hook.f.) Kalkman. J. Ethnopharmacol. 2023;305:305. doi: 10.1016/j.jep.2022.116004. [DOI] [PubMed] [Google Scholar]
- 25.RStudio Team . Integrated Development for R. RStudio, PBC; Boston, MA: 2020. RStudio.http://www.rstudio.com/ URL. [Google Scholar]
- 26.Zingue D., Bouam A., Tian R.B.D., Drancourt M. Buruli ulcer, a prototype for ecosystem-related infection, caused by Mycobacterium ulcerans. Clin Microbiol. 2018;31(1):1–52. doi: 10.1128/CMR.00045-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boccarossa A., Degnonvi H., Brou T.Y., Robbe-Saule M., Esnault L., Boucaud Y., Eveillard M., Gnimavo R., Hounsou S., Djenontin A., Johnson C.R., Fleuret S., Marion E. A combined field study of Buruli ulcer disease in southeast Benin proposing preventive strategies based on epidemiological, geographic, behavioural and environmental analyses. PLOS Global Public Health. 2022;2(1) doi: 10.1371/journal.pgph.0000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabah E.N., Johnson C.R., Degnonvi H., Pluschke G., Röltgen K. Buruli Ulcer: Mycobacterium Ulcerans Disease. 2019. Buruli ulcer in Africa; pp. 43–60. [DOI] [Google Scholar]
- 29.WHO . 2022. Number of New Reported Cases: Data by Country. [Google Scholar]
- 30.Franco-Paredes C., Chastain D.B., Allen L., Henao-Martínez A.F. Overview of cutaneous mycobacterial infections. Current Tropical Medicine Reports. 2018;5(4):228–232. doi: 10.1007/s40475-018-0161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bottai D., Stinear T.P., Supply P., Brosch R. Mycobacterial pathogenomics and evolution. Molecular Genetics of Mycobacteria. 2015:27–47. doi: 10.1128/9781555818845.ch2. [DOI] [PubMed] [Google Scholar]
- 32.Coudereau C., Besnard A., Robbe-Saule M., Bris C., Kempf M., Johnson R.C., Brou T.Y., Gnimavo R., Eyangoh S., Khater F., Marion E. Stable and local reservoirs of mycobacterium ulcerans inferred from the nonrandom distribution of bacterial genotypes, Benin. Emerg. Infect. Dis. 2020;26(3):491–503. doi: 10.3201/eid2603.190573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tobias N.J., Doig K.D., Medema M.H., Chen H., Haring V., Moore R., Seemann T., Stinear T.P. Complete genome sequence of the frog pathogen Mycobacterium ulcerans ecovar Liflandii. J. Bacteriol. 2013;195(3):556–564. doi: 10.1128/JB.02132-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behr M.A., Gordon S.V. Why doesn't Mycobacterium tuberculosis spread in animals? Trends Microbiol. 2015;23(1):1–2. doi: 10.1016/j.tim.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Balamayooran G., Pena M., Sharma R., Truman R.W. The armadillo as an animal model and reservoir host for Mycobacterium leprae. Clin. Dermatol. 2015;33(1):108–115. doi: 10.1016/j.clindermatol.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Franco-Paredes C., Rodriguez-Morales A.J. Unsolved matters in leprosy: a descriptive review and call for further research. Ann. Clin. Microbiol. Antimicrob. 2016;15(1):1. doi: 10.1186/s12941-016-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garchitorena A., Roche B., Kamgang R., Ossomba J., Babonneau J., Landier J., Fontanet A., Flahault A., Eyangoh S., Guégan J.F., Marsollier L. Mycobacterium ulcerans ecological dynamics and its association with freshwater ecosystems and aquatic communities: results from a 12-month environmental survey in Cameroon. PLoS Neglected Trop. Dis. 2014;8(5):5. doi: 10.1371/journal.pntd.0002879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merritt R.W., Walker E.D., Small P.L.C., Wallace J.R., Johnson P.D.R., Benbow M.E., Boakye D.A. Ecology and transmission of buruli ulcer disease: a systematic review. PLoS Neglected Trop. Dis. 2010;4(12):1–15. doi: 10.1371/journal.pntd.0000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO . 2018. Neglected Tropical Diseases.http://www.who.int/neglected_diseases/en/ [Google Scholar]
- 40.Gupta S.K., Drancourt M., Rolain J.M. In silico prediction of antibiotic resistance in mycobacterium ulcerans AGY99 through whole genome sequence analysis. Am. J. Trop. Med. Hyg. 2017;97(3):810–814. doi: 10.4269/ajtmh.16-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franco-Paredes C., Marcos L.A., Henao-Martínez A.F., Rodríguez-Morales A.J., Villamil-Gómez W.E., Gotuzzo E., Bonifaz A. Cutaneous mycobacterial infections. Clin. Microbiol. Rev. 2019;32(1):18–69. doi: 10.1128/CMR.00069-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhungel L., Benbow M.E., Jordan H.R. Linking the Mycobacterium ulcerans environment to Buruli ulcer disease: progress and challenges. One Health. 2021;13(10031):1. doi: 10.1016/j.onehlt.2021.100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doig K.D., Holt K.E., Fyfe J.A.M., Lavender C.J., Eddyani M., Portaels F., Yeboah-Manu D., Pluschke G., Seemann T., Stinear T.P. On the origin of Mycobacterium ulcerans, the causative agent of Buruli ulcer. BMC Genom. 2012;13(1):1. doi: 10.1186/1471-2164-13-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida M., Miyamoto Y., Ogur Y., Hayashi T., Hoshino Y. Complete chromosome sequence of a mycolactoneproducing mycobacterium, Mycobacterium pseudoshottsii. Genome Announc. 2017;5(48):48. doi: 10.1128/genomeA.01363-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida M., Fukano H., Ogura Y., Kazumi Y., Mitarai S., Hayashi T., Hoshino Y. Complete genome sequence of Mycobacterium shigaense. Genome Announc. 2018;6(25):25. doi: 10.1128/genomeA.00552-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pidot S.J., Asiedu K., Käser M., Fyfe J.A.M., Stinear T.P. Mycobacterium ulcerans and other Mycolactone-producing mycobacteria should be considered a single species. PLoS Neglected Trop. Dis. 2010;4(7):7. doi: 10.1371/journal.pntd.0000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stinear T., Johnson P.D.R. First isolation of Mycobacterium ulcerans from an aquatic environment. PLoS Neglected Trop. Dis. 2008;2(3) doi: 10.1371/journal.pntd.0000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boakye-Appiah J., Hall B., Reljic R., Simmonds R.E. In: Vaccines for Neglected Pathogens: Strategies, Achievements and Challenges. Christodoulides M., editor. Springer; 2023. Current progress and prospects for a buruli ulcer vaccine; pp. 71–95. [Google Scholar]
- 49.Röltgen K., Pluschke G. Buruli Ulcer: Mycobacterium Ulcerans Disease. 2019. Buruli ulcer: history and disease burden; pp. 1–41. [DOI] [Google Scholar]
- 50.Fyfe J.A.M., Lavender C.J., Handasyde K.A., Legione A.R., O'Brien C.R., Stinear T.P., Pidot S.J., Seemann T., Benbow M.E., Wallace J.R., McCowan C., Johnson P.D.R. A major role for mammals in the ecology of Mycobacterium ulcerans. PLoS Neglected Trop. Dis. 2010;4(8):1–12. doi: 10.1371/journal.pntd.0000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallace J.R., Gordon M.C., Hartsell L., Mosi L., Benbow M.E., Merritt R.W., Small P.L.C. Interaction of mycobacterium ulcerans with mosquito species: implications for transmission and trophic relationships. Appl. Environ. Microbiol. 2010;76(18):6215–6222. doi: 10.1128/AEM.00340-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farhan M., Shah Z., Jan R., Islam S. A fractional modeling approach of Buruli ulcer in Possum mammals. Phys. Scripta. 2023;98(6) [Google Scholar]
- 53.Vandelannoote K., Buultjens A.H., Porter J.L., Velink A., Wallace J.R., Blasdell K.R.…Stinear T.P. Statistical modeling based on structured surveys of Australian native possum excreta harboring Mycobacterium ulcerans predicts Buruli ulcer occurrence in humans. Elife. 2023;12 doi: 10.7554/eLife.84983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ayerakwa E.A., Abban M.K., Isawumi A., Mosi L. Profiling Mycobacterium ulcerans: sporulation, survival strategy and response to environmental factors. Future Science OA. 2023;9(3):9. doi: 10.2144/fsoa-2022-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh A., McBride W.J.H., Govan B., Pearson M., Ritchie S.A. A survey on Mycobacterium ulcerans in Mosquitoes and March flies captured from endemic areas of Northern Queensland, Australia. PLoS Neglected Trop. Dis. 2019;13(2) doi: 10.1371/journal.pntd.0006745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lavender C.J., Fyfe J.A.M., Azuolas J., Brown K., Evans R.N., Ray L.R., Johnson P.D.R. Risk of Buruli ulcer and detection of Mycobacterium ulcerans in mosquitoes in Southeastern Australia. PLoS Neglected Trop. Dis. 2011;5(9):1–6. doi: 10.1371/journal.pntd.0001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson P.D.R., Lavender C.J. Correlation between buruli Ulcer and vector-borne notifiable diseases, Victoria, Australia. Emerg. Infect. Dis. 2009;15(4):614–615. doi: 10.3201/eid1504.081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bamou R., Mayi M.P.A., Djiappi-Tchamen B., Nana-Ndjangwo S.M., Nchoutpouen E., Cornel A.J., Awono-Ambene P., Parola P., Tchuinkam T., Antonio-Nkondjio C. An update on the mosquito fauna and mosquito-borne diseases distribution in Cameroon. Parasites Vectors. 2021;14(1):527. doi: 10.1186/s13071-021-04950-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yerramilli A., Tay L.E., Stewardson A.J., Kelley P.G., B, Jenkin E., G A., S M., T J., H A., F, ND F. D. P. and Johnson, P.D.R. The Location of Australian Buruli Ulcer Lesions---Implications for Unravelling Disease Transmission. 2017;11(18):1–16. doi: 10.1371/journal.pntd.0005800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Demangel C., Stinear T.P., Cole S.T. Buruli ulcer: reductive evolution enhances pathogenicity of Mycobacterium ulcerans. Nat. Rev. Microbiol. 2009;7(1):50–60. doi: 10.1038/nrmicro2077. [DOI] [PubMed] [Google Scholar]
- 61.Djouaka R., Zeukeng F., Daiga Bigoga J., N'Golo Coulibaly D., Tchigossou G., Akoton R., Aboubacar S., Tchebe S.J.E., Nantcho Nguepdjo C., Adeoti R., Djegbe I., Tamo M., Mbacham W.F., Kakou-Ngazoa S.E., Ablordey A. Evidences of the low implication of mosquitoes in the transmission of Mycobacterium ulcerans, the causative agent of buruli ulcer. Can. J. Infect Dis. Med. Microbiol. 2017;2017:1–12. doi: 10.1155/2017/1324310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zogo B., Djenontin A., Carolan K., Babonneau J., Guegan J.F., Eyangoh S., Marion E. A field study in Benin to investigate the role of mosquitoes and other flying insects in the ecology of Mycobacterium ulcerans. PLoS Neglected Trop. Dis. 2015;9(7):1–12. doi: 10.1371/journal.pntd.0003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wansbrough-Jones M., Phillips R. Buruli ulcer: emerging from obscurity. Lancet. 2006;367(9525):1849–1858. doi: 10.1016/S0140-6736(06)68807-7. [DOI] [PubMed] [Google Scholar]
- 64.Willson S.J., Kaufman M.G., Merritt R.W., Williamson H.R., Malakauskas D.M., Benbow M.E. And amphibians as potential reservoirs of Mycobacterium ulcerans. The Causative Agent of Buruli Ulcer Disease. 2013;3 doi: 10.3402/iee.v3i0.19946. F. (n.d.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maman I., Tchacondo T., Kere A.B., Beissner M., Badziklou K., Tedihou E., Nyaku E., Amekuse K., Wiedemann F.X., Karou D.S., Bretzel G. Molecular detection of Mycobacterium ulcerans in the environment and its relationship with Buruli ulcer occurrence in Zio and Yoto districts of maritime region in Togo. PLoS Neglected Trop. Dis. 2018;12(5):5. doi: 10.1371/journal.pntd.0006455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hammoudi N., Dizoe A.S., Regoui S., Davoust B., Drancourt M., Bouam A. Disseminated Mycobacterium ulcerans infection in wild grasscutters (thryonomys swinderianus), Côte d'Ivoire. Am. J. Trop. Med. Hyg. 2019;101(3):491–493. doi: 10.4269/ajtmh.19-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pou A., Jc I., Hn C., Nb E. Risk factors for buruli ulcer in a referral mission hospital in anambra state , Nigeria : a case control study. Edorium Journal of Public Health. 2018;5:1–9. doi: 10.5348/100018P16AP2018OA. [DOI] [Google Scholar]
- 68.Anokye R., Acheampong E., Mprah W.K., Sarpong E. Perceived causes and risk factors of Buruli ulcer among patients at Agogo Presbyterian hospital in Ashanti Region of Ghana. BMC Res. Notes. 2018;11(1) doi: 10.1186/s13104-018-3172-5. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kenu E., Nyarko K.M., Seefeld L., Ganu V., Käser M., Lartey M., Calys-Tagoe B.N.L., Kwodwo K., Adanu R., Razum O., Afari E., Binka F. Risk factors for buruli ulcer in Ghana---A case control study in the suhum-kraboa-coaltar and akuapem south districts of the eastern region. PLoS Neglected Trop. Dis. 2014;8:11. doi: 10.1371/journal.pntd.0003279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Konan D.O., Mosi L., Fokou G., Dassi C., Narh C.A., Quaye C., Saric J.A. N. N. and Bonfoh, B. Buruli Ulcer in Southern Côte D’ivoire: Dynamic Schemes of Perception and Interpretation of Modes of Transmission. 2019;51(4):520–533. doi: 10.1017/S0021932018000317. [DOI] [PubMed] [Google Scholar]
- 71.Fevereiroid J., Sajjadi N., Fraga A.G., Teixeira P.M., Pedrosa J. Individual and clinical variables associated with the risk of buruli ulcer acquisition: a systematic review and meta-analysis. PLoS Neglected Trop. Dis. 2020;14(4):1–21. doi: 10.1371/journal.pntd.0008161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Debacker M., Portaels F., Aguiar J., Steunou C., Zinsou C., Meyers W., Dramaix M. Risk factors for buruli ulcer, Benin. Emerg. Infect. Dis. 2006;12(9):1325–1331. doi: 10.3201/eid1209.050598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakyi S.A., Aboagye S.Y., Darko Otchere I., Yeboah-Manu D. Clinical and laboratory diagnosis of buruli ulcer disease: a systematic review. Can. J. Infect Dis. Med. Microbiol. 2016:1–10. doi: 10.1155/2016/5310718. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Röltgen K., Cruz I., Ndung’u J.M., Pluschke G. Buruli Ulcer: Mycobacterium Ulcerans Disease. 2019. Laboratory diagnosis of buruli ulcer: challenges and future perspectives; pp. 183–202. [DOI] [PubMed] [Google Scholar]
- 75.Eddyani M., Sopoh G.E., Ayelo G., Brun L.V.C., Roux J.J., Barogui Y., Affolabi D., Faber W.R., Boelaert M., Van Rie A., Portaels F., De Jong B.C. Diagnostic accuracy of clinical and microbiological signs in patients with skin lesions resembling buruli ulcer in an endemic region. Clin. Infect. Dis. 2018;67(6):827–834. doi: 10.1093/cid/ciy197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Portaels F. WHO; Geneva: 2014. Laboratory Diagnosis of Buruli Ulcer: a Manual for Health Care Providers. [Google Scholar]
- 77.Denadai R., Toledo A.P., Martinhão Souto L.R. Basic plastic surgery skills training program on inanimate bench models during medical graduation. Plastic Surgery International. 2012;2012(65186):1–12. doi: 10.1155/2012/651863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yotsu R.R., Suzuki K., Simmonds R.E., Bedimo R., Ablordey A., Yeboah-Manu D., Phillips R., Asiedu K. Buruli ulcer: a review of the current knowledge. Current Tropical Medicine Reports. 2018;5(4):247–256. doi: 10.1007/s40475-018-0166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Awah P.K., Boock A.U., Mou F., Koin J.T., Anye E.M., Noumen D., Nichter M. Developing a Buruli ulcer community of practice in Bankim, Cameroon: a model for Buruli ulcer outreach in Africa. PLoS Neglected Trop. Dis. 2018;12(3):3. doi: 10.1371/journal.pntd.0006238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ackumey M.M., Gyapong M., Pappoe M., Maclean C.K., Weiss M.G. Socio-cultural determinants of timely and delayed treatment of Buruli ulcer: implications for disease control. Infectious Diseases of Poverty. 2012;1(Issue 1):1–13. doi: 10.1186/2049-9957-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Brien D.P., Friedman N.D., Walton A., Hughes A., Athan E. Risk factors associated with antibiotic treatment failure of buruli ulcer. Antimicrob. Agents Chemother. 2020;64(9) doi: 10.1128/AAC.00722-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Der Werf T.S., Barogui Y.T., Converse P.J., Phillips R.O., Stienstra Y. Pharmacologic management of Mycobacterium ulcerans infection. Expet Rev. Clin. Pharmacol. 2020;13(4):391–401. doi: 10.1080/17512433.2020.1752663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Okafor C.N., Rewane A., Guerin C. StatPearls [Internet] StatPearls Publishing; Treasure Island (FL: 2023. https://www.ncbi.nlm.nih.gov/books/NBK538185 2022. [Google Scholar]
- 84.Orujyan D., Narinyan W., Rangarajan S., Rangchaikul P., Prasad C., Saviola B., Venketaraman V. Protective efficacy of BCG vaccine against Mycobacterium leprae and non-tuberculous mycobacterial infections. Vaccines. 2022;10(3):179–186. doi: 10.3390/vaccines10030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murase C., Kono M., Nakanaga K., Ishii N., Akiyama M. Buruli ulcer successfully treated with negative-pressurewound therapy. JAMA Dermatology. 2015;151(10):1137–1139. doi: 10.1001/jamadermatol.2015.1567. [DOI] [PubMed] [Google Scholar]
- 86.Bertolotti A., Izzo A., Grigolato P.G., Iabichella M.L. The use of ozone therapy in Buruli ulcer had an excellent outcome. BMJ Case Rep. 2013 doi: 10.1136/bcr-2012-008249. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adam G.O., Kim S.J., Egbuna C., Oh H.G. Ethnopharmacological properties of african medicinal plants for the treatment of neglected tropical diseases. Research Journal of Pharmacognosy. 2022;9(4):59–72. doi: 10.22127/rjp.2022.334065.1864. [DOI] [Google Scholar]
- 88.Onwuka C.D.O., T Nwoke E., EA Nwoke, C Chukwuocha O., U M. Perceptions, preferred treatment methods, and compliance to WHO recommended control regime for buruli ulcer disease in Imo State, Nigeria. Middle European Scientific Bulletin. 2021;12(11–30):2694–9970. [Google Scholar]
- 89.Tsouh P.V.F., Nyarko A.K., Appiah-Opong R., Tchokouaha Yamthe L.R., Ofosuhene M., Boyom F.F. Update on medicinal plants with potency on Mycobacterium ulcerans. BioMed Res. Int. 2015:1–17. doi: 10.1155/2015/917086. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keumoe R., Nguembou M.S., Tsouh F.P.V., Donkeng D.V.F., Dize D., Tchokouaha Y.L.R., Jiatsa M.C.D., Youmsi F.R.D., Ngameni B., Fekam B.F. Antimycobacterial activity of medicinal plants against the causative agent of buruli ulcer: Mycobacterium ulcerans. International Journal of Mycobacteriology. 2016;5:S105. doi: 10.1016/j.ijmyco.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 91.Andreoli A., Mou F., Minyem J.C., Wantong F.G., Noumen D., Awah P.K., Pluschke G., Boock A.U., Bratschi M.W. Complete healing of a laboratory-confirmed buruli ulcer lesion after receiving only herbal household remedies. PLoS Neglected Trop. Dis. 2015;9(11):11. doi: 10.1371/journal.pntd.0004102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ackah R.Y., Amponsah I.K., Ekuadzi E., Baah M.K., Dickson R.A. Antimycobacterial effects of herbal preparations used in buruli ulcer endemic communities in Ghana. ResearchSquare. 2023;14 doi: 10.21203/rs.3.rs-2581383. [DOI] [Google Scholar]
- 93.Yemoa A., Gbenou J., Affolabi D., Moudachirou M., Bigot A., Anagonou S., Portaels F., Martin A., Quetin-Leclercq J. Beninese medicinal plants as a source of antimycobacterial agents: bioguided fractionation and in vitro activity of alkaloids isolated from Holarrhena floribunda used in traditional treatment of buruli ulcer. BioMed Res. Int. 2015 doi: 10.1155/2015/835767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zabo I.A., Tamasala N., Diakedika L. Phytochemical study of medicinal plants used against buruli ulcer by Ntandu people in Kongo Central, DRC. Tropical Plant Reseach. 2019;6(1):49–53. doi: 10.22271/tpr.2019.v6.i1.009. [DOI] [Google Scholar]
- 95.Idrissa A.Z., Ndombe T., Lusala D. Phytochemical study of medicinal plants used against buruli ulcer by Ntandu people in Kongo Central, DRC. Tropical Plant Research. 2019;6(1):49–53. doi: 10.22271/tpr.2019.v6.i1.009. [DOI] [Google Scholar]
- 96.Grabowska K., Buzdygan W., Galanty A., Wróbel-Biedrawa D., Sobolewska D., Podolak I. Phytochemistry Reviews. Phytochemistry Review. 2023. Current knowledge on genus Bassia All.: a comprehensive review on traditional use, phytochemistry, pharmacological activity, and nonmedical applications. [DOI] [Google Scholar]
- 97.Huang M., Lu J.J., Ding J. Natural products in cancer therapy: past, present and future. Natural Products and Bioprospecting. 2021;11(1):5–13. doi: 10.1007/s13659-020-00293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Makagni T.K., Gbekley E.H., Hoekou Y., Issaka M., Pissang P., Agbodeka K., Tchacondo T., Karou S.D., Batawila K. Ethnobotanical study of medicinal plants in the fight against buruli ulcer in the maritime region of Togo. European Scientific Journal ESJ. 2020;16(27):27. doi: 10.19044/esj.2020.v16n27p239. [DOI] [Google Scholar]
- 99.Bamberger D., Jantzer N., Leidner K., Arend J., Efferth T. Fighting mycobacterial infections by antibiotics, phytochemicals and vaccines. Microb. Infect. 2011;13(7):613–623. doi: 10.1016/j.micinf.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 100.Cheuka P.M., Mayoka G., Mutai P., Chibale K. The role of natural products in drug discovery and development against neglected tropical diseases. MoleculesDec. 2016;31:22. doi: 10.3390/molecules22010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nguta J.M., Appiah-Opong R., Nyarko A.K., Yeboah-Manu D., Addo P.G.A., Otchere I., Kissi-Twum A. Antimycobacterial and cytotoxic activity of selected medicinal plant extracts. J. Ethnopharmacol. 2016;182:10–15. doi: 10.1016/j.jep.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kwofie S.K., Dankwa B., Odame E.A., Agamah F.E., Doe L.P.A., Teye J., Agyapong O., Miller W.A., Mosi L., Wilson M.D. In silico screening of isocitrate lyase for novel anti-buruli ulcer natural products originating from Africa. Molecules. 2018;23(7):1550. doi: 10.3390/molecules23071550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oloya B., Namukobe J., Ssengooba W., Afayoa M., Byamukama R. Phytochemical screening, antimycobacterial activity and acute toxicity of crude extracts of selected medicinal plant species used locally in the treatment of tuberculosis in Uganda. Trop. Med. Health. 2022;50(1):50. doi: 10.1186/s41182-022-00406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amponsah I., Atchoglo P., Ackah R., Tsouh Fokou P., Aboagye S., Yeboah-Manu D., Appiah-Opong R., Mensah A. In vitro Anti-Mycobacterium ulcerans and cytotoxic activities of some selected medicinal plants and an indoloquinoline alkaloid. International Journal of Mycobacteriology. 2021;10(1):60–65. doi: 10.4103/ijmy.ijmy_243_20. [DOI] [PubMed] [Google Scholar]
- 105.Kpegba K., Kondo E.T., Simalou O., Togbenou K., Boyode P., Toundou O., Gbandjaba N.Y., Agbonon A., Gbeassor M. A significant antihypertensive effect of Holarrhena floribunda supported by an exploratory phytochemical study. Journal of Herbmed Pharmacology. 2018;7:160–167. [Google Scholar]
- 106.Atchogloa P.K., Amponsah K., Tsouh P.V., Harley B.K., Baahe M.K., Armah F.A., Adjei S. Anti-mycobacterium ulcerans activity and pharmacognostic standardisation of Pycnanthus angolensis (Welw) Warb. Scientific African. 2021;3 doi: 10.1016/j.sciaf.2021.e00935. [DOI] [Google Scholar]
- 107.Kwofie S.K., Anyimadu D.T., Aryee S., Asare B., Kokroko N., Owusu J.A., Afutu B., Agyapong O., Mosi L., Kyei-Baffour E., Enninful K.S., Agoni C., Wilson M.D. BuDb: a curated drug discovery database for buruli ulcer. Journal of Computational Biophysics and Chemistry. 2022;22(1):31–41. doi: 10.1142/S2737416523500011. [DOI] [Google Scholar]
- 108.Nichter M. Buruli Ulcer: Mycobacterium Ulcerans Disease. 2019. Social science contributions to BU focused health service research in West-Africa; pp. 249–272. [DOI] [PubMed] [Google Scholar]
- 109.Owusu A.Y., Adamba C. Household perceptions, treatment-seeking behaviors and health outcomes for buruli ulcer disease in a peri-urban district in Ghana. Adv. Appl. Sociol. 2012;2(3):179–186. doi: 10.4236/aasoci.2012.23024. [DOI] [Google Scholar]
- 110.Francois Portaels (Who) In: World Health Organization. Portaels Françoise., editor. Vol. 14. WHO; 2014. Laboratory diagnosis of buruli ulcer: a manual for health care providers; p. 4. [DOI] [Google Scholar]
- 111.Gyasi S.F., Awuah E., Larbi J.A. Associations of perceived risk factors for the development of buruli ulcer. Asian J. Biologic. Sci. 2011;4(6):483–497. doi: 10.3923/ajbs.2011.483.497. [DOI] [Google Scholar]
- 112.Trébissou Jonhson Noel D., Bla Kouakou B., Yapo Adou F., Yapi Houphouet F., Djaman Allico J., Biosciences U.F.R., Boigny F.H. Therapeutic survey on traditional treatment of Buruli ulcer in Côte d ’ Ivoire. J. Microbiol. Biotechnol. Res. 2014;4(2):52–56. Available online at: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.