Abstract
During anaerobic nitrate respiration Bacillus subtilis reduces nitrate via nitrite to ammonia. No denitrification products were observed. B. subtilis wild-type cells and a nitrate reductase mutant grew anaerobically with nitrite as an electron acceptor. Oxygen-sensitive dissimilatory nitrite reductase activity was demonstrated in cell extracts prepared from both strains with benzyl viologen as an electron donor and nitrite as an electron acceptor. The anaerobic expression of the discovered nitrite reductase activity was dependent on the regulatory system encoded by resDE. Mutation of the gene encoding the regulatory Fnr had no negative effect on dissimilatory nitrite reductase formation.
Changes in oxygen tension are a part of the environmental conditions in the habitat of the soil bacterium Bacillus subtilis. Oxygen is the major electron acceptor for aerobic growth. In the absence of oxygen B. subtilis develops alternative strategies for survival. Two general principles of anaerobic catabolism are known. Reducing equivalents (NADH and reduced flavin adenine dinucleotide) produced mainly by glycolysis and the Krebs cycle during the consumption of energy substrates like glucose are oxidized by membrane-localized electron transport chains. During these respiratory processes a transmembrane proton gradient is generated and used as the energy supply for ATP synthesis and transport of molecules through the cytoplasmic membrane. In the absence of external electron acceptors, the reducing equivalents are reoxidized by endogenous electron acceptors without proton gradient formation. This redox-balanced dismutation of the substrate is coupled directly on the substrate level to energy conservation.
The energetic yields of these fermentation processes are much lower than those of anaerobic respiration. However, anaerobic respiratory electron transport requires the presence and efficient utilization of alternative terminal electron acceptors (1). Recently, the utilization of nitrate as an alternative anaerobic electron acceptor was described for B. subtilis and genes for the respiratory nitrate reductase system (narGHJI) were cloned (2–4). A large variety of bacilli have been identified as respiring nitrate (10, 14). For Bacillus licheniformis, denitrification from nitrate via nitrite, NO, and N2O to N2 was shown (9). Bacillus macerans performs ammonification with the production of ammonia from nitrate (10). For the gram-positive model bacterium B. subtilis, anaerobic nitrate utilization has been demonstrated without elucidation of the biochemical fate of the reduced nitrate. Induction of anaerobic nitrate respiration in B. subtilis is dependent on the regulator Fnr (2). The currently known location of potential Fnr binding sites and the functional analysis of other anaerobically induced genes (6) indicate the limitation of Fnr function in B. subtilis to the regulation of nitrate respiration. Moreover, in contrast to Fnr function in enterobacteria, the B. subtilis fnr gene is under the control of a second regulatory component, the multifunctional ResDE system (6, 8, 13). Besides its recently discovered importance for anaerobic metabolism, the resDE locus is involved in the regulation of various other central cellular functions, such as sporulation, carbon source utilization, the phosphate regulon, heme A synthesis, and formation of the cytochrome bf complex (5, 13).
With this study we identified B. subtilis as an ammonifying bacterium converting nitrate via nitrite into ammonia. A dissimilatory nitrite reductase activity which required resDE but not fnr for its formation under anaerobic growth conditions was identified and measured.
Anaerobic conversion of nitrate via nitrite into ammonia by B. subtilis.
B. subtilis JH642 (pheA1 trpC2) (Bacillus Genetic Stock Center) and THB1 (pheA1 trpC2 ΔnarGH::Tet Tetr) (4) were grown anaerobically at 37°C on Luria-Bertani medium (7) supplemented with 20 mM K3PO4 (pH 7), 2 mM (NH4)2SO4, 1 mM l-glutamic acid, 1 mM l-tryptophan, 0.8 mM l-phenylalanine, 0.005% (wt/vol) ammonium iron(III) citrate, 1 mM glucose, and, when indicated, 10 mM nitrite or nitrate (4, 6). The bacteria were incubated in completely filled flasks with rubber stoppers and with shaking at 180 rpm in an incubation shaker to minimize aggregation of the bacteria. Inoculation was performed aerobically at a 1:100 ratio of aerobically grown overnight culture and prewarmed medium. Anaerobic conditions were achieved after a short time through consumption of residual oxygen by the inoculated bacteria (11). Nitrate and ammonia concentrations in the growth media were monitored with standard enzymatic test systems (Boehringer, Mannheim, Germany). The ammonia concentration of the employed medium was subtracted from the measured values. Nitrite concentrations were measured by the diazotation procedure (12).
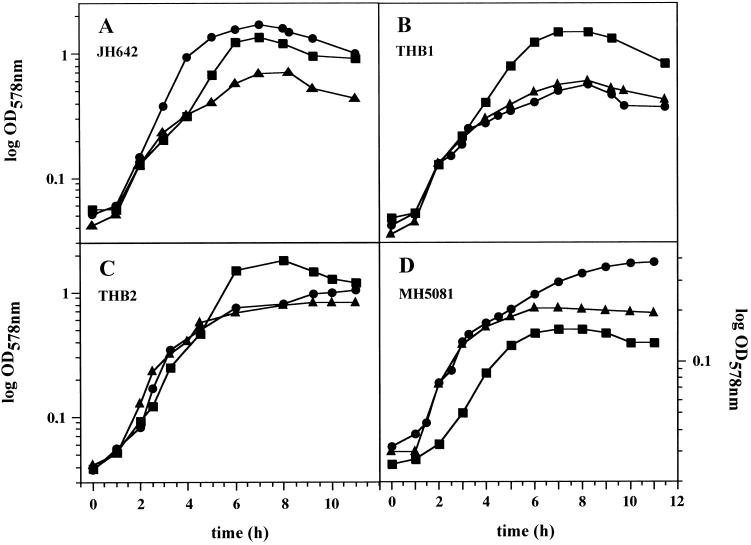
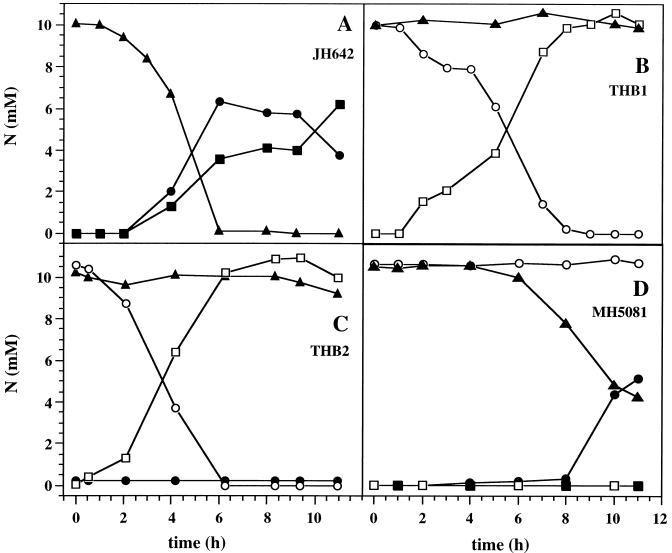
Under anaerobic growth conditions B. subtilis JH642 almost completely converted nitrate into nitrite over a period of 6 h until the end of the log phase (Fig. 1A and 2A). Immediately upon the appearance of nitrite as the product of nitrate reduction, its further conversion into ammonia started (Fig. 2A). Under the employed conditions, approximately 65% of the produced nitrite was converted into ammonia (Fig. 2A). Almost complete conversion of nitrite into ammonia was observed only for the nitrate reductase mutant THB1 (Fig. 2B) and for wild-type cells (data not shown) when nitrite was supplied as the electron acceptor. The gas phase of the B. subtilis growth chamber was carefully analyzed for gases resulting from potential denitrification processes. Growth experiments with denitrifying Pseudomonas aeruginosa (PAO1) and ammonifying Escherichia coli (K-12) served as controls. No significant amounts of N2O or N2 were detected in the gas phase of the B. subtilis and E. coli growth chambers, while the gas phase of the P. aeruginosa growth chamber contained substantial amounts of the various denitrification gases (data not shown). Clearly, B. subtilis should be regarded as an ammonifying facultative anaerobe.
FIG. 1.
Anaerobic growth of B. subtilis JH642 (wild type) (A), THB1 (nitrate reductase-deficient mutant) (B), THB2 (fnr mutant) (C), and MH5081 (resDE mutant) (D) with 10 mM nitrate (•) or 10 mM nitrite (▪) as a terminal electron acceptor and fermentation in the absence of external electron acceptors (▴). Growth was monitored by determination of the optical density at 578 nm (OD578nm) at the indicated time points. Values reported are the averages from at least 10 independent experiments performed in triplicate.
FIG. 2.
Changing concentrations of nitrate (▴), nitrite (• and ○), and ammonia (▪ and □) during anaerobic growth of B. subtilis JH642 (wild type) (A), THB1 (nitrate reductase-deficient mutant) (B), THB2 (fnr mutant) (C), and MH5081 (resDE mutant) (D). The concentrations given were determined at the indicated time points in the media of B. subtilis cultures grown with 10 mM nitrate (closed symbols) or 10 mM nitrite (opened symbols). Values reported are the averages from at least 10 independent experiments performed in triplicate.
In the absence of external terminal electron acceptors, fermentative growth of B. subtilis JH642 up to an optical density at 578 nm of 0.7 was observed (Fig. 1). The exact nature of the observed fermentation processes remains to be determined in future experiments.
B. subtilis contains a dissimilatory nitrite reductase.
To determine whether the conversion of nitrite into ammonia sustains anaerobic growth, B. subtilis JH642 was incubated anaerobically on medium containing nitrite. B. subtilis efficiently grew anaerobically with nitrite as an electron acceptor (Fig. 1A). To exclude any residual or side activity of the nitrate reducing system, the dissimilatory-nitrate-reductase-deficient mutant THB1 was incubated anaerobically with nitrite. As shown in Fig. 1B, THB1 grew efficiently with nitrite as an electron acceptor, demonstrating the presence of a dissimilatory nitrite reductase in B. subtilis. Almost all supplied nitrite was converted into ammonia by THB1 (Fig. 2B). In control experiments, the mutant THB1 failed to grow efficiently on nitrate as an electron acceptor (Fig. 1B) and consequently no nitrate conversion was detected (Fig. 2B). Observed residual growth in the absence of external terminal electron acceptors was on the level of fermentative growth (Fig. 1B).
An oxygen-sensitive benzyl viologen-dependent nitrite reductase activity is present in cell extracts prepared from B. subtilis.
To measure the newly discovered enzymatic activity directly in B. subtilis cell extracts, an in vitro test system had to be established. For this purpose, B. subtilis cultures grown anaerobically in the presence of 10 mM nitrate and nitrite were used at the end of the exponential growth phase. Harvesting, washing, and disruption of cells were performed under strictly anaerobic conditions. Cells were harvested and washed with 10 mM Tris (pH 7.4) containing 1 mg of MgCl2·6H2O per ml. Cells were anaerobically disrupted with a French press (20,000 lb/in2). To obtain cell extracts, the suspensions were filtered through a 0.2-μm-pore-size sterile filter. Dissimilatory nitrite reductase activity was measured spectroscopically by the nitrite-dependent oxidation of reduced benzyl viologen in anoxic cuvettes containing 50 mM Tris (pH 7.4), 2 mM benzyl viologen reduced with 0.2 μM sodium dithionite, 1 mM KNO2, and 5 to 50 μl of cell extract (containing 2 mg of protein per ml) at room temperature. Reaction mixtures were incubated in the absence of external electron acceptors to determine the endogenous rate of benzyl viologen oxidation. No obvious background activities were observed. Reactions were started by the addition of nitrite. Enzymatic oxidation of benzyl viologen was monitored spectroscopically at a wavelength of 578 nm. The extinction coefficient of benzyl viologen was used to determine the amount of reduced nitrite. One unit of activity corresponded to the reduction of 1 μmol of nitrite per min. Important for successful enzyme activity measurements was the strictly anaerobic handling during extract preparation. The nitrite reductase activity of approximately 5.6 U/mg of protein in cell extracts prepared from B. subtilis THB1 (Table 1) was reduced to approximately 2.9 U/mg of protein after 15 min of aerobic treatment and further to approximately 0.7 U/mg of protein after 90 min of exposure to aerobic conditions.
TABLE 1.
Enzymatic determination of benzyl viologen-dependent nitrate and nitrite reductase activities
| B. subtilis strain | Avg activity ± SD (10−3 U/mg of protein)a
|
|
|---|---|---|
| Nitrate reductase | Nitrite reductase | |
| JH642 (wild type) | 330 ± 10 | 6,100 ± 220 |
| THB1 (narG mutant) | ND | 5,600 ± 280 |
| THB2 (fnr mutant) | ND | 9,900 ± 310 |
| MH5081 (resDE mutant) | 50 ± 10 | ND |
Benzyl viologen-dependent dissimilatory activities determined in vitro with cell extracts prepared from the B. subtilis strains after anaerobic growth on nitrate- and nitrite-containing medium. One unit of activity corresponded to the reduction of 1 μmol of nitrite or nitrate per min. See the text for detailed assay conditions. Values reported are the averages from at least three independent experiments performed in triplicate. ND, not detectable.
For all analyzed B. subtilis strains respiratory nitrate reductase activities were also measured. Since this enzyme activity was much less oxygen sensitive, cell extracts were prepared aerobically. However, no obvious benzyl viologen-dependent nitrite reductase activity was measured under these conditions. Strictly anaerobic handling of the protein fractions did not significantly increase nitrate reductase activities (data not shown). Cells were aerobically harvested, washed, disrupted with a French press (20,000 lb/in2), and subsequently centrifuged to give cell extracts. Respiratory nitrate reductase activity was measured spectroscopically by the nitrate-dependent oxidation of reduced benzyl viologen in anoxic cuvettes containing 50 mM Tris (pH 7.4), 2 mM benzyl viologen reduced with 0.2 μM sodium dithionite, 1 mM KNO3, and 100 to 200 μl of cell extract (containing 2 mg of protein per ml) at room temperature (10). Reactions were started by the addition of nitrate. Enzymatic oxidation of benzyl viologen was monitored spectroscopically at a wavelength of 578 nm. The extinction coefficient of benzyl viologen was used to determine the amount of reduced nitrate. One unit of activity corresponded to the reduction of 1 μmol of nitrate per min.
As shown in Table 1, wild-type B. subtilis (JH642) contained both dissimilatory nitrite and nitrate reductase activities. Control reactions with boiled enzyme fraction and without the addition of nitrite or nitrate clearly demonstrated the enzymatic nature of the observed reaction and its electron acceptor dependence (data not shown). The electron acceptor specificity of the used nitrite reductase assay was tested in a nitrate reductase-free background with extracts prepared from the nitrate reductase-deficient mutant THB1. THB1 contained only nitrite reductase activity (Table 1). These results demonstrated the presence of a dissimilatory nitrite reductase activity in B. subtilis.
The regulatory system resDE but not fnr is required for induction of dissimilatory nitrite reductase activity.
The two-component system encoded by resDE is responsible for induction of respiratory nitrate reductase partially via transcriptional activation of fnr (8, 13). To determine the influence of resDE and fnr on dissimilatory nitrite reductase formation, the resDE mutant MH5081 (pheA1 trpC2 resDE::pRC22 Tetr) (13) and the fnr mutant THB2 (pheA1 trpC2 fnr::spec Specr) were incubated anaerobically in the presence of nitrite.
The overall anaerobic growth of the resDE mutant in the absence of external electron acceptors was already reduced compared to that of wild-type B. subtilis, indicating a potential role of resDE in the regulation of fermentative processes (Fig. 1D). Moreover, the pleiotropic phenotype of the resDE mutation might contribute to the observed reduced growth. No increase of anaerobic growth with nitrite as the electron acceptor was detected, while a basal level even below normal fermentative growth remained (Fig. 1D). Moreover, the nitrite concentration in the growth media remained unchanged over the whole incubation period of 13 h (Fig. 2D). In vitro dissimilatory nitrite reductase activity tests (Table 1) using cell extract prepared from the resDE mutant failed to detect significant enzyme activity. These results demonstrated the dependence of nitrite reductase activity formation on resDE.
In control experiments the mutant MH5081 was incubated with nitrate (Fig. 1D and 2D). From the previously published experiments concerning resDE-dependent narGHJI expression, a result similar to that of the growth experiment using nitrite was expected (8, 13). Interestingly, the resDE mutant MH5081 started to grow on nitrate after 6 h and at the same time conversion of nitrate into nitrite and in vitro nitrate reductase activity were observed (Fig. 1D and 2D; Table 1). In agreement with results of nitrite growth experiments described above, no further conversion of the produced nitrite into ammonia was observed, since the respiratory nitrite reductase was still not activated. Since the previous investigations concerning the resDE influence on respiratory nitrate reductase formation were ended 5 h after inoculation, the authors might have missed this late low-level expression of the enzyme (8).
The fnr mutant of B. subtilis JH642, THB2, was constructed as described before (2). Anaerobic growth of the fnr mutant with nitrite as an electron acceptor was slightly better than growth of the wild-type strain (Fig. 1C). Consequently, efficient nitrite conversion by the fnr mutant (Fig. 2C) and an increased respiratory nitrite reductase activity in cell extracts prepared from the fnr mutant were observed (Table 1). In agreement with results of previous investigations (2), fnr mutation totally abolished respiratory growth using nitrate as an electron acceptor (Fig. 1C). No nitrate reductase activity was measured in cell extracts of the fnr mutant (Table 1). These experiments exclude a stimulatory role of fnr on dissimilatory nitrite reductase expression as observed for the narGHJI genes encoding nitrate reductase.
Acknowledgments
We thank F. M. Hulett (University of Illinois at Chicago) for the gift of the B. subtilis resDE mutant and P. Glaser (Institut Pasteur, Paris, France) for the gift of plasmids helpful for the construction of the fnr mutant. We are indebted to I. Arth, P. Frenzel, R. Conrad (Max-Planck-Institut, Marburg, Germany) for the analyses of the gas phases of the cultures for denitrification intermediates. We thank R. K. Thauer and his group (Max-Planck-Institut, Marburg, Germany) for continous support, helpful discussions, and technical advice.
This work was supported by grants from the Sonderforschungsbereich 395 of Deutsche Forschungsgemeinschaft, the Max-Planck-Gesellschaft, the Institut für Organische Chemie und Biochemie of the University of Freiburg, and the Fonds der Chemischen Industrie.
Footnotes
Corresponding author. Mailing address: Institut für Organische Chemie und Biochemie, Albert-Ludwigs-Universität Freiburg, Albertstr. 21, 79104 Freiburg, Germany. Phone: 49 (0)761-2036060. Fax: 49 (0)761-2036096. E-mail: jahndiet@ruf.uni-freiburg.de.
REFERENCES
- 1.Cole J. Nitrate reduction to ammonia by enteric bacteria: redundancy, or a strategy for survival during oxygen starvation? FEMS Microbiol Lett. 1996;136:1–11. doi: 10.1111/j.1574-6968.1996.tb08017.x. [DOI] [PubMed] [Google Scholar]
- 2.Cruz Ramos H, Boursier L, Mozer I, Kunst F, Danchin A, Glaser P. Anaerobic transcription activation in Bacillus subtilis: identification of destinct FNR-dependent and -independent regulatory mechanisms. EMBO J. 1995;14:5984–5994. doi: 10.1002/j.1460-2075.1995.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glaser P, Danchin A, Kunst F, Zuber P, Nakano M M. Identification and isolation of a gene required for nitrate assimilation and anaerobic growth of Bacillus subtilis. J Bacteriol. 1995;177:1112–1115. doi: 10.1128/jb.177.4.1112-1115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann T, Troup B, Szabo A, Hungerer C, Jahn D. The anaerobic life of Bacillus subtilis. Cloning and characterization of the genes encoding the respiratory nitrate reductase system. FEMS Microbiol Lett. 1995;131:219–225. doi: 10.1111/j.1574-6968.1995.tb07780.x. [DOI] [PubMed] [Google Scholar]
- 5.Hulett F M. The signal-transduction network for Pho regulation in Bacillus subtilis. Mol Microbiol. 1996;19:933–939. doi: 10.1046/j.1365-2958.1996.421953.x. [DOI] [PubMed] [Google Scholar]
- 6.LaCelle M, Kumano M, Kurita K, Yamane K, Zuber P, Nakano M M. Oxygen-controlled regulation of the flavohemoglobin gene in Bacillus subtilis. J Bacteriol. 1996;178:3803–3808. doi: 10.1128/jb.178.13.3803-3808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 8.Nakano M M, Zuber P, Glaser P, Danchin A, Hulett F M. Two-component regulatory proteins ResD-ResE are required for transcriptional activation of fnr upon oxygen limitation in Bacillus subtilis. J Bacteriol. 1996;178:3796–3802. doi: 10.1128/jb.178.13.3796-3802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pichinoty F, Garcia J-L, Job C, Dierand M. La denitrification chez Bacillus licheniformis. Can J Microbiol. 1978;24:45–49. doi: 10.1139/m78-008. [DOI] [PubMed] [Google Scholar]
- 10.Schirawski J, Unden G. Anaerobic respiration of Bacillus macerans with fumarate, TMAO, nitrate and nitrite and regulation of the pathways by oxygen and nitrate. Arch Microbiol. 1995;163:148–154. [Google Scholar]
- 11.Schulp J A, Stouthamer A H. The influence of oxygen, glucose and nitrate upon the formation of nitrate reductase and the respiratory system in Bacillus licheniformis. J Gen Microbiol. 1970;64:195–203. doi: 10.1099/00221287-64-2-195. [DOI] [PubMed] [Google Scholar]
- 12.Snell F D, Snell C T. Colorimetric methods of analysis 3. New York, N.Y: Van Nostrand; 1949. pp. 804–805. [Google Scholar]
- 13.Sun G, Sharkova E, Chesnuth R, Birkey S, Duggan M F, Sorokin A, Pujic P, Ehrlich S D, Hulett F M. Regulators of aerobic and anaerobic respiration in Bacillus subtilis. J Bacteriol. 1996;178:1374–1385. doi: 10.1128/jb.178.5.1374-1385.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zumft W G. The denitrifying prokaryotes. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. A handbook on the biology of bacteria, ecophysiology, isolation, identification, application. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 554–582. [Google Scholar]