Summary
Background
The increasing incidence of syphilis and the limitations of first-line treatment with penicillin, particularly in neurosyphilis, neonatal syphilis, and pregnancy, highlight the need to expand the therapeutic repertoire for effective management of this disease. We assessed the in-vitro efficacy of 18 antibiotics from several classes on Treponema pallidum subspecies pallidum (T pallidum), the syphilis bacteria.
Methods
Using the in-vitro culture system for T pallidum, we exposed the pathogen to a concentration range of each tested antibiotic. After a 7-day incubation, the treponemal burden was evaluated by quantitative PCR targeting the T pallidum tp0574 gene. The primary outcome was the minimum inhibitory concentration (MIC) at which the quantitative PCR values were not significantly higher than the inoculum wells. We also investigated the susceptibility of macrolide-resistant strains to high concentrations of azithromycin, and the possibility of developing resistance to linezolid, a proposed candidate for syphilis treatment.
Findings
Amoxicillin, ceftriaxone, several oral cephalosporins, tedizolid, and dalbavancin exhibited anti-treponemal activity at concentrations achievable in human plasma following regular dosing regimens. The experiments revealed a MIC for amoxicillin at 0·02 mg/L, ceftriaxone at 0·0025 mg/L, cephalexin at 0·25 mg/L, cefetamet and cefixime at 0·0313 mg/L, cefuroxime at 0·0156 mg/L, tedizolid at 0·0625 mg/L, spectinomycin at 0·1 mg/L, and dalbavancin at 0·125 mg/L. The MIC for zoliflodacin and balofloxacin was 2 mg/L. Ertapenem, isoniazid, pyrazinamide, and metronidazole had either a poor or no effect. Azithromycin concentrations up to 2 mg/L (64 times the MIC) were ineffective against strains carrying mutations associated to macrolide resistance. Exposure to subtherapeutic doses of linezolid for 10 weeks did not induce phenotypic or genotypic resistance.
Interpretation
Cephalosporins and oxazolidinones are potential candidates for expanding the current therapeutic repertoire for syphilis. Our findings warrant testing efficacy in animal models and, if successful, clinical assessment of efficacy.
Funding
European Research Council.
Introduction
According to WHO, the global burden of syphilis ranges between 18 million and 36 million cases, with an incidence of 5·6–11·0 million new infections per year in adults.1, 2, 3 Although most of these cases occur in low-income and middle-income countries, there has also been a steady resurgence of syphilis over the past two decades in high-income countries (unpublished).4, 5, 6 Penicillin is the preferred treatment for syphilis;7 however, neurosyphilis requires intravenous infusions or injections every 4 h for up to 14 days, neonatal syphilis also requires intravenous infusions every 8 h or 12 h for 10 days,8 and no treatment options exist for pregnant women allergic to penicillin in whom doxycycline is contraindicated because of teratogenicity and who are at risk for congenital transmission. Furthermore, there are temporal shortages in production,9 and rural areas often do not have specialised personnel to administer penicillin intramuscular injections and properly store the drug.10 The aforementioned scenario offers a compelling argument for research endeavours to broaden the therapeutic options for syphilis (unpublished).
A major barrier for testing alternative antibiotics for syphilis was the inability to culture the causative agent of the disease, Treponema pallidum subspecies pallidum (T pallidum). In a recent breakthrough in June, 2018, however, continuous long-term culture of T pallidum was achieved by cocultivation with rabbit epithelial cells in a microaerophilic atmosphere.11 As a result, it is now possible to test T pallidum susceptibility to antimicrobial agents to determine the minimum inhibitory concentrations (MICs) associated with each compound. Using this system, we had previously shown that treponemal growth was inhibited by penicillin G at concentrations of 0·003 mg/L or more and by linezolid concentrations of 0·5 mg/L or more.12 The same study showed no anti-treponemal activity for moxifloxacin (up to 2 mg/L), and clofazimine (up to 2 mg/L). Additionally, a study based on the same cultivation system showed a MIC of 0·1 mg/L for doxycycline,13 and a separate publication reported low MIC values for four penicillin derivatives and four cephalosporins.14 Overall, there is a large knowledge gap concerning the antibacterial activity of a wider range of drug classes in wild-type strains of T pallidum.
Research in context.
Evidence before this study
On Sept 1, 2022, before submitting our study, we searched the PubMed database for articles published from inception to Sept 1, 2022, reporting antibiotics with in-vitro anti-treponemal activity. Our search using the key terms “syphilis”, OR “Treponema pallidum”, AND “susceptibility testing” with no language restrictions, retrieved 19 publications. Most of these articles focused on azithromycin resistance-conferring mutations. However, two articles had used a tissue-culture system established in 2018, which facilitated long-term multiplication of Treponema pallidum subspecies pallidum (T pallidum) for studying its drug susceptibility profile. Our own study, done by our team, confirmed the anti-treponemal activity of penicillin and linezolid as evidenced by the minimum inhibitory concentration (MIC) value estimate, whereas moxifloxacin and clofazimine did not exhibit activity. Another group of researchers also reported the anti-treponemal activity of doxycycline. Concurrently, while our manuscript was undergoing peer review, a separate publication in June, 2023 screened 100 β-lactams and reported the MIC values for four penicillin derivatives and four cephalosporins, indicating their potential for treating syphilis.
Added value of this study
This study holds important value by providing drug-susceptibility measurements for all commonly used classes of antibiotics, distinguishing it from previous studies that either focused on a limited number of compounds or screened numerous compounds from a single antibiotic family, specifically β-lactams. Notably, all four studies, including ours, used similar culture methods with slight variations in outcome measurement tools. Our results regarding β-lactams demonstrate a promising alignment and replicability with the findings of the other research group, as both sets of data exhibit MIC values in the same order of magnitude. Moreover, our study prioritised readily available commercialised compounds that have potential for quick translation into clinical practice if positive results are obtained in clinical trials. Additionally, we considered relevant pharmacokinetic and pharmacodynamic aspects to aid in the interpretation of MIC results and provide a more comprehensive understanding of the efficacy of antibiotics' efficacy, dosing regimen, and potential treatment outcomes. We identified compounds that exhibited anti-treponemal activity in vitro at achievable concentrations in human plasma, such as amoxicillin, ceftriaxone, oral cephalosporins, tedizolid, and dalbavancin. In addition, we have done long-term propagation of T pallidum in subtherapeutic concentrations of linezolid, previously shown to be active, and we have seen no evidence of selection of phenotypic or genotypic resistance.
Implications of all the available evidence
The cumulative experience from various studies, including our own, offers a valuable new approach for a deeper understanding of T pallidum drug susceptibility and has important implications for the field. The results of our study show the clinical potential of several approved drugs, including β-lactams and oxazolidinones, that could be repurposed for treating syphilis and other treponemal infections.
In this study, we tested a broad range of antibiotics belonging to the classes of aminopenicillins, cephalosporins, carbapenems, fluoroquinolones, oxazolidinones, lipoglycopeptides, aminoglycosides, macrolides, antimycobacterials, antiparasitics, and spiropyrimidinetrione against T pallidum. Furthermore, given that previous work showed the efficacy of linezolid for syphilis treatment,12 we propagated T pallidum in subtherapeutic concentrations of linezolid for 10 weeks to assess the potential for selection of a resistant strain.
Methods
Study design and T pallidum strains
For this in-vitro study, we used three T pallidum strains (SS14, UW330B, and Chicago C) for plate inoculation to perform antibiotic testing. The SS14 strain was used to test all antimicrobials, whereas UW330B and Chicago C were used to test azithromycin only.
We aimed to test at least one US Food and Drug Administration-approved drug from each class and subclass of antibiotics. Two prioritisation criteria were applied to selecting the antibiotics for testing: first, pharmacological properties that would make an antibiotic suitable for repurposing to treat syphilis; and second, antibiotics used for other common conditions, regardless of their pharmacological properties, to gain a better understanding of their potential effects on syphilis.
The detailed origin of the T pallidum strains used in this study (appendix p 2) and the antibiotic selection process (appendix p 2) have been provided.
In-vitro cultivation of T pallidum
Procedures for the in-vitro cultivation of T pallidum were done as described previously.11 Briefly, two sets of cultures were prepared, one for the susceptibility assay and one for the bactericidal and recovery assay. The susceptibility assay involved testing drug concentration in 96-well plates (8 × 12 format; Corning, NY, USA). Each drug concentration was treated as a separate experimental group and tested eight times in eight replicate wells. Five control groups without antibiotics were included, each tested in eight replicate wells. Four control groups were harvested at different times (at day 0, day 1, day 4, and day 7) after inoculation, and one group containing the antibiotic solvent (dimethyl sulfoxide or water) instead of the test drug was harvested on day 7.
Wells were seeded with 3 × 103 rabbit Sf1Ep cells in 150 μL minimum essential media and incubated overnight. The next day, minimum essential media was removed and 150 μL T pallidum culture media 2 (TpCM2; equilibrated overnight in a 34°C trigas incubator) was added for 3 h to acclimate cells to low oxygen. After removing TpCM2, 148·5 μL of a 3·3 × 105 T pallidum cells per mL inoculum was added to each well (5 × 104 T pallidum cells per well). Antibiotic solutions (1·5 μL) were added from 100-times concentrated stocks without altering volume followed by incubation at 34°C in a trigas incubator until harvest. The tested concentration range for each drug is reported (table) along with the key microbiological and pharmacokinetic values of tested antibiotics and the standard of care benzathine penicillin G. Experimental wells with varying antibiotic concentrations were harvested after a week for DNA quantification. Control wells without antibiotics were harvested at 1 day, 4 days, and 7 days, while a control with solvent alone was harvested after 1 week. Treponemal burden was assessed using quantitative PCR targeting the T pallidum-specific tp0574 gene (sensitivity of about ten treponemal genomes per reaction).13
Table.
MIC and literature plasma concentration values
| Range tested (mg/L) |
Primary MIC |
Secondary MIC | MBC (mg/L) |
Drug plasma concentrations* |
|||
|---|---|---|---|---|---|---|---|
| Primary MIC (mg/L)† | Secondary MIC (mg/L)† | Cmin (mg/L) | Dose administered for the Cmin calculation | Unbound fraction | |||
| Natural penicillins | |||||||
| Benzathine penicillin G | 0·0001–0·06‡ | Not tested in this study | 0·003‡ | 0·003‡ | 0·012§ | 1·2 million units single dose, IM | 0·55–0·72 |
| Aminopenicillins | |||||||
| Amoxicillin | 0·0025–0·16 | 0·02 | 0·01 | 0·01 | >0·2¶ | 500 mg single dose, PO | 0·83 |
| Cephalosporins | |||||||
| Ceftriaxone | 0·00063–1 | 0·0025 | 0·0025 | 0·0025 | 29·7 | 1000 mg/24 h, IM | 0·50 |
| Cephalexin | 0·0625–8 | 0·25 | 0·25 | 0·25 | 0·30¶ | 1000 mg single dose, PO | 0·85–0·90 |
| Cefetamet | 0·0039–0·25 | 0·0313 | 0·0625 | 0·0625 | >0·3 | 500 mg/12 h, PO | 0·78 |
| Cefuroxime | 0·0039–0·25 | 0·0156 | 0·0156 | 0·0156 | 0·20¶ | 250 mg single dose, PO | 0·50 |
| Cefixime | 0·0039–0·25 | 0·0313 | 0·0313 | 0·0313 | 0·08 | 400 mg/24 h, PO | 0·34 |
| Carbapenems | |||||||
| Ertapenem | 0·00375–2 | >2 | >2 | >2 | 0·8 | 1 g/24 h, IV | 0·05 |
| Tetracyclines | |||||||
| Doxycycline | 0·004–2·5‖ | 0·1‖ | Not determined in this study | 0·1‖ | >1 | 100 mg/24 h, PO | 0·07–0·18 |
| Fluoroquinolones | |||||||
| Moxifloxacin | 0·06–2‡ | Not determined in this study | 2‡ | >2‡ | 0·4–0·6 | 400 mg/24 h, PO | 0·50 |
| Balofloxacin | 0·25–16 | 2 | 2 | >2 | 0·23 | 100 mg/12 h, PO | .. |
| Macrolides | |||||||
| Azithromycin | 0·0313–2 | <0·0313** | 0·125** | <0·0313** | 0·05 | 250 mg/24 h, PO | 0·5–0·9 |
| Oxazolidinones | |||||||
| Linezolid | 0·0156–2 | 0·5 | 0·125 | 0·125 | 6·2 | 600 mg/12 h, PO | 0·69 |
| Tedizolid | 0·0078–0·5 | 0·0625 | 0·313 | 0·0156–0·0313 | 0·41 | 200 mg/24 h, PO | 0·10–0·30 |
| Lipoglycopeptides | |||||||
| Dalbavancin | 0·0039–0·25 | 0·125 | 0·125 | 0·125 | 19·5†† | 1500 mg single dose, IV | 0·07 |
| Aminoglycosides | |||||||
| Spectinomycin | 0·02–2 | 0·1 | 0·1 | 0·25 | 15¶ | 2000 mg single dose, IM | .. |
| Antimycobacterials | |||||||
| Isoniazid | 0·0078–0·5 | >0·5 | >0·5 | >0·5 | Undetectable | 300 mg/24 h, PO | .. |
| Pyrazinamide | 1·0–64 | >64 | >64 | >64 | 7 | 1500 mg/24 h, PO | .. |
| Clofazimine | 0·06–2‡ | Not determined in this study | 1‡ | 1‡ | 0·02§§ | 200 mg single dose, PO | .. |
| Antiparasitics | |||||||
| Ivermectin | 0·125–40 | MIC threshold unattained‡‡ | MIC threshold unattained‡‡ | MIC threshold unattained‡‡ | 0·01§§ | 12 mg single dose, PO | .. |
| Nitroimidazoles | |||||||
| Metronidazole | 0·0313–2 | >2 | >2 | >2 | 11·8 | 500 mg/8 h, PO | 0·8 |
| Spiropyrimidinetrione | |||||||
| Zoliflodacin | 0·250–4 | 2 | 1 | 2 | 1§§ | 3000 mg single dose, PO | .. |
Cmin=minimum blood plasma concentration. IM=injection into a muscle. IV=injection into a vein. MBC=minimum bactericidal concentration. MIC=minimum inhibitory concentration. PO=oral administration.
The appendix (p 7) provides the literature sources used as a reference for pharmacokinetic information.
The primary MIC was defined as the lowest antibiotic dilution at which the tp0574 qPCR values were not significantly higher than the inoculum wells (day 0 control group), as previously defined by Edmondson and colleagues.13 A secondary MIC was defined as the lowest antibiotic dilution at which the tp0574 qPCR values were significantly lower than the positive control wells (day 7 control group), which more closely follows the broth dilution procedure.
Haynes and colleagues.12
22 days after single dose administration.
8 h after administration.
Edmondson and colleagues.13
Only for susceptible strains.
168 h after administration.
Because of ivermectin toxicity to Sf1Ep cells.
24 h after administration.
A second set of plates was prepared for the bactericidal and recovery assay. Treponemes exposed to the drug concentration were subcultured into antibiotic-free recovery plates. These plates were incubated for 7 more days before DNA extraction.
In addition to the regular controls for T pallidum cultures, we assessed whether the tested antimicrobials showed toxicity on rabbit Sf1Ep cells (cocultured with T pallidum), which are essential for the adequate survival and growth of spirochetes in vitro. A detailed description of our experiments on cell culture, harvest, and DNA extraction and quantification, and cytotoxicity assays is provided (appendix pp 2–4).
T pallidum incubation with a subtherapeutic concentration of linezolid
To investigate whether prolonged exposure to a subtherapeutic concentration of linezolid could select for a less susceptible or resistant T pallidum strain, or induce genetic changes associated with linezolid resistance, the SS14 strain was grown for an extended duration in six-well culture plates with and without linezolid added to the cell culture media. Preliminary studies12 showed that linezolid was effective at limiting treponemal growth at a concentration of 0·5 mg/L or higher. In this experiment, TpCM2 media containing a concentration of 0·2 mg/L of linezolid was used for 2 weeks of propagation to exert antibiotic pressure. The rationale and calculations supporting the selected period of antibiotic pressure (appendix p 4–5) and the protocol used for whole-genome sequencing to identify linezolid resistance mutations (appendix p 5) have been provided.
Outcomes
The primary MIC for each experiment was defined as the lowest antibiotic dilution at which the tp0574 quantitative PCR values were not significantly higher than the inoculum wells (day 0, control group), as previously defined by Edmondson and colleagues.15 Additionally, we analysed a secondary MIC, which was defined as the lowest antibiotic dilution at which the tp0574 quantitative PCR values were significantly lower than the positive control (day 7, control group). The secondary MIC definition was more similar to the broth dilution procedure and was used previously by Haynes and colleagues.12 The minimum bactericidal concentration (MBC) was defined as the lowest concentration at which there was no bacterial growth after subculturing into the antibiotic-free media.
The sample size consisted of eight replicates per drug concentration and control group. Each sample represented a technical replicate from the same source mixture. Rigorous experimental conditions minimised substantial variations among samples.
Statistical analysis
The statistical analyses that we did for this study are reported in detail (appendix p 6). We used the Kruskal–Wallis mean-rank test to compare the distribution of quantitative PCR values among independent groups with different antibiotic concentrations and control groups. Dunn's test was used for pairwise comparisons between specific antibiotic groups and control groups at day 0 or day 7. A false discovery rate Benjamin–Hochberg correction (p<0·05) was applied for multiple comparisons. In the cytotoxicity experiment, absorbance was compared between the antibiotic and control groups, with the median blank value of media-containing wells subtracted from the experimental readings.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit the Article for publication.
Results
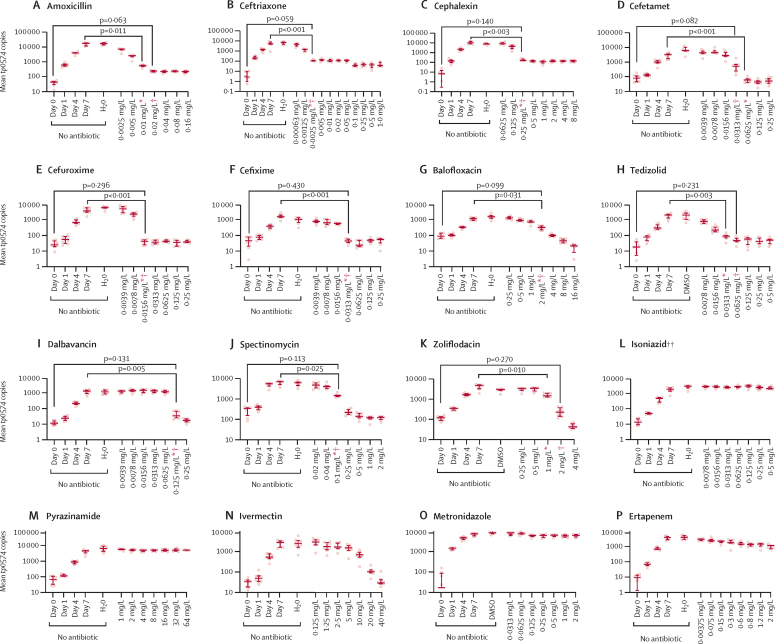
Treponemal growth in the absence of antimicrobial agents progressed as expected (ie, a consistent increase in the number of tp0574 copies detected, ranging from 1·5 to 3·0 logarithmic units increase (figure 1A–K). Overall, no differences were seen when comparing treponemal growth in the antibiotic-free control group wells at day 7 to the H2O and dimethyl sulfoxide wells, despite some variability in yield. All p values for Dunn's test were higher than 0·13, except for isoniazid (p=0·032).
Figure 1.
Treponema pallidum susceptibility to antimicrobials
Non-antibiotic control wells represent treponemal growth in the absence of antibiotic from day 0 (inoculum) to day 7 after plate inoculation. DMSO and H2O bars are relative to Sf1Ep cell cultures to which the compound solvent was added instead of the tested antibiotic. In the bar chart, the middle line represents the median tp0574 gene copies per unit of volume from eight biological replicates, the length of the bar represents the IQR, and the dots represent the individual values. *Secondary MIC: p values (for the Dunn's test) are provided for the comparison between the lowest antibiotic dilution at which the tp0574 qPCR values were significantly lower than the positive control (day 7 control group). †Primary MIC: p values (for the Dunn's test) are provided for the comparison between the lowest antibiotic dilution at which the tp0574 qPCR values were not significantly higher than the inoculum wells (day 0 control group). DMSO=dimethyl sulfoxide. MIC=minimum inhibitory concentration. ‡Isoniazid, pyrazinamide, metronidazole, and ertapenem do not have p values because the MIC was unattained (ie, the MIC value is higher than the highest concentration tested). In the case of ivermectin, we showed that the reduction in the growth of T pallidum at a concentration of 10 mg/L or higher actually reflects toxicity to Sf1Ep cells and therefore cannot be considered an MIC.
Several of the antimicrobials tested in this study showed a primary MIC for T pallidum at concentrations achievable in human plasma (table), including amoxicillin (0·02 mg/L), ceftriaxone (0·0025 mg/L), cephalexin (0·25 mg/L), cefetamet and cefixime (0·0313 mg/L), cefuroxime (0·0156 mg/L), tedizolid (0·0625 mg/L), spectinomycin (0·1 mg/L), and dalbavancin (0·125 mg/L; figure 1 A–F, H, I, J). The MIC values of these antibiotics were of the same order or lower than concentrations achieved in humans after the administration of standard dose regimens (table 1). Balofloxacin and zoliflodacin presented inhibitory activity at 2 mg/L (figure 1G, K). Isoniazid, pyrazinamide, and metronidazole had no effect on T pallidum viability at the tested concentrations (figure 1L, M, O), whereas ertapenem reduced treponemal growth at concentrations higher than 0·3 mg/L compared to the day 7 control group, but none of the concentrations up to 2 mg/L met the criteria for the primary MIC (figure 1P). Ivermectin (figure 1N) only had apparent efficacy against T pallidum, but a steadily declining metabolic activity of Sf1Ep cells noted upon exposure to ivermectin (appendix p 8) supported that this effect was mostly caused by toxicity exerted by this antiparasitic on the cells supporting T pallidum growth, rather than on the pathogen itself. In analyses to establish the secondary MIC, which corresponds to the minimum antibiotic dilution at which the tp0574 quantitative PCR values were significantly lower than the day 7 control wells, the cutoff concentration values either remained the same as the primary MIC or were slightly lower, except for cefetamet (figure 1A–K; table).
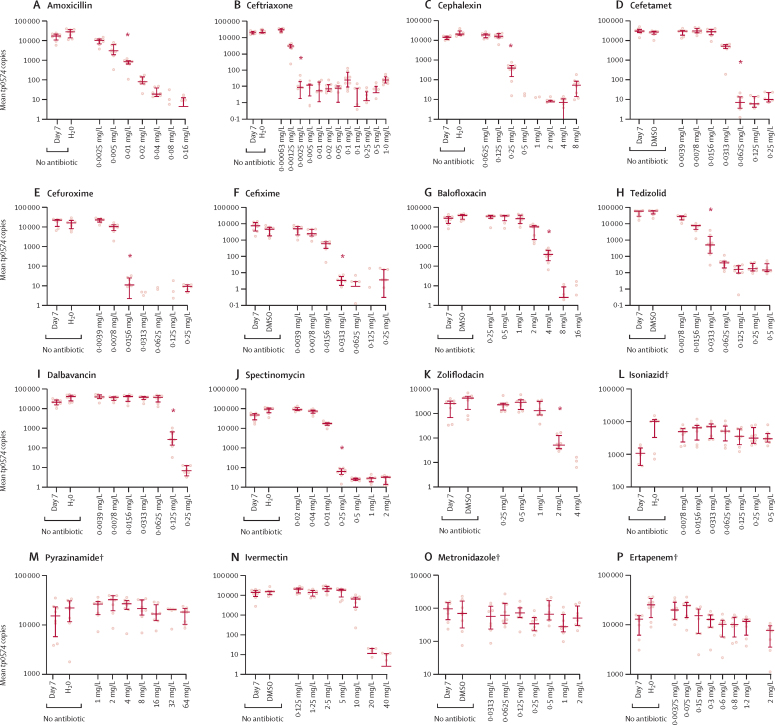
The results of the abovementioned compounds were further supported by their MBC, manifested as the absence of growth when treponemes exposed to these antibiotics were subcultured without the antibiotics (figure 2). Subculturing results demonstrated low MBC values for amoxicillin (0·01 mg/L), ceftriaxone (0·0025 mg/L), cephalexin (0·25 mg/L), cefetamet (0·0625 mg/L), cefuroxime (0·0156 mg/L), cefixime and tedizolid (0·0313 mg/L), dalbavancin (0·125 mg/L), spectinomycin (0·25 mg/L; figure 2 A–F, H–J). The MBC for zoliflodacin was 2 mg/L, and for balofloxacin was 4 mg/L (figure 2G, K). The absence of effect was confirmed for isoniazid, metronidazole, pyrazinamide, and ertapenem (figure 2L, M, O, P).
Figure 2.
Recovery assays following antibiotic removal
In the bar chart, the middle line represents median tp0574 gene copies per unit of volume from eight biological replicates, the length of the bar represents the IQR, and the dots represent the individual values. DMSO=dimethyl sulfoxide. MBC=minimum bactericidal concentration. MIC=minimum inhibitory concentration. *MBC. †Isoniazid, pyrazinamide, metronidazole, and ertapenem do not have p values because the MIC was unattained (ie, the MIC value was higher than the highest concentration tested). In the case of ivermectin, we showed that the reduction in the growth of T pallidum at a concentration of 10 mg/L or higher actually reflects toxicity to Sf1Ep cells and therefore cannot be considered an MIC.
The water-soluble tetrazolium assay which we did to rule out cytotoxic activity of the antibiotics to cultured cells showed that none of the tested concentrations of cephalosporins, tedizolid and dalbavancin were toxic for the Sf1Ep cells (appendix p 8). Moreover, none of the tested concentrations of isoniazid, metronidazole, and pyrazinamide were cytotoxic to Sf1Ep cells (appendix p 8). Although ivermectin apparently suppressed T pallidum growth at concentrations of 20 mg/L or higher (figure 1N), it also affected Sf1Ep cell homoeostasis at these high concentrations (appendix p 8), suggesting that absence of treponemal growth was not caused by specific activity on the pathogen.
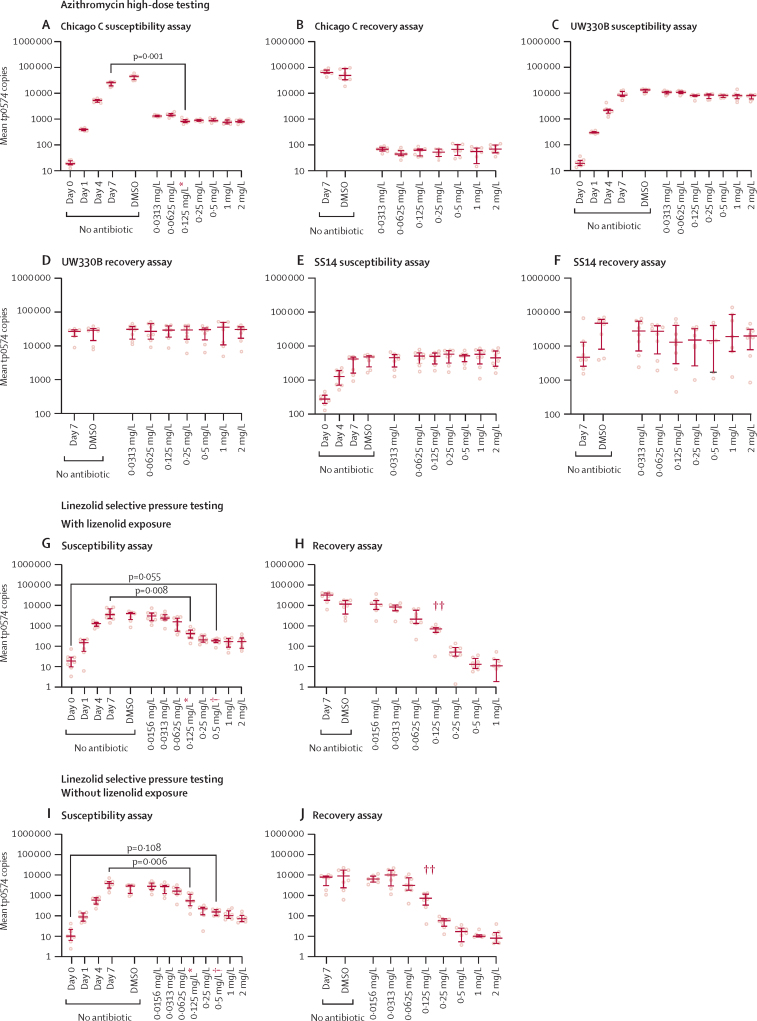
The experiments carried out to evaluate T pallidum resistance to macrolides showed that azithromycin was effective against T pallidum strains that did not have either of the 23S rRNA gene mutations (A2058G or A2059G) conferring resistance to macrolides, such as Chicago C. For this strain, azithromycin had a primary MIC and MBC lower than 0·031 mg/L (figure 3A, B). Azithromycin, however, remained ineffective for two strains (SS14 and UW330B) carrying either one of the aforementioned mutations, at least up to 2·0 mg/L (figure 3C–F); therefore, increasing macrolide dosage would not be a viable strategy to overcome the well documented and widespread genetic resistance of T pallidum to this class of compounds.
Figure 3.
Susceptibility and recovery assays of Chicago C, UW330B, and SS14 to azithromycin, and susceptibility and recovery assays with and without linezolid exposure
Non-antibiotic control wells represent treponemal growth in absence of antibiotic from day 0 (inoculum) to day 7 after plate inoculation. DMSO bars are relative to Sf1Ep cell cultures to which the compound solvent was added instead of the tested antibiotic. Susceptibility and recovery assays of the SS14 strain propagated in the presence of subtherapeutic concentration of linezolid (G, H), or absence of antibiotic (I, J). In the bar chart, the middle line represents the median tp0574 gene copies per unit of volume from eight biological replicates, the length of the bar represents the IQR, and the dots represent the individual values. DMSO=dimethyl sulfoxide. MBC=minimum bactericidal concentration. *Secondary MIC: p values (for the Dunn's test) are provided for the comparison between the lowest antibiotic dilution at which the tp0574 qPCR values were significantly lower than the positive control (day 7 control group). †Primary MIC: p values (for the Dunn's test) are provided for the comparison between the lowest antibiotic dilution at which the tp0574 qPCR values were not significantly higher than the inoculum wells (day 0 control group). ‡MBC.
Lastly, we did experiments to evaluate whether T pallidum propagation employing selective pressure with linezolid concentrations of 0·2 mg/L for 2 weeks followed by propagation at 0·03 mg/L for 8 additional weeks would select for a less susceptible (or fully resistant) strain. The results of the linezolid susceptibility assay done with the SS14 strain propagated with (figure 3G, H) and without (figure 3I, J) linezolid, and their respective treponemal recovery assay graphs have been presented. Overall, susceptibility to linezolid remained the same in both cases (primary MIC value 0·5 mg/L). Mutations mapping to the 23S rRNA genes, as well as to the L3, L4, and L22 50S ribosomal proteins, have been established as a linezolid resistance mechanism.15 These targets correspond to tp0189 (L3), tp0190 (L4), and tp0194 (L22) in T pallidum genes. Sequencing of the SS14 strain propagated in subtherapeutic linezolid concentrations did not have any genetic differences compared with the strain propagated in the absence of antibiotic, including the rRNA-encoding and protein-encoding targets. Genomic data are available on GenBank under bioproject PRJNA885511.
Discussion
On the basis of in-vitro culture of T pallidum strains, MIC values of less than 0·1 mg/L were demonstrated for amoxicillin, several cephalosporins, tedizolid, and dalbavancin. At these concentrations, the organism showed no growth in the presence of the antibiotics or after subculturing on antibiotic-free media. These MIC values add to the existing data on penicillin, doxycycline, and linezolid and represent valuable information for the optimisation and expansion of the treatment options for syphilis.
Although the MIC indicates the susceptibility of the pathogen to the antibiotic, clinical outcomes also depend on achievable drug concentrations at the infection site. Pharmacokinetic–pharmacodynamic analysis integrates both antibiotic exposure (ie, pharmacokinetics) and antimicrobial activity (ie, pharmacodynamics). However, the absence of pharmacokinetic–pharmacodynamic models for T pallidum hinders the definition of pharmacokinetic and pharmacodynamic targets that correlate with clinical efficacy. Consequently, we have compared MIC values obtained in our in-vitro model with trough plasma concentrations achieved in individuals receiving current dosing recommendations to determine whether plasma concentrations are greater than the MIC during the entire dosing interval (percentage of time between two doses during which the unbound fraction of the drug concentration remains above the MIC).
Our results confirm the potential effectiveness of penicillin (treatment of choice), doxycycline, and ceftriaxone (treatment alternatives) for primary, secondary, or latent syphilis. Oral amoxicillin and oral cephalosporins might also be effective treatment options, even when considering the free fraction (not bound to plasma proteins), which indicates the concentration at the site of infection, although further studies are needed to confirm the clinical utility of these antibiotics. In the case of neurosyphilis, the differential ability of these molecules to penetrate the CNS requires individual consideration.
Our study provides MIC values for β-lactams used to treat syphilis, including amoxicillin and ceftriaxone. Amoxicillin results are consistent with the probability of reaching the clinical efficacy target set by EUCAST,16 which is 100% for MIC values up to 0·5 mg/L with the 500 mg per 8 h regimen. In observational studies, amoxicillin has been shown to be effective in treating early syphilis with a 95% success rate.17, 18 Ceftriaxone is recommended to treat early syphilis,19 and some evidence from a retrospective study involving 24 patients suggested that ceftriaxone might be a potential option for treating neurosyphilis.20 The penetration of all β-lactam antibiotics into the CNS in the absence of meningeal inflammation is generally poor (ie, cerebrospinal fluid vs serum ratio of 0·15). However, a daily dose of 1 g to 2 g ceftriaxone achieves concentrations in the CNS of 0·4 mg/L, more than 160 times higher than the MIC value (0·0025 mg/L) we reported. No conclusive results have been reported with cefixime (87% and 56% curative results in the per-protocol and intention-to-treat populations of a study on people with early syphilis).21 Ertapenem, a broad-spectrum β-lactam carbapenem, was overall ineffective against T pallidum in vitro after a 1-week-long incubation. One could hypothesise that ertapenem has reduced inhibitory activity on the only known T pallidum penicillin-binding protein with β-lactamase activity, the 47 kDa lipoprotein, compared with other β-lactams.22 However, this hypothesis requires further studies to be corroborated. Additional experiments done with other carbapenems showed that both doripenem and biapenem are not effective against T pallidum up to 2 mg/L in vitro (p>0·05 vs control wells with no antibiotic) after a 1 week-long incubation, whereas imipenem and meropenem significantly inhibit T pallidum growth at 2 mg/L, but are not completely treponemicidal.
The low MIC values and extended half-life of dalbavancin (ie, 145 h) suggest that a single infusion could maintain high and prolonged plasma concentrations, potentially leading to syphilis cure. However, this agent might not have a substantial impact on CNS infections because of scarce penetration of the blood–brain barrier23, 24 in the animal model (2% in rabbit with non-inflamed meninges and 5% in rabbit with inflamed meninges).
The anti-treponemal activity of zoliflodacin could be explained by comparing the sequences of the DNA gyrase subunit B (GyrB) protein of T pallidum (TP0116) with that of Neisseria gonorrhoeae GyrB protein (NG1772). Although there is only 51% sequence identity between these two enzymes, conservation of key amino-acid residues in the T pallidum GyrB previously identified in the cognate N gonorrhoeae enzyme might account for the results presented here, even though further studies are needed to evaluate this hypothesis.25
Our experiment did not reveal phenotypical or genetic changes potentially related to development of resistance to linezolid following culturing of the pathogen in subtherapeutic concentrations of this antibiotic. The experiment design that incorporated a 2-week period of elevated antibiotic pressure (followed by additional weeks at lower pressure) for selection of resistance mutants in treponemes was subjected to thorough and meticulous consideration (appendix p 4–5), taking into account the usual range of mutation rates for bacteria, given that there is no information on the actual mutation rate of T pallidum.26, 27 Doing further experiments with longer cultivation periods and higher concentrations of linezolid could provide additional value and insights. The pharmacodynamic and pharmacokinetic-based breakpoint of linezolid in gram-positive bacteria is 1 mg/L, representing the highest MIC value at which there is a high likelihood of achieving clinical efficacy.28 In our study the MIC for linezolid was 0·5 mg/L which is lower that the pharmacodynamic and pharmacokinetic breakpoint. Additionally, linezolid has a favourable CNS penetration (38% in rabbit model)29 and has been effective in treating CNS infections caused by other bacterial species. These findings suggest that it is an excellent candidate for clinical evaluation in the treatment of syphilis, including neurosyphilis.30
The in-vitro culture method of T pallidum for determining antimicrobial susceptibility is not devoid of technical limitations that make antibiotic testing still procedurally complex for this pathogen. This method is similar to the broth dilution procedure commonly used with other bacteria,31 but the two systems differ in some important ways. First, the presence of rabbit epithelial cells is necessary to promote the long-term survival and multiplication of T pallidum; 7 days of incubation are used instead of the 16–20 h typically used in other bacteria because the doubling time is about 40 h, and bacterial quantification by quantitative PCR needs to be used instead of visual inspection of turbidity, which does not increase in parallel to T pallidum concentration in the culture media. However, in this and previous studies,12, 13 the culture method of T pallidum yielded reproducible results, and therefore we believe that this assay provides an accurate assessment of MIC values. Second, standardised methods for determining MICs and MBCs in culture were adapted for the purpose of a different culture method altogether; therefore our results need to be interpreted in this context and based on the proposed outcome definitions. Another limitation of our study is the potential for a moderate level of variability in DNA measurement results among replicates because of the limited number of target organisms when growing T pallidum in very small volumes, and the multistep process involved in molecular detection. This drug-susceptibility testing method is more challenging than simply counting colonies of less difficult pathogens on a petri dish, which is not possible with T pallidum. The format adopted in our study, however, allowed us to test eight replicates for various concentrations of each antibiotic, resulting in a more precise determination or redetermination of the MIC of several antibiotics, including linezolid, compared with previous methods used. Another limitation of our study includes that susceptibility testing was only conducted with one T pallidum strain. Replicating the testing with several strains from both the Nichols and SS14 clades, and geographical regions would be more laborious than a normal MIC, but it would increase the robustness and generalisability of data. Additionally, we did not include penicillin as an internal comparator in our assays, instead relying on data from previous experiments12 that were not done simultaneously with the current study. Lastly, this study is limited to in-vitro testing, which is only the first step towards exploring new therapies for syphilis. Nonetheless, given that the studied molecules have been approved for use in humans and their safety is well established, their repurposing could move rapidly to the next stages of preclinical and clinical research.
In conclusion, according to our assessment, cephalosporins and oxazolidinones would be potential candidates for expanding the current therapeutic repertoire for syphilis. The compounds we have identified could transition into preclinical trials in the rabbit model of the disease and onto clinical validation as viable therapeutic options for syphilis. On the basis of our findings and those of similar studies,14 and the fact that these molecules have already been approved, we recommend that clinical guidelines in their section of unmet needs should advocate for randomised clinical trials to evaluate their efficacy in treating syphilis in humans and surveillance of antimicrobial resistance in T pallidum.
Data sharing
The data that support the findings of this study are available from a repository at Harvard Dataverse (https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/Y6LSXT). Genomic data are available on GenBank under bioproject PRJNA885511.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We would like to thank Gerard Carot-Sans for editing the final version of the manuscript and Santiago Ramon Garcia (Department of Microbiology, Faculty of Medicine, University of Zaragoza, Spain) for providing valuable feedback on our research. We also thank Laia Bertran and Miquel Angel Rodríguez for the operational and financial management of the project. This project has been primarily funded by the European Research Council under the EU's Horizon 2020 research and innovation programme (grant agreement number 850450; grant holder OM). KT received support to conduct statistical analyses from the University of Washington under award numbers AI027757, AI144133, and AI027757 from the National Institutes of Health.
Contributors
OM, MVM, and LG conceived, planned, and supervised the study. CP-M and OM did the literature search, drug selection, and dose calculations. LCT and NAPL did the experimental procedures, organised the data, and contributed to data analysis. OM, CS, and LG also contributed to data analysis. KT provided biostatistical support. FG-C did the genomic analysis. ARG and AC contributed to clinical pharmacology data interpretation and modellling. LG and OM accessed and verified all the data reported in this study. All authors contributed to manuscript preparation and critically reviewed and approved the manuscript before submission
Supplementary Material
References
- 1.WHO Prevalence and incidence of selected sexually transmitted infections, Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis: methods and results used by WHO to generate 2005 estimates. 2011. https://apps.who.int/iris/handle/10665/44735
- 2.Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savage EJ, Hughes G, Ison C, Lowndes CM, the European Surveillance of Sexually Transmitted Infections network Syphilis and gonorrhoea in men who have sex with men: a European overview. Euro Surveill. 2009;14 doi: 10.2807/ese.14.47.19417-en. [DOI] [PubMed] [Google Scholar]
- 5.Tucker JD, Cohen MS. China's syphilis epidemic: epidemiology, proximate determinants of spread, and control responses. Curr Opin Infect Dis. 2011;24:50–55. doi: 10.1097/QCO.0b013e32834204bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Centers for Disease Control and Prevention Sexually transmitted disease surveillance. 2018. https://www.cdc.gov/std/stats18/STDSurveillance2018-full-report.pdf
- 7.Idsoe O, Guthe T, Willcox RR. Penicillin in the treatment of syphilis. The experience of three decades. Bull World Health Organ. 1972;47(suppl):1–68. [PMC free article] [PubMed] [Google Scholar]
- 8.Moseley P, Bamford A, Eisen S, et al. Resurgence of congenital syphilis: new strategies against an old foe. Lancet Infect Dis. 2023 doi: 10.1016/S1473-3099(23)00314-6. published online Aug 18. [DOI] [PubMed] [Google Scholar]
- 9.Nelson R. Syphilis rates soar in the USA amid penicillin shortage. Lancet. 2023;402:515. doi: 10.1016/S0140-6736(23)01665-3. [DOI] [PubMed] [Google Scholar]
- 10.US Center for Disease Control and Prevention STD treatment guidelines: syphilis. 2021. https://wwwcdcgov/std/treatment-guidelines/syphilishtm
- 11.Edmondson DG, Hu B, Norris SJ. Long-term in vitro culture of the syphilis spirochete Treponema pallidum subsp pallidum. MBio. 2018;9:e01153–e01158. doi: 10.1128/mBio.01153-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haynes AM, Giacani L, Mayans MV, et al. Efficacy of linezolid on Treponema pallidum, the syphilis agent: a preclinical study. EBioMedicine. 2021;65 doi: 10.1016/j.ebiom.2021.103281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edmondson DG, Wormser GP, Norris SJ. In vitro susceptibility of Treponema pallidum subsp pallidum to doxycycline. Antimicrob Agents Chemother. 2020;64:e00979–e01020. doi: 10.1128/AAC.00979-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes KA, Dressler JM, Norris SJ, Edmondson DG, Jutras BL. A large screen identifies beta-lactam antibiotics which can be repurposed to target the syphilis agent. Antimicrob Resist. 2023;1:4. doi: 10.1038/s44259-023-00006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu BG, Yuan XL, He DD, Hu GZ, Miao MS, Xu EP. Research progress on the oxazolidinone drug linezolid resistance. Eur Rev Med Pharmacol Sci. 2020;24:9274–9281. doi: 10.26355/eurrev_202009_23009. [DOI] [PubMed] [Google Scholar]
- 16.EUCAST Amoxicillin. Rationale for the EUCAST clinical breakpoints. Version 1.0. 2010. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Amikacin_rationale_1.2_0906.pdf
- 17.Nishijima T, Kawana K, Fukasawa I, et al. Effectiveness and tolerability of oral amoxicillin in pregnant women with active syphilis, Japan, 2010–2018. Emerg Infect Dis. 2020;26:1192–1200. doi: 10.3201/eid2606.191300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanizaki R, Nishijima T, Aoki T, et al. High-dose oral amoxicillin plus probenecid is highly effective for syphilis in patients with HIV infection. Clin Infect Dis. 2015;61:177–183. doi: 10.1093/cid/civ270. [DOI] [PubMed] [Google Scholar]
- 19.Cao Y, Su X, Wang Q, et al. A multicenter study evaluating ceftriaxone and benzathine penicillin G as treatment agents for early ayphilis in Jiangsu, China. Clin Infect Dis. 2017;65:1683–1688. doi: 10.1093/cid/cix611. [DOI] [PubMed] [Google Scholar]
- 20.Bettuzzi T, Jourdes A, Robineau O, et al. Ceftriaxone compared with benzylpenicillin in the treatment of neurosyphilis in France: a retrospective multicentre study. Lancet Infect Dis. 2021;21:1441–1447. doi: 10.1016/S1473-3099(20)30857-4. [DOI] [PubMed] [Google Scholar]
- 21.Stafylis C, Keith K, Mehta S, et al. Clinical efficacy of cefixime for the treatment of early syphilis. Clin Infect Dis. 2021;73:907–910. doi: 10.1093/cid/ciab187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weigel LM, Radolf JD, Norgard MV. The 47-kDa major lipoprotein immunogen of Treponema pallidum is a penicillin-binding protein with carboxypeptidase activity. Proc Natl Acad Sci USA. 1994;91:11611–11615. doi: 10.1073/pnas.91.24.11611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janier M, Unemo M, Dupin N, Tiplica GS, Potočnik M, Patel R. 2020 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol. 2021;35:574–588. doi: 10.1111/jdv.16946. [DOI] [PubMed] [Google Scholar]
- 24.Cavaleri M, Riva S, Valagussa A, et al. Pharmacokinetics and excretion of dalbavancin in the rat. J Antimicrob Chemother. 2005;55(suppl 2):ii31–ii35. doi: 10.1093/jac/dki006. [DOI] [PubMed] [Google Scholar]
- 25.Alm RA, Lahiri SD, Kutschke A, et al. Characterization of the novel DNA gyrase inhibitor AZD0914: low resistance potential and lack of cross-resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2015;59:1478–1486. doi: 10.1128/AAC.04456-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlsson M, Fellström C, Heldtander MU, Johansson KE, Franklin A. Genetic basis of macrolide and lincosamide resistance in Brachyspira (Serpulina) hyodysenteriae. FEMS Microbiol Lett. 1999;172:255–260. doi: 10.1111/j.1574-6968.1999.tb13476.x. [DOI] [PubMed] [Google Scholar]
- 27.Prapasarakul N, Ochi K, Adachi Y. In vitro susceptibility and a new point mutation associated with tylosin-resistance in Japanese canine intestinal spirochetes. J Vet Med Sci. 2003;65:1275–1280. doi: 10.1292/jvms.65.1275. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez-Gascón A, Aguirre-Quiñonero A, Aspiazu MAS, Canut-Blasco A. Pharmacokinetic/pharmacodynamic analysis of tedizolid phosphate compared to linezolid for the treatment of infections caused by gram-positive bacteria. Antibiotics. 2021;10:755. doi: 10.3390/antibiotics10070755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cottagnoud P, Gerber CM, Acosta F, Cottagnoud M, Neftel K, Täuber MG. Linezolid against penicillin-sensitive and -resistant pneumococci in the rabbit meningitis model. J Antimicrob Chemother. 2000;46:981–985. doi: 10.1093/jac/46.6.981. [DOI] [PubMed] [Google Scholar]
- 30.Shaikh ZH, Peloquin CA, Ericsson CD. Successful treatment of vancomycin-resistant Enterococcus faecium meningitis with linezolid: case report and literature review. Scand J Infect Dis. 2001;33:375–379. doi: 10.1080/003655401750174048. [DOI] [PubMed] [Google Scholar]
- 31.Clinical and Laboratory Standards Institute . Clinical and Laboratory Standards Institute; Wayne, PA: 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th edn. CLSI standard M07. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from a repository at Harvard Dataverse (https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/Y6LSXT). Genomic data are available on GenBank under bioproject PRJNA885511.