Abstract
Salmonella is a foodborne zoonotic bacterium, and the antimicrobial-resistant strains of Salmonella are a worldwide health concern. Herein, we employed a meta-analysis to determine the pooled prevalence of Salmonella and its antimicrobial resistance status in human, animal, and environmental isolates in South Asia. To this end, we followed the standard guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements for searching literature in three databases namely PubMed, Google Scholar, and CAB abstracts, and a total of 100 eligible datasets were finally included which were published from January 2010 to June 2021. In the pooled prevalence of Salmonella in South Asia, the random model effect was 14.47% (95% CI: 10.17–20.19) with a high degree of heterogeneity (I2, 99.8%) and overall antimicrobial resistance was 70% (95% CI: 63.0–76.0) with a heterogeneity of 23.6%. The temporal distribution of the overall antimicrobial resistance (%) against Salmonella was increased from 53 to 77% within 10 years. Out of 18 distinct Salmonella serotypes, S. enterica was highly prevalent (14.22%, 95% CI: 4.02–39.64) followed by S. pullorum (13.50%, 95% CI: 5.64–29.93) with antimicrobial resistance (%) were 86.26 and 90.06, respectively. Noteworthy, nalidixic acid (74.25%) and tetracycline (37.64%) were found mostly resistant to Salmonella whereas ceftriaxone (1.07%) and cefixime (1.24%) were sensitive. This systematic review demonstrated that overall antibiotic resistance profiles of Salmonella are increasing over time in South Asia. Thus, adequate hygienic practices, proper use of antimicrobials, and implementation of antibiotic stewardship are imperative for halting the Salmonella spread and its antimicrobial resistance.
Supplementary Information
The online version contains supplementary material available at 10.1007/s44197-023-00160-x.
Keywords: Prevalence, Antimicrobial resistance, Salmonella, South Asia
Introduction
Salmonella is a Gram-negative, rod-shaped, non-spore-forming, and facultative anaerobic bacterium belonging to the Enterobacteriaceae family that can produce diseases in humans and animals [1]. Under the genus Salmonella, there are two species, namely S. bongori and S. enterica. Although S. bongori is primarily found in cold-blooded animals, this species also can infect humans [2]. On the other hand, Salmonella enterica is widely abundant and comprises over 2600 serovars which are categorized into typhoidal and non-typhoidal groups. Although these two groups have a resemblance genetically, they can produce various diseases with distinct immune responses [3]. Among all non-typhoidal Salmonella (NTS) serovars globally, around 50% of human isolates were found from S. enterica serovar Typhimurium and S. enterica serovar Enteritidis [4].
S. enterica causes a wide range of food and water-borne diseases both in humans and animals [5]. In humans, approximately 93.8 million cases of foodborne illness and/or gastroenteritis and 155,000 death cases are reported yearly due to non-typhoidal Salmonella [6, 7]. The global prevalence of Salmonella is high, and non-typhoidal Salmonella infections are reported across the world. However, the prevalence of Salmonella varies from region to region [8]. For instance, in the USA, NTS has been reported as the second-most causal bacteria for foodborne illness [9] whereas typhoidal salmonellosis is highly prevalent in South and South-East Asia. Again, invasive non-typhoidal Salmonella has been recorded to cause bacteremia with high morbidity in the sub-Saharan African region [8].
Salmonella has a wide host range such as reptiles, avian species, and mammals including humans [10]. NTS is usually transmitted to humans by contaminated foods, animals, animal products, and manures [11, 12]. Some studies found an association between Salmonella-contaminated fruits and vegetables with food poisoning. Pathogenicity studies showed that Salmonella has some unique properties to cross a larger number of barriers and invade different cells [5]. NTS is often reported as an important foodborne pathogen causing gastrointestinal disorders, different localized infections, and bacteremia. These bacteria can develop worse conditions in immunosuppressive humans [13], especially malaria-infected patients, malnourished children, and human immunodeficiency virus (HIV) patients [4]. Notwithstanding treatment and preventive strategies being implemented, millions of new typhoid infections are being reported globally every year [14]. Interestingly, S. typhimurium not only infects humans and animals but also can use plants as their alternative hosts. Infections in humans, animals, and plants by Salmonella have raised questions about their host specificity [15].
Salmonella is one of the most reported zoonotic pathogens, and the antimicrobial-resistant (AMR) strains of Salmonella are a big concern for public health [16]. Even though NTS commonly causes gastrointestinal infections worldwide, most of the strains cause mild gastroenteritis which is usually not required to treat with antibiotics [17]. However, there are some factors that help Salmonella to be more pathogenic and a threat to public health. For instance, genetic modification and genomic evolution in Salmonella have increased virulence and have made them resistant to multiple drugs [17]. Antibiotic-induced selective pressures cause mutations in chromosomal genes and plasmid leading to continuous genetic evolution in Salmonella. Again, horizontal gene transfer may also contribute to the spread of AMR genes. The acquisition and spread of resistant genes are significantly affected by the exchange between plasmid(s) and the bacterial chromosome as well as the integration of resistant genes into specialized genetic components known as integrons [17, 18]. In fact, poultry that has never been raised with antibiotics has meat contaminated with antibiotic-resistant Salmonella [9]. Moreover, wastewater in hospitals is a hotspot for AMR pathogens, and hospitals are contributing to the spread of resistant pathogens [19]. Overall, there are several underlying factors for developing AMR, and the root causes are complex, particularly in developing countries. Inappropriate use of antimicrobials and lack of knowledge about antibiotics usages are crucial factors. The use of antimicrobials in animals and plants is also generating resistant bacteria [20]. AMR serotypes of Salmonella can also be transmitted with wild birds such as vultures [16]. AMR is generating serious challenges for health and the economy. It is estimated that ten million deaths may occur by 2050 due to AMR. The World Bank has also estimated that by 2050, the global GDP may fall by 1.1–3.8% due to the independent impacts of AMR [21].
South Asia is at high risk in terms of the emergence and spread of antimicrobial resistance [22]. Despite the increasing knowledge of the prevalence of Salmonella and its AMR profile which is mostly reported by individual and local surveillance study(s), comprehensive and robust study of the prevalence and AMR pattern in South Asia is poorly characterized. Thus, this meta-analysis includes a comprehensive evaluation of scientific literature published between January 2010 to June 2021 on antimicrobial resistance by species-specific Salmonella serotypes isolated from the environment, animals, and humans in South Asia.
Methodology
Study Design and Systematic Review Protocol
The outcome of interest in this study includes any species under the genus of Salmonella isolated from humans or animals or environment using cultural, immunological, or molecular diagnostic methods, and AMR patterns of Salmonella. The outcome measure is the prevalence of Salmonella based on the peer-reviewed publication which may contain one or more datasets, depending on different sampling techniques, sources, time, and locality. We used three different databases for searching literature: PubMed (https://pubmed.ncbi.nlm.nih.gov/), Google Scholar (https://scholar.google.com/), and CAB abstracts (https://www.cabi.org/publishing-products/cab-abstracts/). The search key was Prevalence OR Incidence OR Occurrence AND Salmonella OR Salmonellosis AND antimicrobial resistance OR antibiotic resistance AND (Bangladesh/India/Pakistan/Nepal/Bhutan/Maldives/Sri Lanka/Afghanistan). Articles published from January 2010 to June 2021 were included in this study. The last search was conducted on May 24, 2021. This meta-analysis was conducted by following the standard guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements [23].
Selection Criteria
The inclusion criteria for all the eligible studies included in the meta-analysis were: articles published primarily on the quantitative prevalence of Salmonella spp. in humans, animals, and environment in South Asia (Bangladesh, India, Pakistan, Nepal, Bhutan, Maldives, Sri Lanka, and Afghanistan); the study included descriptive (surveys) or observational (cross-sectional, case–control and cohort) and not clinical trials; and articles only reported in English on antibiotic resistance of Salmonella published between January 2010 to June 2021.
Studies excluded from this meta-analysis were book and book chapters, review papers, unpublished studies, proceedings, and theoretical models. Studies were further excluded if the diagnostic test was not mentioned and had overlapping data with another included study.
Data Extraction
Based on the ‘search keywords’, the titles and abstracts were initially examined, and full-text articles were downloaded to determine the eligibility. Primary information including author(s) name, year of publication, location, total sample size, number of positive samples, species of Salmonella, and its antibiotic resistance profile were collected individually from the publication and entered into Microsoft Excel. Two authors independently reviewed and evaluated the full texts for the eligibility of final inclusion. In our study, we examined a large dataset of 822,120 isolates. We found a total of 28,810 isolates that tested positive for Salmonella. It is important to note that we were able to specifically identify 18 distinct Salmonella serotypes out of these positive cases, whereas the remaining cases were non-specific in terms of serotype identification. In these studies, different types of samples were collected such as blood, feces, gut, and anal swab from animals; food, insect, soil, and water from the environment; blood, serum, stool, and hand swab from humans (Supplementary Table 2). Diagnostic approaches used for these studies were culture, biochemical, PCR, and serological. All antimicrobial susceptibility tests (AST) were done by using disc diffusion method; therefore, no subgroup analysis was performed on AST.
Statistical Analysis
A random-effect meta-analysis was carried out to estimate the prevalence of Salmonella with 95% Confidence Interval (CI) in South Asia. Between-study variations were identified by using Cochran’s Q test to measure the heterogeneity which indicates whether the variation in the studies is more than the expected level by chance (P < 0.05 were considered significant heterogeneity). Higgins’s I2 value was used to determine the percentage of total variance in effect estimates among the studies which was attributable to heterogeneity rather than chance. I2 values of more than 50% were considered as high heterogeneity [24]. Publication bias may affect the pooled effect estimation. Therefore, we also performed Egger’s test and funnel plot to explore the potential publication bias. Antimicrobial resistance percentage was defined as the number of antimicrobials were found resistant divided by total number of antimicrobials were tested. A subgroup meta-analysis was conducted on different subsets of data to find out the prevalence of Salmonella in different sources, locations, countries, and time-periods. The test for subgroup differences was based on Cochran’s Q value which indicates the presence of heterogeneity among subgroups. The prevalence estimates of different datasets were pooled using the DerSaimonian-Laird random-effect method [25]. Additionally, meta-regression analysis was used to further investigate the heterogeneity in different subsets of the group. Four possible groups were examined: Country, Source (Human, Animal, or Environment), Location (Rural, Urban, Both), and Time (2010–2013, 2014–2017, 2018–2021). Initially, a univariate meta-regression model was employed to determine the association between different groups and the prevalence of Salmonella. Groups with P < 0.2 in univariate analysis were included in the final multivariate model. All the analyses were performed using the ‘Meta’, ‘Metafor’, and ‘DmetaR’ packages of the open-source R (version 4.0.3).
Results
Study Selection
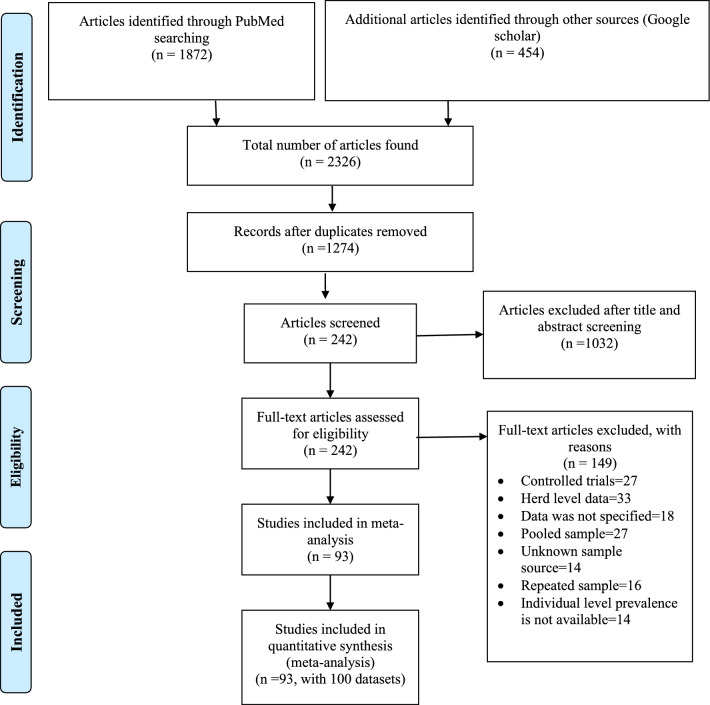
Figure 1 shows the overall selection processes of the eligible articles used in this study. A total of 1872 articles were identified in the PubMed database and 454 articles in Google Scholar and CAB abstracts. We selected a total of 1274 articles after removal of duplicates or triplicates. After screening the titles and abstracts, only 242 papers were included. Finally, 93 articles (containing 100 datasets) were included to determine the prevalence of Salmonella in the South Asian Region.
Fig. 1.
Study selection process for meta-analysis of Salmonella
Overall Prevalence of Salmonella
The prevalence distributions of Salmonella in different categories are depicted in Table 1. A total of 100 datasets of 93 articles from South-Asian countries are presented for the prevalence of Salmonella. The overall prevalence of Salmonella in the random model effect was 14.47% (95% CI: 10.17–20.19) with a high degree of heterogeneity (I2 = 99.8%) (Fig. 2).
Table 1.
Prevalence distribution of Salmonella in different categories
| Variables (P-value) | Observations | Number of positive isolates | Sample size | No. of study | Sub-group analysis | ||
|---|---|---|---|---|---|---|---|
| Prevalence (%) | Q | I2 | |||||
| Country | Bangladesh (Ref) | 17,558 | 126,607 | 41 | 34.23 (23.73–46.56) | 6062.77 | 99.3% |
| India | 4375 | 561,526 | 31 | 3.28 (1.84–5.77) | 9420.78 | 99.7% | |
| Pakistan | 6204 | 124,072 | 14 | 26.15 (15.04–41.47) | 6230.43 | 99.8% | |
| Nepal | 648 | 9637 | 12 | 10.36 (3.73–25.63) | 906.70 | 98.8% | |
| Bhutan | 23 | 180 | 1 | 12.78 (8.64–18.50) | – | – | |
| Sri Lanka | 2 | 98 | 1 | 2.04 (0.51–7.79) | – | – | |
| Source | Animal (Ref) | 2283 | 12,377 | 43 | 22.66 (15.41–32.03) | 1926.99 | 97.8% |
| Environment | 1539 | 12,507 | 19 | 27.81 (12.01–52.07) | 987.58 | 98.2% | |
| Human | 24,988 | 797,236 | 38 | 5.81 (3.17–10.41) | 35,583.25 | 99.9% | |
| Locality | Both (Ref) | 1200 | 4297 | 7 | 35.77 (20.91–54.00) | 178.67 | 96.6% |
| Rural | 3415 | 399,544 | 35 | 8.85 (5.10–14.91) | 9761.82 | 99.7% | |
| Urban | 24,195 | 418,279 | 58 | 17.02 (10.42–26.56) | 25,003.42 | 99.8% | |
| Time | 2010–2013 (Ref) | 924 | 6646 | 19 | 17.88 (8.25–34.50) | 779.49 | 97.7% |
| 2014–2017 | 20,990 | 411,997 | 36 | 11.02 (5.66–20.36) | 16,282.64 | 99.8% | |
| 2018–2021 | 6896 | 403,477 | 45 | 16.41 (10.08–25.58) | 24,789.37 | 99.8% | |
| Test | Culture (Ref) | 5793 | 412,051 | 46 | 18.39 (10.44– 30.36) | 24,540.96 | 99.8% |
| PCR | 1808 | 60,285 | 19 | 11.65 (5.62–22.60) | 2394.48 | 99.2% | |
| Culture and Biochemical | 20,394 | 247,994 | 25 | 12.00 (6.57–20.93) | 9645.64 | 99.8% | |
| Culture and PCR | 73 | 190 | 2 | 30.14 (2.80–86.62) | 61.93 | 98.4% | |
| Biochemical | 708 | 101,535 | 7 | 6.33 (1.44; 23.73) | 1717.80 | 99.7% | |
| Antisera | 34 | 65 | 1 | 52.31 (40.27; 64.09) | 0.00 | – | |
*Statistically significant (P value < 0.05)
Fig. 2.

Forest plot of meta-analysis showing pooled prevalence of Salmonella in studies conducted in South Asia
Country
The highest prevalence of Salmonella in South Asia was observed in Bangladesh as 34.23% (95% CI: 23.73–46.56) with a high degree of heterogeneity (I2 = 99.3%). Pakistan also had a relatively higher prevalence of 26.15% (95% CI: 15.04–41.47). Only a single study was identified in Sri Lanka with the lowest prevalence (2.04%, 95% CI: 0.51–7.79) among South Asian countries. India also had a lower prevalence of 3.04% but a very high variation in between-study (I2 = 99.7%). Nepal (10.36%) and Bhutan (12.78%) both had a prevalence around the pooled prevalence of South Asia. Noteworthy, we did not find any articles on Maldives and Afghanistan in our search.
Source
Based on sample origin, we categorized the prevalence of Salmonella into Human, Animal, and Environment. The highest prevalence was found in environment (27.81%, 95% CI: 12.01–52.07, I2 = 98.2%) followed by animal-source (22.66%, 95% CI: 15.41–32.03, I2 = 97.8%) and human origin (5.81%, 95% CI: 3.17–10.41, I2 = 99.9%).
Locality
In this study, we considered Salmonella prevalence into three categories based on locality: rural, urban, and semi-urban areas. From rural areas, 35 studies demonstrated a high degree of heterogeneity (99.7%), and the prevalence was 8.85% (95% CI: 5.10–14.91). In the urban region, 58 studies out of 100 showed Salmonella positivity which was almost double (17.02%, 95% CI: 10.42–26.56) than rural areas. The prevalence of Salmonella was 35.77% (95% CI: 20.91–54.00) in semi-urban areas.
Time
The temporal pattern of Salmonella prevalence was irregular. It was 17.88% in 2010–2013, then decreased to 11.02% (2014–2017), and further increased to 16.41% in recent years (2018–2021).
Diagnostic Test
In this study, the variation of detection of Salmonella showed that some methods had higher rates than others. Using an antisera method resulted in the highest rate as 52.31% (95% CI: 40.27–64.09) of cases being detected in this way followed by combining two methods, culture and PCR as 30.14% (95% CI: 2.80–86.62). On the other hand, biochemical test had the lowest rate, 6.33% (95% CI: 1.44–23.73) of cases.
Source of Heterogeneity
To measure the prevalence of Salmonella in South Asia, two sources of heterogeneity were identified as significant in univariate meta-regression: Country (P < 0.0001) and Source (P < 0.0001). The heterogeneity of locality was borderline significant (P = 0.0547). In multivariate meta-regression (Table 2), both country and source were found to be significant. There was no significant correlation found between groups, thus no interactions were added in the multivariate model. In multivariate, the prevalence in India and Nepal were significantly different from others. Studies in which the sources of samples were humans were borderline significant (P-value = 0.0529) from other sources of samples.
Table 2.
Final multivariate meta-analysis with different variables of Salmonella
| Variables (P-value) | Observations | Univariate meta-regression | Multivariate meta-regression | ||
|---|---|---|---|---|---|
| Co-efficient | P-value | Co-efficient | P-value | ||
| Country (< 0.0001*) | Bangladesh (Ref) | – | – | – | – |
| India | −2.73 (−3.50, −1.96) | < 0.0001 | −0.22 (−0.35, −0.09) | 0.0012* | |
| Pakistan | −0.38 (−1.38, 0.61) | 0.4495 | 0.11 (−0.10, 0.31) | 0.2993 | |
| Nepal | −1.50 (−2.56, −0.44) | 0.0054 | 0.004 (−0.19, 0.18) | 0.9712 | |
| Bhutan | −1.29 (−4.55, 1.97) | 0.0041 | −0.35 (−0.80, 0.09) | 0.1212 | |
| Sri Lanka | −3.43 (−7.00, 0.14) | 0.0600 | −0.36 (−0.81, 0.08) | 0.1076 | |
| Source (0.0001*) | Animal (Ref) | – | – | – | – |
| Environment | 0.26 (−0.77, 1.28) | 0.6253 | −0.004 (−0.13, 0.12) | 0.9500 | |
| Human | −1.55 (−2.37, −0.73) | 0.0002 | −0.09 (−0.21, 0.02) | 0.1038 | |
| Locality (0.0547*) | Both (Ref) | – | – | ||
| Rural | −1.76 (−3.37, −0.15) | 0.0325 | −0.09 (−0.29, 0.12) | 0.4065 | |
| Urban | −1.01 (−2.57, 0.54) | 0.2021 | 0.008 (−0.18, 0.19) | 0.9315 | |
| Time (0.4953) | 2010–2013 (Ref) | – | – | – | – |
| 2014–2017 | −0.57 (−1.70, 0.56) | 0.3252 | |||
| 2018–2021 | −0.10 (−1.20, 0.99) | 0.8535 | |||
| Test (< 0.0001*) | PCR (Ref) | – | – | – | – |
| Culture | 0.19 (−0.04, 0.24) | 0.1435 | 0.07 (−0.05, 0.20) | 0.2781 | |
| Culture and biochemical | 0.002 (−0.15, 0.15) | 0.9768 | −19 (−0.39, 0.010) | 0.0615 | |
| Culture and PCR | 0.19 (−0.18, 0.57) | 0.3173 | −0.01 (−0.35, 0.33) | 0.9575 | |
| Biochemical | −0.04 (−0.27, 0.18) | 0.7056 | −0.05 (−0.24, 0.14) | 0.6247 | |
| Antisera | 0.32 (−0.22, 0.85) | 0.2420 | 0.11 (−0.40, 0.61) | 0.6877 | |
*Statistically significant (P value < 0.05)
Pooled Prevalence and Distribution of Salmonella Serotypes
Table 3 shows the pooled prevalence and distribution of Salmonella serotypes. The prevalence of S. enterica was 14.22% (95% CI: 4.02–39.64) followed by S. pullorum 13.50% (95% CI: 5.64–29.93), S. indica 5.05% (95% CI: 0.52–35.19), S. salamae 3.74% (95% CI: 2.08–6.63), S. typhi 3.24% (95% CI: 1.58–6.51), S. Paratyphi B 2.66% (95% CI: 1.72–4.10), S. typhimurium 2.43% (95% CI: 0.73–7.74), and S. houtenae 2.06% (95% CI: 0.78–5.36) (Table 3). There is a 20.88% (95% CI: 13.17–31.49) prevalence of non-specific Salmonella in the population. The prevalence of the remaining Salmonella species is less than 2%. Prevalence of different Salmonella serovar in different countries are depicted in supplementary Fig. 1. The prevalence of Salmonella varies considerably between different sources (Table 3). In human cases, non-specific Salmonella were observed at 3.06% followed by S. enterica (2.92%). S. enterica was highly prevalent in animals (34.71%) followed by S. pullorum (22.67%). Considering environmental sources, non-specific Salmonella was observed as 33.13% followed by S. enteritidis (16.00%).
Table 3.
Pooled prevalence and distribution of Salmonella serotypes
| Species | No. of study | Prevalence % (95% CI) | Q | I2 | Source-specific prevalence % (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Human | Animal | Environment | |||||
| S. typhi | 27 | 3.24 (1.58–6.51) | 13,329.44 | 99.8% | 1.48 (1.45–1.51) | 4.08 (2.54–6.41) | 9.25 (8.28–10.32) |
| S. Paratyphi A | 18 | 1.18 (0.56–2.48) | 4884.89 | 99.7% | 0.59 (0.57–0.61) | 1.00 (0.05–6.24) | 3.07 (2.49–3.76) |
| S. Paratyphi B | 4 | 2.66 (1.72–4.10) | 13.25 | 77.4% | 1.81 (1.18–2.74) | 3.33 (1.36–7.45) | 4.07 (3.40–4.85) |
| S. typhimurium | 7 | 2.43 (0.73–7.74) | 193.72 | 96.9% | 0.09 (0.04–0.21) | 3.66 (2.82–4.73) | 10.55 (9.50–11.70) |
| S. enterica | 7 | 14.22 (4.02–39.64) | 615.17 | 99.0% | 2.92 (2.40–3.55) | 34.71 (31.01–38.58) | 14.42 (9.69–24.98) |
| S. enteritidis | 5 | 1.24 (0.10–13.57) | 78.09 | 94.9% | 0.011 (0.001–0.07) | 4.27 (2.97–6.08) | 16.00 (9.70–24.99) |
| S. pullorum | 2 | 13.50 (5.64–29.93) | 9.64 | 89.6% | – | 22.67 (16.41–30.36) | 7.00 (3.10–14.38) |
| S. Kentucky | 4 | 1.17 (0.34–4.00) | 192.35 | 98.4% | 0.42 (0.31–0.57) | 4.19 (3.29–5.30) | – |
| S. Virchow | 3 | 0.73 (0.21–2.46) | 34.82 | 94.3% | 0.21 (0.13–0.33) | 1.67 (0.95–2.85) | – |
| S. indica | 2 | 5.05 (0.52–35.19) | 9.30 | 89.2% | – | 5.05 (0.52–35.19) | – |
| S. salamae | 2 | 3.74 (2.08–6.63) | 0.03 | 0.0% | – | 3.74 (2.08–6.63) | – |
| S. Weltevereden | 1 | 0.011 (0.001–0.08) | 0.0 | – | 0.011 (0.001–0.08) | ||
| S. Bareilly | 1 | 0.011 (0.001–0.08) | 0.0 | – | 0.011 (0.001–0.08) | – | – |
| S. Stanley | 1 | 0.011 (0.001–0.08) | 0.0 | – | 0.011 (0.001–0.08) | – | – |
| S. Worthington | 1 | 0.011 (0.001–0.08) | 0.0 | – | 0.011 (0.001–0.08) | – | – |
| S. houtenae | 1 | 2.06 (0.78–5.36) | 0.0 | – | – | 2.06 (0.78–5.36) | – |
| S. bongori | 1 | 1.00 (0.14–6.75) | 0.0 | – | – | 1.00 (0.14–6.75) | – |
| S. Choleraesuis | 1 | 1.00 (0.14–6.75) | 0.0 | – | – | 1.00 (0.14–6.75) | – |
| Nonspecific Salmonella | 56 | 20.88 (13.17–31.49) | 13,425.59 | 99.6% | 3.06 (2.94–3.19) | 14.46 (13.67–15.28) | 33.13 (31.35–34.96) |
The Prevalence of Antibiotic Resistance in Salmonella Species
This study investigated the antimicrobial resistance rates of different Salmonella serotypes (Table 4). S. pullorum had a high resistance rate of 90.06% (95% CI: 5.96–99.92) followed by S. enterica as 86.26% (95% CI: 50.07–97.51). However, due to the paucity of studies on these serotypes, limited data were available for less common strains such as S. Weltevereden, S. Bareilly, S. Stanley, S. Worthington, and S. houtenae, with a resistance rate of 0.00%. S. Virchow had the highest level of antimicrobial resistance among human cases with a significant higher rate of 80% (95% CI: 44.22–96.46). S. pullorum had the highest resistance rate in animals, reaching 100% (95% CI: 69.87–100). Notably, S. enterica exhibited a startlingly high resistance rate of 100% (95% CI: 71.66–100) in environmental samples. We analyzed the resistance status of Salmonella against different antibiotics (Supplementary Table 1) of which most tested antibiotics are presented (Table 5). Table 5 shows the highest pooled percentage of Salmonella-resistance against nalidixic acid (74.25%) followed by tetracycline (37.64%), trimethoprim/sulfamethoxazole (32.92%), amoxicillin (32.18%), azithromycin (31.05%), chloramphenicol (22.45%), gentamicin (15.94%), and ampicillin (12.12%). Conversely, the lowest percentage of resistance was found against ceftriaxone (1.07%) followed by cefixime (1.24%), co-trimoxazole (3.92%), and ciprofloxacin (7.58%).
Table 4.
Prevalence of AMR percentage in Salmonella species depending on source-specific samples
| Species | No. of study | AMR Prevalence (95% CI) | Q | I2 | Source-specific AMR prevalence (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Human | Animal | Environment | |||||
| S. typhi | 27 | 67.70% (57.51–76.45) | 38.50 | 32.5% | 67.96% (61.06–74.18) | 40% 19.98–63.59) | 60.53% (43.45–75.51) |
| S. Paratyphi A | 18 | 54.59% (39.74–68.64) | 30.57 | 44.4% | 49.63% (40.96–58.32) | 50% (23.66–76.34) | 76.34% (69.23–38.88) |
| S. Paratyphi B | 4 | 59.94% (40.11–76.97) | 4.74 | 36.7% | 60.71% (40.73–77.87) | 66.67% (24.11–94.0) | 61.54% (32.28–84.87) |
| S. typhimurium | 7 | 70.57% (58.03–80.61) | 7.93 | 24.3% | 66.67% (24.11–94) | 75% (61.88–84.89) | 59.26% (39.01–76.99) |
| S. enterica | 7 | 86.26% (50.07–97.51) | 8.90 | 32.6% | 39.13% (20.47–61.22) | 90.48% (68.17–98.33) | 100% (71.66–100) |
| S. enteritidis | 5 | 60.37% 26.46–86.57) | 8.01 | 50.1% | 40% (13.69–72.63) | 64.29% (47.99–78.00) | 42.86% (18.81–70.35) |
| S. pullorum | 2 | 90.06% (5.96–99.92) | 0.00 | 0.00% | – | 100% (69.87–100) | 42.86% (18.81–70.35) |
| S. Kentucky | 4 | 53.42% (7.84–93.93) | 4.83 | 37.9% | 41.18% (19.43–66.55) | 68.42% (43.50–86.44) | – |
| S. Virchow | 3 | 66.11% (0.12–99.97) | 0.00 | 0.0% | 80% (44.22–96.46) | 52.17% (31.08–72.58) | – |
| S. indica | 2 | 44.44% (23.99–66.96) | 0.28 | 0.0% | – | 44.44% (23.99–66.96) | – |
| S. salamae | 2 | 44.59% (6.02–90.99) | 6.28 | 84.1% | – | 50% (29.03–70.97) | – |
| S. Weltevereden | 1 | 0.00 | 0.00 | – | 0.00 | – | – |
| S. Bareilly | 1 | 0.00 | 0.00 | – | 0.00 | – | – |
| S. Stanley | 1 | 0.00 | 0.00 | – | 0.00 | – | – |
| S. Worthington | 1 | 0.00 | 0.00 | – | 0.00 | – | – |
| S. houtenae | 1 | 25.00% (6.30–62.29) | 0.00 | – | – | 25.00% (6.30–62.29) | – |
| S. bongori | 1 | 50.00% (22.45–77.54) | 0.00 | – | – | 50.00% (22.45–77.54) | – |
| S. Choleraesuis | 1 | 10.00% (1.39–46.72) | 0.00 | – | – | 10.00% (1.39–46.72) | – |
| Nonspecific Salmonella | 52 | 83.75% (75.02–89.83) | 47.19 | 0.0% | 74.44% (63.97–82.80) | 73.90% (68.42–78.74) | 83.110% (75.69–88.66) |
Table 5.
Resistance percentage of Salmonella against most used antibiotics
| Antibiotics | Number of samples tested | Number of resistant | Percentage of resistance with 95% CI |
|---|---|---|---|
| Ampicillin | 40,216 | 4875 | 12.12 (11.80–12.45) |
| Amoxycillin | 3297 | 1061 | 32.18 (30.59–33.81) |
| Azithromycin | 4840 | 1503 | 31.05 (29.75–32.38) |
| Ciprofloxacin | 37,196 | 2820 | 7.58 (7.31–7.86) |
| Ceftriaxone | 38,386 | 410 | 1.07 (0.97–1.18) |
| Cefixime | 36,046 | 446 | 1.24 (1.13–1.36) |
| Chloramphenicol | 13,580 | 3049 | 22.45 (21.75–23.16) |
| Cotrimoxazole | 33,735 | 1323 | 3.92 (3.72–4.13) |
| Gentamicin | 2911 | 464 | 15.94 (14.63–17.32) |
| Tetracycline | 2858 | 1076 | 37.65 (35.87–39.45) |
| Nalidixic acid | 4819 | 3578 | 74.25 (72.99–75.48) |
| Trimethoprim/sulfamethoxazole | 6392 | 2104 | 32.92 (31.76–34.08) |
Among different classes of antibiotics, the highest resistance percentage was against fluoroquinolone (67.67%). On the other hand, the lowest resistance percentage was in aminoglycoside (0.25%) followed by macrolide (0.83%) (Supplementary Table 1). The resistance pattern of specific Salmonella serotypes according to different antibiotics is described in Table 6. The resistance rate of S. typhi to nalidixic acid was 88.12% (95% CI: 62.58–97.05), highlighting the antibiotic's limited efficacy against this serotype. Similarly, S. Paratyphi A was resistant to nalidixic acid at a rate of 91.32% (95% CI: 78.25–96.85). Resistance to cefixime reached 60% (95% CI: 20.04–89.97) in S. Paratyphi B which indicate moderate resistance. Furthermore, S. typhimurium was resistant to tetracycline at 87.80% (95% CI: 73.85–94.83).
Table 6.
AMR pattern of Salmonella serotypes against different antibiotics
| Serovar | AMR percentage (95% confidence interval) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | Amoxycillin | Azithromycin | Ciprofloxacin | Ceftriaxone | Cefixime | Chloramphenicol | Cotrimoxazole | Gentamicin | Tetracycline | Nalidixic acid | Trimethoprim/sulfamethoxazole | |
| S. typhi | 33.28 (15.22–58.06) | 9.10 (1.46–40.32) | 2.76 (0.29–21.58) | 12.16 (3.73–33.09) | 0.08 (0.006–0.99) | 0.11 (0.005–2.27) | 12.81 (4.89–29.58) | 13.42 (6.86–24.59) | 2.77 (0.35–18.70) | 50.72 (19.37–81.51) | 88.12 (62.58–97.05) | 83.99 (21.92–98.99) |
| S. Paratyphi A | 2.95 (0.76–10.78) | 17.20 (1.60–72.60) | 6.64 (4.22–10.29) | 12.65 (2.22–48.00) | 0.009 (0.0001–1.53) | 0.008 (0.00–2.15) | 2.24 (0.66–7.28) | 2.55 (0.85–7.39) | 6.47 (1.92–19.63) | 8.81 (0.00–99.98) | 91.32 (78.25–96.85) | 32.39 (2.84–88.70) |
| S. Paratyphi B | 10.61 (7.33–15.13) | – | 40 (10.02–79.96) | 3.26 (1.64–6.39) | 6.21 (0.09–82.08) | 60 (20.04–89.97 | 7.19 (2.20–21.10) | 4.31 (0.28–41.59) | 2.41 (0.04–59,82) | – | 19.42 (12.88–28,19) | |
| S. typhimurium | 67.24 (41.25–85.71) | – | 9.37 (3.05–25.35) | 57.23 (18.85–88.52) | 6.25 (1.57–21.81) | 9.06 (6.52–12.48) | – | 28.60 (5.61–72.99) | 87.80 (73.85–94.83) | 66.41 (43.60–83.49) | 5.20 (0.91–24.67) | |
| S. enterica | 16.72 (0.001–99.97) | 0.00 | 2.71 (0.75–9.25) | 9.66 (6.98–13.21) | 0.70 (0.05–8.81) | 0.00 | 4.52 (0.97–18.57) | – | 1.67 (0.03–45.31) | 86.59 (34.33–98.76) | 85.15 (35.54–98.35) | |
| S. enteritidis | 93.74 (62.03–99.27) | – | 20 (2.72–69.10) | 34.72 (1.25–95.73) | 30.77 (16.19–50.55) | 14.28 (4.68–36.13) | 2.78 (0.01–85.77) | – | 27.44 (1.36–91.19) | 79.76 (53.69–93.05) | 85.02 (36.78–98.23) | 6.25 (0.87–33.54) |
| S. pullorum | 100 | – | – | 16.46 (0.27–93.35) | 23.53 (12.23–40.46) | 23.53 (12.22–40.46) | 16.59 (0.19–95.39) | – | 16.50 (0.22–94.45) | 82.64 (17.94–99.04) | 91.50 (62.76–98.57) | 28.57 (7.20–67.33) |
| S. Kentucky | 86.12 (62.35–95.87) | – | 0.00 | 97.36 (92.16–99.15) | 0.00 | 0.00 | 0.00 | 25.64 (14.39–41.64) | 100 | 97.78 (61.51–99.92) | – | |
| S. Virchow | 0.00 | 0.00 | – | 44.47 (21.32–70.13) | 5.56 (0.78–30.65) | 5.55 (0.77–30.65) | 0.00 | 83.33 (59.14–94.52) | 100 | 100 | 0.00 | |
| S. indica | 11.11 (4.23–26.11) | – | – | 0.00 | – | 0.00 | 2.77 (0.38–17.25) | 0.00 | 0.00 | – | – | |
| S. salamae | 100 | – | – | 0.00 | – | 0.00 | 0.00 | 0.00 | 0.00 | – | ||
| S. Weltevereden | 0.00 | – | – | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | – | 0.00 | – | |
| S. Bareilly | 0.00 | – | – | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | – | 0.00 | – | |
| S. Stanley | 0.00 | – | – | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | – | 0.00 | – | |
| S. Worthington | 0.00 | – | – | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | – | 0.00 | – | |
| S. houtenae | 25 (3.35–76.21) | – | – | 0.00 | – | 0.00 | 0.00 | 0.00 | 25 (3.35–76.21) | – | – | |
| S. bongori | – | – | – | – | – | – | – | – | – | – | – | |
| S. Choleraesuis | – | – | – | – | – | – | – | – | – | – | – | |
| Nonspecific Salmonella | 61.19 (35.02–82.18) | 44.06 (22.83–67.70) | 60.77 (23.16–88.84) | 12.58 (5.88–24.86) | 8.59 (2.53–25.39) | 46.75 (25.04–69.77) | 7.08 (1.92–22.90) | 13.29 (6.45–25.42) | 8.96 (4.46–17.15) | 49.42 (18.23–81.06) | 38.45 (10.77–76.37) | 32.51 (13.85–59.08) |
– No study found
Overall Antimicrobial Drug Resistance Percentage
In this present paper, the overall antimicrobial drug resistance percentage was 70% (95% CI: 63.0–76.0) with a heterogeneity of 23.6%. The overall antimicrobial drug percentage of Salmonella with different variables are depicted in Table 7. The overall antimicrobial resistance patterns of different antibiotics are also given in Supplementary Table 1.
Table 7.
Overall antimicrobial drug resistance in Salmonella in different categories
| Variables (P-value) | Observations | No. of study | Sub-group analysis | Univariate Meta-regression | |||
|---|---|---|---|---|---|---|---|
| Prevalence | Q | I2 | Coeff | P-value | |||
| Source (0.0316*) | Animal (Ref) | 56 | 77% (65–86) | 75.24 | 26.9% | – | |
| Environment | 23 | 78% (65–88) | 21.2 | 0.0% | 0.24 (−0.63, 1.12) | 0.5884 | |
| Human | 61 | 60% (50–69) | 84.23 | 28.8% | −0.72 (−1.37, 0.06) | 0.0315 | |
| Locality (0.005*) | Semi-urban (Ref) | 13 | 96% (79–99) | 1.69 | 0.0% | – | |
| Rural | 43 | 61% (47–73) | 64.80 | 35.2% | −2.40 (−3.61, −1.18) | 0.0001 | |
| Urban | 84 | 69% (60–76) | 105.01 | 21.0% | −2.03 (−3.19, −0.88) | 0.0006 | |
| Time (0.0756) | 2010–2013 (Ref) | 23 | 53% (41–65) | 40.83 | 46.1% | – | |
| 2014–2017 | 44 | 68% (58–76) | 65.66 | 34.5% | 0.69 (−0.19, 1.57) | 0.1259 | |
| 2018–2021 | 73 | 77% (66–86) | 68.52 | 0.0% | 0.94 (0.13, 1.76) | 0.023 | |
*Statistically significant (P value < 0.05)
Source
The prevalence of overall antimicrobial resistance percentage was highest in the samples from the environmental origin (78%, 95% CI: 65.0–88.0), with a zero heterogeneity (I2 = 0.00%), followed by samples from animal sources (77%, 95% CI: 65.0–86.0, I2 = 26.9%) and human origin (60%, 95% CI; 50.0–69.0, I2 = 28.8%) (Table 7).
Locality
The drug resistance of semi-urban areas was 96% (95% CI: 79.0–99.0, I2 = 0.0%), meaning that 96% of all Salmonella are resistant to antimicrobials. Rural and urban areas had a resistance of 61% (95% CI: 47.0–73.0, I2 = 35.2%) and 69% (95% CI: 60.0–76.0, I2 = 21%), respectively.
Time
The temporal distribution of the overall antimicrobial resistance percentage against Salmonella shows an increasing pattern. It was 53% in between 2010 and 2013, then increased to 68% in 2014–2017, and finally rose to 77% in 2018–2021.
Source of Heterogeneity in Overall Antimicrobial Resistance Percentage
In univariate meta-regression, two sources of heterogeneity were identified as significant in the occurrence of the overall antimicrobial resistance: Source (P = 0.0315) and Locality (P = 0.005). The group time was found borderline significant (P = 0.0756); thus, it was included in the multivariate meta-regression. No interactions were included in the multivariate due to a lack of significant correlation among them. In multivariate meta-regression (Table 8), source, locality, and time were found to be significant. Samples of human origin (P = 0.0083) were significantly different from animal and environmental sources. Considering locality, the overall antimicrobial resistance in both rural (P = 0.0022) and urban areas (P = 0.0112) varied significantly from the semi-urban area. In the case of time, drug resistance varied significantly in recent years than the past.
Table 8.
Final multivariate meta-regression analysis with different variables of antimicrobial-resistant Salmonella
| Variables | Observations | Co-efficient | P-value |
|---|---|---|---|
| Source | Animal (Ref) | – | |
| Environment | 0.02 (−0.12, 0.16) | 0.7750 | |
| Human | −0.13 (−0.24, −0.02) | 0.0188* | |
| Location | Semi-urban (Ref) | – | |
| Rural | −0.18 (−0.38, −0.006) | 0.0576 | |
| Urban | −0.12 (−0.29, 0.04) | 0.1466 | |
| Time | 2010–2013 (Ref) | – | |
| 2014–2017 | 0.19 (0.04, 0.33) | 0.0155* | |
| 2018–2021 | 0.17 (0.03, 0.30) | 0.0148* | |
| Test | PCR (Ref) | – | |
| Culture | 0.07 (−0.05, 0.20) | 0.2565 | |
| Culture and biochemical | 0.09 (−0.05, 0.23) | 0.2257 | |
| Culture and PCR | 0.15 (−0.08, 0.38) | 0.1909 | |
| Biochemical | 0.07 (−0.12, 0.26) | 0.4636 | |
| Antisera | 0.29 (−0.28, 0.86) | 0.3191 |
*Statistically significant (P value < 0.05)
Publication Bias
The funnel plot indicates that there are some publication biases present in this meta-analysis (Fig. 3), however, Egger’s test result indicates that the funnel plot asymmetry is not significant (P = 0.3130). Thus, we can reject the concern of significant publication bias which might mask the original prevalence of Salmonella. The contour funnel indicates a substantial contribution of the studies to the overall meta-analysis in different levels of significance.
Fig. 3.
Funnel plot of meta-analysis showing publication bias in studies reporting the prevalence of Salmonella
Discussion
AMR is a worldwide health concern affecting significantly low- and middle-income countries, though high-income countries are also facing the threat of AMR at an alarming level. Antibiotic-resistant bacterial infections caused an estimated 1.2 million deaths in 2019, more deaths than AIDS or malaria [26]. Salmonella is globally one of the leading causes of human death among diarrheal diseases. Understanding the epidemiological status of Salmonella is thus crucial for controlling this pathogen [27]. This meta-analysis focuses on a comprehensive and robust assessment of current primary research on the prevalence of Salmonella in humans, animals, and the environment and its antimicrobial resistance status in South Asia.
This study indicates that the pooled prevalence of Salmonella was 14.47% (95% CI: 10.17–20.19) in South Asia. The subgroup analysis indicates the highest pooled prevalence of Salmonella in Bangladesh (34.24%, 95% CI: 23.73–46.56) followed by Pakistan (26.15%, 95% CI: 15.04–41.47), and Nepal (10.36%, 95% CI: 3.73–25.63). On the other hand, India had the lowest prevalence of Salmonella (P-value < 0.0001). This variation might be due to the difference in temperature, humidity, and other weather-related events [28], food habits [29], and availability of migratory birds [30]. The number of studies included might be a possible reason for the country-wise pooled prevalence variation. For instance, only a single study was found in Sri Lanka and Bhutan which may not reflect the actual pooled prevalence. The pooled prevalence of Salmonella among human samples was 5.81% (95% CI: 3.17–10.40). A study in the Middle East and Northern Africa reported almost similar pooled prevalence of Salmonella in humans [31]. The prevalence of Salmonella in humans was lower than in animals (22.66%, 95% CI: 15.41–32.03) and in the environment (27.81%, 95% CI: 12.01–52.07). One of the possible reasons for the comparatively higher prevalence of Salmonella in the environment is that they can survive both in soil and water and even may transfer with flies from different hosts to the environment [32]. Moreover, not all the Salmonella species can transmit from the environment to animals as well as animals to humans [33]. Additionally, unhygienic animal husbandry practices were recorded in South Asia which may be a potential cause of higher prevalence in animals [34, 35]. When categorizing into regions, the prevalence of Salmonella was found higher in semi-urban regions (35.77%, 95% CI: 8.25–34.50) than in the rural (8.85%, 95% CI: 5.10–14.91) and urban regions (17.02%, 95% CI: 10.42–26.56). Though rural communities have higher exposure to zoonotic pathogens due to frequent contact with wild and domesticated animals, there are limited disease surveillance programs in rural areas, and they receive less healthcare support [36]. Nevertheless, we found comparatively less prevalence in rural areas than in semi-urban and urban regions. This might be due to high population density in urban areas [37] which leads to greater transmission of infectious agents [38]. This meta-analysis found 18 different types of Salmonella serovar. This study found that enteric Salmonella is more evident than other serovars in South Asia. Some other serovars like S. Kentucky, S. salamae, S. houtenae, etc. are also present in fewer numbers, however, they may arise as a significant threat [39, 40]. The transmission of Salmonella enterica Serovar Kentucky has a travel association and Southeast Asia is at major risk [41]. Likewise, local S. typhi and S. Paratyphi A strains were identified in Nepal which had close genetic relatives in other South Asian countries, which highlights a major public health concern with inter and intra-country transmission [42].
Salmonella has a broad-spectrum host range, hence is considered as a universal pathogen. Each serovar has a different ability to adapt to the host environment and cause virulency. Some Salmonella serovars are restricted within one host whereas some have broad host spectrum [43]. For instance, Salmonella enterica serovars were isolated in majority of cases (99.5%) from animals and humans [43]. With no exception, in this study, we found that Salmonella enterica serovar was highly prevalent in humans (2.92%), animals (34.71%) and the environment (14.42%). Similarly, S. enteritidis and S. typhimurium were observed in all source-specific samples, even though not highly prevalent. On the other hand, S. pullorum is known to host-restrictive serovar only in poultry as primary host [44]. Of note, in this study, no human cases of S. pullorum are found but highly prevalent in animal-specific samples (22.67%). Therefore, the ability of a pathogen to spread disease in populations in many respects is influenced by host adaptation. Asymptomatic animals can shed the bacterium continually via feces, and Salmonella regardless of serovar can persist in dry environments as well as in water for many weeks to months. These animals can contaminate environment and directly transmit pathogens to susceptible hosts [44]. Thus, in general sense, environment-specific samples may serve as a good source of respective bacterium and become a risk to susceptible hosts.
We analyzed the resistance percentage of Salmonella against different antibiotics. In the present study, the resistance of Salmonella to nalidixic acid was 74.25%, fluoroquinolone 67.67%, tetracycline 37.64%, trimethoprim/sulfamethoxazole 32.92%, and amoxicillin 32.18%. There was an increasing trend in the prevalence (53–77%) of overall antimicrobial-resistant Salmonella from 2010–2013 to 2018–2021. Indiscriminate application of antibiotics in human and animal health, and food production and subsequently leaching of the antibiotics into the environment are contributing to the increased AMR bacteria [22]. In South Asia, S. typhi and S. Paratyphi were reported as endemic, and several antibiotics were used for enteric fever resulting in the development of antimicrobial resistance by these antibiotics [45]. Due to the resistance of most Salmonella species to first-line antibiotics in clinical cases, critically important antibiotics such as fluoroquinolones, third and fourth generation cephalosporins, macrolide, etc. have become a choice for the treatment of invasive Salmonella infections [8, 46]. As a consequence of increasing resistance, WHO enlisted fluoroquinolone-resistant Salmonella spp. in the list of priority pathogens describing the urgent need for antibiotics against these bacteria [8].
In our result, the variability in the prevalence of Salmonella is strongly evident (I2 > 96%). A meta-regression model was used to assess the influence of different variables as well as methodological variation on the prevalence estimation of Salmonella. Different variables i.e., different sources of sample, country, and location might be responsible for the between-study variation which is obvious in our final multivariable model. Different countries and locations have diverse geographic patterns, seasonal variations, different food habits of people, and economic disparities which are some contributing factors to the variance in the prevalence of Salmonella [47]. Furthermore, different sample sources, for instance, environmental samples are often affected by various climatic events (e.g., temperature and rainfall), water temperature, soil moisture, soil types, presence of plants, etc. Again, types of animal farms may also add variations in the prevalence. For example, Salmonella was reported to be identified more frequently in swine farms compared to dairy and poultry farms [48]. The difference in the estimation of the prevalence may be partly due to the sorts of specimens obtained, for instance, blood culture, rectal swabs, feces from intestines, feces from rectums, voided feces, and mixed samples. On the other hand, global heterogeneity in the prevalence of Salmonella in animals was detected which may be due to the variation in the methodological procedures used in the isolation and identification of the organism (Supplementary Data). For instance, the isolation of Salmonella from whole feces may be different from the isolation from fecal swabs in terms of sensitivity. Also, culture-based testing of bacteria may not often reflect the actual prevalence (i.e., bacteria may present as a viable but non-culturable state) which might also add variation in the pooled prevalence [48]. With that in mind, random-effect model analysis is usually recommended in this situation assuming real differences in sampling variability.
The funnel plot was used to quantify and illustrate the extent of publication bias in the selected studies. The funnel is evidently not symmetrical and some of the points fall outside of it which indicates the presence of publication bias. The sources of the funnel plot asymmetry were tested by the Egger test to confirm the small study effects. The estimated bias coefficient was 6.79 with a standard error of 0.084 (P = 0.31). As a result, the test reveals a deficiency of evidence supporting the small study effects present. However, there are many different possible factors for funnel plot asymmetry, namely selection bias, true heterogeneity, data irregularities, artifacts as well as by-chance [49]. In this meta-analysis, unpublished papers, conference abstracts, and government reports were excluded since they seldom contain enough information to allow for relevant screening, data extraction, and analysis.
Conclusion
In conclusion, the pooled prevalence of Salmonella was 14.47% from 2010 to 2021 in South Asia. The random effects pooled prevalence in Bangladesh, India, Pakistan, Nepal, Bhutan, and Sri Lanka were 34.24%, 3.28%, 26.15%, 10.36%, 12.78%, and 2.04% respectively, although there was heterogeneity between studies in most of these regions. The prevalence of overall antimicrobial resistance Salmonella was increased from 2010–2013 to 2018–2021 as 53–77%. Resistance to quinolones, tetracycline, trimethoprim/sulfamethoxazole, and amoxicillin was comparatively higher. This study indicates that the prevalence of AMR Salmonella is increasing with time in South Asia, and there are multiple potential reasons for this. Therefore, proper use of antimicrobials, regular surveillance of AMR, implementing antibiotic stewardship, and policy making, and implementation is necessary for controlling the spread of antibiotic resistant bacteria.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Abbreviations
- AIDS
Acquired immunodeficiency syndrome (AIDS)
- AST
Antimicrobial susceptibility testing
- AMR
Antimicrobial resistant
- CDC
Centers for disease control and prevention
- CI
Confidence interval
- GDP
Gross domestic product
- HIV
Human immunodeficiency virus
- NTS
Non-typhoidal Salmonella (NTS)
- PCR
Polymerase chain reaction
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- USA
United States of America
- WHO
World Health Organization
Author Contributions
Conception and design: HT, SAR, KD, BS, JA, SR. Conducted research and data acquisition: HT, SAR, KD, BS, SR. Analysis and interpretations of data: HT, SAR, KD, BS, JA, SR. Manuscript writing—original draft preparation: HT, JA, and SR. Manuscript review and editing: JA and SR. All authors reviewed and approved the finalized manuscript.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of Data and Materials
All data generated and analyzed during this study are included in the main manuscript or supplementary files.
Declarations
Conflict of Interest
Authors declare no conflict of interest.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All authors consented to submission for publication.
Footnotes
Shamsul Alam Roky, Konad Debnath, and Binayok Sharma contributed equally.
References
- 1.Punchihewage-Don AJ, Hawkins J, Adnan AM, et al. The outbreaks and prevalence of antimicrobial resistant Salmonella in poultry in the United States: an overview. Heliyon. 2022;8:e11571. doi: 10.1016/J.HELIYON.2022.E11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fookes M, Schroeder GN, Langridge GC, et al. Salmonella bongori provides insights into the evolution of the Salmonellae. PLoS Pathog. 2011;7:e1002191. doi: 10.1371/JOURNAL.PPAT.1002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gal-Mor O, Boyle EC, Grassl GA. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol. 2014;5:391. doi: 10.3389/FMICB.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tennant SM, MacLennan CA, Simon R, et al. Nontyphoidal salmonella disease: current status of vaccine research and development. Vaccine. 2016;34:2907–2910. doi: 10.1016/J.VACCINE.2016.03.072. [DOI] [PubMed] [Google Scholar]
- 5.Wiedemann A, Virlogeux-Payant I, Chauss A-M, et al. Interactions of Salmonella with animals and plants. Front Microbiol. 2015;5:791. doi: 10.3389/fmicb.2014.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eng SK, Pusparajah P, Ab Mutalib NS, et al. Salmonella: a review on pathogenesis, epidemiology and antibiotic resistance. Front Life Sci. 2015;8:284–293. doi: 10.1080/21553769.2015.1051243. [DOI] [Google Scholar]
- 7.Gong B, Li H, Feng Y, et al. Prevalence, serotype distribution and antimicrobial resistance of non-typhoidal Salmonella in hospitalized patients in Conghua district of Guangzhou, China. Front Cell Infect Microbiol. 2022;12:805384. doi: 10.3389/FCIMB.2022.805384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuypers WL, Jacobs J, Wong V, et al. Fluoroquinolone resistance in Salmonella: insights by whole genome sequencing. Microb Genom. 2018;4:e000195. doi: 10.1099/mgen.0.000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novoa Rama E, Bailey M, Kumar S, et al. Prevalence and antimicrobial resistance of Salmonella in conventional and no antibiotics ever broiler farms in the United States. Food Control. 2022;135:108738. doi: 10.1016/J.FOODCONT.2021.108738. [DOI] [Google Scholar]
- 10.Lou L, Zhang P, Piao R, Wang Y. Salmonella Pathogenicity Island 1 (SPI-1) and its complex regulatory network. Front Cell Infect Microbiol. 2019;9:270. doi: 10.3389/FCIMB.2019.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faulder KE, Simmonds K, Robinson JL. The epidemiology of childhood Salmonella infections in Alberta, Canada. Foodborne Pathog Dis. 2017;14:364–369. doi: 10.1089/FPD.2016.2259. [DOI] [PubMed] [Google Scholar]
- 12.Kimura AC, Reddy V, Marcus R, et al. Chicken consumption is a newly identified risk factor for sporadic Salmonella enterica serotype enteritidis infections in the United States: a case–control study in FoodNet sites. Clin Infect Dis. 2004;38:S244–S252. doi: 10.1086/381576. [DOI] [PubMed] [Google Scholar]
- 13.Acheson D, Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis. 2001;32:263–269. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Escobedo G, Marshall JM, Gunn JS. Chronic and acute infection of the gall bladder by Salmonella typhi: understanding the carrier state. Nat Rev Microbiol. 2011;9:9–14. doi: 10.1038/NRMICRO2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schikora A, Garcia AV, Hirt H. Plants as alternative hosts for Salmonella. Trends Plant Sci. 2012;17:245–249. doi: 10.1016/J.TPLANTS.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Espunyes J, Illera L, Dias-Alves A, et al. Eurasian griffon vultures carry widespread antimicrobial resistant Salmonella and Campylobacter of public health concern. Sci Total Environ. 2022;844:157189. doi: 10.1016/J.SCITOTENV.2022.157189. [DOI] [PubMed] [Google Scholar]
- 17.Chen HM, Wang Y, Su LH, Chiu CH. Nontyphoid salmonella infection: microbiology, clinical features, and antimicrobial therapy. Pediatr Neonatol. 2013;54:147–152. doi: 10.1016/J.PEDNEO.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Carattoli A. Plasmid-mediated antimicrobial resistance in Salmonella enterica. Curr Issues Mol Biol. 2003;5:113–122. doi: 10.21775/cimb.005.113. [DOI] [PubMed] [Google Scholar]
- 19.Sakkas H, Bozidis P, Ilia A, et al. Antimicrobial resistance in bacterial pathogens and detection of carbapenemases in Klebsiella pneumoniae isolates from hospital wastewater. Antibiotics (Basel) 2019;8:85. doi: 10.3390/ANTIBIOTICS8030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control. 2017;6:1–8. doi: 10.1186/S13756-017-0208-X/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medina M, Legido-Quigley H, Hsu LY. Antimicrobial resistance in one health. In: Masys AJ, Izurieta R, Reina Ortiz M, editors. Global health security. Advanced sciences and technologies for security applications. Cham: Springer; 2020. pp. 209–229. [Google Scholar]
- 22.Chereau F, Opatowski L, Tourdjman M, Vong S. Risk assessment for antibiotic resistance in South East Asia. BMJ. 2017;358:2–8. doi: 10.1136/BMJ.J3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:332–336. doi: 10.1136/BMJ.B2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/BMJ.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Murray CJ, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrari RG, Rosario DKA, Cunha-Neto A, et al. Worldwide epidemiology of Salmonella serovars in animal-based foods: a meta-analysis. Appl Environ Microbiol. 2019;85:e00591–e619. doi: 10.1128/AEM.00591-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edelson PJ, Harold R, Ackelsberg J, et al. Climate change and the epidemiology of infectious diseases in the United States. Clin Infect Dis. 2023;76:950–956. doi: 10.1093/CID/CIAC697. [DOI] [PubMed] [Google Scholar]
- 29.Ehuwa O, Jaiswal AK, Jaiswal S. Salmonella, food safety and food handling practices. Foods. 2021;10:907. doi: 10.3390/FOODS10050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saiful Islam M, Paul A, Talukder M, et al. Migratory birds travelling to Bangladesh are potential carriers of multi-drug resistant Enterococcus spp., Salmonella spp., and Vibrio spp. Saudi J Biol Sci. 2021;28:5963–5970. doi: 10.1016/J.SJBS.2021.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Rifai RH, Chaabna K, Denagamage T, Alali WQ. Prevalence of enteric non-typhoidal Salmonella in humans in the Middle East and North Africa: a systematic review and meta-analysis. Zoonoses Public Health. 2019;66:701–728. doi: 10.1111/ZPH.12631. [DOI] [PubMed] [Google Scholar]
- 32.Winfield MD, Groisman EA. Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Appl Environ Microbiol. 2003;69:3687–3694. doi: 10.1128/AEM.69.7.3687-3694.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spanò S. Host restriction in Salmonella: insights from Rab GTPases. Cell Microbiol. 2014;16:1321–1328. doi: 10.1111/CMI.12327. [DOI] [PubMed] [Google Scholar]
- 34.Parkunan T, Ashutosh M, Sukumar B, et al. Antibiotic resistance: a cross-sectional study on knowledge, attitude, and practices among veterinarians of Haryana state in India. Vet World. 2019;12:258–265. doi: 10.14202/VETWORLD.2019.258-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roess AA, Winch PJ, Akhter A, et al. Household animal and human medicine use and animal husbandry practices in rural Bangladesh: risk factors for emerging zoonotic disease and antibiotic resistance. Zoonoses Public Health. 2015;62:569–578. doi: 10.1111/ZPH.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worsley-Tonks KEL, Bender JB, Deem SL, et al. Strengthening global health security by improving disease surveillance in remote rural areas of low-income and middle-income countries. Lancet Glob Health. 2022;10:e579–e584. doi: 10.1016/S2214-109X(22)00031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Streatfield PK, Karar ZA. Population challenges for Bangladesh in the coming decades. J Health Popul Nutr. 2008;26:261. doi: 10.3329/jhpn.v26i3.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu H, Nigmatulina K, Eckhoff P. The scaling of contact rates with population density for the infectious disease models. Math Biosci. 2013;244:125–134. doi: 10.1016/J.MBS.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 39.Haley BJ, Kim SW, Haendiges J, et al. Salmonella enterica serovar Kentucky recovered from human clinical cases in Maryland, USA (2011–2015) Zoonoses Public Health. 2019;66:382–392. doi: 10.1111/ZPH.12571. [DOI] [PubMed] [Google Scholar]
- 40.Andruzzi MN, Krath ML, Lawhon SD, Boudreau B. Salmonella enterica subspecies houtenae as an opportunistic pathogen in a case of meningoencephalomyelitis and bacteriuria in a dog. BMC Vet Res. 2020;16:437. doi: 10.1186/S12917-020-02652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park AK, Shin E, Kim S, et al. Traveller-associated high-level ciprofloxacin-resistant Salmonella enterica Serovar Kentucky in the Republic of Korea. J Glob Antimicrob Resist. 2020;22:190–194. doi: 10.1016/J.JGAR.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 42.Britto CD, Dyson ZA, Duchene S, et al. Laboratory and molecular surveillance of paediatric typhoidal Salmonella in Nepal: ANTIMICROBIAL resistance and implications for vaccine policy. PLoS Negl Trop Dis. 2018;12:e0006408. doi: 10.1371/JOURNAL.PNTD.0006408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evangelopoulou G, Kritas S, Govaris A, Burriel AR. Animal salmonelloses: a brief review of “host adaptation and host specificity” of Salmonella spp. Vet World. 2013;6:703–708. doi: 10.14202/vetworld.2013.703-708. [DOI] [Google Scholar]
- 44.Jajere SM. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and adaptation and antimicrobial resistance including multidrug resistance. Vet World. 2019;12:504–521. doi: 10.14202/vetworld.2019.504-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akhtar S, Sarker MR, Jabeen K, et al. Antimicrobial resistance in Salmonella enterica serovar typhi and paratyphi in South Asia-current status, issues and prospects. Crit Rev Microbiol. 2015;41:536–545. doi: 10.3109/1040841X.2014.880662. [DOI] [PubMed] [Google Scholar]
- 46.Collignon PC, Conly JM, Andremont A, et al. World Health Organization ranking of antimicrobials according to their importance in human medicine: a critical step for developing risk management strategies to control antimicrobial resistance from food animal production. Clin Infect Dis. 2016;63:1087–1093. doi: 10.1093/CID/CIW475. [DOI] [PubMed] [Google Scholar]
- 47.Akil L, Ahmad HA, Reddy RS. Effects of climate change on Salmonella infections. Foodborne Pathog Dis. 2014;11:974–980. doi: 10.1089/fpd.2014.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bondo KJ, Pearl DL, Janecko N, et al. Impact of season, demographic and environmental factors on Salmonella occurrence in Raccoons (Procyon lotor) from swine farms and conservation areas in Southern Ontario. PLoS ONE. 2016;11:e0161497. doi: 10.1371/journal.pone.0161497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/BMJ.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed during this study are included in the main manuscript or supplementary files.