Abstract
Background
Molnupiravir is an orally administered antiviral authorized for COVID-19 treatment in adults at high risk of progression to severe disease. Here, we report secondary and post hoc analyses of participants’ self-reported symptoms in the MOVe-OUT trial, which evaluated molnupiravir initiated within 5 days of symptom onset in nonhospitalized, unvaccinated adults with mild-to-moderate, laboratory-confirmed COVID-19.
Methods
Eligible participants completed a 15-item symptom diary daily from day 1 (randomization) through day 29, rating symptom severity as “none,” “mild,” “moderate,” or “severe”; loss of smell and loss of taste were rated as “yes” or “no.” Time to sustained symptom resolution/improvement was defined as the number of days from randomization to the first of 3 consecutive days of reduced severity, without subsequent relapse. Time to symptom progression was defined as the number of days from randomization to the first of 2 consecutive days of worsening severity. The Kaplan-Meier method was used to estimate event rates at various time points. The Cox proportional hazards model was used to estimate the hazard ratio between molnupiravir and placebo.
Results
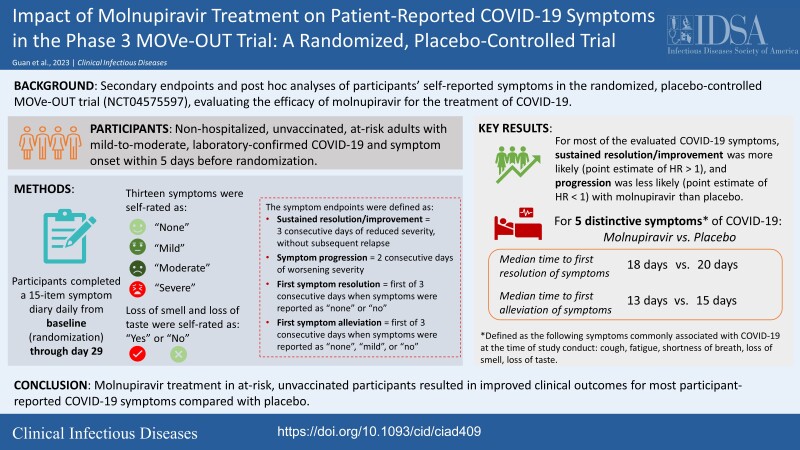
For most targeted COVID-19 symptoms, sustained resolution/improvement was more likely, and progression was less likely, in the molnupiravir versus placebo group through day 29. When evaluating 5 distinctive symptoms of COVID-19, molnupiravir participants had a shorter median time to first resolution (18 vs 20 d) and first alleviation (13 vs 15 d) of symptoms compared with placebo.
Conclusions
Molnupiravir treatment in at-risk, unvaccinated patients resulted in improved clinical outcomes for most participant-reported COVID-19 symptoms compared with placebo.
Clinical Trials Registration. ClinicalTrials.gov: NCT04575597.
Keywords: SARS-CoV-2, antiviral, β-D-N4-hydroxycytidine, clinical trial, public health
Secondary and post hoc analyses of patient-reported outcome data from the phase 3 component of MOVe-OUT demonstrated improved clinical outcomes with molnupiravir treatment—both for sustained resolution/improvement and progression—for most targeted COVID-19 symptoms compared with placebo.
Graphical abstract
Graphical Abstract.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can be asymptomatic or may develop into coronavirus disease 2019 (COVID-19) with a variable clinical course ranging from mild to critical illness. There is a need for more effective drugs against COVID-19 that not only improve patient outcomes but that can be self-administered in the outpatient setting to reduce the impact of SARS-CoV-2 infection. Molnupiravir is an orally administered prodrug of the small-molecule, nucleoside-analogue β-D-N4-hydroxycytidine (NHC), which has demonstrated broad-spectrum antiviral activity against SARS-CoV-2 and its variants [1–7]. In the global phase 3, randomized, placebo-controlled, double-blind, multicenter MOVe-OUT trial assessing the safety and efficacy of molnupiravir in at-risk, unvaccinated, nonhospitalized adults with laboratory-confirmed COVID-19, molnupiravir demonstrated a clinically meaningful reduction in the risk of all-cause hospitalization or death [8].
Patient-reported outcomes (PROs), which refer to any report of the status of a patient's health condition that comes directly from the patient, are useful because they enhance the understanding of patients’ experiences with the study intervention compared with investigator-assessed or biomedical measures alone [9]. Another advantage of evaluating PROs in clinical trials is improved communication between patients and healthcare providers/investigators, which may further support adherence and provide better understanding of treatment benefits [9, 10]. The increased use of PROs in clinical trials in recent years may be attributed to the recognition of PROs as appropriate measures of treatment efficacy and/or supporting data for efficacy outcomes and encouragement for use by regulatory bodies such as the US Food and Drug Administration (FDA) and the European Medicines Agency [9, 10].
The COVID-19 pandemic highlighted the importance of PRO endpoints that can address various aspects of patient health, including symptoms, quality of life, and functionality [11]. Furthermore, symptom-based assessments are becoming more insightful for the evaluation of COVID-19 treatments because of the decrease in hospitalizations or deaths due to emerging viral variants that are less virulent and higher rates of preexisting vaccine- and infection-induced host immunity [12, 13]. For clinical trials evaluating drugs for the prevention or treatment of COVID-19 in outpatient adults and adolescents, the FDA has recommended systematically assessing key COVID-19‒related symptoms reported by patients to provide an accurate evaluation of treatment benefit [14].
To assess the impact of molnupiravir treatment on COVID-19 symptoms reported directly from patients, we present participant-reported symptoms data from the phase 3 component of MOVe-OUT. Key secondary objectives of MOVe-OUT were to evaluate the efficacy of molnupiravir compared with placebo as assessed by time to sustained resolution/improvement, and time to progression, of targeted self-reported COVID-19 symptoms daily from baseline through day 29.
METHODS
The trial protocol (no. MK-4482-002) and all amendments were approved by the appropriate institutional review boards. The trial was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent prior to enrollment.
Trial Design
Details of MOVe-OUT (ClinicalTrials.gov NCT04575597) have been previously reported [8, 15]. This paper reports on the participant-reported symptom outcomes in the phase 3 component of the trial conducted between May and November 2021. Participants aged 18 years and older with laboratory-confirmed SARS-CoV-2 infection 5 or fewer days prior to enrollment were randomized in a 1:1 ratio to receive oral molnupiravir 800 mg or placebo twice daily for 5 days. The first dose of the trial drug was administered within 24 hours of randomization. The modified intention-to-treat (MITT) population, which is the primary population for evaluation of efficacy, included all randomized participants who received at least 1 dose of the trial drug and were not hospitalized before the first dose. Trial visits occurred at screening and on days 1 (baseline), 3, 5 (end of therapy [EOT]), 10, 15, and 29.
Key inclusion criteria included having at least 1 risk factor for progression to severe COVID-19 (>60 y old, active cancer, chronic kidney disease, chronic obstructive pulmonary disease, body mass index [BMI] ≥30 kg/m2, serious heart conditions [including heart failure, coronary artery disease, or cardiomyopathies], or diabetes mellitus) and at least 1 symptom attributable to COVID-19 with onset 5 or fewer days prior to randomization. Key exclusion criteria included current hospitalization or an expected need for hospitalization due to COVID-19 within 48 hours of randomization, receipt of certain therapies indicated specifically for the treatment of COVID-19, or SARS-CoV-2 vaccination.
Outcomes
Participants completed a 15-item paper symptom diary daily starting from baseline through day 29. The daily symptom diary content was based on a common COVID-19 symptom list from the US Centers for Disease Control and Prevention [16] and refined based on feedback from clinicians and regulatory agencies. The format and wording (instructions, symptom description, and response options) were based on internal PRO development experience using patient-friendly language. The final diary was aligned with the FDA guidance released in September 2020 [14]. The symptoms evaluated included cough, sore throat, nasal congestion (stuffy nose), runny nose, shortness of breath or difficulty breathing, muscle or body aches, fatigue (tiredness), feeling hot or feverish, chills, headache, nausea, vomiting, diarrhea, loss of smell, and loss of taste. Symptom severity was rated as “none,” “mild,” “moderate,” or “severe”; loss of smell and loss of taste were rated as “yes” or “no.”
On day 1, participants were required to complete the symptom diary prior to the first dose of the trial drug. Completion of the symptom diary was observed at the trial site or in-home visit and documented, including time of participant completion, by trial staff/home healthcare professional(s). Following day 1, participants were requested to complete the symptom diary daily at approximately the same time through day 29, recording and rating each symptom during the prior 24 hours at its worst (ie, none, mild, moderate, or severe) for 13 symptoms and indicating symptom presence (ie, no, yes) for loss of smell and loss of taste. If a participant was unable to record their responses on the symptom diary by themselves, the trial staff would support completion using an interview script. To support compliance with diary completion, the symptom diary was reviewed by trial staff with each participant at each visit, and participants were provided with regular reminders (eg, telephone call, e-mail, text messages) following the EOT visit, every other day through day 29.
Time to sustained resolution/improvement of symptoms was defined per protocol as the number of days from baseline to the first of 3 consecutive days of reduced severity, without subsequent relapse (ie, ≥2 consecutive days without returning to baseline severity, or worse than baseline severity) by day 29. Time to progression was defined per protocol as the number of days from baseline to the first of 2 consecutive days of worsening severity. Additionally, 5 distinctive symptoms commonly associated with COVID-19 at the time of trial conduct (ie, shortness of breath or difficulty breathing, cough, fatigue, loss of smell, and loss of taste) were evaluated post hoc to investigate the timing of symptom resolution/improvement, or symptom progression, early in the disease course and the proportion of participants reporting “moderate” and “severe” symptom severity over time.
Additional post hoc endpoints, including time to first symptom resolution (ie, the number of days from baseline to the first of 3 consecutive days when symptoms were reported as “none” or “no”) and time to first symptom alleviation (ie, the number of days from baseline to the first of 3 consecutive days when symptoms were scored as “moderate” or “severe” at baseline and subsequently as “none” or “mild,” or scored as “mild” at baseline and subsequently as “none”), were also assessed for these 5 distinctive symptoms. For loss of smell and loss of taste, alleviation was defined as the first day of 3 consecutive days when symptoms scored as “yes” at baseline were scored as “no.” Participants who reported “none” or “no” symptoms at baseline and throughout day 29 were censored at time 0. For participants who reported “none” or “no” symptoms at baseline but who developed symptoms post-baseline, time to first symptom resolution was defined as the number of days from baseline to the first of 3 consecutive days when symptoms were reported as “none” or “no” and time to first symptom alleviation was defined as the number of days from baseline to the first of 3 consecutive days when symptoms were alleviated.
The Platform Adaptive Trial of Novel Antivirals for Early Treatment of COVID-19 in the Community (PANORAMIC) study is an ongoing, UK-based, open-label, prospective, randomized, platform, adaptive, controlled clinical study that evaluated over 25 000 at-risk patients with COVID-19 and included PROs as key secondary outcome measures to evaluate the efficacy of molnupiravir plus usual care compared with usual care alone in the treatment of COVID-19 [17]. Since this study consisted of a large dataset and demonstrated use of symptom-based endpoints as outcome measures, we conducted similar post hoc analyses, including time to alleviation and time to sustained alleviation, of all 15 symptoms. Time to alleviation of all 15 symptoms was defined as the time from randomization to the first day that all 15 symptoms were alleviated (symptoms scored as “moderate” or “severe” at baseline and subsequently scored as “none” or “mild”; for loss of smell and loss of taste, symptoms scored as “yes” at baseline and subsequently scored as “no”). Time to sustained alleviation of all 15 symptoms was defined as the time from randomization to the first day that all 15 symptoms were alleviated (as defined above), without subsequently being reported as “moderate” or “severe” or subsequently reported as “yes” for loss of smell and loss of taste. For individual symptoms, participants who did not have symptom alleviation were censored at the last date they recorded that specific symptom. Participants who died were censored at day 29.
Statistical Analysis
Time to sustained resolution/improvement and time to progression, and the additional post hoc analyses of time to event (ie, first symptom resolution/improvement, first symptom alleviation, alleviation of all reported symptoms, and sustained alleviation of all reported symptoms), were evaluated based on Kaplan-Meier estimates. Hazard ratios (HRs) between molnupiravir and placebo were estimated based on a stratified Cox regression model, which was adjusted for the randomization stratum (ie, participants’ time from symptom onset prior to the day of randomization [≤3 d, >3 d]). Analyses were descriptive and not adjusted for multiplicity. Missing symptom diary data were imputed using the last-observation-carried-forward method.
RESULTS
The MITT population in MOVe-OUT included 1408 participants: 709 received molnupiravir and 699 received placebo. The median age was 43.0 (range: 18–90) years, and more than half of participants (51.3%) were female. The median time from symptom onset to randomization was 4 (range: 1–5) days. The most common risk factors for severe COVID-19 were obesity (BMI ≥30 kg/m2; 74.0%), aged older than 60 years (17.4%), and diabetes mellitus (15.9%). Of the 5 distinctive symptoms commonly associated with COVID-19 at the time of trial conduct, the frequency reported at baseline were cough (81.7%), fatigue (76.1%), loss of smell (45.9%), shortness of breath or difficulty breathing (37.0%), and loss of taste (36.1%) (Table 1). Compliance with the symptom diary was high, with completion rates of more than 97% from baseline through day 5 (EOT) and more than 92% from baseline through day 29 (Table 2).
Table 1.
Baseline Demographics and Clinical Characteristics in the MITT Population
| Characteristics | Molnupiravir (n = 709) | Placebo (n = 699) | Total (N = 1408) |
|---|---|---|---|
| Female, n (%) | 379 (53.5) | 344 (49.2) | 723 (51.3) |
| Median (range) age, y | 43 (18–90) | 44 (18–88) | 43 (18–90) |
| Median (range) time from symptom onset to randomization, d | 4 (1–5) | 4 (1–5) | 4 (1–5) |
| Risk factors for progression to severe COVID-19, n (%) | |||
| BMI ≥30 kg/m2 | 535 (75.5) | 507 (72.5) | 1042 (74.0) |
| Age >60 y | 118 (16.6) | 127 (18.2) | 245 (17.4) |
| Diabetes mellitus | 107 (15.1) | 117 (16.7) | 224 (15.9) |
| Serious heart condition | 86 (12.1) | 78 (11.2) | 164 (11.6) |
| Chronic kidney disease | 38 (5.4) | 43 (6.2) | 81 (5.8) |
| Moderate renal impairment | 41 (5.8) | 48 (6.9) | 89 (6.3) |
| Chronic obstructive pulmonary disease | 22 (3.1) | 34 (4.9) | 56 (4.0) |
| Race, n (%) | |||
| White | 397 (56.0) | 405 (57.9) | 802 (57.0) |
| Multiple | 188 (26.5) | 195 (27.9) | 383 (27.2) |
| American Indian or Alaska Native | 60 (8.5) | 43 (6.2) | 103 (7.3) |
| Black or African-American | 39 (5.5) | 34 (4.9) | 73 (5.2) |
| Asian | 25 (3.5) | 22 (3.1) | 47 (3.3) |
| Five distinctive symptoms commonly associated with COVID-19 at the time of trial conduct, n/N (%) | |||
| Cough | 570/706 (80.7) | 574/694 (82.7) | 1144/1400 (81.7) |
| Fatigue | 538/705 (76.3) | 528/695 (76.0) | 1066/1400 (76.1) |
| Loss of smell | 318/703 (45.2) | 323/695 (46.5) | 641/1398 (45.9) |
| Shortness of breath or difficulty breathing | 258/705 (36.6) | 260/694 (37.5) | 518/1399 (37.0) |
| Loss of taste | 242/703 (34.4) | 262/695 (37.7) | 504/1398 (36.1) |
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; MITT, modified intention-to-treat.
Table 2.
Summary of Symptom Diary Completion Rate for Any Symptom Through Day 29 (MITT Population, N = 1408)
| Visit | Molnupiravir (n = 709), % | Placebo (n = 699), % | ||
|---|---|---|---|---|
| Minimuma | Maximumb | Minimuma | Maximumb | |
| Day 1 (baseline) | 99.0 | 99.7 | 99.3 | 99.6 |
| Day 3 | 99.2 | 99.6 | 99.0 | 99.1 |
| Day 5 (EOT) | 97.7 | 98.2 | 97.3 | 97.6 |
| Day 10 | 95.2 | 95.5 | 95.7 | 95.9 |
| Day 15 | 94.4 | 94.4 | 95.0 | 95.1 |
| Day 29 | 92.9 | 93.2 | 94.1 | 94.4 |
The completion rate for each symptom = number of participants who completed the symptom/number of participants in the MITT population. On any given trial day, each of the 15 symptoms may have a different completion rate due to the implementations of a paper diary (eg, a participant completed the severity of some symptoms but not the others).
Abbreviations: EOT, end of therapy; MITT, modified intention-to-treat.
aMinimum = completion rate of a symptom that was completed by the lowest proportion of participants on that trial day.
bMaximum = completion rate of a symptom that was completed by the highest proportion of participants on that trial day.
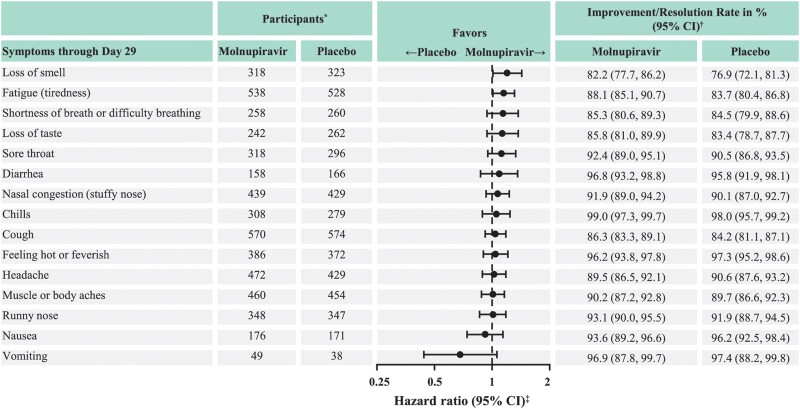
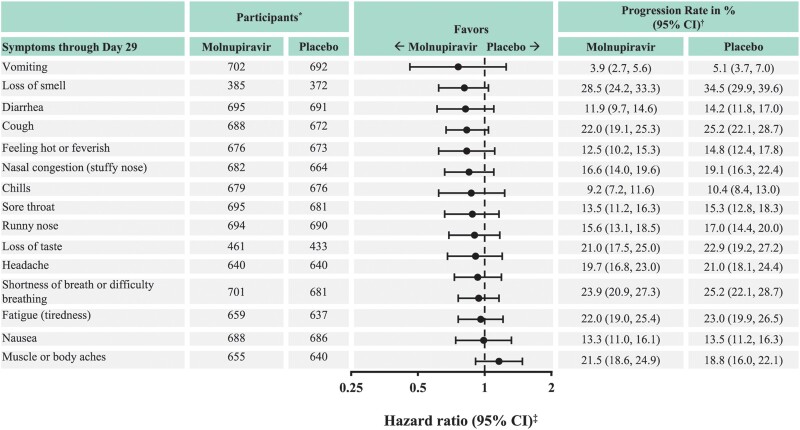
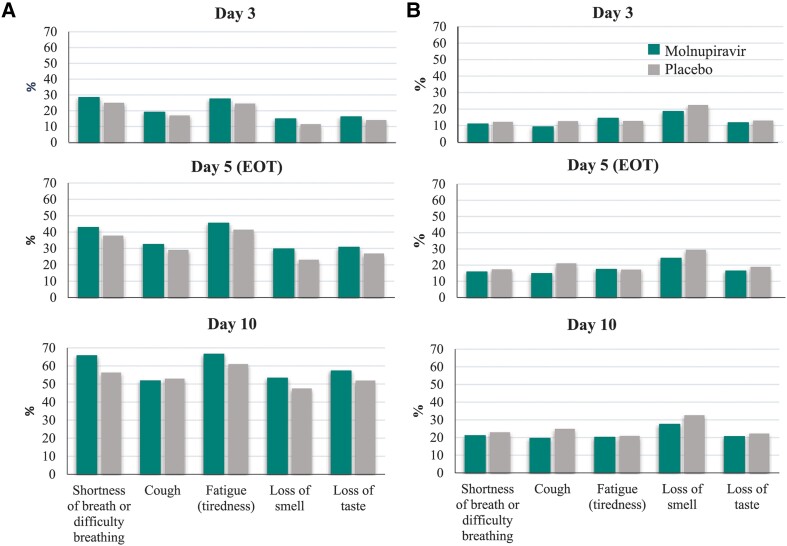
For most COVID-19 symptoms, sustained resolution/improvement was more likely in the molnupiravir group than in the placebo group through day 29 (point estimate of HR >1; the 95% confidence interval [CI] included 1 for most symptoms) (Figure 1). Among the 5 distinctive symptoms commonly associated with COVID-19 at the time of trial conduct, the observed median time to resolution/improvement was shorter for participants in the molnupiravir versus the placebo group for fatigue (tiredness), shortness of breath or difficulty breathing, loss of taste, and loss of smell; the 95% CI included 1 for shortness of breath or difficulty breathing and loss of taste (Table 3). Similarly, for most COVID-19 symptoms, progression was less likely in the molnupiravir group than in the placebo group through day 29 (point estimate of HR <1; the 95% CI included 1 for all symptoms) (Figure 2). Among the 5 distinctive COVID-19 symptoms, participants in the molnupiravir group were more likely to achieve sustained resolution/improvement by day 3, day 5 (EOT), and day 10 (Figure 3A) for all symptoms, except for cough on day 10. Progression of symptoms was also less likely in the molnupiravir group by day 3, day 5 (EOT), and day 10 for all symptoms, except for fatigue on day 3 and day 5 (EOT) (Figure 3B).
Figure 1.
Sustained resolution/improvement of COVID-19 symptoms through day 29 (MITT population). Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; MITT, modified intention-to-treat. *Number of participants eligible for sustained resolution/improvement (ie, those who had the corresponding symptom at baseline) in the MITT population. †From the product-limit (Kaplan-Meier) method for censored data. ‡Based on stratified Cox regression model with Efron's method of tie handling with treatment as covariates and randomization stratum as the stratification factor. A hazard ratio >1 favors the molnupiravir group.
Table 3.
Time to Sustained Resolution/Improvement of Distinctive COVID-19 Symptoms Through Day 29 (MITT Population, N = 1408)
| Sign/Symptom | Median Time to Resolution/Improvement (95% CI), d | HR (95% CI) | |
|---|---|---|---|
| Molnupiravir (n = 709) | Placebo (n = 699) | ||
| Fatigue (tiredness) | 6.0 (6.0–7.0) | 7.0 (6.0–8.0) | 1.15 (1.01–1.31) |
| Shortness of breath or difficulty breathing | 6.0 (6.0–8.0) | 9.0 (6.0–10.0) | 1.14 (.94–1.37) |
| Loss of taste | 9.0 (8.0–10.0) | 10.0 (8.0–12.0) | 1.13 (.94–1.37) |
| Cough | 10.0 (9.0–11.0) | 10.0 (8.0–11.0) | 1.04 (.92–1.18) |
| Loss of smell | 10.0 (9.0–11.0) | 11.0 (9.0–14.0) | 1.2 (1.01–1.43) |
Five symptoms commonly associated with COVID-19 at the time of trial conduct are shown. An HR >1 favors the molnupiravir group.
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio; MITT, modified intention-to-treat.
Figure 2.
Progression of COVID-19 symptoms through day 29 (MITT population). Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; MITT, modified intention-to-treat. *Number of participants eligible for sustained resolution/improvement (ie, those who had the corresponding symptom at baseline) in the MITT population. †From the product-limit (Kaplan-Meier) method for censored data. ‡Based on stratified Cox regression model with Efron's method of tie handling with treatment as covariates and randomization stratum as the stratification factor. A hazard ratio <1 favors the molnupiravir group.
Figure 3.
Proportion of participants with (A) sustained resolution/improvement or (B) progression of distinctive COVID-19 symptoms at day 3, day 5 (EOT), and day 10 (MITT population). Distinctive symptoms = five symptoms commonly associated with COVID-19 at the time of trial conduct. In panel A, a higher percentage indicates more favorable outcomes (sustained resolution/improvement), while in panel B, a higher percentage indicates less favorable outcomes (progression of symptoms). Abbreviations: COVID-19, coronavirus disease 2019; EOT, end of treatment; MITT, modified intention-to-treat.
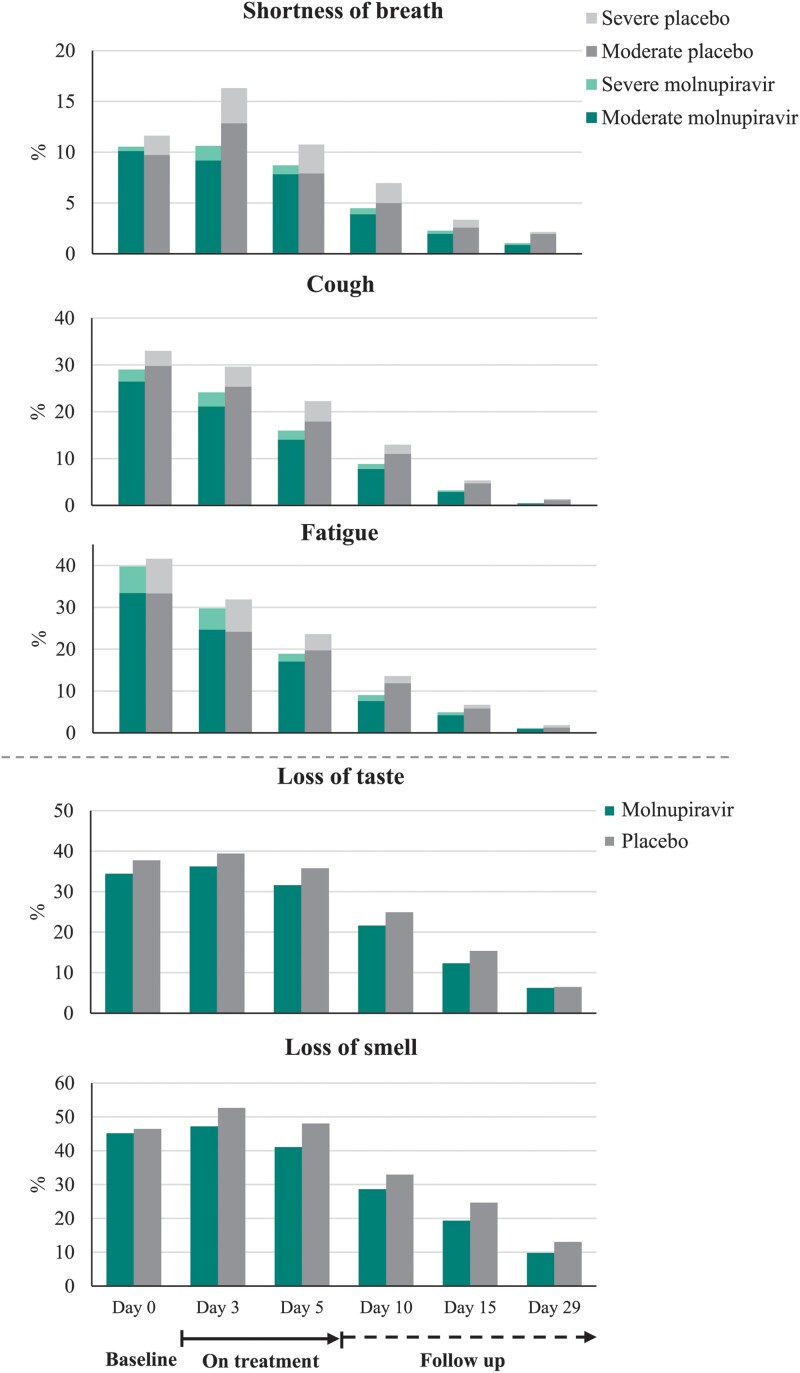
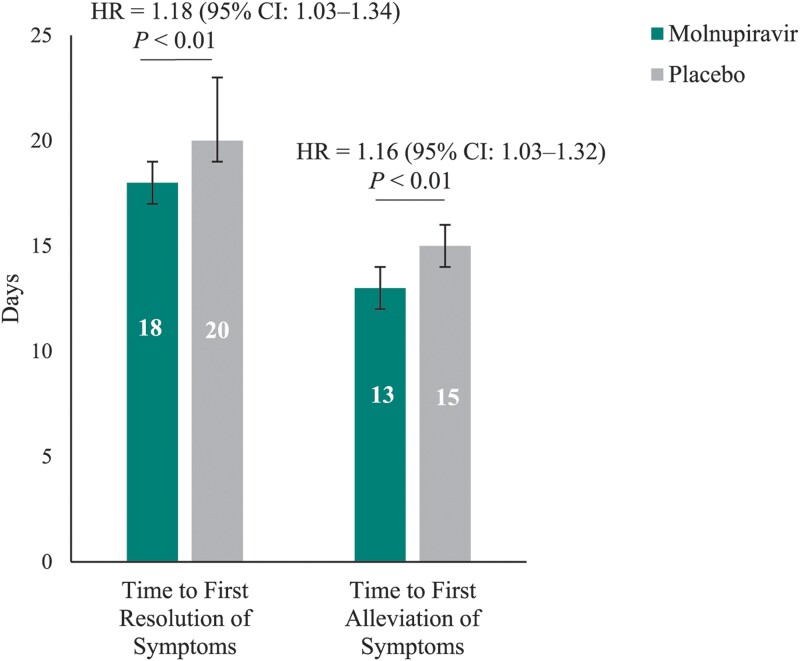
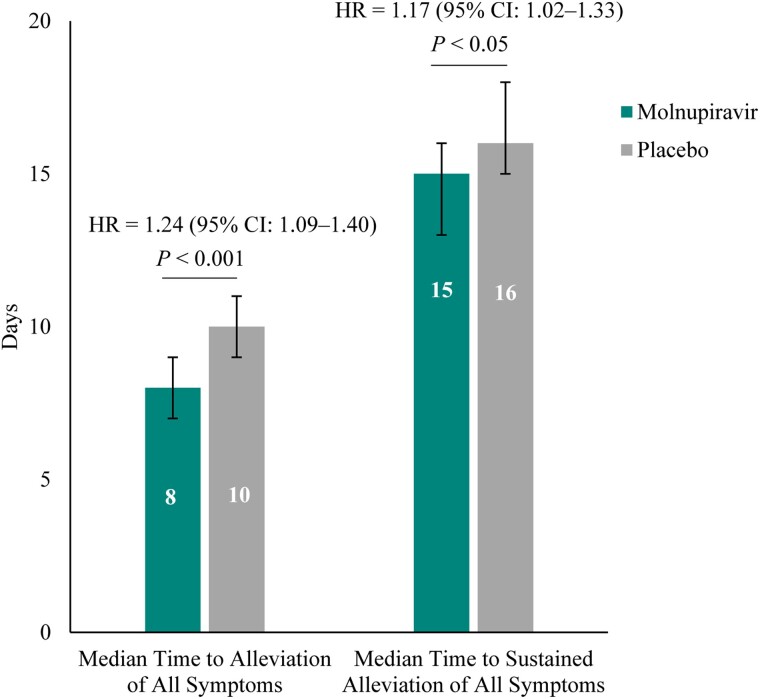
Post hoc analyses of the 5 distinctive COVID-19 symptoms demonstrated that the following were less common in the molnupiravir group compared with placebo through day 29: progression to moderate and severe symptoms for shortness of breath or difficulty breathing, fatigue, cough, and new occurrences of loss of smell and loss of taste (Figure 4). Furthermore, the median time to first resolution of symptoms, and first alleviation of symptoms, for the 5 distinctive COVID-19 symptoms was shorter in the molnupiravir group compared with placebo (18 d [95% CI: 17–19] vs 20 d [95% CI: 19–23] and 13 d [95% CI: 12–14] vs 15 d [95% CI: 14–16], respectively) (Figure 5). Finally, in the post hoc analyses similar to those of the PANORAMIC study, we observed reduced median time to alleviation, and sustained alleviation, of all 15 symptoms in the molnupiravir group compared with placebo (8 d [95% CI: 7–9] vs 10 d [95% CI: 9–11] and 15 d [95% CI: 13–16] vs 16 d [95% CI: 15–18], respectively) through day 29 (Figure 6).
Figure 4.
Proportion of participants with moderate to severe rating for distinctive COVID-19 symptoms through day 29 (MITT population). Participants with available data at each time point are shown. Distinctive symptoms = five symptoms commonly associated with COVID-19 at the time of trial conduct. For “loss of smell” and “loss of taste,” the figure demonstrates proportion of participants who reported “yes” at each time point. Abbreviations: COVID-19, coronavirus disease 2019; MITT, modified intention-to-treat.
Figure 5.
Median time to first resolution and time to first alleviation of distinctive COVID-19 symptoms through day 29 (MITT population). Median time calculated as the average for the 5 symptoms from the product-limit (Kaplan-Meier) method for censored data. Distinctive symptoms = five symptoms commonly associated with COVID-19 at the time of trial conduct (ie, shortness of breath or difficulty breathing, cough, fatigue, loss of smell, and loss of taste). Error bars represent the 95% confidence interval. One-sided P values based on log-rank test stratified by randomization stratification stratum. An HR >1 favors the molnupiravir group. Based on stratified Cox regression model with Efron's method of tie handling with treatment as covariates and randomization stratum as the stratification factor. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio; MITT, modified intention-to-treat.
Figure 6.
Median time to alleviation and time to sustained alleviation of all 15 targeted signs and symptoms through day 29 (MITT population). Median time calculated as the average of all 15 targeted symptoms from the product-limit (Kaplan-Meier) method for censored data. Error bars represent the 95% confidence interval. One-sided P values based on log-rank test stratified by randomization stratification stratum. An HR >1 favors the molnupiravir group. Based on stratified Cox regression model with Efron's method of tie handling with treatment as covariates and randomization stratum as the stratification factor. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio; MITT, modified intention-to-treat.
DISCUSSION
In MOVe-OUT, treatment with molnupiravir resulted in improved outcomes for most participant-reported COVID-19 symptoms compared with placebo. When evaluating 5 symptoms commonly associated with COVID-19 at the time of trial conduct (prior to the Omicron wave), molnupiravir-treated participants were more likely to achieve sustained resolution/improvement and less likely to experience progression of symptoms than those receiving placebo at nearly all time points. In addition, the median time to first resolution and first alleviation of these distinctive COVID-19 symptoms was shorter in participants treated with molnupiravir through day 29 when compared with the placebo group. These results are consistent with other exploratory and post hoc endpoints from MOVe-OUT (eg, evaluation of biomarkers, respiratory interventions, and use of medical services), which demonstrated additional clinical benefits beyond a reduction in hospitalization/deaths in participants treated with molnupiravir [18].
Recently published real-world evidence supports the impact of molnupiravir on patient-reported symptom data, including in vaccinated patients during the Omicron wave [17]. In PANORAMIC, a vaccinated, lower-risk patient population than in MOVe-OUT was enrolled, with consequent low rates of hospitalization and/or death (<1%) during the study, and no effect was seen for the primary endpoint regarding reducing the number of hospitalizations or deaths. However, PANORAMIC demonstrated treatment benefit of molnupiravir in secondary symptom-based endpoints based on a broad range of PROs, including a faster time to sustained recovery, with a clinically meaningful difference of 4.2 days compared with standard-of-care treatment alone [17]. Consistent with findings from the PANORAMIC study, post hoc analyses from MOVe-OUT demonstrated reduced median time to alleviation, and sustained alleviation, of all 15 targeted symptoms through day 29 in participants treated with molnupiravir compared with placebo.
The PRO endpoints provide information on treatment efficacy that is not attained through objective markers or clinical assessments. The current SARS-CoV-2 variants and the increase in preexisting immunity from vaccination and/or prior SARS-CoV-2 infection have resulted in lower overall rates of hospitalization and mortality [19–21], emphasizing the importance of symptom data in informing the treatment benefits of new SARS-CoV-2 therapeutics. Although there have been efforts to develop disease-specific PROs to assess COVID-19 symptoms and treatment impact, there is still a lack of consensus on how to evaluate symptom-based endpoints in the dynamic landscape of COVID-19 therapeutics [22, 23].
Strengths of this trial include its global conduct at 107 sites and the high rates of symptom diary completion. A large and diverse cohort can provide a better understanding of the efficacy of molnupiravir therapy as reported directly from patients across multiple racial and ethnic groups. In this trial, the 15-item symptom diary was consistent with FDA guidance [14] and the definition of sustained resolution/improvement excluded those patients who had symptom relapse through day 29. Another strength of this trial is the double-blinding, which prevents any researcher or participant bias, particularly with PRO measures that rely on participants’ own reporting of their symptoms.
An important limitation is that some of the analyses were not predefined in the protocol and were performed post hoc. In many analyses, the HR point estimates favored molnupiravir versus placebo, although the 95% CI often included 1. However, the study was not powered to demonstrate a difference for these symptom-based endpoint analyses. Furthermore, symptom data were collected only through day 29; therefore, due to the short-term nature of the evaluation, any potential assessment of post-acute sequelae of COVID-19 was not in the scope of this trial. Another limitation is that participants in MOVe-OUT were not vaccinated against SARS-CoV-2; therefore, any potential effects of vaccination on the results obtained in this trial could not be assessed. Finally, symptom diary development was based on existing evidence at the time of trial initiation and the content was not directly confirmed with patient feedback. This was challenging at the time of trial initiation because COVID-19 was a novel disease being studied during an unprecedented global pandemic; however, confirmation of content validity of the symptom diary through patient feedback is ongoing.
Conclusions
The PRO data from the phase 3 MOVe-OUT trial suggest that treatment with molnupiravir resulted in improved outcomes—both for sustained resolution/improvement and progression—for most COVID-19 symptoms compared with placebo. These results are consistent with the overall results from MOVe-OUT, where molnupiravir demonstrated a benefit in reducing hospitalization and/or death and was well tolerated in nonhospitalized adults with mild-to-moderate, laboratory-confirmed COVID-19. These data contribute to the growing body of evidence demonstrating the benefits of molnupiravir on clinical outcomes in patients with mild-to-moderate COVID-19.
Contributor Information
Yanfen Guan, Merck & Co, Inc, Rahway, New Jersey, USA.
Amy Puenpatom, Merck & Co, Inc, Rahway, New Jersey, USA.
Matthew G Johnson, Merck & Co, Inc, Rahway, New Jersey, USA.
Ying Zhang, Merck & Co, Inc, Rahway, New Jersey, USA.
Yujie Zhao, Merck & Co, Inc, Rahway, New Jersey, USA.
Joseph Surber, Centricity Research, Columbus, Georgia, USA.
Aaron Weinberg, Carbon Health Technologies, Inc, North Hollywood, California, USA.
Carlos Brotons, EAP Sardenya, Biomedical Research Institute Sant Pau, Barcelona, Spain.
Roman Kozlov, Smolensk State Medical University, Smolensk, Russia.
Rudy Lopez, Clínica Médica Especialista en Pediatría e Infectología Pediátrica, Guatemala City, Guatemala.
Kathleen Coetzee, Paarl Research Centre, Paarl, South Africa.
Joel Santiaguel, Philippine General Hospital, University of the Philippines Manila, Manila, Philippines.
Jiejun Du, Merck & Co, Inc, Rahway, New Jersey, USA.
Angela Williams-Diaz, Merck & Co, Inc, Rahway, New Jersey, USA.
Michelle Brown, Merck & Co, Inc, Rahway, New Jersey, USA.
Amanda Paschke, Merck & Co, Inc, Rahway, New Jersey, USA.
Carisa De Anda, Merck & Co, Inc, Rahway, New Jersey, USA.
Josephine M Norquist, Merck & Co, Inc, Rahway, New Jersey, USA.
Notes
Author Contributions. All authors contributed substantially to the conception, design, or planning of the study and/or the acquisition or analysis of the data and/or interpretation of the results and drafting of the manuscript and/or critically reviewing or revising it for important intellectual content. All authors reviewed the final version of the manuscript to be submitted and are in agreement with its content and submission, had access to all the relevant study data and related analyses, and vouch for the completeness and accuracy of the data presented.
Acknowledgments. The authors thank the patients and their families and caregivers for participating in this trial, along with all investigators and site personnel. Medical writing assistance was provided by Ascentia Seboko, PhD, of ApotheCom, London, United Kingdom. This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, New Jersey, USA.
Financial support. This work was supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, New Jersey, USA.
Data sharing statement. The following data will be made available with publication: deidentified participant data and clinical trial reports. (The data sharing policy, including restrictions, of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, USA is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical trial data can be submitted through the Engage Zone site or via e-mail to dataaccess@merck.com). No supporting documents will be made available. The data will be made available to qualified scientific researchers for specific purposes outlined in a proposal after the researcher enters into a standard data sharing agreement and the proposal is approved. Researchers must commit to transparency in publication.
References
- 1. Abdelnabi R, Foo CS, De Jonghe S, Maes P, Weynand B, Neyts J. Molnupiravir inhibits replication of the emerging SARS-CoV-2 variants of concern in a hamster infection model. J Infect Dis 2021; 224:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pourkarim F, Pourtaghi-Anvarian S, Rezaee H. Molnupiravir: a new candidate for COVID-19 treatment. Pharmacol Res Perspect 2022; 10:e00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheahan TP, Sims AC, Zhou S, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med 2020; 12:eabb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2. N Engl J Med 2022; 386:1475–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takashita E, Yamayoshi S, Simon V, et al. Efficacy of antibodies and antiviral drugs against omicron BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med 2022; 387:468–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wahl A, Gralinski LE, Johnson CE, et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature 2021; 591:451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoon JJ, Toots M, Lee S, et al. Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses. Antimicrob Agents Chemother 2018; 62:e00766-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med 2022; 386:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas 2018; 9:353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kluzek S, Dean B, Wartolowska KA. Patient-reported outcome measures (PROMs) as proof of treatment efficacy. BMJ Evid Based Med 2022; 27:153–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong AW, Shah AS, Johnston JC, Carlsten C, Ryerson CJ. Patient-reported outcome measures after COVID-19: a prospective cohort study. Eur Respir J 2020; 56:2003276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adjei S, Hong K, Molinari NAM, et al. Mortality risk among patients hospitalized primarily for COVID-19 during the omicron and Delta variant pandemic periods—United States, April 2020-June 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jassat W, Abdool Karim SS, Mudara C, et al. Clinical severity of COVID-19 in patients admitted to hospital during the omicron wave in South Africa: a retrospective observational study. Lancet Glob Health 2022; 10:e961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. US Food and Drug Administration. Assessing COVID-19-related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment: guidance for industry. 2020. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/assessing-covid-19-related-symptoms-outpatient-adult-and-adolescent-subjects-clinical-trials-drugs. Accessed 19 June 2023.
- 15. Caraco Y, Crofoot GE, Moncada PA, et al. Phase 2/3 trial of molnupiravir for treatment of COVID-19 in nonhospitalized adults. NEJM Evidence 2022; 1:EVIDoa2100043. [DOI] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention . Symptoms of COVID-19. 2022. Available at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed 19 June 2023.
- 17. Butler CC, Hobbs FDR, Gbinigie OA, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet 2023; 401:281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson MG, Puenpatom A, Moncada PA, et al. Effect of molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19: a randomized, placebo-controlled trial. Ann Intern Med 2022; 175:1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis 2022; 22:1293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Madhi SA-O, Kwatra G, Myers JE, et al. Population immunity and COVID-19 severity with omicron variant in South Africa. N Engl J Med 2022;386:1314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danza P, Koo TH, Haddix M, et al. SARS-CoV-2 infection and hospitalization among adults aged ≥18 years, by vaccination status, before and during SARS-CoV-2 B. 1.1. 529 (omicron) variant predominance—Los Angeles County, California, November 7, 2021–January 8, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amdal CD, Taylor K, Kuliś D, et al. Health-related quality of life in patients with COVID-19; international development of a patient-reported outcome measure. J Patient Rep Outcomes 2022; 6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Romano C, Fehnel S, Stoddard J, et al. Development of a novel patient-reported outcome measure to assess signs and symptoms of COVID-19. J Patient Rep Outcomes 2022; 6:85. [DOI] [PMC free article] [PubMed] [Google Scholar]