Abstract
Background
Although comorbidities are risk factors for recurrent Clostridioides difficile infection (rCDI), many clinical trials exclude patients with medical conditions such as malignancy or immunosuppression. In a phase 3, double-blind, placebo-controlled, randomized trial (ECOSPOR III), fecal microbiota spores, live (VOWST, Seres Therapeutics; hereafter “VOS,” formerly SER-109), an oral microbiota therapeutic, significantly reduced the risk of rCDI at week 8. We evaluated the efficacy of VOS compared with placebo in patients with comorbidities and other risk factors for rCDI.
Methods
Adults with rCDI were randomized to receive VOS or placebo (4 capsules daily for 3 days) following standard-of-care antibiotics. In this post hoc analysis, the rate of rCDI through week 8 was assessed in VOS-treated participants compared with placebo for subgroups including (i) Charlson comorbidity index (CCI) score category (0, 1–2, 3–4, ≥5); (ii) baseline creatinine clearance (<30, 30–50, >50 to 80, or >80 mL/minute); (iii) number of CDI episodes, inclusive of the qualifying episode (3 and ≥4); (iv) exposure to non-CDI-targeted antibiotics after dosing; and (v) acid-suppressing medication use at baseline.
Results
Of 281 participants screened, 182 were randomized (59.9% female; mean age, 65.5 years). Comorbidities were common with a mean overall baseline age-adjusted CCI score of 4.1 (4.1 in the VOS arm and 4.2 in the placebo arm). Across all subgroups analyzed, VOS-treated participants had a lower relative risk of recurrence compared with placebo.
Conclusions
In this post hoc analysis, VOS reduced the risk of rCDI compared with placebo, regardless of baseline characteristics, concomitant medications, or comorbidities.
Keywords: Clostridioides difficile infection, microbiome therapeutics, SER-109, VOWST, comorbidities
In a post hoc analysis, a microbiota-based therapeutic, VOS, reduced recurrent Clostridioides difficile infection (rCDI) risk versus placebo, regardless of comorbidities. Since most risk factors for rCDI are nonmodifiable, these data highlight the potential benefit of VOS in a broad patient population.
Graphical Abstract
Graphical Abstract.
Clostridioides difficile infection (CDI) is differentiated from many other bacterial infections due to its recurrent nature. Approximately 20%–25% of patients with primary infection experience recurrent disease [1], and those with 2 or more recurrences have ≥40% risk of further episodes [2, 3]. The main risk factor for CDI is exposure to broad-spectrum antibiotics, which cause collateral damage to beneficial microbes that normally reside within the gastrointestinal microbiome, our first line of host defense. Although C. difficile–targeted antibiotics rapidly kill vegetative toxin-producing bacteria, they do not eradicate C. difficile spores that may germinate in a disrupted gastrointestinal microbiome, leading to recurrent infection [4].
Epidemiologic studies have identified a variety of demographic risk factors for recurrent CDI (rCDI) including older age, female sex, Charlson comorbidity index (CCI) score, and medications, such as reexposure to broad-spectrum antibiotics, which further exacerbate microbiome disruption [5–10]. Elderly patients may have senescence of the microbiome, compounding the impact of antibiotic exposure due to lack of resilience [11]. The underlying reason that females are at higher risk of recurrence is not well understood. Use of acid-suppressing medications (ASMs), particularly proton pump inhibitors (PPIs), is also associated with increased risk of recurrence [12, 13]. However, whether ASM use is an independent risk factor for recurrence is unclear from epidemiologic studies since these medications are often prescribed in older patients with comorbidities, who are also at risk for rCDI [14]. One of the greatest risk factors is a history of recurrence, with >40% of patients experiencing another episode on antibiotics alone [5], highlighting the importance of microbiome restoration in this patient subgroup.
Comorbidities, such as cardiovascular disease, renal insufficiency, chronic obstructive pulmonary disease, cancer, immunosuppression, and other chronic diseases, have been strongly associated with increased risk of rCDI [8, 15–20]. The prevalence of these comorbidities may also contribute to the high rates of all-cause hospitalization and mortality observed within 30 to 90 days following rCDI, particularly in the elderly. Although patients with comorbidities are at high risk for recurrence, they are often excluded from clinical trials, particularly those with malignancy or immunosuppression.
Fecal microbiota spores, live (VOWST, Seres Therapeutics; formerly SER-109 and hereafter referred to as VOS for VOWST Oral Spores), a microbiota-based oral therapeutic comprised of Firmicutes bacterial spores, was developed to reduce CDI recurrence. ECOSPOR III, a phase 3, double-blind, randomized trial, evaluated the safety and efficacy of VOS compared to placebo for treatment of patients with a history of rCDI. VOS was superior to placebo at week 8, the primary endpoint achieving a 68% relative risk reduction in recurrence rates compared to those treated with placebo (12.4% vs 39.8%, respectively; relative risk, 0.32 [95% confidence interval, .18–.58; P < .001]) [21]. VOS was generally well tolerated and no serious adverse events were directly attributed to drug by the blinded investigators.
In a post hoc analysis, we assessed (i) the prevalence of comorbidities and age-adjusted CCI scores in this high-risk population of patients with a history of rCDI and (ii) the efficacy of VOS, compared to placebo, in subgroups of patients by antibiotic and ASM use, and demographic and baseline characteristics that placed them at high risk of recurrence, including CCI score category.
METHODS
ECOSPOR III (NCT03183128) was a double-blind, randomized, multicenter trial conducted at 56 US and Canadian sites from July 2017 to September 2020. The protocol and amendments were reviewed and approved by local or central investigational review boards, and participants provided written informed consent prior to screening.
The study included adults ≥18 years of age with ≥3 CDI episodes within 12 months, inclusive of the qualifying acute episode, which was defined as (i) ≥3 unformed bowel movements over 2 consecutive days; (ii) a positive C. difficile toxin test by enzyme immunoassay (EIA) or reflex cytotoxicity neutralization assay (CCNA); and (iii) symptom resolution after 10–21 days of standard-of-care antibiotics. A full list of inclusion and exclusion criteria is published elsewhere [21].
In brief, patients were stratified by age (<65 or ≥65 years) and antibiotic received for their qualifying CDI (ie, vancomycin or fidaxomicin) and were randomly assigned 1:1 to VOS (approximately 3 × 107 spore colony-forming units per day) or matching placebo administered as 4 oral capsules once daily over 3 consecutive days [21, 22]. Patients were instructed to take 296 mL (10 oz) of magnesium citrate or 250 mL of polyethylene glycol 1 day prior to treatment initiation. Donor screening and manufacturing of VOS are described elsewhere [21].
Endpoints
On-study CDI recurrence was determined by the presence of 3 components: (i) ≥3 unformed stools per day over 2 consecutive days with the requirement that participants continue to have diarrhea until antibiotic treatment was initiated; (ii) a positive C. difficile stool toxin result (EIA or reflex CCNA); and (iii) investigator assessment that antibiotic treatment was warranted.
The primary endpoint (ie, CDI recurrence rate at 8 weeks in VOS- vs placebo-treated participants) was met and the superiority of VOS versus placebo was previously reported [21]. Secondary endpoints, including CDI recurrence rates in VOS- versus placebo-treated participants at 4, 12, and 24 weeks after initiation of treatment and time to recurrence, are also reported elsewhere [22]. Those who did not have a recurrence were considered to have a sustained clinical response. Subjects who were lost to follow-up, terminated the study prematurely, or died without a recorded recurrence before the end of the time interval were assumed to have had a recurrence. Handling of other types of missing data was provided in the statistical analysis plan.
In this post hoc exploratory analysis, we assessed the rate of CDI recurrence among VOS-treated participants compared to placebo for the following subgroups: (i) number of prior CDI episodes, inclusive of the qualifying episode (3 and ≥4); (ii) concomitant medications such as exposure to non-CDI-targeted antibiotics after dosing, and ASM usage at baseline (ie, PPIs and/or H2-receptor antagonists) versus no ASM use; and (iii) CCI score category (0, 1–2, 3–4, ≥5).
We also analyzed the rate of CDI recurrence by age (≥65 and <65 years), sex, and creatinine clearance rate at baseline (<30, 30–50, >50 to 80, and >80 mL/minute), which were prespecified. In addition, we summarized the presence of specific comorbid conditions (using the age-adjusted CCI score) by ASM subgroup and treatment arm.
Safety and tolerability were analyzed through 24 weeks and are reported elsewhere [21, 22].
Statistical Analysis
All efficacy analyses, including subgroup analyses, were performed on the intention-to-treat (ITT) population, which included all randomized participants, and analyzed according to intended assignment, regardless of treatment received.
For analysis of the overall population, relative risk was defined as the percentage of patients with recurrence in the VOS group divided by the percentage in the placebo group adjusted for stratification factors (age group [<65 years or ≥65 years] and antibiotic regimen received for the qualifying acute episode [vancomycin or fidaxomicin]) using Cochran–Mantel–Haenszel method. Confidence intervals were calculated using the Greenland and Robins variance estimate for the natural log of the common relative risk. For analysis of subgroups, relative risk was defined as the percentage of patients with recurrence in the VOS group divided by the percentage in the placebo group. Confidence intervals were calculated from the natural logarithm of the relative risk and its variance estimate.
Medical history was obtained through self-report or health records and all data were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 23.1. Components of the CCI were derived from MedDRA v23.1-coded medical history terms (see Supplementary Material). The CCI was originally developed in 1987 to be used in different populations as a weighted prognostic index to predict mortality, where higher scores indicate greater mortality risk [23]. CCI scores presented reflect weighting methods with consideration of age as described by Quan et al [24, 25].
All statistical analyses were performed using SAS version 9.4 software (SAS Institute, Cary, North Carolina).
RESULTS
Demographics, Baseline Characteristics, and Prevalence of Comorbidities
Of 281 patients screened, 182 were enrolled; females made up 59.9% (n = 109) of the study population, and more than half of participants (n = 103 [56.6%]) were aged ≥65 years. Demographics were balanced across treatment arms, although there were more females in the VOS arm than the placebo arm (67.4% vs 52.7%).
Chronic medical conditions were determined from the screening medical history or concomitant medications, including psychiatric disorders, gastroesophageal reflux disease, cardiovascular disease, diabetes, malignancy, renal failure and/or insufficiency, and immunocompromised status, which were highly prevalent in the overall study population (Table 1; see also Supplementary Table 1). Mean CCI scores were 4.1 (standard deviation [SD], 2.4) and 4.2 (SD, 2.6), in the VOS and placebo arms, respectively [22], and 73.1% of the overall population had a CCI score of ≥3.
Table 1.
Baseline Chronic Medical Conditions (Intention-to-Treat Population)
| Baseline Condition | Total (N = 182) |
|---|---|
| Psychiatric disorders | 89 (48.9) |
| Gastroesophageal reflux disease | 78 (42.9) |
| Respiratory disease | 66 (36.3) |
| Cardiovascular disease | 59 (32.4) |
| Immunocompromised status | 54 (29.7) |
| Diabetes | 43 (23.6) |
| Malignancy | 33 (18.1) |
| Renal impairment or failure | 27 (14.8) |
| Neurologic disease | 23 (12.6) |
| Ulcerative colitis, microscopic colitis, irritable bowel syndrome | 23 (12.6) |
Data are presented as No. (%). Chronic conditions were determined from the screening medical history. More information can be found in Supplementary Table 1. Patients may have had >1 chronic medical condition.
In addition, based on the screening medical history, 89 participants (48.9%) had a history of psychiatric disorders; 47 (25.8%) had a history of depression, and 37 (20.3%) had a history of anxiety. In addition, 38 participants (20.9%) had a history of insomnia. Of note, these psychiatric disorders appeared to be ongoing at the time of enrollment with 76 of these 89 (85.4%) participants taking a range of concomitant psychotropic medications including antidepressants, anxiolytics, and sedatives/hypnotics.
Relative to other medications of interest, 25 of 182 (13.7%) patients took non-CDI-targeted antibiotics after dosing with VOS or placebo while 74 patients (40.7%) were actively taking ASMs at baseline (PPIs alone [n = 45], H2-receptor antagonists alone [n = 24], or both PPIs and H2-receptor antagonists [n = 5]). Additional medical history data relevant to ASM use included dyspepsia (n = 9), gastric ulcer (n = 8), gastritis (n = 7), and peptic ulcer (n = 2). The average age of patients taking ASMs was higher compared to those who were not (mean, 67.5 years vs 64.2 years).
Efficacy of VOS Versus Placebo in High-Risk Demographic Groups
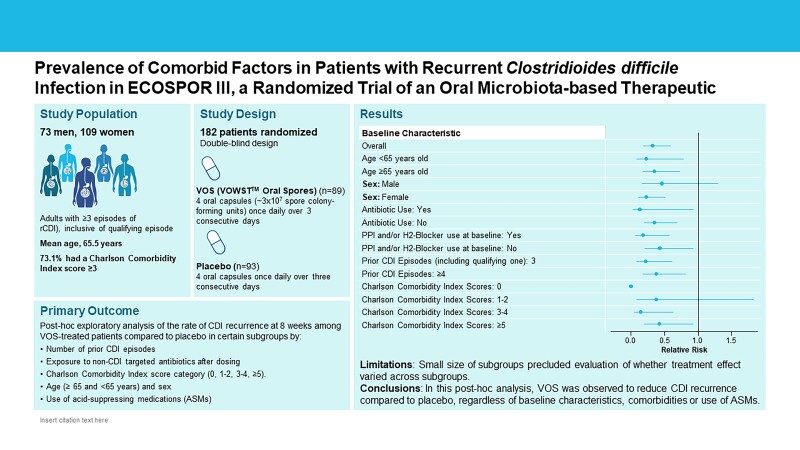
In the subgroup analysis, VOS-treated participants had a lower relative risk of CDI recurrence at week 8 compared to placebo for all subgroups analyzed, including age, sex, number of prior episodes, creatinine clearance at baseline, non-CDI antibiotic usage, use of ASMs at baseline, and CCI score categories of 0, 1–2, 3–4, and ≥5 (Figure 1).
Figure 1.
Forest plot of relative risks of recurrence at week 8 for selected baseline characteristics (intention-to-treat population). Subjects who were lost to follow-up, terminated the study prematurely, or died without a recorded recurrence were assumed to have had a recurrence. Relative risk for the overall population was defined as the percentage of patients with recurrence in the fecal microbiota spores, live (VOS) group divided by the percentage in the placebo group adjusted for stratification factors (age group [<65 or ≥65 years] and antibiotic regimen received for the qualifying acute episode [vancomycin or fidaxomicin]) using Cochran–Mantel–Haenszel method. Confidence intervals (CIs) were calculated using the Greenland and Robins variance estimate for the natural log of the common relative risk. For analysis of subgroups, relative risk was defined as the percentage of patients with recurrence in the VOS group divided by the percentage in the placebo group. CIs were calculated from the natural logarithm of the relative risk and its variance estimate. Antibiotic use refers to those antibiotics other than for Clostridioides difficile infection (CDI) recurrence. Charlson comorbidity index scores were adjusted for age (Quan et al [25]). One participant in the VOS arm had missing data for number of prior CDI episodes. Abbreviations: CCI, Charlson comorbidity index; events, on-study Clostridioides difficile infection recurrence; NE, non-estimable; No., number of patients; PPI, proton pump inhibitor; VOS, fecal microbiota spores, live (VOWST, formerly SER-109).
CDI Recurrence Rates by CCI Score Category
The percentage of patients with CDI recurrence in the placebo arm rose from 20% among those with a CCI score of 0, to >45.7% among those with a CCI score of ≥5, whereas CDI recurrence rates in VOS-treated patients were lower across all CCI score categories, ranging from 0% to 20%. Since CDI recurrence is associated with hospitalization [22], we also evaluated hospitalization by CCI score category and observed that 18 of 23 patients who were hospitalized through week 8 (78.3%) had CCI scores of ≥5 (Table 2). However, only 2 of confirmed CDI recurrences would have been categorized as “hospital-acquired CDI” (per Centers for Disease Control and Prevention definition) [10], suggesting that exposure to the hospital environment was not a confounding factor for recurrent infection.
Table 2.
Hospitalizations Through Week 8 by Charlson Comorbidity Index Score Category (Intention-to-Treat Population of 182 patients)
| CCI Score Category | Nonhospitalized Patients, No. (%) | Hospitalized Patients, No. (%) |
|---|---|---|
| 0 | 17 (9.3) | 0 (0.0) |
| 1–2 | 30 (16.5) | 2 (1.1) |
| 3–4 | 60 (33.0) | 3 (1.7) |
| ≥5 | 52 (28.6) | 18 (9.9) |
| Total | 159 (87.4) | 23 (12.6) |
CDI Recurrence Rates by Use of ASMs
In subgroups defined by ASM use, recurrence rates among those randomized to placebo were higher in those taking ASMs versus those who were not (48.8% vs 32.7%, respectively). This trend was not observed among those randomized to VOS, where CDI recurrence rates were similarly low (9.1% vs 14.3%, for those taking vs not taking ASMs, respectively). Although sample sizes are limited, when analyzed by specific class of ASM, recurrence rates were also numerically lower in the VOS arm versus placebo for patients taking PPIs (15.0% [3/20] vs 46.7% [14/30]) and for patients taking H2-receptor antagonists (0% [0/14] vs 46.7% [7/15]).
Since some data suggest that use of ASM is a risk factor for recurrence that may be confounded by prevalence of comorbidities, we also examined ASM use by age-adjusted CCI score. In fact, CCI scores were higher in those taking versus not taking ASMs in both treatment arms and in the overall population (Table 3), although all 95% confidence intervals overlapped.
Table 3.
Association of Acid-Suppressing Medication Use at Baseline With Age-Adjusted Charlson Comorbidity Index Score (Intention-to-Treat Population)
| Treatment Arm | PPI and/or H2-Receptor Antagonist Use at Baseline | No. | Age-Adjusted CCI Score, Mean (SD) [95% CI] |
|---|---|---|---|
| Placebo | Yes | 41 | 4.7 (2.6) [3.8–5.5] |
| No | 52 | 3.8 (2.6) [3.1–4.5] | |
| VOS | Yes | 33 | 4.7 (2.3) [3.8–5.5] |
| No | 56 | 3.7 (2.4) [3.1–4.4] | |
| Overall population | Yes | 74 | 4.7 (2.5) [4.1–5.2] |
| No | 108 | 3.7 (2.5) [3.3–4.2] |
Abbreviations: CCI, Charlson comorbidity index; CI, confidence interval; PPI, proton pump inhibitor; SD, standard deviation; VOS, fecal microbiota spores, live (VOWST, formerly SER-109).
DISCUSSION
Recurrence of CDI is the hallmark of a disrupted microbiome, signaling the need for a comprehensive therapeutic approach, beyond antibiotics alone. Regardless of demographics or baseline risk factors, an oral microbiota therapeutic, VOS, reduced recurrence of CDI compared to placebo in this high-risk population with a history of rCDI. Reduction of the risk of recurrence with VOS compared to placebo was observed in all risk groups, including those with an age-adjusted CCI score of ≥5, highlighting the importance of microbiome restoration after treatment of rCDI with antibiotics.
Importantly, most of these risk factors are nonmodifiable, such as older age, female sex, chronic comorbidities, and a history of recurrence. Comorbidities associated with lower host defenses have been consistently observed as a risk factor for CDI including immunosuppression, malignancy, and renal failure [16–18]. Yet clinical trials in rCDI have often excluded such patients. Most participants in ECOSPOR III had comorbidities consistent with the broad inclusion criteria in this phase 3 trial, reflective of real-world patient populations. In fact, 73.1% of the overall study population had a CCI score of ≥3, and higher CCI scores have been associated with greater risk of rCDI and hospitalization, as observed in the literature and in this clinical trial [26]. However, VOS reduced recurrence of CDI compared to placebo in each subgroup, including those with an age-adjusted CCI score of ≥5, demonstrating efficacy regardless of presence of comorbidities. Aging populations were also well-represented in ECOSPOR III with 56.6% of the study population being ≥65 years of age [27, 28]. Due to the well-documented association of CDI with all-cause hospitalizations, particularly among the elderly with multiple comorbidities [29, 30], the reduction of CDI recurrence may potentially lessen future healthcare costs and morbidity [27, 28].
A striking observation was the high proportion of patients who had a history of depression and anxiety at enrollment, which are medical conditions excluded from the CCI. It is unknown if these underlying mental health issues existed prior to CDI or followed recurrent infections. It is well-established that CDI is associated with a low quality of life, due to disabling symptoms, anxiety, and social isolation, whereas nonrecurrence after treatment is associated with improved quality of life [31, 32]. Furthermore, those with primary CDI have a higher quality of life than those with rCDI, who have significantly higher anxiety scores on the Cdiff32, a disease-specific survey [31, 33]. Severe anxiety regarding any loose bowel movement as a sign of anticipated recurrence has led some experts to refer to this hypervigilance as “CDI-related posttraumatic syndrome” [34]. Physicians should be cognizant of the high prevalence of anxiety and depression in patients with rCDI, as observed in this trial, and query patients regarding relevant symptoms.
Use of ASMs was highly prevalent, particularly in those with higher CCI scores; thus, whether ASM use is an independent risk factor for CDI recurrence is still unclear as comorbidities appear to be an important confounder in both the literature and within this clinical trial. However, lower CDI recurrence rates in VOS versus placebo-treated patients were consistently observed in patients taking versus not taking ASMs at baseline. Although ASM usage is a modifiable risk factor, these data indicate that VOS may be efficacious, regardless of ASM use, giving clinicians greater options for management of underlying acid-related gastrointestinal disorders.
Following resolution of CDI symptoms, after standard-of-care antibiotics, reexposure to antibiotics for other infections, such as pneumonia or urinary tract infections, is a clear risk factor for rCDI due to their negative impact on beneficial microbiota, which play an important role in preventing C. difficile spore germination. Only 25 patients were exposed to antibiotics after dosing with placebo or VOS and, as a postrandomization event, could not be controlled for in the analysis. Furthermore, a variety of antibiotics were used that vary in risk of inducing CDI. Thus, we are unable draw conclusions about the effectiveness of VOS compared to placebo in patients exposed to antibiotics posttreatment, although the trends are reassuring. If antibiotics are needed after clinical resolution of CDI, clinicians should consider not only the resistance profile of the pathogen or the spectrum of antibacterial activity of the antibiotic, but also drug concentrations in stool, to lessen the negative impact on the gastrointestinal microbiome, whenever feasible. Although some clinicians have supported use of vancomycin following antibiotic exposure as a prophylactic measure to prevent CDI recurrence, this approach has become less tenable in light of the known negative impact of vancomycin on the microbiome and the low quality of evidence [35].
There are strengths and limitations to these data. We had low representation of patients with active inflammatory bowel disease, so we are unable to draw conclusions in this subgroup, which is at higher risk for recurrence, hospitalization, and fulminant disease [36]. In addition, the relatively small sample size did not allow evaluation of whether the treatment effect of VOS varied across subgroups. It is also not possible to rule out potential confounding factors within each subgroup that may also contribute to the variability in response. The strengths of the data are that the efficacy observed overall was consistent among subgroups with nonmodifiable risk factors, such as those with a history of recurrence, elderly persons, females, and those with comorbidities, suggesting broad benefit in a wide range of vulnerable patients with chronic medical diseases associated with risk of rCDI. Finally, all patients entered the study with an acute CDI episode and a history of recurrent infection, one of the most significant risk factors for future episodes, underscoring that all study participants were at high risk for recurrence [5].
CONCLUSIONS
In this post hoc analysis, VOS was observed to reduce CDI recurrence compared to placebo in this phase 3 trial, regardless of baseline characteristics, concomitant medications, or comorbidities. Most patients in ECOSPOR III had comorbidities consistent with the broad inclusion criteria in this phase 3 trial and more than half were ≥65 years of age. Since most risk factors for CDI are nonmodifiable, these efficacy data help to inform the potential benefit of VOS in vulnerable patients with recurrent infection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Charles S Berenson, Veterans Affairs Western New York Healthcare System, University at Buffalo, New York, New York, USA.
Bret Lashner, Gastroenterology Division, Cleveland Clinic, Ohio, USA.
Louis Y Korman, Gastroenterology and Hepatology, Chevy Chase Clinical Research, Chevy Chase, Maryland, USA.
Elizabeth Hohmann, Infectious Diseases Division, Massachusetts General Hospital, Boston, Massachusetts, USA.
Abhishek Deshpande, Gastroenterology Division, Cleveland Clinic, Ohio, USA.
Thomas J Louie, Department of Microbiology and Infectious Diseases, Cumming School of Medicine, University of Calgary, Alberta, Canada.
Matthew Sims, Section of Infectious Diseases and International Medicine, Department of Internal Medicine, Beaumont Royal Oak, Royal Oak, Michigan, USA; Departments of Internal Medicine and Foundational Medical Studies, William Beaumont School of Medicine, Oakland University, Rochester, Michigan, USA.
Darrell Pardi, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota, USA.
Colleen S Kraft, Division of Infectious Diseases, Department of Pathology and Laboratory Medicine, Emory University, Atlanta, Georgia, USA.
Elaine E L Wang, Clinical Development, Seres Therapeutics, Cambridge, Massachusetts, USA.
Stuart H Cohen, Division of Infectious Diseases, University of California, Davis Health, Sacramento, California, USA.
Paul Feuerstadt, Division of Digestive Disease, Yale University School of Medicine, New Haven, Connecticut, USA; Division of Gastroenterology, Yale University and PACT-Gastroenterology Center, Hamden, Connecticut, USA.
Caterina Oneto, Vanguard Gastroenterology, New York, New York, USA.
Bharat Misra, Borland-Groover Clinic, P.A., Jacksonville, Florida, USA.
John Pullman, Mercury Street Medical, Butte, Montana, USA.
Ananya De, Clinical Development, Seres Therapeutics, Cambridge, Massachusetts, USA.
Asli Memisoglu, Clinical Development, Seres Therapeutics, Cambridge, Massachusetts, USA.
David A Lombardi, Clinical Development, Seres Therapeutics, Cambridge, Massachusetts, USA.
Brooke R Hasson, Clinical Development, Seres Therapeutics, Cambridge, Massachusetts, USA.
Barbara H McGovern, Clinical Development, Seres Therapeutics, Cambridge, Massachusetts, USA.
Lisa von Moltke, Clinical Development, Seres Therapeutics, Cambridge, Massachusetts, USA.
Christine H Lee, Department of Microbiology and Infectious Diseases, Island Medical Program, University of British Columbia and University of Victoria, British Columbia, Canada.
Notes
Author contributions. Concept and design: E. E. L. W. and B. H. M. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: B. H. M. and B. R. H. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: A. M. and D. A. L. Obtained funding: L. v. M. Supervision: E. E. L. W. and L. v. M.
Financial support. This work was supported by Seres Therapeutics.
References
- 1. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–31. [DOI] [PubMed] [Google Scholar]
- 2. Pepin J. Improving the treatment of Clostridium difficile–associated disease: where should we start? Clin Infect Dis 2006; 43:553–5. [DOI] [PubMed] [Google Scholar]
- 3. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012; 12:281–9. [DOI] [PubMed] [Google Scholar]
- 4. Theriot CM, Young VB. Interactions between the gastrointestinal microbiome and Clostridium difficile. Annu Rev Microbiol 2015; 69:445–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gerding DN, Kelly CP, Rahav G, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis 2018; 67:649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garey KW, Sethi S, Yadav Y, DuPont HL. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect 2008; 70:298–304. [DOI] [PubMed] [Google Scholar]
- 8. Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2015; 36:452–60. [DOI] [PubMed] [Google Scholar]
- 9. Finn E, Andersson FL, Madin-Warburton M. Burden of Clostridioides difficile infection (CDI)—a systematic review of the epidemiology of primary and recurrent CDI. BMC Infect Dis 2021; 21:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guh AY, Mu Y, Winston LG, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med 2020; 382:1320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jump RL. Clostridium difficile infection in older adults. Aging Health 2013; 9:403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehta P, Nahass RG, Brunetti L. Acid suppression medications during hospitalization as a risk factor for recurrence of Clostridioides difficile infection: systematic review and meta-analysis. Clin Infect Dis 2020; 73:e62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deshpande A, Pant C, Pasupuleti V, et al. Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis. Clin Gastroenterol Hepatol 2012; 10:225–33. [DOI] [PubMed] [Google Scholar]
- 14. Bauer SR, O’Malley P. Withholding proton pump inhibitors to prevent recurrent Clostridium difficile: time for a randomized trial. JAMA Intern Med 2017; 177:791. [DOI] [PubMed] [Google Scholar]
- 15. Shakov R, Salazar RS, Kagunye SK, Baddoura WJ, DeBari VA. Diabetes mellitus as a risk factor for recurrence of Clostridium difficile infection in the acute care hospital setting. Am J Infect Control 2011; 39:194–8. [DOI] [PubMed] [Google Scholar]
- 16. Pant C, Deshpande A, Anderson MP, Sferra TJ. Clostridium difficile infection is associated with poor outcomes in end-stage renal disease. J Invest Med 2012; 60:529–32. [DOI] [PubMed] [Google Scholar]
- 17. Donnelly JP, Wang HE, Locke JE, Mannon RB, Safford MM, Baddley JW. Hospital-onset Clostridium difficile infection among solid organ transplant recipients. Am J Transplant 2015; 15:2970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abu-Sbeih H, Choi K, Tran CN, et al. Recurrent Clostridium difficile infection is associated with treatment failure and prolonged illness in cancer patients. Eur J Gastroen Hepat 2018; 31:128–34. [DOI] [PubMed] [Google Scholar]
- 19. Saffouri G, Gupta A, Loftus EV Jr, Baddour LM, Pardi DS, Khanna S. The incidence and outcomes from Clostridium difficile infection in hospitalized adults with inflammatory bowel disease. Scand J Gastroenterol 2017; 52:1–8. [DOI] [PubMed] [Google Scholar]
- 20. Negrut N, Bungau S, Behl T, et al. Risk factors associated with recurrent Clostridioides difficile infection. Healthcare (Basel) 2020; 8:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feuerstadt P, Louie TJ, Lashner B, et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N Engl J Med 2022; 386:220–9. [DOI] [PubMed] [Google Scholar]
- 22. Cohen SH, Louie TJ, Sims M, et al. Extended follow-up of microbiome therapeutic SER-109 through 24 weeks for recurrent Clostridioides difficile infection in a randomized clinical trial. JAMA 2022; 328:2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 24. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 25. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173:676–82. [DOI] [PubMed] [Google Scholar]
- 26. Khanna S, Pardi DS, Aronson SL, Kammer PP, Baddour LM. Outcomes in community-acquired Clostridium difficile infection. Aliment Pharm Therap 2012; 35:613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pechal A, Lin K, Allen S, Reveles K. National age group trends in Clostridium difficile infection incidence and health outcomes in United States community hospitals. BMC Infect Dis 2016; 16:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Solanki D, Kichloo A, El-Amir Z, et al. Clostridium difficile infection hospitalizations in the United States: insights from the 2017 National Inpatient Sample. Gastroenterology Res 2021; 14:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elixhauser A, Steiner C, Gould C. Readmissions following hospitalizations with Clostridium difficile infections, 2009. In: Healthcare Cost and Utilization Project (HCUP) statistical briefs. Rockville, MD: Agency for Healthcare Research and Quality, 2012. [PubMed] [Google Scholar]
- 30. Lucado J, Gould C, Elixhauser A. Clostridium difficile infections (CDI) in hospital stays, 2009. In: Healthcare Cost and Utilization Project (HCUP) statistical briefs. Rockville, MD: Agency for Healthcare Research and Quality, 2012. [PubMed] [Google Scholar]
- 31. Hengel RL, Schroeder CP, Jo J, et al. Recurrent Clostridioides difficile infection worsens anxiety-related patient-reported quality of life. J Patient Rep Outcomes 2022; 6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heinrich K, Harnett J, Vietri J, Chambers R, Yu H, Zilberberg M. Impaired quality of life, work, and activities among adults with Clostridium difficile infection: a multinational survey. Digest Dis Sci 2018; 63:2864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garey KW, Jo J, Gonzales-Luna AJ, et al. Assessment of quality of life among patients with recurrent Clostridioides difficile infection treated with investigational oral microbiome therapeutic SER-109. JAMA Netw Open 2023; 6:e2253570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hohmann EL. Are microbial politics local? Ann Intern Med 2016; 165:667. [DOI] [PubMed] [Google Scholar]
- 35. Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis 2021; 73:e1029–44. [DOI] [PubMed] [Google Scholar]
- 36. Khanna S, Pardi DS. Poor outcomes after Clostridium difficile infection in IBD. Nat Rev Gastroenterol Hepatol 2012; 9:307–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.