Abstract
In the renal collecting ducts, chloride reabsorption occurs through both transcellular and paracellular pathways. Recent literature highlights a functional interplay between both pathways. We recently showed that in polarized inner medullary collecting duct cells, expression of the basolateral kidney anion exchanger 1 (kAE1) results in a decreased transepithelial electrical resistance (TEER), in a claudin-4 dependent pathway. Claudin-4 is a paracellular sodium blocker and chloride pore. Here, we show that kAE1 expression in mouse inner medullary collecting duct cells triggers WNK4, SPAK and claudin-4 phosphorylation. Expression of a functionally dead kAE1 E681Q mutant has no effect on phosphorylation of these proteins. Expression of a catalytically inactive WNK4 D321A or chloride-insensitive WNK4 L319F mutant abolishes kAE1 effect on TEER, supporting a contribution of WNK4 to the process. We propose that variations of the cytosolic pH and chloride concentration upon kAE1 expression alter WNK4 kinase activity and tight junction properties.
Keywords: Kidney, Claudin, Transporters, Membrane protein, Epithelium, Tight junctions, Sodium conservation, Chloride conservation, Blood pressure, Paracellular proteins, Distal nephron, Collecting duct, Intercalated cells
1. Introduction
Fine-tuning of urine composition occurs in the renal collecting ducts. Collecting ducts are composed of a mixture of principal cells and intercalated cells. Principal cells are mainly responsible for transcellular water and Na+ reabsorption through aquaporin 2 (AQP2) and epithelial Na+ channel (ENaC), and potassium secretion through the renal outer medullary K+ channel, ROMK [1]. On the other hand, type-A and type-B intercalated cells work in harmony to sustain systemic acid/base balance. Type-A IC cells express apical V–H+ ATPase to acidify the urine, and basolateral kidney anion exchanger 1 (kAE1) to reabsorb HCO3− to the bloodstream in exchange with Cl−. Conversely, neighbor type-B IC cells express basolateral membrane V–H+-ATPase to reabsorb H+, and apical pendrin, to secrete HCO3− into the urine [1]. The efficiency of these transcellular fluxes also depends on the paracellular permeability of the epithelium, which is dictated by the tight junction claudins. In humans, claudins form a 26-member family of transmembrane proteins that are localized in tight junctions [2]. They span the membrane 4 times and determine the tight junctions’ selective permeability. Both principal cells and intercalated cells express claudin-4 protein [3,4]. The main function of claudin-4 in the collecting ducts is to provide a Cl− paracellular shunt to facilitate Cl− reabsorption and block paracellular Na+ back flux to the urine [5,6].
In the collecting duct, claudin-4 has been indirectly implicated in pseudohypoaldosteronism type II (PHA II), a disease characterised by hypertension, hyperkalemia and hyperchloremic acidosis [7]. One cause of the disease has been genetically linked to two members of with-no-lysine kinase (WNK) family members, WNK1 and WNK4: a deletion mutation in the first intron of the gene encoding WNK1 or other missense mutations in the WNK4-encoding gene have been found to cause PHA II [8,9].
WNK1 and 4 are highly expressed in the distal nephron. They regulate salt and potassium homeostasis transcellularly by regulating the membrane expression and activities of epithelial sodium channel ENaC [10], the Na+/Cl− cotransporter NCC [11] and renal outer medullary potassium channel ROMK [12], through downstream effectors Ste20-related proline alanine rich kinase/oxidative stress responsive kinase 1 (SPAK/OSR1 kinases) [13]. WNK4 inhibits ENaC and ROMK activity by decreasing their membrane expression, but it activates NCC by phosphorylating SPAK. WNK1 has a similar effect on ENaC and ROMK, however, it counteracts the WNK4 inhibitory effect on NCC when co-expressed in Xenopus oocytes [14]. Additionally, WNK1 and 4 also regulate the paracellular ionic flux in the distal nephron by increasing claudin-4 phosphorylation [7,15,16].
We have previously reported that the type-A intercalated cell-specific kAE1 expression affects TJ properties via claudin-4 [3]. Encoded by SLC4A1, the 14 transmembrane domain glycoprotein kAE1 exchanges Cl− for bicarbonate and when mutated, causes distal renal tubular acidosis (dRTA), a disease characterized by a metabolic acidosis, hyperchloremia, hypokalemia, nephrocalcinosis, and renal failure if untreated [17]. When expressed in polarized Madin-Darby canine kidney (MDCKI) cells, kAE1 results in a leaky epithelium that becomes permeable to apically applied fluorescent biotin [18]. In our recent publication [3], we investigated this effect by expressing kAE1 wild-type (WT) or inactive kAE1 E681Q mutant in inducible inner medullary collecting duct (mIMCD3) cells and assessing their effect on TJ properties. We observed that expression of kAE1 WT but not the inactive mutant kAE1 E681Q, resulted in a decrease in transepithelial electrical resistance (TEER) and that this effect was mediated by claudin-4. In that manuscript, we also reported that kAE1 expression neither affected claudin-4 abundance nor plasma or basolateral membrane expression. However, the mechanism by which kAE1 influences claudin-4 and TJ properties remains unknown.
In this manuscript, we hypothesized that kAE1 function affects intracellular Cl− concentration, which triggers WNK4 kinase regulatory pathway that in turn modulates claudin-4 phosphorylation and activity. Our results show that: (i) kAE1 expression and function result in increased phosphorylation of WNK4, SPAK, and claudin-4; and (ii) the catalytically inactive WNK4 D321A or constitutively active WNK4 L319F mutant abolish kAE1 effect on tight junctions, supporting a WNK4-dependent effect of kAE1 on tight junction properties.
2. Methods
2.1. Plasmid constructs and antibodies
The pLVX TRE3G kAE1 WT and functionally inactive E681Q, both with an extracellular hemagglutinin (HA) epitope in position 557 (eAE1 numbering) have been previously published [3]. The HA epitope in the extracellular loop neither affects kAE1 localization nor function [19]. Previously described pcDNA3 vector containing human WNK4 WT and mutants L319F and D321A (all carrying an HA epitope) were used [20,21]. A polyclonal rabbit antibody from InVitrogen (Cat # 36-4800), or a monoclonal mouse anti-claudin-4 antibody coupled to AlexaFluor488 (3E2C1) (Thermoscientific, Cat # 329488) were used to detect claudin-4 in IMCD cells. Murine claudin-3 and claudin-8 were detected using rabbit polyclonal antibodies from InVitrogen (Cat # 34-1700 and 40–2600, respectively). The specificity of the polyclonal rabbit anti-claudin-4 antibody has been verified in our previous publication [3]. Polyclonal rabbit anti-WNK4 was obtained from AbCam (Cat # ab126477), polyclonal rabbit anti-phospho WNK4 (pWNK4) is a kind gift from Maria Castañeda-Bueno (Instituto Nacional de Nutrición Salvador Zubirán), polyclonal sheep anti-SPAK/OSR1 (S365D) and polyclonal sheep anti- S373 phospho SPAK/OSR1 (S670B) (pSPAK/OSR1) antibodies were obtained from Dundee University (https://www.ppu.mrc.ac.uk/). Monoclonal mouse anti-HA antibody was purchased from BioLegend (Cat # 901513) or Cell Signalling (Cat # 2367).
2.2. Cell culture and transient transfection
Immortalized mouse inner medullary collecting duct cells expressing kAE1 have been previously reported [3]. Briefly, 7 μg of either pLVX-Tet3G regulator plasmid, pLVX-TRE3G kAE1 WT-HA (later referred as kAE1) or pLVX-TRE3G kAE1 E681Q-HA cDNA were transfected in 70 % confluent Lenti-X 293T cells (Clontech) using Lenti-X HTX Packaging mix and Xfect Transfection Reagent (Clontech), as per the manufacturer's instructions. Following a 48 h incubation at 37 °C in serum-free OptiMEM medium (Gibco), supernatants were collected and filtered through 0.45 μm filters. The filtered supernatants with lentiviruses carrying both regulator (pLVX-Tet3G) and response (pLVX-TRE3G kAE1 WT-HA, pLVX-TRE3G kAE1 mutant, or pLVX-TRE3G EV) were next used to co-infect 70 % confluent mIMCD3 (ATCC, CRL-2123) in the presence of 8 μg/ml of polybrene (Sigma-Aldrich, USA) for 48 h. The selection of infected cells was achieved by addition of 4 μg/ml Puromycin and 2 mg/ml G418 to the growth medium of infected cells. kAE1 expression was induced by incubation with 0.5 μg/ml of doxycycline for 24 h kAE1 protein expression remained similar even after multiple passages of the cells. For transient transfection of WNK4 WT or mutants, mIMCD3 cells stably expressing inducible kAE1 WT-HA (1-2X104) were grown for 8 days on semi permeable filters, and the growth medium was changed every other day. Forty-eight hours before the start of the experiment, cells were transiently transfected with 10 μg of WNK4 cDNA and 3.75 μl Lipofectamine 3000 (Invitrogen) (added apically) according to the manufacturer's instructions. The addition of 0.5 μg/ml doxycycline (+Dox) 48 to 20 h prior to the start of the experiment resulted in kAE1 protein expression.
2.3. Immunoblotting
Lysates from kAE1-HA-expressing cells were prepared in RIPA buffer (0.3 M NaCl, 20 mM Tris/HCl pH 7.5, 2 mM EDTA, 2 % Deoxycholate, 2 % Triton X-100, 0.2 % SDS, pH 7.4) containing Complete EDTA-free protease inhibitors (Roche), and PhoSTOP phosphatase inhibitor (Roche) where indicated. After measurement of protein concentration using a BCA assay (Pierce, Rockford, IL, USA), 20 or 60 (where indicated) μg of proteins were loaded on a SDS-PAGE gel. Proteins of interest, including kAE1, claudin-4, WNK4, phospho-WNK4, SPAK, phospho-SPAK and β-actin proteins were detected using a mouse anti-HA, rabbit anti-claudin-4, rabbit anti-WNK, rabbit anti-pWNK4, sheep anti-SPAK, sheep anti-pSPAK or mouse anti-β-actin antibodies, respectively, overnight at 4 °C, followed by secondary antibodies coupled to horseradish peroxidase (HRP) (Cell Signalling) for 1 h at room temperature. The bands of interest were detected with a BioRad Imager (and ImageLab software (BioRad)), using Enhanced chemiluminescence (ECL prime western blotting detection reagent from GE Healthcare, Wauwatosa, WI, USA).
2.4. Ussing chambers experiments
Confluent kAE1-HA expressing mIMCD3 cells were grown for 10 days on 0.45 μm semi-permeable Transwell filters (Costar, Cat # 07200225). One day before the start of the experiment, these cells were transiently transfected using lipofectamine 3000 with cDNA encoding either WNK4-WT-HA or WNK4-D321A-HA. Expression of kAE1 protein was induced by the addition of 0.5 μg/ml of doxycycline to the cells or cells were kept untreated. The filters were then mounted in Ussing chambers maintained at 37 °C in solution A (145 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES, pH 7.4), followed by current clamping with a DVC 1000 I/V clamp (World Precision Instruments, Sarasota, FL) and filling electrodes with 3 M KCl. PowerLab (ADInstruments, Colorado Springs, CO) and Chart 4.0 software were used to record the data. We started by applying 90 μA pulses across the epithelial monolayer, followed by induction of a dilution potential by switching basolateral solution A to an iso-osmotic solution B with reduced NaCl to 80 mM (Solution B: 80 mM NaCl, 130 mM mannitol, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES, pH 7.4). Transepithelial electrical resistance (TEER), ion permeability ratios and absolute permeabilities of the epithelium to sodium and chloride were then calculated with the Goldman-Hodgkin Katz and Kimizuka Koketsu equations, as previously published [3,22,23].
2.5. Immobilized iron affinity electrophoresis
A modified SDS-PAGE gel protocol was used to assess claudin-4 protein phosphorylation as previously described [24]. The principle of this technique is based on the ability of iron chloride to capture negatively charged phosphorylated proteins. This results in the retention of phosphorylated proteins in the well of the gel while non-phosphorylated proteins can migrate to their expected molecular weight. By comparing the intensity of the claudin-4 band in the “iron gel” with that in the “normal gel”, one can determine the ratio of phosphorylated claudin-4 protein. Cells were washed with 1 X TBS prior to lysing, lysates were prepared with a Tris-based buffer lysis buffer containing PhoSTOP phosphatase inhibitor (Roche), and the SDS-PAGE gels did not include a stacking gel but only a resolving one with 10 % acrylamide. After full polymerization and removal of the combs, 35 μl/well of 10 % resolving gel without 20 mM FeCl3 was added to the bottom of half of the wells and 35 μl/well of 10 % resolving gel with 20 mM FeCl3 was added to the bottom of the remaining half of the wells and left to polymerize. Each sample was loaded twice on the same gel, once in a FeCl3-containing well and once in a FeCl3-free well, where the FeCl3-free wells served as controls. Standard immunoblot assay described above was next performed. To dephosphorylate proteins, cell lysates prepared without phosphatase inhibitors were treated with 1 unit of Calf Intestinal Alkaline Phosphatase (Invitrogen) per μg of protein and incubated at 37 °C for 30 min. Claudin-4 proteins (total and non-phosphorylated) were detected using rabbit anti-Claudin-4 antibodies. Of note, loading controls were not performed as they may be affected by phosphorylation and thus our analysis consisted in measuring the band density ratio of phosphorylated over total protein loaded on the same gel.
2.6. Statistical analysis
Graphs provided in this manuscript correspond to experiments that have been repeated a minimum of three independent times. Results are expressed as mean values ± standard error of the mean (SEM). The number of independent experimental repeats is represented by the “n” values provided. Unpaired Student t-test, Welch t-test, one-way or two-way ANOVA (where appropriate) were used in statistical analyses, P < 0.05 was considered significant. Normality of the data was verified using a Shapiro-Wilk test.
3. Results
3.1. Transport-competent kAE1 expression results in increased claudin-4 phosphorylation
We have previously reported that kAE1 expression affects TJs properties in mIMCD3 cells through claudin-4 and that kAE1 function is essential to this effect [3]. As claudin-4 phosphorylation alters TJs properties [7,16,[25], [26], [27]], we assessed the effect of kAE1 WT or inactive kAE1 E681Q mutant expression [28,29] on claudin-4 phosphorylation. Since anti-phosphorylated claudin-4 antibodies are not available, we used an immobilized iron affinity electrophoresis to detect changes in claudin-4 phosphorylation [24,30]. As shown in Fig. 1 A & B, by calculating the ratio of claudin-4 abundance in iron-containing gel over that of iron-free gel, we found that kAE1 expression resulted in a 21 ± 3 % (n = 5, ±SEM) increase in claudin-4 phosphorylation, although total claudin-4 protein abundance was not altered upon kAE1 expression [3]. However, upon kAE1 E681Q expression, the band density ratio of claudin-4 in the iron-containing lane was not significantly different from that of the iron-free lane (−6.8 ± 14.5 % (n = 6, ±SEM)) (Fig. 1 A & B), indicating that claudin-4 phosphorylation was not affected upon kAE1 E681Q mutant expression. Dephosphorylation of kAE1 WT lysates with a phosphatase treatment prior to immobilized iron affinity electrophoresis loading abolished the effect of kAE1 expression on claudin-4 phosphorylation (% phosphorylated claudin-4: 14.9 ± 8.3 % (n = 4, ±SEM) versus 4.1 ± 7.6 % (n = 4, ±SEM) in kAE1 WT cells - and + Dox, respectively). Since claudin-4 interacts with claudin-8, we assessed claudin-8 abundance in cells expressing kAE1 or not. As shown on Fig. 1C & D, there was no difference in claudin-8 abundance in cells expressing kAE1 WT or E681Q mutant. Similarly, the abundance of claudin-3 was unchanged in cells expressing kAE1 WT, kAE1 E681Q or not (Fig. 1 E & F). Hence, expression of kAE1 WT, but not functionally dead kAE1 E681Q, increases claudin-4 phosphorylation in mIMCD3 cells. These results indicate that the function of kAE1 is essential for the effect seen on claudin-4 phosphorylation.
Fig. 1.
Effect of expressing kAE1 WT or E681Q protein on claudin-4 phosphorylation
A, Representative FeCl3-free or -containing immunoblots showing claudin-4 phosphorylation in the presence or absence of kAE1 WT (top) or E681Q proteins (bottom). Samples from mIMCD3 cells expressing kAE1 WT or E681Q (+Dox) or not (-Dox) were run on 10 % acrylamide gels that either contain iron Cl− or not (See material and Methods for further details). The claudin-4 bands were detected with rabbit anti-claudin-4 antibody and on the “iron gel” represent the non-phosphorylated fraction of claudin-4 in these lysates. B, quantification of phosphorylated claudin-4 normalized to total claudin-4. C, immunoblots showing the abundance of claudin-8 in kAE1 WT (top) or E681Q (bottom)-expressing cells. D, quantification of claudin-8 from immunoblots shown in C. E, immunoblots showing abundance of claudin-3 in kAE1 WT (top) or E681Q (bottom)-expressing cells. F, quantification of claudin-3 from immunoblots shown in E. Error bars correspond to means ± SEM, n = 4–6. Not significant (n.s.), **P < 0.01 using a Student's t-test. Normality of the data was verified using a Shapiro-Wilk test. Uncropped blots are provided in Supplementary Figures.
3.2. WNK4 is endogenously expressed in mIMCD3 cells and expression of transport competent kAE1 increases WNK4 phosphorylation
As WNK4 is sensitive to Cl− [20,31,32], an anion that is also a kAE1 substrate, and since kAE1 affects claudin-4 phosphorylation (Fig. 1), we hypothesized that WNK4 is involved in kAE1 effect on claudin-4 phosphorylation. We first confirmed that mIMCD3 cells express WNK4 endogenously by immunoblotting using rabbit anti-WNK4 antibodies (Fig. 2 An upper panels). Quantification of the relative amount of total WNK4 protein abundance in mIMCD3 cells in the presence and absence of kAE1 protein showed that kAE1 expression (+Dox) had no effect on WNK4 protein abundance (Fig. 2 B left panel).
Fig. 2.
mIMCD3 cells express endogenous WNK4, and kAE1 WT but not E681Q mutant protein expression increases WNK4 phosphorylation.
A, C, Immunoblots using rabbit anti-total WNK4, rabbit anti-phospho (S1196) WNK4 or mouse anti β-Actin antibodies to detect total, phospho- WNK4 or β-Actin, in kAE1 WT- or E681Q-expressing mIMCD3 cells, respectively, in the presence or absence of kAE1 WT proteins. B, D, quantification of total and phospho-WNK4 normalized to β-actin. Error bars correspond to means ± SEM, n = 4. Not significant (n.s.), *P < 0.05, **P < 0.01 using a Student's t-test or a Welch t-test (phospho-WNK4 in WT cells). Normality of the data was verified using a Shapiro-Wilk test. Note that additional bands may appear on the actin blot, corresponding to detection of the proteins with antibodies incubated prior to the anti-actin antibody. Uncropped blots are provided in Supplementary Figures.
We next assessed the effect of kAE1 expression on WNK4 phosphorylation, using an anti-phospho-WNK4 (S1196) antibody. As shown in Fig. 2 A & B (bottom and right panels, respectively), WNK4 phosphorylation increased by 32 ± 8 % (n = 4, ±SEM) upon kAE1 WT expression in mIMCD3 cells. To determine whether the effect of kAE1 on WNK4 phosphorylation is merely due to kAE1 expression or whether its function is necessary, we assessed WNK4 abundance and phosphorylation upon expression of the inactive kAE1 E681Q mutant in mIMCD3 cells. As shown in Fig. 2C & D, although kAE1 E681Q expression slightly increased total WNK4 abundance, its phosphorylation level remained unchanged. These results indicate that kAE1 function is required to affect WNK4 phosphorylation.
3.3. Transport-competent kAE1 increases SPAK/OSR1 phosphorylation
To further investigate and confirm kAE1's effect on WNK4, we next determined the phosphorylation status of a known WNK4 downstream substrate, SPAK/OSR1, by using anti-phospho-SPAK/OSR1 (S373) antibodies. SPAK is phosphorylated by WNK4 and is involved in the regulatory pathway of the Na+/Cl− cotransporter NCC [13]. As demonstrated in Fig. 3 A & B, kAE1 WT expression resulted in increased phosphorylation of SPAK/OSR1 by 31 ± 12 % (n = 4, ±SEM), in line with WNK4's increased phosphorylation. However, neither total nor phosphorylated SPAK were significantly changed upon kAE1 E681Q expression (Fig. 3C & D). Altogether, these results confirm that expression of a transport-competent kAE1 leads to an increase in WNK4 phosphorylation as well as the phosphorylation of its downstream effector SPAK.
Fig. 3.
kAE1 WT but not E681Q mutant protein expression increases SPAK/OSR1 phosphorylation
A, C, Immunoblots using rabbit anti-total or phospho-SPAK/OSR1, or mouse anti β-Actin antibodies in kAE1 WT- (A) or E681Q-expressing (C) mIMCD3 cells, respectively, in the presence or absence of kAE1 WT proteins. Note that the immunoblot for housekeeping protein actin in panel C (bottom) is the same as in Fig. 2C (bottom). B, D, quantification of total or phospho-SPAK/OSR1 normalized to β-actin. Error bars correspond to means ± SEM, n = 3 to 8. Not significant (n.s.), *P < 0.05 using a Student's t-test. Normality of the data was verified using a Shapiro-Wilk test. Uncropped blots are provided in Supplementary Figures.
3.4. Chloride-insensitive, constitutively active WNK4 L319F mutant migrates faster than WT or catalytically inactive WNK4 D321A mutant WNK4
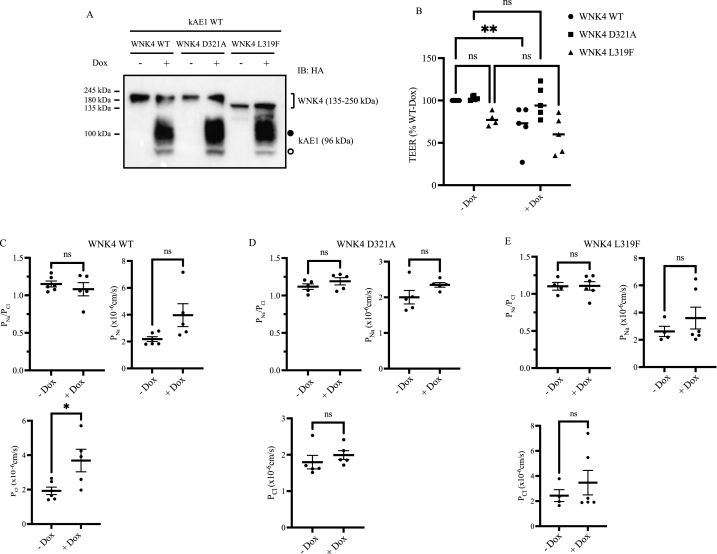
To conclusively assess whether kAE1 effect on TJ properties is mediated by WNK4, we reasoned that transfecting a constitutively inactive WNK4 mutant in mIMCD3 cells should abolish kAE1 effect on lowering TEER. We transiently expressed WT or catalytically dead WNK4 D321A mutant [20,33,34] in polarized mIMCD3 cells expressing (+Dox) or not (-Dox) kAE1 protein (Fig. 4). As an additional control, we included the constitutively active, chloride-insensitive WNK4 L319F WNK4 mutant [21,35,36]. WNK4 D321A mutant is kinase incompetent and is therefore unable to phosphorylate SPAK/OSR1 and downstream effectors [20], while WKN4 L319F mutant cannot be inhibited by chloride and is therefore constitutively active [21]. We first confirmed transfected WNK4 protein expression using a mouse anti-HA antibody since both kAE1 and WNK4 carry a HA epitope. As shown on Fig. 4 A, kAE1 expression was induced upon doxycycline incubation as expected. As previously reported, kAE1 appears as a double band, with the upper, thick band corresponding to complex glycosylated kAE1 (black circle) and the bottom band to high mannose glycosylated kAE1 (white circle) [3].
Fig. 4.
Kinase dead WNK4 D321A or chloride insensitive WNK4 L319F abolish kAE1 effect on tight junctions.
A, Immunoblot using mouse anti-HA antibody to detect both WNK4 WT or mutants and kAE1 protein in mIMCD3 cells inducibly expressing kAE1 and transiently transfected with cDNA encoding WNK4 WT, WNK4 D321A or WNK4 L319F. B, Percentage of transepithelial electrical resistance relative to mIMCD3 cells without Dox measured in cells expressing kAE1 (+ or – Dox) and transiently transfected with cDNA encoding WNK4 WT, WNK4 D321A or WNK4 L319F. C, PNa/PCl ratio, PNa and PCl measured for IMCD cells transfected with WNK4 WT. D, PNa/PCl ratio, PNa and PCl measured for IMCD cells transfected with WNK4 D321A. E, PNa/PCl ratio, PNa and PCl measured for IMCD cells transfected with WNK4 L319F. Error bars correspond to means ± SEM, n = 4–6. *P < 0.05, **P < 0.01, not significant (n.s.) using two-way ANOVA and a Fisher's LSD test. Uncropped blots are provided in Supplementary Figures.
WNK4 WT, L319F and D321A cDNA were efficiently transfected in the polarized mIMCD3 cells. Interestingly, both WT and WNK4 D321A mutants migrated at the same molecular weight (around 250 kDa) but the WNK4 L319F mutant migrated at a lower molecular weight (approximately 150 kDa). Nevertheless, this result indicates a successful transfection in polarized mIMCD3 cells.
3.5. Both chloride-insensitive, constitutively active WNK4 L319F or catalytically dead WNK4 D321A mutants abolish kAE1 effect on tight junctions
We next assessed the effect of WNK4 WT or mutants on the TEER of polarized monolayers expressing or not kAE1. We hypothesized that 1) WNK4 L319F mutant will induce a lower TEER than WNK4 WT in mIMCD3 cells as this mutant is constitutively active, 2) expression of catalytically inactive WNK4 D321A mutant will not affect TEER as it is inactive, and 3) the effect of kAE1 expression on TEER will be abolished in cells expressing the catalytically inactive D321A or constitutively active WNK4 mutants.
As shown in Fig. 4 B, in contrast to catalytically dead WNK4 D321A, expression of constitutively active WNK4 L319F resulted in a trend for decreased TEER values, suggesting a loosening of tight junctions. The decrease in TEER is similar to that observed when kAE1 is expressed in WT cells.
In cells expressing WNK4 WT, kAE1 expression causes a significant decrease in TEER compared to cells that do not express kAE1, as reported previously [3]. However, in cells expressing the constitutively active WNK4 L319F or catalytically dead WNK4 D321A, kAE1 no longer had an effect on TEER values as there was no significant difference between “– Dox” and “+ Dox” conditions. In cells expressing WNK4 WT, no change in PNa/PCl ratio was observed upon Dox incubation, however PNa trended upward (not significant) and PCl was significantly higher. PNa/PCl ratio, PNa and PCl were not significantly different after Dox incubation in both WNK4 L319F or WNK4 D321A cell lines (Fig. 4 D & E). These findings suggest that in cells expressing either a chloride insensitive WNK4 or a catalytically dead one, kAE1 no longer has an effect on TEER, thus supporting the involvement of WNK4 in the pathway between kAE1 and claudin-4.
4. Discussion
We previously showed that transport-competent kAE1 expression lowers TEER in polarized mIMCD3 cells and this protein physically interacts with claudin-4 in mIMCD3 cells [3]. In that manuscript, we also reported that kAE1 expression neither affected claudin-4 abundance nor plasma or basolateral membrane expression. We further showed that kAE1 effect on tight junction was abolished when claudin-4 was knocked down. Given that claudin-4 physically interacts with chloride-sensor WNK4 in Madin-Darby canine kidney (MDCK) cells and that this interaction increases claudin-4 phosphorylation [7], we hypothesized that via its Cl− transport function, kAE1 alters WNK4 kinase activity, which in turn phosphorylates claudin-4 and results in decreased TEER. We have previously verified that kAE1 WT expression in mIMCD3 cells affects cytosolic pH and extracellular chloride concentration [3]. Our results demonstrate that the expression of kAE1 WT promotes the phosphorylation of claudin-4 and activates the WNK4-SPAK pathway by promoting the phosphorylation of WNK4 and its downstream effector SPAK/OSR1. This effect was abolished in cells expressing the functionally dead kAE1 E681Q mutant. Expression of either a functionally dead WNK4 mutant or a constitutively active WNK4 mutant abolished kAE1 effect on tight junctions. Our results support that (i) kAE1 effect is dependent on cytosolic chloride concentration, and (ii) WNK4 is in the signaling pathway between kAE1 and claudin-4-driven effect on tight junction properties.
In our previous publication, we reported that although kAE1 expression and function affects TJ properties through claudin-4, neither claudin-4 abundance nor plasma membrane expression were affected by kAE1 [3]. In presence or absence of kAE1 in confluent mIMCD3 cells, claudin-4 did not relocate away either from the TJ [3]. Thus, the mechanism by which kAE1 affects TJ properties remained unknown. In the current manuscript, we report that transport-competent kAE1 expression results in increased claudin-4 phosphorylation. The lack of claudin-4 relocation despite increased phosphorylation has been seen by another research group investigating WNK4 D564A mutant related to PHA II disease [7]. WNK4 WT and D564A mutant were found to increase claudin-4 phosphorylation without affecting its localization at the plasma membrane. Our data, in agreement with Yamauchi and colleagues’ data, suggest that claudin-4 phosphorylation may modify its function at the TJ without altering its targeting to, or removal from the TJs.
In the literature, claudin-4 is reported to undergo several post-transcriptional modifications that affect its function, including phosphorylation [37]. The C-terminus of claudin-4 carries several possible phosphorylation sites by different kinases [38,39]. In MDCK cells, claudin-4 phosphorylation by EphA2 leads to its removal from the TJ, and as a result decreases TEER [27]. Similarly, protein kinase C phosphorylates claudin-4 in ovarian cancer cells which leads to defective barrier function due to claudin-4 delocalization [26]. Comparably, extracellular signal-regulated protein kinase 1 and 2 (ERK 1/2) phosphorylate claudin-4 and leads to its ubiquitination and degradation in rat salivary epithelial cells [40]. However, in keratinocyte cells, claudin-4 phosphorylation by atypical PKC targets claudin-4 to the TJ, which causes a transient increase in TEER during TJ formation [25]. Therefore, conflicting effects of claudin-4 phosphorylation are reported and likely depend on the expression system used in these studies. It is possible that claudin-4 interaction with other claudins is affected by its kAE1-induced phosphorylation and possibly by its interaction with kAE1. Claudin-4 is known to interact with claudin-8, and together, they form a paracellular Cl− pore in the collecting ducts [41]. This interaction is crucial for claudin-4 localization into the TJs. kAE1-induced phosphorylation of claudin-4 may cause a conformational change that could disrupt its interaction with claudin-8, resulting in modified TJ properties. Alternatively, phosphorylation may cause conformational changes within the first extracellular loop leading to a modification in claudin-4 ion selectivity, and subsequently TJ properties. Note that it is possible that claudin-4 behaves differently in the hyper-osmotic environment of the renal medulla which is not fully reflected in the iso-osmotic cell growth conditions in our experiments. In fact, we previously reported that in mouse kidney sections, claudin-4 appears basolaterally located in addition to its expected tight junction localization [3].
Our results indicate that expression of transport competent kAE1 results in higher WNK4 phosphorylation level. Changes in intracellular Cl− concentration influence the function of the protein kinase WNK family [32]. Except for the KS-WNK1 isoform which is only expressed in the kidney, all members (1–4) of the WNK family contain a Cl− binding site. This site is located in the kinase domain, which is highly conserved among WNK family members [42,43]. Cl− bound to WNK1 inhibits its autophosphorylation, thus, inhibits its kinase activity [42]. In vitro kinase assays showed that WNK4, WNK1 and WNK3 are inhibited by a serial increase in Cl− concentration [32]. WNK4 kinase activity responds to Cl− concentration variations ranging from 0 to 40 mmol98, which are within the physiological range of epithelial cells. These findings were further confirmed by in vivo studies that identified WNK4 as a physiological intracellular Cl− sensor [31]. As Cl− is a kAE1 substrate, we hypothesized that kAE1 transport influences WNK4 activity, and subsequently, claudin-4 phosphorylation. Our results indeed showed that transport-competent kAE1 resulted in increased claudin-4, WNK4 and SPAK/OSR1 phosphorylation, in agreement with our hypothesis. To further confirm the involvement of WNK4 in the pathway, we assessed the effect of kAE1 in cells expressing either WT WNK4, a constitutively active (WNK4 L319F) or a kinase dead (D321A) WNK4 mutant [20,21,35,36,44]. WNK4 L319F protein migrated at a lower molecular weight, compared to WNK WT or D321A. The reason for this shift is unknown, however it is possible that the point mutation causes a SDS-resistant modification of the overall WNK4 conformation and results in a faster migration. kAE1 expression in WNK4-expressing cells significantly reduced TEER as previously observed [3]. Expression of inactive WNK4 D321A mutant did not affect TEER values compared to WNK4 WT transfected cells (Fig. 4). In contrast with WNK4 WT-expressing cells, induction of kAE1 expression did not significantly reduce TEER, PNa/PCl, PNa or PCl in WNK4 D321A mutant expressing cells. This indicates that WNK4 kinase activity is required for kAE1 effect on tight junctions.
Expression of constitutively active WNK4 L319F mutant significantly reduced TEER, suggesting that at basal state, endogenous WNK4 is likely inactive since mIMCD3 cells have a higher TEER. kAE1 expression neither significantly reduced TEER, nor affected PNa/PCl, PNa or PCl further, thus kAE1 effect is abolished when WNK4 is constitutively active. This could indicate that permeability of the epithelium is already maximal when WNK4 L319F mutant is expressed, and that alteration of cytosolic Cl− by kAE1 no longer has an effect on WNK4 activity. Overall, these experiments with both mutants support that WNK4 is involved in the pathway between kAE1 and claudin-4-mediated effect on tight junctions. Importantly, our experiments focused on WNK4 but did not assess kAE1 effect on other kinases such as L-WNK1, or KS-WNK1, which are expressed in these cells. The role of these proteins will be assessed in further research.
Overall, our results support that kAE1 effect on tight junction properties occurs in a claudin-4- and a WNK4-dependent mechanism. This work also emphasizes further a functional relationship between transcellular transporters and paracellular pathways in regulating epithelial permeability. As the collecting duct is an epithelium with high TEER, variations in permeability of this epithelium can have important consequences on the final composition of urine.
Grants
This work was supported by a CIHR Project Grant (PJT168871) and a Kidney Health Research grant from the Kidney Foundation of Canada (2020KHRG-666615). R.L. received a graduate studentship from the Libyan Government and from a CREATE Program funded by the Natural Sciences and Engineering Research Council (NSERC). GE received a Community Engagement Scholarship and a partial Graduate Teaching Assistantship from the University of Alberta. MC-C is supported by CONACyT Mexicon grant no. 87794 and 845144; and from PAPIIT UNAM, grant no. IA208522.
Data availability statement
Data associated with this study have not been deposited into a publicly available repository as they are included in Supplementary Material in the article.
CRediT authorship contribution statement
Rawad Lashhab: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. Grace Essuman: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Maria Chavez-Canales: Conceptualization, Methodology, Resources, Writing – original draft, Writing – review & editing. R. Todd Alexander: Conceptualization, Resources, Validation, Writing – original draft, Writing – review & editing. Emmanuelle Cordat: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Emmanuelle Cordat reports financial support was provided by Canadian Institutes of Health Research. Rawad Lashhab reports financial support was provided by Natural Sciences and Engineering Research Council of Canada. Maria Chavez-Canales reports financial support was provided by CONACyT Mexicon. Maria Chavez-Canales reports financial support was provided by PAPIIT UNAM.
Acknowledgements
We thank Kris MacNaughton for excellent technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22280.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Roy A., Al-bataineh M.M., Pastor-Soler N.M. Collecting duct intercalated cell function and regulation. Clin. J. Am. Soc. Nephrol. 2015;10:305–324. doi: 10.2215/CJN.08880914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mineta K., Yamamoto Y., Yamazaki Y., Tanaka H., Tada Y., Saito K., Tamura A., Igarashi M., Endo T., Takeuchi K., Tsukita S. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585:606–612. doi: 10.1016/J.FEBSLET.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Lashhab R., Rumley A.C., Arutyunov D., Rizvi M., You C., Dimke H., Touret N., Zimmermann R., Jung M., Chen X.-Z., Alexander T., Cordat E. The kidney anion exchanger 1 affects tight junction properties via claudin-4. Sci. Rep. 2019;9:3099. doi: 10.1038/s41598-019-39430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiuchi-Saishin Y., Gotoh S., Furuse M., Takasuga A., Tano Y., Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J. Am. Soc. Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. http://www.ncbi.nlm.nih.gov/pubmed/11912246 [DOI] [PubMed] [Google Scholar]
- 5.Hou J., Renigunta A., Yang J., Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc. Natl. Acad. Sci. USA. 2010;107:18010–18015. doi: 10.1073/pnas.1009399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou J., Rajagopal M., Yu A.S. Claudins and the kidney. Annu. Rev. Physiol. 2013;75:479–501. doi: 10.1146/annurev-physiol-030212-183705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamauchi K., Rai T., Kobayashi K., Sohara E., Suzuki T., Itoh T., Suda S., Hayama A., Sasaki S., Uchida S. Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins. Proc. Natl. Acad. Sci. U.S.A. 2004;101:4690–4694. doi: 10.1073/PNAS.0306924101/SUPPL_FILE/06924FIG7.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis-Dit-Picard H., Kouranti I., Rafael C., Loisel-Ferreira I., Chavez-Canales M., Abdel-Khalek W., Argaiz E.R., Baron S., Vacle S., Migeon T., Coleman R., Do Cruzeiro M., Hureaux M., Thurairajasingam N., Decramer S., Girerd X., O'Shaugnessy K., Mulatero P., Roussey G., Tack I., Unwin R., Vargas-Poussou R., Staub O., Grimm R., Welling P.A., Gamba G., Clauser E., Hadchouel J., Jeunemaitre X. Mutation affecting the conserved acidic WNK1 motif causes inherited hyperkalemic hyperchloremic acidosis. J. Clin. Invest. 2020;130:6379–6394. doi: 10.1172/JCI94171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson F.H., Disse-Nicodeme S., Choate K.A., Ishikawa K., Nelson-Williams C., Desitter I., Gunel M., V Milford D., Lipkin G.W., Achard J.M., Feely M.P., Dussol B., Berland Y., Unwin R.J., Mayan H., Simon D.B., Farfel Z., Jeunemaitre X., Lifton R.P. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. 80- [DOI] [PubMed] [Google Scholar]
- 10.Ring A.M., Cheng S.X., Leng Q., Kahle K.T., Rinehart J., Lalioti M.D., Volkman H.M., Wilson F.H., Hebert S.C., Lifton R.P. WNK4 regulates activity of the epithelial Na+ channel in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 2007;104:4020–4024. doi: 10.1073/PNAS.0611727104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang C.L., Angell J., Mitchell R., Ellison D.H. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J. Clin. Invest. 2003;111:1039–1045. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahle K.T., Wilson F.H., Leng Q., Lalioti M.D., O'Connell A.D., Dong K., Rapson A.K., MacGregor G.G., Giebisch G., Hebert S.C., Lifton R.P. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat. Genet. 2003;35:372–376. doi: 10.1038/NG1271. [DOI] [PubMed] [Google Scholar]
- 13.Alessi D.R., Zhang J., Khanna A., Hochdörfer T., Shang Y., Kahle K.T. The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci. Signal. 2014;7 doi: 10.1126/SCISIGNAL.2005365. [DOI] [PubMed] [Google Scholar]
- 14.Yang C.L., Zhu X., Wang Z., Subramanya A.R., Ellison D.H. Mechanisms of WNK1 and WNK4 interaction in the regulation of thiazide-sensitive NaCl cotransport. J. Clin. Invest. 2005;115:1379–1387. doi: 10.1172/JCI22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahle K.T., MacGregor G.G., Wilson F.H., Van Hoek A.N., Brown D., Ardito T., Kashgarian M., Giebisch G., Hebert S.C., Boulpaep E.L., Lifton R.P. Paracellular Cl- permeability is regulated by WNK4 kinase: insight into normal physiology and hypertension. Proc. Natl. Acad. Sci. USA. 2004;101:14877–14882. doi: 10.1073/pnas.0406172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohta A., Yang S.-S., Rai T., Chiga M., Sasaki S., Uchida S. Overexpression of human WNK1 increases paracellular chloride permeability and phosphorylation of claudin-4 in MDCKII cells. Biochem. Biophys. Res. Commun. 2006;349:804–808. doi: 10.1016/j.bbrc.2006.08.101. [DOI] [PubMed] [Google Scholar]
- 17.Almomani E.Y.Y., Chu C.Y.S.Y., Cordat E. Mis-trafficking of bicarbonate transporters: implications to human diseases. Biochem. Cell. Biol. 2011;89:157–177. doi: 10.1139/o10-153. o10-153[pii]10.1139/o10-153. [DOI] [PubMed] [Google Scholar]
- 18.Toye A.M., Banting G., Tanner M.J.A. Regions of human kidney anion exchanger 1 (kAE1) required for basolateral targeting of kAE1 in polarized kidney cells: mis-targeting explains dominant renal tubular acidosis (dRTA) J. Cell Sci. 2004;117:1399–1410. doi: 10.1242/jcs.00974. [DOI] [PubMed] [Google Scholar]
- 19.Cordat E., Kittanakom S., Yenchitsomanus P.T., Li J., Du K., Lukacs G.L., Reithmeier R.A.F. Dominant and recessive distal renal tubular acidosis mutations of kidney anion exchanger 1 induce distinct trafficking defects in MDCK cells. Traffic. 2006;7:117–128. doi: 10.1111/J.1600-0854.2005.00366.X. [DOI] [PubMed] [Google Scholar]
- 20.Bazúa-Valenti S., Chávez-Canales M., Rojas-Vega L., González-Rodríguez X., Vázquez N., Rodríguez-Gama A., Argaiz E.R., Melo Z., Plata C., Ellison D.H., García-Valdés J., Hadchouel J., Gamba G. The effect of WNK4 on the Na+-Cl- cotransporter is modulated by intracellular chloride. J. Am. Soc. Nephrol. 2015;26:1781–1786. doi: 10.1681/ASN.2014050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castañeda-Bueno M., Arroyo J.P., Zhang J., Puthumana J., Yarborough O., Shibata S., Rojas-Vega L., Gamba G., Rinehart J., Lifton R.P. Phosphorylation by PKC and PKA regulate the kinase activity and downstream signaling of WNK4. Proc. Natl. Acad. Sci. U.S.A. 2017;114:E879–E886. doi: 10.1073/pnas.1620315114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borovac J., Barker R.S., Rievaj J., Rasmussen A., Pan W., Wevrick R., Alexander R.T. Claudin-4 forms a paracellular barrier, revealing the interdependence of claudin expression in the loose epithelial cell culture model opossum kidney cells. Am. J. Physiol. Cell Physiol. 2012;303:C1278–C1291. doi: 10.1152/ajpcell.00434.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimizuka H., Koketsu K. Ion transport through cell membrane. J. Theor. Biol. 1964;6:290–305. doi: 10.1016/0022-5193(64)90035-9. [DOI] [PubMed] [Google Scholar]
- 24.Lee B.S., Lasanthi G.D., Jayathilaka P., Huang J.S., Gupta S. Immobilized metal affinity electrophoresis: a novel method of capturing phosphoproteins by electrophoresis. J. Biomol. Tech. 2008;19:106–108. https://pubmed.ncbi.nlm.nih.gov/19137092/ [PMC free article] [PubMed] [Google Scholar]
- 25.Aono S., Hirai Y. Phosphorylation of claudin-4 is required for tight junction formation in a human keratinocyte cell line. Exp. Cell Res. 2008;314:3326–3339. doi: 10.1016/j.yexcr.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 26.D'Souza T., Indig F.E., Morin P.J. Phosphorylation of claudin-4 by PKCepsilon regulates tight junction barrier function in ovarian cancer cells. Exp. Cell Res. 2007;313:3364–3375. doi: 10.1016/J.YEXCR.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka M., Kamata R., Sakai R. EphA2 phosphorylates the cytoplasmic tail of Claudin-4 and mediates paracellular permeability. J. Biol. Chem. 2005;280:42375–42382. doi: 10.1074/JBC.M503786200. [DOI] [PubMed] [Google Scholar]
- 28.Cheung J.C., Cordat E., Reithmeier R.A. Trafficking defects of the Southeast Asian ovalocytosis deletion mutant of anionexchanger 1 membrane proteins. Biochem. J. 2005;392:425–434. doi: 10.1042/BJ20051076. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16107207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jennings M.L., Smith J.S. Anion-proton cotransport through the human red blood cell band 3 protein. Role of glutamate 681. J. Biol. Chem. 1992;267:13964–13971. http://www.ncbi.nlm.nih.gov/pubmed/1352774 [PubMed] [Google Scholar]
- 30.Lee B.-S., Jayathilaka L.P., Huang J.-S., Gupta S. Applications of immobilized metal affinity electrophoresis. Methods Mol. Biol. 2019:371–385. doi: 10.1007/978-1-4939-8793-1_32. [DOI] [PubMed] [Google Scholar]
- 31.Chen J.C., Lo Y.F., Lin Y.W., Lin S.H., Huang C.L., Cheng C.J. WNK4 kinase is a physiological intracellular chloride sensor. Proc. Natl. Acad. Sci. U.S.A. 2019;116:4502–4507. doi: 10.1073/pnas.1817220116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terker A.S., Zhang C., Erspamer K.J., Gamba G., Yang C.L., Ellison D.H. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int. 2016;89:127–134. doi: 10.1038/KI.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vitari A.C., Deak M., Morrice N.A., Alessi D.R. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem. J. 2005;391:17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai H., Cebotaru V., Wang Y.H., Zhang X.M., Cebotaru L., Guggino S.E., Guggino W.B. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int. 2006;69:2162–2170. doi: 10.1038/SJ.KI.5000333. [DOI] [PubMed] [Google Scholar]
- 35.Murillo-de-Ozores A.R., Rodríguez-Gama A., Bazúa-Valenti S., Leyva-Ríos K., Vázquez N., Pacheco-Álvarez D., De La Rosa-Velázquez I.A., Wengi A., Stone K.L., Zhang J., Loffing J., Lifton R.P., Yang C.L., Ellison D.H., Gamba G., Castañeda-Bueno M. C-terminally truncated, kidney-specific variants of the WNK4 kinase lack several sites that regulate its activity. J. Biol. Chem. 2018;293:12209–12221. doi: 10.1074/JBC.RA118.003037/ATTACHMENT/12C71CA6-0635-4A07-96E0-CBC17FC42498/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mongin A.A., Sun D., Cai H., Castañeda-Bueno M., Murillo-De-Ozores A.R., Chávez-Canales M., De Los Heros P., Gamba G. 2020. Physiological Processes Modulated by the Chloride-Sensitive WNK-SPAK/OSR1 Kinase Signaling Pathway and the Cation-Coupled Chloride Cotransporters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Günzel D., Yu A.S.L. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013;93:525–569. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.González-Mariscal L., Garay E., Quirós M. Regulation of claudins by posttranslational modifications and cell-signaling cascades. Curr. Top. Membr. 2010;65:113–150. doi: 10.1016/S1063-5823(10)65006-5. [DOI] [Google Scholar]
- 39.Butt A.M., Khan I.B., Hussain M., Idress M., Lu J., Tong Y. Role of post translational modifications and novel crosstalk between phosphorylation and O-beta-GlcNAc modifications in human claudin-1, -3 and -4. Mol. Biol. Rep. 2012;39:1359–1369. doi: 10.1007/S11033-011-0870-7. [DOI] [PubMed] [Google Scholar]
- 40.Cong X., Zhang Y., Li J., Mei M., Ding C., Xiang R.-L., Zhang L.-W., Wang Y., Wu L.-L., Yu G.-Y. Claudin-4 is required for modulation of paracellular permeability by muscarinic acetylcholine receptor in epithelial cells. J. Cell Sci. 2015;128:2271–2286. doi: 10.1242/jcs.165878. [DOI] [PubMed] [Google Scholar]
- 41.Hou J., Renigunta A., Yang J., Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc. Natl. Acad. Sci. U.S.A. 2010;107 doi: 10.1073/pnas.1009399107. –18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piala A.T., Moon T.M., Akella R., He H., Cobb M.H., Goldsmith E.J. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci. Signal. 2014;7 doi: 10.1126/SCISIGNAL.2005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu B.e., Min X., Stippec S., Lee B.H., Goldsmith E.J., Cobb M.H. Regulation of WNK1 by an autoinhibitory domain and autophosphorylation. J. Biol. Chem. 2002;277:48456–48462. doi: 10.1074/jbc.M207917200. [DOI] [PubMed] [Google Scholar]
- 44.Murillo-de-Ozores A.R., Carbajal-Contreras H., Magaña-Ávila G.R., Valdés R., Grajeda-Medina L.I., Vázquez N., Zariñán T., López-Saavedra A., Sharma A., Lin D.H., Wang W.H., Delpire E., Ellison D.H., Gamba G., Castañeda-Bueno M. Multiple molecular mechanisms are involved in the activation of the kidney sodium-chloride cotransporter by hypokalemia. Kidney Int. 2022;102:1030–1041. doi: 10.1016/J.KINT.2022.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study have not been deposited into a publicly available repository as they are included in Supplementary Material in the article.