Abstract
Leptospirosis is a zoonotic bacterial disease caused by infection of spirochetes of the genus Leptospira. While typically self-limiting and non-fatal, severe manifestations can arise, including various neurological complications that are often overlooked. This case study presents a 59-year-old man with serologically positive Leptospirosis, who subsequently developed asymmetrical progressive leg weakness, severe back pain, and overflow incontinence suggestive of mononeuritis multiplex. Doxycycline treatment was started and intended to last for seven days. The patient had ongoing paraparesis, but all other problems were disappeared. The present case emphasizes the significance of identifying and treating neurological problems brought on by leptospirosis. To improve suitable treatment plans and patient outcomes, more research on these problems is necessary.
Keywords: Leptospirosis, Paraparesis, Jaundice, Thrombocytopenia, Renal abnormality
1. Introduction
Leptospirosis, a highly prevalent zoonotic endemic disease in tropical and subtropical regions, demonstrate a relatively low documented burden in Indonesia compared to other tropical diseases, such as dengue fever [1], despite the country's total population of 274 million. Its impact is particularly significant in areas with high humidity, heavy rainfall, and frequent flooding, such as Central and East Java, while occurrences in West Java are relatively rare [[2], [3], [4]]. According to official reports from the Ministry of Health, the incidence of Leptospirosis in Indonesia ranged from 239 to 1170 cases per year between 2012 and 2021 [4]. However, Costa et al. estimated a higher incidence of 55.54 cases per 100,000 population (95 % CI 20.32–99.53) in Southeast Asia [5].
The transmission of spirochetes occurs through direct contact with infected rodents' urine, blood, or tissue, and infection can occur in humans through water intake, mucous membrane, percutaneous, or conjunctival routes [3,6]. Following an incubation period of 1–2 weeks, a leptospiraemic phase lasting at least 3–7 days is observed, followed by an immune phase that extends from 4 to 30 days [3,6]. Among all cases of leptospirosis, neurological manifestations occur in approximately 10–15 % [6], with neuropathy being one of the least common presentations [7,8].
The clinical manifestations of leptospirosis are diverse, often leading to its under-recognition as the primary etiology, particularly when patients present with classical risk factors for a primary neurological disorder. Limited occurrences and insufficient diagnostic modalities have further hindered the identification of this disease, particularly in low-income countries. This report presents a rare case of leptospirosis from West Java, with the unusual manifestation of mononeuritis multiplex. Rather than conducting a classical workup solely centered on the neurological deficit, the identification of renal dysfunction, thrombocytopenia, and hepatic insufficiency prompted a comprehensive investigation into neuroleptospirosis. This underscores the significance of taking these conditions into account in similar cases.
2. Background
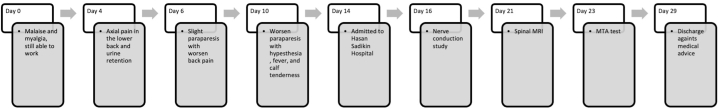
A 59-year-old male presented to the emergency unit at Hasan Sadikin Hospital in Bandung, Indonesia, with symptoms of paraparesis. Motor weakness was more pronounced in the distal extremities compared to the proximal ones and had been present for ten days prior to admission. The patient experienced acute, sharp pain in the low back area without radiating sensations, rated 8–9 on a numerical rating scale. There was no reported history of chronic low back pain, with or without radicular pain. Over the course of six days, the paraparesis worsened to the point where the patient was unable to lift his lower extremities, and he also experienced overflow incontinence during the four days leading up to admission. Additionally, the patient reported influenza-like symptoms, including malaise and myalgia, over the past two weeks. Fever was present for the previous four days, coinciding with calf tenderness (Fig. 1). The patient worked as a farmer and had a history of significant exposure to mud and an environment with a high risk of infection. There were no comorbidities such as diabetes mellitus, and no history of spinal cord trauma, autoimmune disease, nor history of familial disorders.
Fig. 1.
History of clinical timeline.
During the physical examination, the patient exhibited a Glasgow Coma Scale (GCS) score of 15 (E4M6V5) along with a mild fever (37.8 °C), a pulse rate of 105 beats per minute, and mild hypertension (140/90 mmHg). He appeared to be clinically well-hydrated but displayed signs of jaundice. There is no headache, seizures, conjunctival suffusion, eyes abnormalities, lymphadenopathy or rash. Neurological examination revealed negative Lasègue's/Kernig signs. There was evidence of hypotonic inferior paraparesis, with a motor strength score of 1–2 (maximum score 5) observed in bilateral knee flexion and knee extension, and a motor strength level of zero observed in bilateral dorsoplantar, plantar inversion, and plantar eversion movements. The sensory evaluation yielded inconsistent findings. Deep tendon reflexes were diminished, and no abnormal plantar reflexes were noted. The anal reflex and toe flexion were positive, while saddle anesthesia was absent.
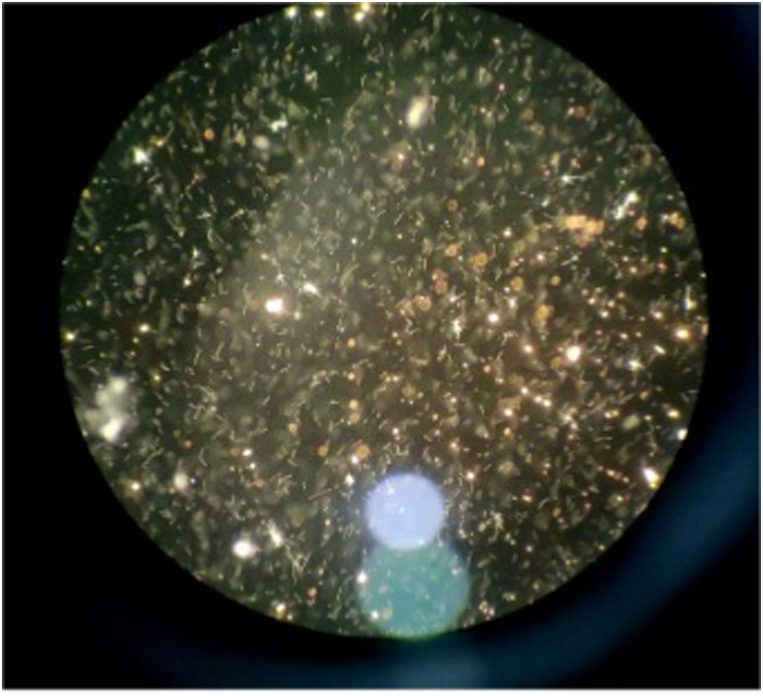
Laboratory investigations revealed anemia (hemoglobin: 7.7 g/dL) and thrombocytopenia (thrombocytes: 67,000/μL), along with renal dysfunction (urea: 122.5 mg/dL, creatinine: 1.99 mg/dL), and liver dysfunction (aspartate aminotransferase: 76 U/L, alanine aminotransferase: 70 U/L, total bilirubin: 2.614 mg/dL, direct bilirubin: 1.531 mg/dL, indirect bilirubin: 1.083 mg/dL) (Table 2). The non-structural (NS) 1 rapid test for dengue was non-reactive, hepatitis B antigen (HBsAg) was non-reactive, and the immunoglobulin M antibody test for leptospira was also negative. Microscopic Agglutination Test (MAT) was performed in Department of Microbiology Diponegoro University, Semarang, Indonesia. The results showed seropositivity for serovars Pyrogenes and Patox from serogroup Pyrogenes, Semaranga, strain Sallnem, and Patoc 1, with a titer of 1:320 (Fig. 2). Additionally, a lower titer of 1:160 was observed for strain Ballico from serovar Australis (species L. interrogans), strain Sarmin from serovar Sarmin (species L. weilii), and strain M84 serovar Sejroe and strain Perpelitsin from serovar Tarassovi (species L. borgpetersenii).
Table 2.
Hematology and biochemistry test.
| Parameter | Baseline | Discharge | Normal reference | Parameter | Baseline | Discharge | Normal reference |
|---|---|---|---|---|---|---|---|
| Hemoglobin | 7,7 g/dl | 9,6 g/dl | 14,0–17,4 | Calcium | 4,33 mg/dL | 4,54 mg/dL | 8,6–10,3 |
| Leukocyte | 6540/μL | 18210/μL | 4,4–11,3 | Magnesium | 2,9 mg/dL | – | 1,8-2,6 |
| Hematocrit | 24,71 % | 28,6 % | 41,5–50,4 | ALT | 70 U/L | 41 | <30 |
| Thrombocyte | 67x103/μL | 418x103/μL | (150–400) x103 | AST | 76 U/L | 32 | <25 |
| MCV | 75,31 fl | 82,2 fl | 80–96 | Total Bilirubin | 2614 mg/dL | 1792 | 0,25-1,0 |
| MCH | 23,51 pg | 27,6 pg | 27,5–33,2 | Direct bilirubin | 1.531 mg/dL | 1407 | 0,0-0,25 |
| MCHC | 31.21 g/dL | 33,6 g/dL | 33,4–35,5 | Urea | 122.5 mg/dL | 27,9 | 8–25 |
| Basophils | 0 | 0 | 0–1 | Creatinine | 1.99 U/L | 0,63 | 70–160 |
| Eosinophils | 0 | 0 | 0–4 | BUN | 56.93 | – | 6–27 mg/dL |
| Neutrophils | 70 % | 88 | 45–73 | Sodium | 126 mmol/L | 133 | 135–145 |
| Lymphocyte | 16 % | 2 | 18–44 | Potassium | 4.11 mmol/L | 4,3 | 3,5-5,0 |
| Monocytes | 11 % | 4 | 3–8 | Blood glucose | 113 mg/dL | – | 70–100 |
| NLR | 4.54 | 46,53 | 0,78-3,53 | BUN/CR | 28.6 | – | 6–27 |
| HBsAg | – | Non-reactive | Non-reactive | INR | – | 0,98 | 35,1–43,9 |
ALT: alanine transaminase; AST: aspartate aminotransferase; NLR: neutrophil-to-lymphocyte ratio; BUN: blood urea nitrogen; BUN/CR: BUN to creatinine ratio; MCV: mean corpuscular volume; MCH: mean corpuscular haemoglobin; MCHC: mean corpuscular haemoglobin concentration.
Fig. 2.
Microscopic Agglutination Test (MAT)
Positive microscopic agglutination test (MAT) at a titre of 1:320.
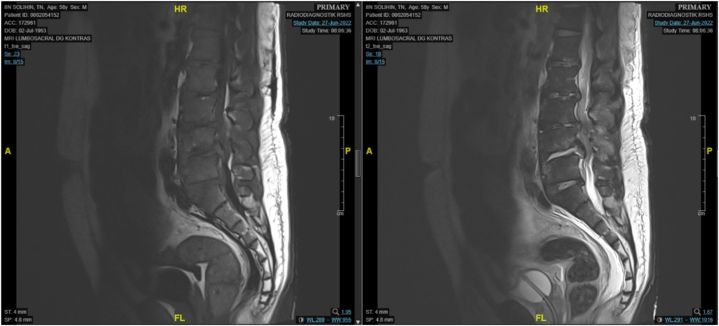
Chest X-ray results were normal, while lumbar Magnetic Resonance Imaging (MRI) indicated signal changes at the intervertebral discs, suggestive of a degenerative process. Specifically, there was a disc bulge at the first and second levels, extruded discs at the fourth and fifth intervertebral spaces, and herniation of the nucleus pulposus posteriorly at the first level of the sacral segment (Fig. 3).
Fig. 3.
Spinal Magnetic Resonance Imaging
Sagittal MRI of the lumbosacral region (A) T1 and (B) T2, showing a protruded disc at levels second, third, and fifth, and the first level of sacral segment leading to herniation of nucleus pulposus to the posterior.
The WHO modified Faine's assessment score for leptospirosis was 31, indicating a presumptive diagnosis (Table 1) [9,10]. The presence of fever (score: 2), muscle pain (score: 4), jaundice (score: 1), residing in a rainfall area (score: 5), contact with a contaminated environment (score: 4), and positive microscopic agglutination test (score: 15) contributed to the total score. A nerve conduction study (NCS) revealed significant prolonged distal latency (6.0 ms and 7.2 ms) and reduced amplitude (0.2 mV and 0.3 mV) in bilateral motor peroneal nerves and 4.0 mv in the left tibial motor nerve. The left tibial motor nerve showed prolonged distal onset latency (7.2 ms), and reduced amplitude (4,0 mV), whereas all sensory nerves remain normal. F wave studies indicate no response suggestive of a severe axonal demyelinated motor neuropathy of bilateral peroneal and left tibial nerves (Table 3). H-reflex studies showed no response in both the left and right tibial nerves. Given the polyneuropathy features of motor weakness, electromyography assessment was not performed.
Table 1.
| PART A: Clinical Data | ||
|---|---|---|
| Headache | 2 | 0 |
| Fever | 2 | 2 |
| Temp >39 °C | 2 | 0 |
| Conjunctival suffusion | 4 | 0 |
| Meningism | 4 | 0 |
| Muscle pain | 4 | 4 |
| Conjunctival suffusion + Meningism + Muscle pain | 10 | 0 |
| Jaundice | 1 | 1 |
| Albuminuria/Nitrogen Retention | 2 | 0 |
| Haemoptysis/dyspnoea | 2 | 0 |
| PART B: Epidemiological Factorsa, b, c, d, e, f | ||
| Rainfall | 5 | 5 |
| Contact with contaminated Environment | 4 | 4 |
| Animal contact | 1 | 0 |
| PART C: Bacteriological and Lab Findings | ||
| Isolation of leptospira in culture - Diagnosis certain | ||
| PCRa | 25 | 0 |
| Positive Serology | ||
| ELISAb IgM Positive*f | 15 | 0 |
| SATc Positivef | 15 | 0 |
| Other rapid testsd,f | 15 | 0 |
| MATe – Single positive in high titerf | 15 | 15 |
| MATe – Rising titer/seroconversion (Paired sera) | 25 | 0 |
| Total | 31 | |
Polymerase Chain Reaction.
Enzyme Linked Immunosorbant Assay.
Slide Agglutination Test.
Other rapid tests—Latex agglutination test/Leptodipstick/LeptoTek lateral flow/Lepto Tek Dri Dot test.
Microscopic Agglutination Test.
Any one of the tests only should be scored. A presumptive diagnosis of leptospirosis may be made if: (i) Score of Part A + Part B = 26 or more (Part C laboratory report is usually not available before fifth day of illness; thus, it is mainly a clinical and epidemiologic diagnosis during early part of disease) or Part A + Part B + Part C ≥ 25. A score between 20 and 25: Suggests a possible but unconfirmed diagnosis of leptospirosis.
Table 3.
Nerve conduction study (NCS).
| Motor nerve conduction studies | |||||
|---|---|---|---|---|---|
| Stimulation Site | NR | Onset (ms) | Normal Onset (ms) | O–P* Amp (mV) | Normal O–P Amp |
| Left Peroneal Motor (Ext Dig Brev) | |||||
| Ankle | 6 | <4,8 | 0,2 | >3,0 | |
| Poplitea | 14 | 0,1 | |||
| Right Peroneal Motor (Ext Dig Brev) | |||||
| Ankle | 4,3 | <4,8 | 0,3 | >3,0 | |
| Poplitea | 11,4 | 0,2 | |||
| Left Tibial Motor (Abd Hall Brev) | |||||
| Ankle | 7,2 | <5,0 | 4 | >6,0 | |
| Knee | 16,6 | 2,8 | |||
| Right Tibial Motor (Abd Hall Brev) | |||||
| Ankle | 4,1 | <5,0 | 6,5 | >6,0 | |
| Knee | 12,3 | 4,6 | |||
| F Wave Studies | |||||
| NR | F-Latency (ms) | Latency Norm (ms) | L-R F-Latency (ms) | L-R Latency Norm | |
| Left Peroneal (Markers) (EDB) | |||||
| NR | <60 | <5,1 | |||
| Right Peroneal (Markers) (EDB) | |||||
| NR | <60 | <5,1 | |||
| Left Tibial (Markers) (Abd Hallucis) | |||||
| 51,81 | <61 | 12,16 | <5,7 | ||
| Right Tibial (Markers) (Abd Hallucis) | |||||
| 39,65 | <61 | 12,16 | <5,7 | ||
| H Reflex Studies | |||||
| NR | H-Latency (ms) | Latency Norm (ms) | L-R H-Latency (ms) | L-R Latency Norm | |
| Left Tibial (Gastrocnemius) | |||||
| NR | <32 | <2,0 | |||
| Right Tibial (Gastrocnemius) | |||||
| NR | <32 | <2,0 | |||
NR: no response; ms: mili second; Ext Dig Brev: extensor digiti brevis; Abd Hall Brev: abductor hallucis brevis; Amp: amplitudo; mv: mili volt; R: right; L: left; EDB: extensor digitorum brevis; O–P: onset to peak.
Following a seven-day oral administration of doxycycline 100 mg twice daily, the patient experienced alleviation of all symptoms, accompanied by normalization of hemoglobin and platelet levels, as well as kidney and liver enzyme values. Mecobalamin 500 mcg was administered twice daily following the identification of motor nerve abnormalities through NCS. However, despite these improvements, the paraparesis persisted. Upon hospitalization, the patient's Modified Rankin Scale (MRS) score was five, signifying severe disability and the requirement for ongoing care and attention due to immobility and incontinence. Regrettably, due to financial limitations, the patient declined further physical rehabilitation. Informed consent was obtained to publish this case report from the patient.
3. Discussion
The diagnosis of leptospirosis in this patient was established based on the presence of fever, muscle pain, and jaundice, along with relevant risk factors such as residing in a high-rainfall area and exposure to environments contaminated with rat urine or feces. Additionally, seropositive results from MAT on blood preparations were obtained, yielding a score of 31 according to the WHO Modified Faine's Score, supporting the presumptive diagnosis of leptospirosis [9,10].
The patient's clinical presentation, including asymmetrical paraparesis, axial pain, and bladder dysfunction, along with the significant reduction in response amplitudes observed in the left tibial and bilateral peroneal motor studies, is indicative of mononeuritis multiplex. However, we carefully considered other potential diagnoses that could explain the observed clinical findings. It is worth noting that the disc degeneration observed in the lumbar MRI is likely associated with the patient's age but may not be the underlying cause of the paraparesis in this case. Classic radiculopathy due to degenerative causes typically presents with unilateral symptoms and a more gradual onset, often accompanied by a history of sensory and strength disturbances that are exacerbated by activities such as standing, walking, and hyperextension [11]. In the moderate or severe stages, radicular pain typically precedes the worsening degenerative process by several months or even years. While MRI can reveal underlying pathologies related to low back pain, it may not always correlate with the clinical findings [12]. Considering the abrupt onset of spinal cord dysfunction, characterized by bilateral weakness of the lower extremities and impaired bowel and bladder control, acute transverse myelitis was considered as a possible diagnosis. However, the abnormal findings in the peripheral nerve conduction studies and preserved sensory function did not align with this diagnosis. It is important to note that the absence of inflammation signals in the spinal MRI, as observed in this patient, does not definitively exclude the possibility of acute transverse myelitis and has been reported in 17.2 %–30 % of cases [13,14].
This patient is likely in the transitional phase from leptospirosis to the immune or immune-leptospiraemic phase. The exact time of infection with leptospira in this patient is unknown, making it difficult to determine the precise incubation period. After prodromal symptoms, which lasted for approximately four days, paraparesis developed four days after the onset of the initial symptoms. During the leptospiraemic phase, visceral involvement can occur, affecting organs such as the liver, kidneys, hematological, and respiratory systems. Conversely, neurological involvement is attributed to the immune response of the body against the organism, indicating the immune phase [6]. A previous study on neuromuscular biopsy revealed evidence of Wallerian degeneration and perivascular inflammatory infiltration in the epineural vessels. It is hypothesized that antibodies produced in response to leptospiral antigens may cross-react with peripheral nerves, leading to systemic vasculitis and the formation of circulating immune complexes [3,15].
The blood IgM-detectable enzyme-linked immunosorbent assay (IgM-ELISA), using a rapid immunochromatographic assay kit (Biomedika, Pakar BioMedika, Indonesia) in this patient yielded a negative result, which could be attributed to various factors, including the timing of the test. In theory, ELISA is capable of detecting antibodies during the initial week of illness, enabling prompt diagnosis confirmation and initiation of treatment [16]. However, MAT is a gold standard for diagnosing Leptospirosis [3,16,17] and despite the sensitivity and specificity of IgM ELISA compared to MAT were 90.6%–100 % and 92–97.5 %, respectively [18,19], its correlation with MAT is frequently abysmal; antibody levels are generally low or absent during early infection [16]. MAT, a resource-intensive diagnostic method necessitating well-trained personnel [20], is exclusively accessible at only three centers in all of Indonesia [1]. In additional, we used the WHO modified Faine's criteria developed in India to help diagnose leptospirosis [10,16]. Sensitivity was 58 %, specificity was 97.4 %, and PPV/NPV of 85.7 % and 89.9 %, respectively, p < 0.001 [9].
Leptospira PCR was not available in our setting, and it is most likely to yield a positive result during the first week of infection. Limited access to diagnostic modalities in Indonesia, including Rickettsia testing available at a single center primarily for research purposes, restricted our ability to explore alternative differential diagnoses, such as Rickettsia and Hanta virus infections. Notably, the absence of hallmark symptoms like headache and rash [20], which are characteristic of Rickettsia infections, influenced our decision. Furthermore, it's important to emphasize that peripheral nerve abnormalities are more commonly associated with leptospirosis compared to Rickettsia infections [1]. Consequently, we did not conduct the PCR test or evaluate Rickettsia or Hanta virus infection, which is a limitation commonly encountered in resource-constrained regions where leptospirosis is prevalent [1].
The first strain of leptospirosis discovered in Indonesia in 1937 was Veldrat Semarang, which was initially isolated by Sardjito. However, subsequent investigations confirmed that it belongs to the non-pathogenic biflexa species. A study conducted by Litbang P2B2 Banjarnegara reported that out of 63 blood samples from Indonesia, 60.4 % were infected with strain Patoc 1 [21]. Unlike in some other settings, in Indonesia, a titer of 1:320 is considered positive based on personal communication with local microbiologists. Currently, it remains unknown which specific strains have a predilection for the nervous system. In a study of 94 cases of leptospirosis in Bulgaria, 20 cases (21.3 %) involved the central nervous system (CNS), with L.tropica (15 %) and L.tsaratsovo (15 %) being the predominant strains identified [22]. High levels of anti-leptospiral IgG have been shown to predict disease severity regardless of the strain. However, it is important to note that both studies did not specifically mention the involvement of the nervous system in severe leptospirosis [23].
Several significant limitations should be taken into account. Firstly, the patient's refusal to undergo lumbar puncture resulted in limited information regarding the presence of inflammation or infectious agents in the cerebrospinal fluid (CSF). However, it should be noted that pathogen isolates are less likely to be detected during the immune phase [3,16]and were more often cultured from tissue or cerebrospinal fluid (CSF) within the first ten days [16]. Secondly, this patient did not undergo electromyography (EMG) and did not have paired sera testing by MAT assay. The decision to prioritize selected examinations due to limited budget for health insurance reflects the situation in most countries with a high burden of leptospirosis. Furthermore, the incidence of undiagnosed leptospirosis cases in Indonesia is not uncommon, underscoring the diagnostic challenges associated with this disease, characterized by non-specific clinical presentations and the limited availability of diagnostic resources in routine healthcare services. Notably, within a substantial cohort of acute fever cases in Indonesia, more than half (56.9 %) of the 51 leptospirosis cases were initially overlooked during hospitalization but were subsequently identified through research-oriented examinations of archived samples in which no pathogens had been initially detected [1].
In this patient, the clinical and laboratory findings were typical of leptospirosis. The complete clinical recovery, along with chronological correlation with the immune leptospiremic phase, strongly suggests that the paraparesis reported in this case was attributable to leptospirosis. The presence of severe axonal impairment may have contributed to the irreversible paraparesis observed. This case serves as an important reminder that mononeuritis multiplex with blood and hepatorenal abnormalities can be a potential clinical feature of leptospirosis. To arrive at an accurate diagnosis, it is essential to conduct a comprehensive neurological examination and a thorough general assessment. Furthermore, antibiotic administration and implementing long-term care and rehabilitation measures can play a vital role in preventing patients from experiencing irreversible disability.
4. Conclusion
Neuroleptospirosis is a rare condition that is often under-reported in Indonesia. It is crucial to thoroughly investigate and evaluate for neuroleptospirosis in patients presenting with neurological abnormalities, along with accompanying features such as hepatorenal syndrome, jaundice, and thrombocytopenia, even in the absence of clear exposure to animals or water contaminated by an infected animal.
Funding
This study was supported by National Institutes of Health (R01AI145781) and Medical Research Council UK (MR/S004963/1).
Data availability statement
Data will be made available upon personal contact to authors.
CRediT authorship contribution statement
Sofiati Dian: Writing – review & editing, Writing – original draft, Methodology, Data curation, Conceptualization. Ni Made Gitaria Sylvana Ratmadewi: Writing – review & editing, Data curation. Suryani Gunadharma: Writing – review & editing, Data curation. Ahmad Rizal Ganiem: Writing – review & editing, Supervision, Funding acquisition, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We thank dr. Iva Puspitasari, SpMK from Universitas Diponegoro, Semarang, for the MAT examination, and Rubiah Adawiyah, MD for arranging the sample shipment.
References
- 1.Gasem M.H., et al. Leptospirosis in Indonesia: diagnostic challenges associated with atypical clinical manifestations and limited laboratory capacity. BMC Infect. Dis. 2020;20(1):179. doi: 10.1186/s12879-020-4903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gasem M.H., et al. An observational prospective cohort study of the epidemiology of hospitalized patients with acute febrile illness in Indonesia. PLoS Neglected Trop. Dis. 2020;14(1) doi: 10.1371/journal.pntd.0007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levett P.N. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kk R. Jakarta; 2021. Profil Kesehatan Indonesia 2021. [Google Scholar]
- 5.Costa F., et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Neglected Trop. Dis. 2015;9(9) doi: 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panicker J.N., Mammachan R., Jayakumar R.V. Primary neuroleptospirosis. Postgrad. Med. 2001;77(911):589–590. doi: 10.1136/pmj.77.911.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azouvi P., et al. [Acute and reversible axonal polyneuropathy in post-leptospirosis] Rev. Neurol. (Paris) 1989;145(11):805–807. [PubMed] [Google Scholar]
- 8.Hancox R.J., Karalus N., Singh V. Mononeuritis multiplex in leptospirosis. Scand. J. Infect. Dis. 1991;23(3):395–396. doi: 10.3109/00365549109024332. [DOI] [PubMed] [Google Scholar]
- 9.Shivakumar S., Shareek P.S. Diagnosis of leptospirosis utilizing modified Faine's criteria. J. Assoc. Phys. India. 2004;52:678–679. [PubMed] [Google Scholar]
- 10.Bandara K., et al. Utility of modified Faine's criteria in diagnosis of leptospirosis. BMC Infect. Dis. 2016;16(1):446. doi: 10.1186/s12879-016-1791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benoist M. The natural history of lumbar disc herniation and radiculopathy. Joint Bone Spine. 2002;69(2):155–160. doi: 10.1016/s1297-319x(02)00385-8. [DOI] [PubMed] [Google Scholar]
- 12.Endean A., Palmer K.T., Coggon D. Potential of magnetic resonance imaging findings to refine case definition for mechanical low back pain in epidemiological studies: a systematic review. Spine. 2011;36(2):160–169. doi: 10.1097/BRS.0b013e3181cd9adb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbatemarco J.R., et al. Modern look at transverse myelitis and inflammatory myelopathy: epidemiology of the national veterans health administration population. Neurol Neuroimmunol Neuroinflamm. 2021;8(6) doi: 10.1212/NXI.0000000000001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goh C., Desmond P.M., Phal P.M. MRI in transverse myelitis. J. Magn. Reson. Imag. 2014;40(6):1267–1279. doi: 10.1002/jmri.24563. [DOI] [PubMed] [Google Scholar]
- 15.de Souza A.L., et al. Peripheral nerve palsy in a case of leptospirosis. Trans. R. Soc. Trop. Med. Hyg. 2006;100(7):701–703. doi: 10.1016/j.trstmh.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Budihal S.V., Perwez K. Leptospirosis diagnosis: competancy of various laboratory tests. J. Clin. Diagn. Res. 2014;8(1):199–202. doi: 10.7860/JCDR/2014/6593.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niloofa R., et al. Diagnosis of leptospirosis: comparison between microscopic agglutination test, IgM-ELISA and IgM rapid immunochromatography test. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0129236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behera S.K., et al. Diagnosis of human leptospirosis: comparison of microscopic agglutination test with recombinant LigA/B antigen-based in-house IgM Dot ELISA dipstick test and latex agglutination test using bayesian latent class model and MAT as gold standard. Diagnostics. 2022;12(6) doi: 10.3390/diagnostics12061455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shekatkar S., et al. Comparison of an in-house latex agglutination test with IgM ELISA and MAT in the diagnosis of leptospirosis. Indian J. Med. Microbiol. 2010;28(3):238–240. doi: 10.4103/0255-0857.66484. [DOI] [PubMed] [Google Scholar]
- 20.Bharti A.R., et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 2003;3(12):757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 21.Ikawati B., Marbawati D. Bakteri Leptospira Patoc 1 merupakan strain bakteri dalam kelompok non patogen yang sering ditemukan pada penderita leptospirosis. BALABA. 2010;6(1):12–16. [Google Scholar]
- 22.Gancheva G.I., Kostadinova M.A., Kostadinova P.I. Involvement of central nervous system in leptospirosis. J Biomed Clin Res Volume. 2009;2(2):109–114. [Google Scholar]
- 23.Lessa-Aquino C., et al. Distinct antibody responses of patients with mild and severe leptospirosis determined by whole proteome microarray analysis. PLoS Neglected Trop. Dis. 2017;11(1) doi: 10.1371/journal.pntd.0005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon personal contact to authors.