Abstract
The aim of the study was to identify predictive patient characteristics for Henoch Schoenlein Purpura (HSPN) relapse in childhood HSPN. One hundred and thirty-five Chinese children with HSPN were enrolled in this study, mean age 10.25 ± 3.39 years. The pathology of HSPN was according to the International Study of Kidney Disease in Children criteria.(ISKDC); ISKDC II(mesangial proliferation (MP)) AND ISKDC III (MP with <50 % crescents).Recurrence of HSPN was observed in 66.3 % patients; male to female ratio (2:1)Statistically significant correlation existed between biopsy grade(p < 0.001), gender(p < 0.001),age ranges(p = 0.002) and treatment regimen (p < 0.001)in the frequency of recurrent HSPN episodes. We identified some significant predictors for HSPN relapse such as the severity of HSPN, adjunctive therapies administered to these patients,and close attention should be paid in patients between the ages 7 and 12 years old. In addition, the use of mycophenolate mofetil as an adjunctive therapy in the treatment of HSPN may reduce the frequency of HSPN relapse episodes in children.
Keywords: Henoch-schoenlein purpura nephritis, Vasculitis, Recurrence, Relapse, Children
1. Introduction
Henoch Schoenlein Purpura (HSP) also known as Immunoglobulin A vasculitis, is an autoimmune disease of the small blood vessels, characterized by the presence of palpable purpura and multi-organ involvement. Henoch-Schoenlein Purpura Nephritis (HSPN) progresses from HSP, as a result of renal involvement [1]. HSPN's renal involvement may lead to chronic and end-stage renal disease [2]. There have been some reports on the risk factors for HSPN, but finding predictors for frequent HSPN relapse episodes would be an important issue for the clinician caring for children with HSPN and, until now, few studies have reported on the HSPN relapse [[3], [4], [5], [6], [7], [8]]. Therefore, we examined the parameters of HSPN patients presented upon hospitalization in relation to the recurrence rates of HSPN and determined whether correlations existed between these parameters with the frequency of relapses. We identified some significant parameters for HSPN relapses that are useful for both patients and clinicians in predicting a recurrent HSPN episode.
2. Patients and methods
2.1. Study group
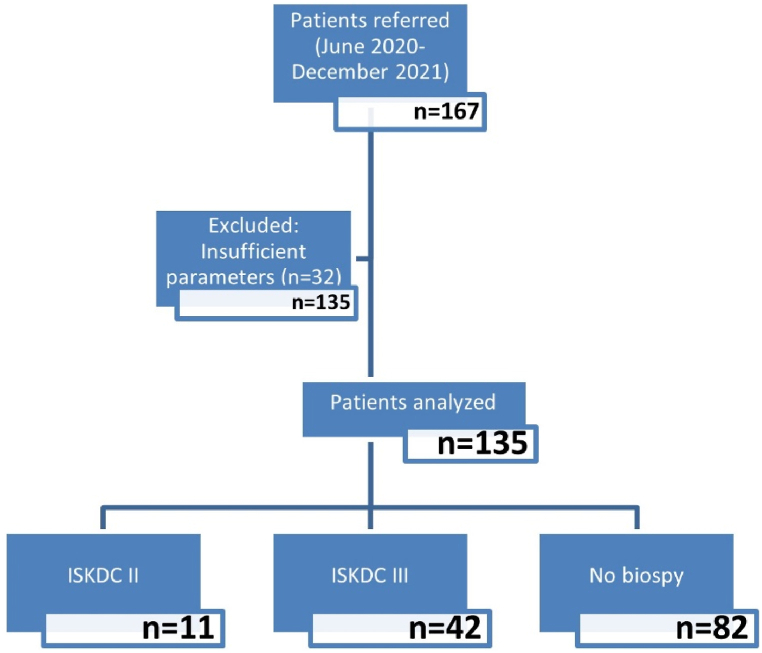
This was a retrospective study conducted in Sir Run Run Hospital Nanjing Medical University, Department of Pediatrics, Nanjing, China, from June 2020 to December 2021.One hundred and sixty-seven children were referred from the HSPN patients registry at the hospital but only one hundred and thirty-fivechildren were included in this study(91 males,44 females) as shown in Fig. 1.The inclusion criteria included the following: patient age less than 18 years, diagnosis being HSPN, parental consent approval, availability of complete medical records. Renal biopsy was performed in patients with kidney dysfunction presenting with either hematuria or heavy proteinuria. Meanwhile, in patients without kidney dysfunction, biopsy was determined by the amount of proteinuria(Alb<30 g/dl).Patients with ISKDC stage I; mesangial proliferation (MP) (n = 10), ISKDC stage IV; MP with 50–75%crescents (n = 11) and ISKDC stage V; MP with >75 % crescents (n = 1) were not analyzed and excluded from the study due to the small number of patients in these groups. Patients with missing data on drugs administered upon hospitalization (n = 10) were also excluded, as well as refusal of parental consent. Information about HSPN children's’ demographic values (age and sex), weight, height, hospitalization date, hospitalization number, onset time, pathology, cortisol levels, number of relapses, serum vitamin D status, and drugs administered(prednisone acetate, mycophenolate mofetil, tacrolimus were obtained from the subjects' medical records. The registry showed that patients only received systematic treatment which was in line with common local clinical practice and did not receive other nephroprotective treatments. Patient data obtained for analysis is presented in Table 1. The data were then examined to determine if any associations or correlations existed between recurrence of HSPN and various patient characteristics (BMI, gender, age, and drugs administered).
Fig. 1.
Flow diagram of study process.
Table 1.
Demographic and treatment modalities of the patients at time of hospitalization.
| Patient characteristics | Patients n (%) n = 135 |
|---|---|
| Gender | |
| Male | 91(67.4) |
| Female | 44(32.6) |
| Age, yearsa | 10.42 ± 3.380 |
| Cortisol ug/dLa | 5.3229 ± 5.01754 |
| 25 -hydroxyvitamin D ng/mla | 20.46 ± 7.42563 |
| 25 -hydroxyvitamin D2 ng/mla | 26.18 ± 7.36408 |
| 25 -hydroxyvitamin D3 ng/mla | 33.30 ± 5.57159 |
| Age ranges | |
| 0–6 years | 18(13.3) |
| 7–12 years | 78(57.8) |
| 13–17 years | 39(28.9) |
| Biopsy grade | |
| ISKDC II: mesangial proliferation (MP) | 11(8.2) |
| ISKDC III: MP with <50 % crescents | 42(31.1) |
| No biopsy | 82(60.7) |
| Number of HSPN relapse | |
| 0 (no relapse) | 41(30.4) |
| 1 | 47(34.8) |
| 2 | 32(23.7) |
| 3 | 7(5.2) |
| >4(more than 4 times) | 8(5.9) |
| Treatment | |
| PA only | 41(30.4) |
| MMF only | 3(2.2) |
| PA + Ta | 32(23.7) |
| PA + MMF | 15(11.1) |
| Ta + MMF | 3(2.2) |
| PA + MMF + Ta | 41(30.4) |
Data expressed as mean ± standard deviation.PA = Prednisone Acetate, Ta = Tacrolimus, MMF = Mycophenolate Mofetil.
2.2. Definitions
Nephritis was defined by the presence of macroscopic or microscopic hematuria (>5 RBCs a strong microscopic field in a centrifuged sample), with or without proteinuria [9]. Significant proteinuria was defined as proteinuria equal to or greater than 20 mg/m2/hour [10]. Recurrence of HSPN was defined as the number of times a patient was diagnosed or presented with another episode of HSPN between the period of June 2020 to December 2021 [4].The criteria of HSPN was classified according to the International Study of Kidney Disease in Children (ISKDC) [11]. The main morphological change leading to this classification is the crescents. These lesions, whose pathology has not yet been described, are associated with the destruction of the capillary wall with fibrinoid necrosis associated with intracapillary proliferation and infiltration of inflammatory cells, including macrophages and neutrophils [11,12]. In this study, HSPN criteria was divided into three groups according to ISKDC [11]; ISKDC II: mesangial proliferation (MP),ISKDC III: MP with <50 % crescents and no biopsy. Body Mass Index (BMI) was calculated and categorized according to the BMI age range classification for Asian and South Asian population [13].
2.3. Statistical analysis
Statistical analyses were performed on Statistical Package for the Social Sciences (SPSS), version 21.0 (SPSS Inc., Chicago, IL, USA). Normally distributed continuous data was expressed as mean ± standard deviation. Categorical data was expressed in percentages. Comparisons of the frequencies among groups was analyzed using Chi-square tests. Comparison of mean values between groups was carried out using the Paired-Samples T Test and one-way ANOVA. Post hoc analysis was calculated using the Student–Newman–Keuls test. All P values were 2 sided and P < 0.05 was considered significant.
3. Results
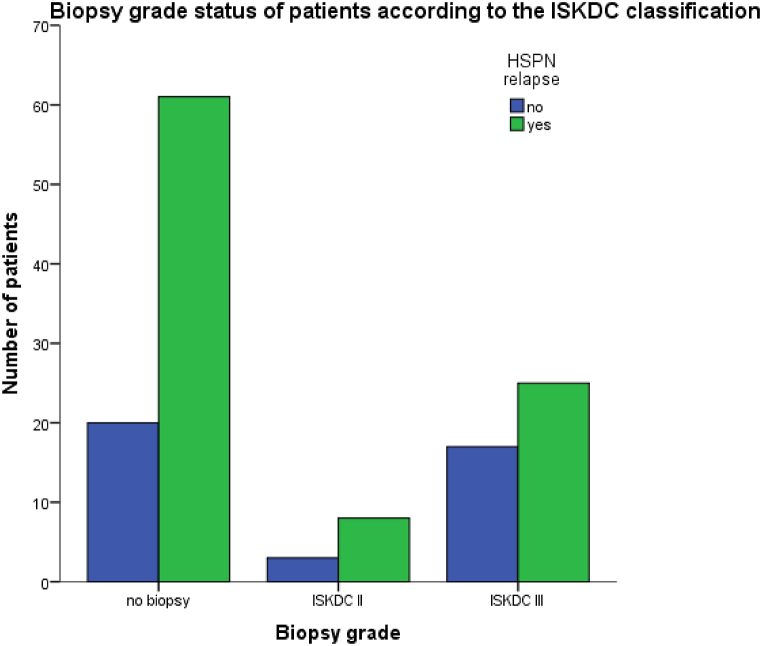
We included 135 patients diagnosed with allergic purpura nephritis. Almost 69 % experienced at least one HSPN relapse episode. The main demographic and parameters of patients with relapsing and non-relapsing HSPN are summarized in Table 2.The biopsy grade status of children according to the ISKDC classifications is shown in Table 2 and illustrated in Fig. 2. As shown in Table 2, patients’ mean age of the HSPN relapsing group was10.40 ± 3.075, and the mean age of the non-relapsing HSPN group was 10.46 ± 3.521. There was no statistically significant difference between the groups in terms of mean age, although there were 64 boys and 30 girls in the relapsing group, and27 boys and 14 girls in the non-relapsing group. Main demographic and parameter differences in patients with HSPN relapses are shown in Table 3.
Table 2.
Main differences in patients’ parameters and treatment options between patients with HSPN who experienced relapses and those who did not suffer this complication.
| Parameters | Relapsing HSPN n(%) | Non-relapsing HSPN n (%) | p |
|---|---|---|---|
| Gender (M/F) | 64(47.4)/30(22.2) | 27(20.0)/14(10.4) | .799 |
| Agea | 10.40 ± 3.075 | 10.46 ± 3.521 | .208 |
| Age ranges | |||
| 0–6 years | 15(11.1) | 3(2.2) | |
| 7–12 years | 50(37.0) | 28(20.7) | <.001 |
| 13–17 years | 29(21.5) | 10(7.4) | |
| BMI classification | |||
| Underweight | 31(31.6) | 21(21.4) | |
| Normal | 15(15.3) | 7(7.1) | <.001 |
| Overweight | 7(7.1) | 1(1.0) | |
| Obese | 10(10.2) | 6(6.1) | |
| 25 -hydroxyvitamin D ng/mla | 12.502 ± 7.177 | 13.424 ± 7.871 | .697 |
| 25-hydroxyvitamin D2 ng/mla | 12.850 ± 7.807 | 8.261 ± 6.122 | .045 |
| 25-hydroxyvitamin D3 ng/mla | 11.393 ± 6.298 | 13.7063 ± 4.436 | .188 |
| Biopsy grade | |||
| ISKDC II | 8(5.9) | 3(2.2) | |
| ISKDC III | 25(18.5) | 17(12.6) | .003 |
| No biopsy | 61(45.2) | 21(15.6) | |
| Treatment | |||
| PA only | 29(21.5) | 12(8.9) | |
| MMF only | 2(1.5) | 1(0.7) | <.001 |
| PA + Ta | 26(19.3) | 6(4.4) | |
| PA + MMF | 8(5.9) | 7(5.2) | |
| Ta + MMF | 2(1.5) | 1(0.7) | |
| PA + MMF + Ta | 27(20.0) | 14(10.4) | |
Data expressed as mean ± standard deviation.PA=Prednisone Acetate, Ta=Tacrolimus, MMF = Mycophenolate Mofetil.
Fig. 2.
Biopsy grade status of patients according to the ISKDC classification.
Table 3.
Patient parameters in relation to the frequency of HSPN relapse.
| Parameters | Frequency of HSPN relapsing value n(%) | p | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | >4 | ||
| Gender | |||||
| Male | 30(31.9) | 21(22.3) | 6.6.4) | 7(7.4) | <.001 |
| Female | 17(18.1) | 11(11.7) | 1(1.1) | 1(1.1) | |
| Age range | |||||
| 0–6 years | 5(5.3) | 5(5.3) | 2(2.1) | 3(3.2) | |
| 7–12 years | 25(26.6) | 19(20.2) | 3(3.2) | 3(3.2) | .002 |
| 13–17 years | 17(18.1) | 8(8.5) | 2(2.1) | 2(2.1) | |
| BMI classification | |||||
| Underweight | 16(25.4) | 10(15.9) | 4(6.3) | 1(1.6) | |
| Normal | 8(12.6) | 4(6.3) | – | 3(4.8) | |
| Overweight | 3(4.8) | 3(4.8) | 1(1.6) | – | .188 |
| Obese | 8(12.7) | 1(1.6) | – | 1(1.6) | |
| Treatment | |||||
| PA only | 12(12.8) | 14(14.9) | 3(3.2) | – | |
| MMF only | 0 | 2(2.1) | 0 | – | |
| PA + Ta | 13(13.8) | 7(7.4) | 2(2.1) | 4(4.3) | |
| PA + MMF | 6(6.4) | 1(1.1) | – | 1(1.1) | <.001 |
| Ta + MMF | 2(2.1) | – | – | – | |
| PA + MMF + Ta | 14(14.9) | 8(8.9) | 2(2.1) | 3(3.2) | |
| Biopsy grade | |||||
| ISKDC II | 3(3.2) | 3(3.2) | 1(1.1) | 1(1.1) | |
| ISKDC III | 12(12.8) | 9(9.6) | – | 4(4.3) | <.001 |
| No biopsy | 32(34.0) | 20(21.3) | 6(6.4) | 3(3.2) | |
PA=Prednisone Acetate, Ta=Tacrolimus, MMF = Mycophenolate Mofetil.
3.1. Main differences in patients’ parameters and treatment options between patients with HSPN who experienced relapses and those who did not relapse
No differences were found by gender or age at diagnosis between HSPN patients who relapsed and those who did not. However, several patient parameter differences were observed between the two groups. Regarding age ranges in these patients, there was a statical difference in the subgroup of patients who later experienced relapses and in patients without relapses(P < 0.001). Age ranges 7–12 years old presented with a highest number of patients with relapses(37 %), followed by 13–17 years(21.5 %) and the least number relapses were observed in ages 0–6years(11.1 %).In addition, there was a statistical significance in BMI classification between the two groups (p < 0.001).Underweight patients(31.6 %) relapsed more and overweight patients relapsed the least(7.1 %).The biopsy grade of patients had a statistical difference between the two groups (p < 0.003).Relapse were observed in patients who did not undergo renal biopsy(45.2 %),patients with ISKDC II(5.9 %) and ISKDC III(18.5 %).The different treatment modalities within the two groups had a statistical significance (p < 0.001).Least number of patients with relapses(1.5 %) were in both the Ta + MMF and MMF only group. However, 25 -hydroxyvitamin D ng/ml levels were only significantly different on day 2(p = 0.045) when compared to day 1(p = 0.697) and day 3(p = 0.188).
3.2. Main difference in patient parameters in relation to the frequency of HSPN relapse
Out of 135 patients,94(69.6 %)patients experienced one or more recurrent HSPN episode(shown in Table 3).Relapse episodes were predominant in boys(68.1 %)when compared to girls(31.9 %),male to female relapse ratio = 2:1.There was a statistically significant correlation between age ranges and the recurrence of HSPN (p = 0.002).Patients aged between 7 and 12 years (53 %) relapsed the most when compared to age range 0–6 years(16 %) and 13–17 years(31 %).However the number patients in each age group decreased as recurrent HSPN episodes increased. No statistical difference was found between BMI and relapse frequencies(p = 0.188). Although more patients who relapsed were underweight, this had no statistical difference. Treatment modalities had a statistical difference in the number of HSPN relapse p < 0.001). Recurrent episodes were more in the PA + Ta group (28 %) and PA + MMF + Ta groups(27 %). However, the number patients in each treatment group decreased as recurrent HSPN episodes increased. There was a statistical difference (P < 0.001) in the recurrence in patients with no biopsy proven HSPN (64.9 %).
4. Discussion
Recurrent HSPN episodes are a huge challenge for clinicians even though patients respond well to initial treatment. In contrast to the extensive literature detailing the clinical manifestations of HSPN, this study paid relatively more attention to the frequency of HSPN recurrence [8].
The male and female ratio of HSPN is consistent through the literature. Uehara E et al., in their single center study in Tokyo, Japan, indicated sex as a significant risk factor of renal involvement in children with HSPN [14].In our study, the male to female ratio was 2:1. Our finding supports the probability of male children having higher chances to experience an HSPN relapse episode when compared to girls. Several studies have also shown the male gender as predominant in this disease, with increased chances of having proteinuria [15,16]. Contrastingly, Kim et al. showed the female was predominant in patients with renal involvement [17].
Precise triggers for the frequent HSPN relapse are unclear,it was generally felt to reflect on the treatment regimen offered to these patients. Administration of tacrolimus to HSPN patients as an immunosuppressant has been mentioned in literature. A more recent randomized controlled trial by Zhang H et al. on 279 children with HSPN showed the effectiveness and safety of tacrolimus regiment for long-term treatment of HSPN. Also, tacrolimus was shown to significantly lower the HSPN recurrence rate [18]. In our study, patients were administered tacrolimus in combination with either prednisone acetate or mycophenolate mofetil or both. We observed a significant reduction in relapse frequencies in patients who were either administered mycophenolate mofetil only or in combination with tacrolimus. Our findings suggests that an adjunct course of mycophenolate mofetil might be useful in the management of HSPN and in reducing the number of relapses. In support to our findings, several studies have also shown MMF as an effective secondary treatment option for children with HSPN [3,19,20].
On the other hand, we observed that patientswho were administered prednisone acetate in combination with either tacrolimus or mycophenolate mofetil experienced a significant number of relapses. This finding is in line with several studies in which relapses occurred more frequently in children treated with an adjunctive steroid therapy [21,22]. Contrastingly, in Chen L et al. study, corticosteroids improved the immune function of children with HSPN and promoted the recovery of renal function [23].In the same line, Hou et al. successfully treated patients with HSPN by administering corticosteroid in combination with leflunomide therapy [24].Our controversial finding highlights the recommendation to limit the use of steroids to a carefully selected group of HSPN children, even though corticosteroids may alleviate the symptoms.
Providing that there was an all-year-round reported cases of HSPN onset our this study, though unevenly distributed across the months, most patients had an onset of HSPN in the summer compared to winter. This may be due to less reported cases of HSP as it is usually considered to be self-limiting and benign, so people don't usually go to the hospital, at the same time HSP might be progressing to HSPN. Moreover, the registration and follow-up of chronic disease might have been lacking so hospitalization would not have been indicated. In contrast to our seasonal findings, other studies reported an increased incidence of HSPN during fall and winter [[25], [26], [27], [28], [29]]. Thus, seasonal variations might be a trigger that promote the onset of this disease.
Another factor to consider in patients with HSPN was the inability to maintain adequate phosphorus and calcium levels. Resultant increases in bone metabolism to release calcium causes bone deformation, pain, and an increased risk of fractures [30]. In our study, 25-hydroxyvitamin D3 [25(OH)D3] levels decreased in children with relapses suggesting predictive values for prognosis and manifestation of another episode of HSPN. This finding aligns with conclusions made by Zhu L et al. [31].
To this end,the association between BMI variability and HSPN is largely obscure. To address the gaps in current knowledge, we evaluated the relationship of BMI variability in patients with HSPN. Our findings suggested that higher BMI variability may influence the incidence of HSPN, though underlying reasons for the association between BMI variability and the risk of HSPN were unclear. According to the results of searches yielded, there is little relevant literature about the influence of BMI on the relapse rates of HSPN, thus BMI was a conflicting factor in our work and further studies are required.
There is a need for development of specific predictive models based on relapse risk and significant renal impact to make effective clinical decisions for individual patients. Clinicians can use this model to predict relapse or severity of renal failure in most children with HSPN. In conclusion, we identified some predictors for relapse in HSPN patients, and care should be taken in patients with risk factors such as age, treatment regimen, and severity of HSPN. More research is needed to determine whether more aggressive treatments for children with HSPN with these conditions will prevent frequent relapse.
To our knowledge, this study is a novel analysis of pediatric patients with frequent HSPN relapses reported thus far, yet we admit this study has some limitations—it's partly retrospective nature and the rather small sample size. Although the registry collected information on pediatric patients with HSPN, not all patients performed kidney biopsies, this may be due to changes in urine. Another possible limitation could be channeling bias because the included patients were seen at one pediatric department, thus, our findings should not be generalized.
Declarations
-
•
This study was reviewed and approved by Affiliated Shaw Hospital of Nanjing Medical University Ethical review of scientific research projects with the approval number: 2020-SR-S034.
-
•
All participants/patients (or their proxies/legal guardians) provided informed consent to participate in the study.
Data availability Statements
Has data associated with your study been deposited into a publicly available repository?
NO.
Has data associated with your study been deposited into a publicly available repository?
Data will be made available on request.
CRediT authorship contribution statement
Lydia Mukanhaire: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Xianguo Ren: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft. Guangling Liu: Data curation, Formal analysis, Investigation, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Ting Wang: Data curation, Formal analysis, Resources, Software, Validation, Visualization, Writing – review & editing. Yeukai Y. Kasumba: Data curation, Formal analysis, Investigation, Software, Validation, Visualization, Writing – review & editing. Xiaohui Zhou: Conceptualization, Data curation, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. Hongjun Peng: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Validation, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Xiaohui Zhou, Email: zhxh@cpu.edu.cn, zhxhedu@163.com.
Hongjun Peng, Email: hjpeng1975@hotmail.com.
References
- 1.Luqmani R.A., et al. Nomenclature and classification of vasculitis - update on the ACR/EULAR diagnosis and classification of vasculitis study (DCVAS) Clin. Exp. Immunol. 2011;164(Suppl 1):11–13. doi: 10.1111/j.1365-2249.2011.04358.x. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizerska-Wasiak M., et al. IgA vasculitis nephritis clinical course and kidney biopsy - national study in children. Pediatr Rheumatol Online J. 2021;19(1):150. doi: 10.1186/s12969-021-00616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hackl A., et al. Mycophenolate mofetil following glucocorticoid treatment in Henoch-Schönlein purpura nephritis: the role of early initiation and therapeutic drug monitoring. Pediatr. Nephrol. 2018;33(4):619–629. doi: 10.1007/s00467-017-3846-6. [DOI] [PubMed] [Google Scholar]
- 4.Shin J.I., et al. Predictive factors for nephritis, relapse, and significant proteinuria in childhood Henoch-Schönlein purpura. Scand. J. Rheumatol. 2006;35(1):56–60. doi: 10.1080/03009740510026841. [DOI] [PubMed] [Google Scholar]
- 5.Xia Y., et al. Clinical outcomes in children with Henoch-Schönlein purpura nephritis grade IIIa or IIIb. Pediatr. Nephrol. 2011;26(7):1083–1088. doi: 10.1007/s00467-011-1834-9. [DOI] [PubMed] [Google Scholar]
- 6.Torun Bayram M., et al. Comparison of clinical, pathological and long-term renal outcomes of children with Henoch-Schonlein purpura nephritis and IgA nephropathy. Int. Urol. Nephrol. 2022;54(8):1925–1932. doi: 10.1007/s11255-021-03063-7. [DOI] [PubMed] [Google Scholar]
- 7.Foster B.J., et al. Effective therapy for severe Henoch-Schonlein purpura nephritis with prednisone and azathioprine: a clinical and histopathologic study. J. Pediatr. 2000;136(3):370–375. doi: 10.1067/mpd.2000.103448. [DOI] [PubMed] [Google Scholar]
- 8.Buscatti I.M., et al. Henoch-Schönlein purpura nephritis: initial risk factors and outcomes in a Latin American tertiary center. Clin. Rheumatol. 2018;37(5):1319–1324. doi: 10.1007/s10067-017-3972-3. [DOI] [PubMed] [Google Scholar]
- 9.Shi D., et al. Risk factors associated with IgA vasculitis with nephritis (Henoch-Schönlein purpura nephritis) progressing to unfavorable outcomes: a meta-analysis. PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0223218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng D., et al. A single-center analysis of Henoch-Schonlein purpura nephritis with nephrotic proteinuria in children. Pediatr Rheumatol Online J. 2017;15(1):15. doi: 10.1186/s12969-017-0146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Counahan R., et al. Prognosis of Henoch-Schönlein nephritis in children. Br. Med. J. 1977;2(6078):11–14. doi: 10.1136/bmj.2.6078.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinoue K., et al. [Crescent formation in children with Henoch-Schönlein purpura nephritis: a pathological and immunohistochemical study] Nihon Jinzo Gakkai Shi. 1996;38(8):364–371. [PubMed] [Google Scholar]
- 13.Stevens J., Nowicki E.M. Body mass index and mortality in asian populations: implications for obesity cut-points. Nutr. Rev. 2003;61(3):104–107. doi: 10.1301/nr.2003.marr.104-107. [DOI] [PubMed] [Google Scholar]
- 14.Elmas A.T., Tabel Y. Platelet counts in children with henoch-schonlein purpura--relationship to renal involvement. J. Clin. Lab. Anal. 2016;30(1):71–74. doi: 10.1002/jcla.21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uehara E., et al. Risk factors of long hospital stay for immunoglobulin A vasculitis: single-center study. Pediatr. Int. 2018;60(10):918–922. doi: 10.1111/ped.13685. [DOI] [PubMed] [Google Scholar]
- 16.Kawashima N., et al. Abnormal urinalysis on day 7 in patients with IgA vasculitis (Henoch-Schönlein purpura) Nagoya J. Med. Sci. 2016;78(4):359–368. doi: 10.18999/nagjms.78.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim W.K., Kim C.J., Yang E.M. Risk factors for renal involvement in Henoch-Schönlein purpura. J. Pediatr. 2021;97(6):646–650. doi: 10.1016/j.jped.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H., et al. Effect and safety evaluation of tacrolimus and tripterygium glycosides combined therapy in treatment of Henoch-Schönlein purpura nephritis. Int. J. Urol. 2021;28(11):1157–1163. doi: 10.1111/iju.14665. [DOI] [PubMed] [Google Scholar]
- 19.Nikibakhsh A.A., et al. Treatment of severe henoch-schonlein purpura nephritis with mycophenolate mofetil. Saudi J Kidney Dis Transpl. 2014;25(4):858–863. doi: 10.4103/1319-2442.135182. [DOI] [PubMed] [Google Scholar]
- 20.Nikibakhsh A.A., et al. Treatment of complicated henoch-schönlein purpura with mycophenolate mofetil: a retrospective case series report. Int J Rheumatol. 2010;2010 doi: 10.1155/2010/254316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anil M., et al. Henoch-Schönlein purpura in children from western Turkey: a retrospective analysis of 430 cases. Turk. J. Pediatr. 2009;51(5):429–436. [PubMed] [Google Scholar]
- 22.Trapani S., et al. Henoch Schonlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature. Semin. Arthritis Rheum. 2005;35(3):143–153. doi: 10.1016/j.semarthrit.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Chen L., et al. Effects of dexamethasone and gamma globulin combined with prednisone on the therapeutic effect and immune function of Henoch-Schonlein purpura nephritis in children. J. Clin. Lab. Anal. 2021;35(1) doi: 10.1002/jcla.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou L., Zhang Z., Du Y. Leflunomide therapy for IgA vasculitis with nephritis in children. BMC Pediatr. 2021;21(1):391. doi: 10.1186/s12887-021-02866-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang H.H., et al. Analysis of seasonal tendencies in pediatric Henoch-Schönlein purpura and comparison with outbreak of infectious diseases. Medicine (Baltim.) 2018;97(36) doi: 10.1097/MD.0000000000012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Y.H., et al. Henoch-Schonlein purpura in children hospitalized at a tertiary hospital during 2004-2015 in korea: epidemiology and clinical management. Pediatr Gastroenterol Hepatol Nutr. 2016;19(3):175–185. doi: 10.5223/pghn.2016.19.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heineke M.H., et al. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schönlein purpura) Autoimmun. Rev. 2017;16(12):1246–1253. doi: 10.1016/j.autrev.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Blanco R., et al. Henoch-Schönlein purpura in adulthood and childhood: two different expressions of the same syndrome. Arthritis Rheum. 1997;40(5):859–864. doi: 10.1002/art.1780400513. [DOI] [PubMed] [Google Scholar]
- 29.Calvo-Río V., et al. Henoch-Schönlein purpura in northern Spain: clinical spectrum of the disease in 417 patients from a single center. Medicine (Baltim.) 2014;93(2):106–113. doi: 10.1097/MD.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekinci R.M.K., et al. Do practical laboratory indices predict the outcomes of children with Henoch-Schönlein purpura? Postgrad. Med. 2019;131(4):295–298. doi: 10.1080/00325481.2019.1609814. [DOI] [PubMed] [Google Scholar]
- 31.Zhu L., et al. Correlations of leukotriene B4 and 25-hydroxyvitamin D3 levels with disease severity in children with henoch-schonlein purpura. Clin. Lab. 2022;68(8) doi: 10.7754/Clin.Lab.2021.211030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Has data associated with your study been deposited into a publicly available repository?
NO.
Has data associated with your study been deposited into a publicly available repository?
Data will be made available on request.