Abstract
A major unsolved question in vertebrate photoreceptor biology is the mechanism of rhodopsin transport to the outer segment. In rhodopsin-like class A G protein–coupled receptors, hydrophobic interactions between C-terminal α-helix 8 (H8), and transmembrane α-helix-1 (TM1) have been shown to be important for transport to the plasma membrane, however whether this interaction is important for rhodopsin transport to ciliary rod outer segments is not known. We examined the crystal structures of vertebrate rhodopsins and class A G protein–coupled receptors and found a conserved network of predicted hydrophobic interactions. In Xenopus rhodopsin (xRho), this interaction corresponds to F313, L317, and L321 in H8 and M57, V61, and L68 in TM1. To evaluate the role of H8–TM1 hydrophobic interactions in rhodopsin transport, we expressed xRho-EGFP where hydrophobic residues were mutated in Xenopus rods and evaluated the efficiency of outer segment enrichment. We found that substituting L317 and M57 with hydrophilic residues had the strongest impact on xRho mislocalization. Substituting hydrophilic amino acids at positions L68, F313, and L321 also had a significant impact. Replacing L317 with M resulted in significant mislocalization, indicating that the hydrophobic interaction between residues 317 and 57 is exquisitely sensitive. The corresponding experiment in bovine rhodopsin expressed in HEK293 cells had a similar effect, showing that the H8-TM1 hydrophobic network is essential for rhodopsin transport in mammalian species. Thus, for the first time, we show that a hydrophobic interaction between H8 and TM1 is critical for efficient rhodopsin transport to the vertebrate photoreceptor ciliary outer segment.
Keywords: photoreceptor, rhodopsin, G protein–coupled receptor (GPCR), intracellular trafficking, cilia
Retinal photoreceptors convert light into electrical signals in the first step of vision (reviewed in (1, 2)). Light transduction depends on opsin proteins (Fig. 1A), canonical seven transmembrane domain G protein–coupled receptors (GPCRs), to be localized at high density within disc membranes of the photoreceptor ciliary outer segment, reaching 3 mM concentration relative to the outer segment plasma membrane envelope (1, 2). The outer segments undergo 3 to 10% daily turnover (depending on species), thus the delivery rate of opsins to this ciliary compartment is exceptionally high. The mechanisms underlying the high opsin transport to, and enrichment within the outer segments, have been the subject of intense study for decades. However, recent studies of opsin transport have arrived at conflicting conclusions, leaving the mechanisms of their transport to the outer segment unsolved.
Figure 1.
The xRhoCT44 probe is transported to Xenopus rod outer segments more efficiently than the zRhoCT44 probe.A, schematic of the bovine rhodopsin structure highlighting H8 and TM1. B, sequence alignment of Xenopus, zebrafish, human, and bovine rhodopsin C-tails highlighting conserved H8 helix residues. C, schematic of construct design for expression of EGFP-RhoCT44 transgenes in Xenopus photoreceptors. D, transmission image (left panel) and confocal image of live rods expressing the xRhoCT44-L317M-EGFP probe (right top panel). Note the dark line circumscribing the cell at the IS-OS junction, thus making this structure easily identifiable. IS and OS compartments are labeled. Colors in the fluorescence image represent an intensity heat map, where yellow is the highest fluorescence and blue is the lowest. Quantification of EGFP axial fluorescence intensity distribution in photoreceptor was achieved using spline analysis of 3D segmented images of individual cells (Experimental procedures). Fluorescence emission intensity values (photons μs−1) along the spline line (black line in the fluorescence image) are plotted as a function of distance from the IS-OS junction (lower right panel). E–H, confocal images of Xenopus retina sections expressing transgenes of (E) EGFP-xRhoCT44, (F) EGFP-xRho-CT44L317M, (G) EGFP-zRhoCT44, and (H) EGFP-zRho-CT44M317L. I, plot of IS mislocalization indices as notched box-whisker plots with bee-swarm overlay of the individual values. The red lines indicate the median mislocalization index (FIS/(FIs + FOS)), the upper and lower bounds of the box represent the upper and lower quartiles, the whiskers represent the minimum and maximum values that are not outliers, outliers are indicated by redcrosses. Nonoverlapping notches indicate the medians are significantly different at p = 0.05. Significant differences amongst the groups are indicated by asterisks (∗), ns indicate not significant. J, mislocalization indices plotted as a function of expression level as determined by total fluorescence (FIS + FOS, photons μs−1). Lines are linear regressions. R, Pearson’s correlation coefficient, and p values of the regression are indicated. Note that except for xRhoCT44, the dependence of the mislocalization index failed to reach significance at p = 0.05. EGFP, enhanced green fluorescent protein; TM1, transmembrane α-helix 1; xRho, Xenopus rhodopsin.
The role of the rhodopsin C-terminal domain in rhodopsin transport to the outer segment is controversial. Tam, Moritz, Hurd, Papermaster (3) identified an outer segment targeting motif consisting of the four C-terminal amino acids (VxPx) that has been recognized as a general sequence for targeting membrane proteins to the outer segment (4). More recently, several studies have identified additional targeting (5) and mislocalization (6) sequences in the rhodopsin C-terminus. Finally, one recent study suggested the entire C-terminal domain is dispensable for rhodopsin localization to the outer segment (7). Interestingly, a previous study showed that a motif consisting of a hydrophobic amino acid and a basic amino acid (HB motif) in C-terminal α-helix 8 (H8) were involved in targeting GPCRs to ciliary membranes (8). These residues correspond to the conserved F313 and R314 in rhodopsins, where it was later dubbed the FR motif and was suggested to be important for binding to the rhodopsin trafficking protein, ASAP1, and by extension rhodopsin trafficking to the outer segment (9). In addition to these targeting sequences, hydrophobic interactions between H8 and transmembrane α-helix 1 (TM1) have been implicated in proper plasma membrane delivery of α- and β-adrenergic receptors (α2B/β2-AR) (10) and vasopressin V2 receptor (V2R) (11), rhodopsin-like class A GPCRs. However, it is not known if this hydrophobic interaction is important for rhodopsin transport to the photoreceptor outer segment.
In the current study, we examined the role of H8–TM1 hydrophobic interactions in the transport of rhodopsin. We examined the available crystal structures of vertebrate rhodopsins (12, 13, 14, 15) and β2AR (16) and found a conserved network of predicted hydrophobic interactions between H8 and TM1. In Xenopus rhodopsin (xRho), this network consists of F313, L317, and L321 in H8 and M57, V61, and L68 in TM1. While F313 is known to be involved in ASAP1 binding, the role of the other hydrophobic residues is unknown. Structural analysis shows that F313 forms an interhelical hydrophobic interaction with L68 in TM1, L317 forms hydrophobic interactions with M57 and V61 of TM1 and that L321 forms with a hydrophobic interaction M57 in TM1. We thus examined the role of the hydrophobic network in xRho transport to rod outer segments and found that its disruption led to inner segment mislocalization. Our results show that mutation of L317 and M57 to hydrophilic amino acids resulted in the strongest disruption of outer segment rhodopsin localization, with mutations of the other players in the network producing smaller, but significant mislocalization. Interestingly, in most other species, the orthologs of L317 and M57 are reversed. Thus, we also examined how the orientation of the hydrophobic interactions between these residues impacts rhodopsin transport. We found that the L317M;M57L double mutant in xRho, and the orthologous M317L;L57M mutation in bovine rhodopsin (bRho) did not result in significant rhodopsin mislocalization. However, a L317M mutation in xRho and M317L or L57M mutations in bRho resulted in significant mislocalization to the inner segment/cell body in the case of xRho in Xenopus rods and to cytoplasmic membranes in the case of bRho in HEK cells. These results indicate that proper opsin transport is exquisitely sensitive to the nature of the hydrophobic interaction between residues 57 in TM1 and 317 in H8. Together, these results show that a hydrophobic network between H8 and TM1 is essential for efficient transport of rhodopsin to the plasma membrane and ultimately to the photoreceptor outer segment.
Results
C-terminal peptides of Xenopus and zebrafish rhodopsins lead to different photoreceptor distribution patterns of GFP probes
It is well accepted that the C-terminus of rhodopsin (Fig. 1B) contains multiple motifs essential for rhodopsin trafficking to the rod outer segment. This function was first demonstrated using C-terminal 44-amino acid peptide, which includes H8, from Xenopus laevis rhodopsin (xRhoCT44) labeled at the N terminus with enhanced green fluorescent protein (EGFP) (3). Interestingly, a recent study (17) showed that the C-terminal 44 amino acids of zebrafish (Danio rerio) rhodopsin (zRhoCT44) was less efficiently localized to the zebrafish outer segment than was xRhoCT44. The C-terminal 44 amino acids of X. laevis and zebrafish rhodopsin are 76% identical (Fig. 1B). Fang, Peden, van Eeden, Malicki (17) showed that the L317 residue found in H8 of xRhoCT44 is responsible for the enhanced outer segment localization efficiency. All other vertebrate species they examined, including zRhoCT44, have M at position 317, Xenopus is the sole species where 317 is L. In order to determine if this sequence variation had a similar impact in Xenopus photoreceptors, we designed constructs like those used by Fang et al. (Fig. 1C) and expressed them transgenically in X. laevis tadpoles under the control of the Xenopus opsin (XOP) promoter (18). We then used quantitative live imaging of retinal slice preparations to evaluate the distribution patterns of the probes in rod cells (Fig. 1, D). Importantly, the C-terminal 44 amino acids of both species contain the VxPx outer segment targeting motif, C322 and C323, the palmitoylated cysteines and the EGFP was fused to the N terminus of the peptides (19).
Live-cell imaging shows that the xRhoCT44 transports EGFP to Xenopus rod outer segments more efficiently than zRhoCT44 (compare Fig. 1, E, G and I), paralleling the results from Fang et al. Specifically, in rods expressing EGFP-zRhoCT44 a substantial quantity of protein appears in the inner segment, approximately uniformly populating membranes from the apical region to the synapse, although the majority of the protein transports to the outer segment (Fig. 1, G and I). In contrast, EGFP-xRhoCT44 is found almost exclusively in the outer segments, with small amounts detected in the inner segment (Fig. 1, E and I). We then made complementary mutations in the Xenopus and zebrafish constructs to verify that position 317 is important for efficient outer segment localization. The L317M mutation in the xRhoCT44 (xRhoCT44L317M) probe resulted in the significant appearance of the probe in the rod inner segment (Fig. 1, F and I). In contrast, the zRhoCT44M317L mutant probe was found to be more strongly localized to the rod outer segment than the WT zRhoCT44 probe (Fig. 1, H and I). In all cases, the fluorescence in the outer segments appears banded due to strong disc membrane binding via the palmitoylation sites. This banding represents the time course of transgene expression variation over the lifetime of the outer segment compartment.
An interesting observation is that the xRhoCT44L317M mutant probe and the WT zRhoCT44 probe (both containing M at position 317) accumulate at the base of the outer segments (Fig. 1, F and G), showing that these probes inefficiently incorporate into more distal regions of the outer segment compartment. This pattern suggests that L at position 317 may be important for the efficient incorporation of these probes into the mature, enclosed outer segment disc membranes.
An important control is to show that the protein distribution patterns and the mislocalization indices are not caused by vastly different expression levels of the transgenes. In Figure 1J, plots of the mislocalization signal as a function of broad ranges of expression level shows that, with the exception of the xRhoCT44 construct which has a significant negative correlation; these parameters are not significantly linked. Thus, the mislocalization indices are bona fide metrics of rhodopsin transport, a conclusion that is not diminished by the negative correlation of xRhoCT44 since its outer segment localization is more efficient with increasing expression level. Similar analyses are presented for the remaining figures. Together, these results show that the H8 helix plays a role in the outer segment targeting of these probes and highlight the importance of residue 317 for their delivery to the distal outer segment.
L317M mutation results in the mislocalization of full-length rhodopsin
Although the results for the 44 C-terminal amino acids of rhodopsin are interesting, the impact of H8 residue 317 on full-length rhodopsin transport to the outer segment was not previously addressed. In order to investigate the possible impact, we compared the distribution patterns of WT xRho to xRho containing the L317M mutation (xRho-L317M). Constructs encoding WT xRho with EGFP fused at the C terminus (xRho-EGFP) or the xRho-L317M-EGFP mutant, where the VxPx motif was repeated after EGFP in both cases, were expressed transgenically in Xenopus rod photoreceptors. Confocal images of live retinal slices showed that xRho-L317M-EGFP is partially but significantly mislocalized to the rod inner segment (Fig. 2, B and C), as compared to WT xRho-EGFP (Fig. 2, A and C). The mislocalized proteins appear to be uniformly present on inner segment membranes, including the plasma membrane and the endomembrane system and reach the synaptic region (Fig. 2B). The mislocalization index does not correlate with expression level (Fig. 2, D and E). Thus, L317 of H8 is critical for proper outer segment localization of full-length rhodopsin.
Figure 2.
The xRho-L317M mutation reduces the outer segment transport efficiency of full-length rhodopsin in Xenopus rods.A and B, left panels: brightfield images of retinal sections. Right panels: live confocal images of a retinas expressing full-length WT xRho (A) and xRhoL317M mutant rhodopsin (B). C, box-whisker plot with bee-swarm overlay of mislocalization indices, as described in Figure 1I. The asterisk (∗) indicates significant differences at p = 0.05. D and E, Mislocalization indices plotted as a function of expression level, as described in Figure 1J. Note that the dependence of mislocalization index (FIS/(FIs + FOS)) on expression did not reach significance for either construct. xRho, Xenopus rhodopsin.
Introduction of hydrophilic residues at L317 enhanced rhodopsin mislocalization
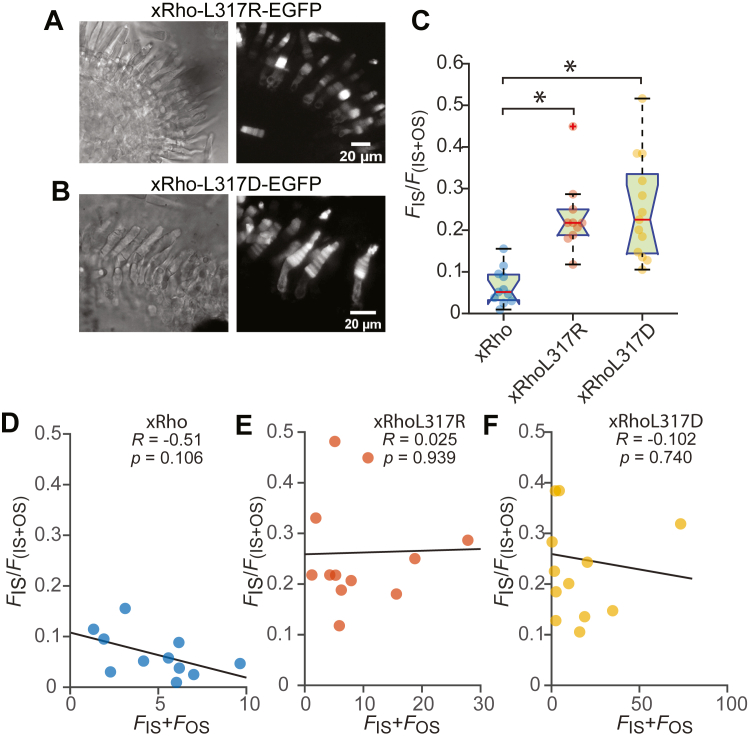
The rhodopsin H8-helix hydrophobic residues are highly conserved among class A GPCRs (10) and are known to play important roles in the trafficking of these proteins to plasma membranes (9, 20). However, there is no current knowledge regarding how the hydrophobic H8 residues impact rhodopsin transport to the ciliary outer segments of photoreceptor cells. To test the hypothesis that the hydrophobic nature of residue 317 in the H8 helix is important for rhodopsin localization, we replaced the hydrophobic L at 317 with the hydrophilic amino acids, aspartic acid, or arginine (xRho-L317R or xRho-L317D). These mutants resulted in more severe rhodopsin mislocalization to the inner segment compartment (Fig. 3) as compared to L317M (Fig. 2). Importantly, secondary structure analysis by the PSIPRED server (21) indicated that introducing these hydrophilic amino acids would not disrupt the helical nature of the H8 structure (Fig. 4C). These results validate the hypothesis that hydrophobic H8 residues are important for rhodopsin transport to the rod outer segment and suggest that H8 interactions with other proteins or membranes is involved in this critical process.
Figure 3.
Replacing L317 with hydrophilic residues enhanced rhodopsin mislocalization in Xenopus photoreceptors.A and B, expression profiles of L317R (A) and L317D (B) rhodopsin mutants, showing strong mislocalization in the photoreceptor inner segment. C, box-whisker plot with bee-swarm overlay of mislocalization indices, as described in Figure 1I. The asterisks (∗) indicate significance at p = 0.05. D–F, mislocalization indices plotted as a function of expression level, as described in Figure 1J. Note that the dependence of mislocalization index (FIS/(FIs + FOS)) on expression did not reach significance for any of the constructs. Panel D is reproduced from Figure 2D.
Figure 4.
Multiple sequence alignment and comparative structural analysis of different rhodopsin proteins.A, multiple sequence alignment of TM1 and C-terminal tail regions of different vertebrate rhodopsin proteins. Both L317 and M57 positions are highly conserved in all species except Xenopus, where they are reversed. B, the 3D structural alignment of Xenopus (modeled structure, magenta) and Bovine (RCSB ID 1L9H, cyan) rhodopsin structures are oriented to show H8 and TM1 helices. C–E, details of H8 and TM1 showing the residues of Xenopus and Bovine rhodopsin that form interhelix hydrophobic interactions. TM1, transmembrane α-helix 1.
C-terminal H8 residues form hydrophobic interactions with TM1 residues
We next investigated the mechanistic role that residue 317 plays in proper outer segment localization. A few studies have indicated that the H8 helix residues in α2B-AR, β2-AR, and V2R receptors interact hydrophobically with TM1 helix residues (10, 11, 22, 23). This H8–TM1 interaction involves the first phenylalanine residue in H8, which was hypothesized to interact with a variety of TM1 hydrophobic residues to stabilize their structures and improve the efficiency of their transport to the plasma membrane of cultured cells. Mutating the first H8 phenylalanine or its corresponding hydrophobic amino acids in TM1 impacts protein folding and causes mislocalization/aggregation of the receptors (10).
In order to determine whether this kind of interaction is important in rhodopsin transport to the outer segment, we examined several rhodopsin sequences that had been submitted to the RCSB database (Fig. 4A; https://www.rcsb.org) (13, 14, 24). Our analysis showed that the H8 helix of all these rhodopsins interact hydrophobically with the TM1 helix, where F313 in H8, which corresponds to the first phenylalanine residues in H8 of the α2B-AR, β2-AR, and V2R receptors, interacts with L68 in TM1 (Fig. 4C), and M317 in H8 interacts with L57 and V61 in TM1 (Fig. 4, C and E). This suggests that multiple hydrophobic interactions between H8 and TM1 may be important for rhodopsin trafficking to the outer segment.
To test this idea, we developed a strategy to interrupt the H8-TM1 hydrophobic network in xRho and determine the outer segment localization efficiency. The 3D structure of xRho is not known; thus, we created a model using an online tool Robetta (25) and compared it to existing bRho structures (13, 14, 24) (Fig. 4B). Analysis of the predicted xRho structure suggests that the F313–L68 interaction is conserved between Xenopus and bovine. However In Xenopus, L317 in H8 is interacting with M57 and V61 in TM1, whereas in bovine M317 interacts with L57 and V61 (Fig. 4, B–E). This analysis indicates that the hydrophobic network is highly conserved, with the exception that the M and L are in opposite positions on H8 and TM1 (Fig. 4, D and E). This hydrophobic interaction provides an explanation for the more dramatic mislocalization of the xRho-M317R/D mutants shown in Figure 3 that replaced hydrophobic amino acids with hydrophilic ones.
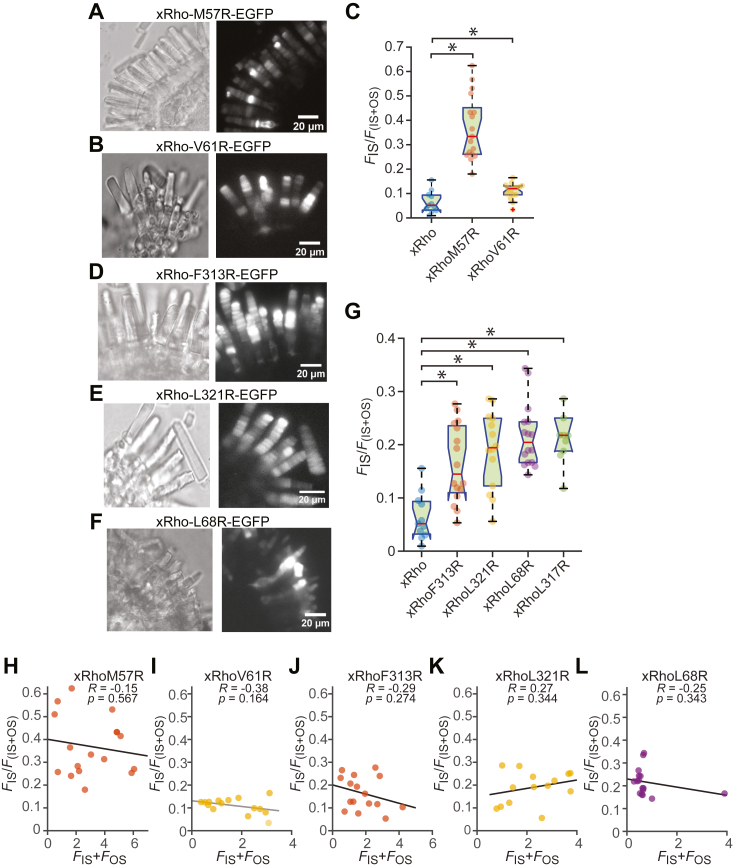
Arginine residues incorporated into the TM1 helix lead to the mislocalization of rhodopsin
To further test the hypothesis that the hydrophobic interaction between H8 and TM1 is required for proper outer segment localization, we replaced hydrophobic residues within TM1 with the hydrophilic amino acid arginine. The M57R mutation, like that of L317R, resulted in significant inner segment mislocalization of rhodopsin, while V61R had a smaller effect (Fig. 5, A–C). The F313R, L321R, and L68R mutations also resulted in significant mislocalization of rhodopsin (Fig. 5, D–G), but to a lower extent compared to L317 R and M57 R mutations suggesting that the L317–M57 interaction plays the major role. The mislocalization index did not correlate with expression level for any of these constructs (Fig. 5, H–L), showing that the mislocalization was not due to over expression of the mutants. Examination of the amino acid hydrophobicity scores (26) shows that L, with a score of 3.8 is more hydrophobic than M, with a score of 1.9. Thus, the xRho-L317M mutation is expected to have reduced hydrophobic interaction between H8 and TM1. This may be an explanation for the mislocalization of this mutant to the inner segment (Fig. 2). Moreover, it provides an explanation for the more dramatic mislocalization of mutants where hydrophilic residues are substituted (Fig. 3). On the other hand, most rhodopsins possess M at the equivalent position 317 in the H8 helix, without significant mislocalization. Interestingly, most other rhodopsin species have L at position 57 (Fig. 4A, arrows), thus showing that there is an L-M “flip” of the hydrophobic residues involved in this H8–TM1 interaction in Xenopus, suggesting that L–M interaction is important, but the direction does not matter.
Figure 5.
xRho-L57R, but not xRho-V61R, results in inner segment mislocalization.A, image of xRho-M57R and B, xRho-V61R expressing retinas. Left panels: transillumination images; right panels: maximum projection confocal images. C, box-whisker plot with bee-swarm overlay of mislocalization indices, as described in Figure 1I. D–F, mutations of the other amino acids predicted to form hydrophobic interactions. Left and right panels as in A and B. G, box-whisker plot with bee-swarm overlay of mislocalization indices, as described in Figure 1I. The asterisks (∗) in C and G indicate significant difference at p = 0.05. H–L, mislocalization indices plotted as a function of expression level, as described in Figure 1J. Note that the dependence of mislocalization index (FIS/(FIs + FOS)) on expression did not reach significance for any of the constructs. xRho, Xenopus rhodopsin.
Outer segment localization of xRho-L317M, M57L double mutant is equally efficient as WT-xRho
To test the idea that the direction of L–M interaction between H8 and TM1 does not matter for efficient rhodopsin localization to the outer segment, we flipped them in a double mutation of the xRho-EGFP construct (xRho-M57L,L317M-EGFP). The distribution pattern of xRho-M57L, L317M-EGFP was not distinguishable from that of WT xRho-EGFP, whereas the single xRho-L317M was significantly more mislocalized to the inner segment (Fig. 6). This result suggests that the L and M interaction between H8 and TM1 is not sensitive to the orientation of interaction.
Figure 6.
The orientation of L and M at positions 317 and 57 in xRho are equally effective for outer segment localization.A, images of rods expressing xRho-M57L,L317M, a double mutation that reverses the orientation of the L and M interaction. Transillumination (left panel), maximum projection confocal image (right panel). B, quantification of double mutant rhodopsin distribution in Xenopus photoreceptors compared to WT xRho and the xRhoL317M single mutation. Box-whisker plot with bee-swarm overlay of mislocalization indices, as described in Figure 1I. The asterisk (∗) indicates a significant difference at p = 0.05. C, mislocalization indices of the xRhoM57L;L317M mutant plotted as a function of expression level, as described in Figure 1J. Note that the dependence of the mislocalization index (FIS/(FIs + FOS)) on expression did not reach significance. xRho, Xenopus rhodopsin; ns, not significant.
Expression of bRho possessing a complementary mutation in H8 and TM1 results in reduced efficiency in plasma membrane delivery when expressed in HEK293T cells
bRho has 82% sequence similarity with xRho. H8 and TM1 are highly conserved, except for the inversion of L and M at positions 317 and 57 (Fig. 4A). Therefore, to explore if hydrophobic interactions between H8 and TM1 are important for rhodopsin transport in mammalian species, we fused bRho with EGFP (bRho-EGFP) and expressed it in HEK293T cells under the cytomegalovirus promoter. HEK293 cells are regarded as a viable substitute for studies of mammalian opsin function and transport (27, 28, 29, 30). They allow rapid evaluation of numerous mutants that would require considerably more time and be prohibitively expensive if performed in a mammalian retina model, like transgenic or knock-in mouse.
WT bRho-EGFP is enriched on the plasma membrane (Fig. 7, A. K, and L). Like Xenopus, bRho-M317L or bRho-L57M mutants result in increased signal in the cytoplasmic organelle membranes relative to the plasma membrane (Fig. 7, B, C, K, and L). This indicates that hydrophobic interactions between H8 and TM1 are also important for the trafficking of bRho. To further explore this similarity, we expressed bRho-L57R and bRho-M317R mutants in HEK293T cells, thus replacing the hydrophobic residues with hydrophilic. These mutations resulted in dramatically higher cytoplasmic membrane fluorescence relative to that of the plasma membrane as well as large cytoplasmic aggregates/inclusions (Fig. 7, D and E, arrowheads and Fig. 7, K and L). The arginine mutations also appeared to reduce HEK cell viability as compared to the methionine and leucine mutations, as judged from loss of coverslip adhesion (not shown). These results show that H8–TM1 hydrophobic interactions are likely important for mammalian rhodopsin delivery to the outer segment as well, and thus that the hydrophobic network is likely of universal importance for GPCR transport. The bRhoM317L;L57M double mutation resulted in lower cytoplasmic rhodopsin accumulation than bRho-M317L or bRho-L57M single mutants (compare Fig. 7F with Fig. 7, B and C; and see Fig. 7, K and L), showing that the L–M interaction is important for efficient plasma membrane enrichment. F313R, L321R, and L68R mutations in bRho also resulted in rhodopsin accumulation in cytoplasmic membranes (Fig. 7, G–I, and K), although apparently to a lower extent than the M317R/L57R mutants, thus paralleling the results for xRho in Xenopus rods.
Figure 7.
H8–TM1 hydrophobic interactions are important for efficient bRho transport to the plasma membrane of HEK293T cells.A–I, representative confocal images of HEK293T cells expressing WT and indicated mutant bRho-EGFP. All images were obtained using identical acquisition conditions. The images are presented with identical lookup table settings, which were optimized for the WT bRho-EGFP expressing cells, thus allowing for accurate qualitative view of the localization patterns among the different constructs. D and E, Arrowheads indicate aggregates or endoplasmic reticulum/Golgi accumulation of mutant rhodopsins. J, segmentation of plasma membrane and cytoplasmic signals in bRho-EGFP–expressing HEK293T cells with a custom MATLAB routine. The top panel shows a cell segmented from neighboring cells using a polygon region of interest tool. The edge of the cell was then found using a Sobel operator and three binary masks were generated corresponding to the entire cell, the cytoplasm (middle panel), and the plasma membrane (lower panel) and the intensity values of voxels under the masks were integrated. The cytoplasmic and plasma membrane localization indices were then calculated as FCP/Ftot and FPM/Ftot, respectively (see Experimental procedures for details). K and L, quantification of bovine rhodopsin distribution between HEK293T cell cytoplasm (K) and plasma membrane (L) where WT bRho and various bRho mutants are as indicated. Box-whisker plot with bee-swarm overlay show the localization indices for the cytoplasm (FCP/Ftot) and plasma membrane (FPM/Ftot) where markers show values for individual cells, the red lines indicate the median localization index, the upper and lower bounds of the box represent the upper and lower quartiles, the whiskers represent the minimum and maximum values that are not outliers, and outliers are indicated by red crosses. Nonoverlapping notches indicate that the medians are significantly different at p = 0.05. Significant differences among the groups are indicated by asterisks (∗), ns indicates not significant. bRho, bovine rhodopsin; EGFP, enhanced green fluorescent protein; TM1, transmembrane α-helix 1.
Discussion
The H8 helix has been implicated in a number of aspects of GPCR function, including protein folding (10, 31), transport, dimerization, and signal transduction (for reviews, see (32, 33)). Previous work has shown that H8 in rhodopsin-like GPCRs, including the adrenergic and vasopressin receptors, possess a conserved pattern of hydrophobic amino acids spaced four amino acids apart such that they are all on the same side of the α helix, oriented toward the membrane (11). The first H8 hydrophobic amino acid was proposed to interact with TM1 hydrophobic residues, and this interaction is essential for proper GPCR transport and enrichment in target membranes where they subserve their signaling roles (10). We show here for the first time that a similar hydrophobic interaction between H8 and TM1 is required for proper delivery of rhodopsin to the ciliary outer segments of vertebrate photoreceptors, thus exposing a conserved role for this interaction across GPCRs that are delivered to different cell structures. Additionally, our structural modeling and mutagenesis experiments revealed a second H8–TM1 hydrophobic interaction that is equally important for proper GPCR transport to target membranes.
The H8 helix in rhodopsin is 11-amino acids long and contains three hydrophobic residues, F313, L/M317, and L321, which are oriented toward the disc membrane. All these residues are located near the TM1 helix, and we propose that they form a hydrophobic interaction with TM1. In the model, F313 is predicted to interact with L68, L/M317 with M/L57;V61, respectively, and L321, located near the palmitoylated C322 and C323, is predicted to interact with M/L57 and may be involved in a hydrophobic interaction with palmitoyl lipids and/or the disc membrane. We suggest that all together these interactions form a tight hydrophobic network that is required for rhodopsin transport. The hydrophobic interaction between the second hydrophobic residue (L/M317 in rhodopsin) and TM1 (M/L57) has not been previously recognized in rhodopsin-like GPCRs (class A), however, our modeling suggests that this interaction is likely a general feature of class A GPCRs, where the second H8 hydrophobic residue is within 4 Å of corresponding TM1 residues.
Our mutagenesis analysis shows that H8 residue 317 and TM1 residue 57 are the main interacting pair for the second hydrophobic amino acid in H8. Interestingly, this interacting pair is different in Xenopus than in most other rhodopsin species, where the pair is L317/M57 in Xenopus and M317/L57 in most other species. The amino acids in the 317/57 interaction appear to be reciprocal, reversing their positions in a given rhodopsin species did not result in significantly reduced efficiency in target membrane enrichment. Interestingly, a few amphibian species possess M317/M57 pair; however, our results would suggest these rhodopsins would be less efficiently transported to the outer segment. Together, our results reveal the second hydrophobic interaction between H8 and TM1 is important for GPCR transport, and that L-M pair is overwhelmingly preferred in rhodopsin.
The hydrophobic interactions between H8 and TM1 likely work in concert to hold rhodopsin’s H8 helix close to the disc membrane. Yet, in rhodopsin-like GPCRs, the hydrophobic residues of the H8 helix are proposed to interact with binding proteins that are involved in GPCR transport, including the BBSome (20) and ASAP1 (9). In rhodopsin, ASAP1 binding is thought to be involved in rhodopsin transport from the Golgi to the base of the outer segment (34). This interaction is proposed to involve F313 and R314 in the H8 helix of rhodopsin, which was dubbed the FR motif (9) and was originally found as a conserved hydrophobic and basic pair of amino acids near the N-terminal end of H8 (HB motif) (8). The BBSome recognizes C-terminal peptides, including the H8 helical domain, of a variety of GPCRs (35) possibly through interaction with the HB motif. This interaction was proposed to be important for GPCR transport out of primary cilia (20). While the FR motif on rhodopsin corresponds to the conserved HB motif of other GPCRs, BBSome knockout in mouse rods has little to no impact on rhodopsin enrichment in the outer segment (36, 37, 38), suggesting that the BBSome is not involved in outer segment transport of rhodopsin in rod photoreceptors. Interestingly, there is evidence that the BBSome is important for the localization of cone opsins, which bear the FR motif in H8, to cone photoreceptor outer segments (36, 39). It, thus, will be interesting to explore the mechanisms of BBSome–cone opsin interactions and the role it plays in anterograde and retrograde cone opsin outer segment transport.
An important unsolved question revealed by our study is how proteins like ASAP1 and the BBSome access and bind to the hydrophobic residues on H8 given that they are likely tightly associated with the membrane and the membrane-buried residues of TM1 and thus not accessible. This is particularly important for rhodopsin and other GPCRS where, in addition to the H8–TM1 hydrophobic interactions, up to two palmitoylated cysteines immediately following the H8 helix further tether H8 to the membrane. These hydrophobic interactions require H8 to be removed from the membrane surface, suggesting that ASAP1 and BBSome binding must follow a disruption of the hydrophobic interactions and removal of the palmitoyl moieties from the membrane, possibly through an unknown activation mechanism of the GPCR. It is important to note that interactions of ASAP1 and BBSome with H8 are based on GPCR C-terminal peptide binding experiments that lacked H8 interactions with TM1 and palmitoyl anchoring to the membrane. This raises a paradox because our results show that disrupting the hydrophobic interaction by replacing hydrophobic residues with hydrophilic ones is deleterious to rhodopsin localization to the outer segment.
It has been proposed that the H8 helix is important for ER exit of a variety of class A GPCRs (10, 31, 40). An endoplasmic reticulum (ER) exit motif was proposed that spans H8, F(X)6 L/IL, where the F is the first phenylalanine in H8, corresponding to F313 in rhodopsin. Mutations of the hydrophobic F and L/IL resulted in significantly reduced GPCR transport to the plasma membrane. F to A mutation in the motif resulted in tighter binding to the ER chaperones calnexin and calreticulin (10), leading to the hypothesis that H8 is important for ER exit. Our results show that mutation in another conserved hydrophobic amino acid four residues downstream from the first F on H8, and not included in this motif, resulted in a marked reduction of bRho enrichment in the plasma membranes of HEK293T cells, where it is instead found in endo membranes. Thus, rather than an ER exit motif, we propose that the hydrophobic network between H8 and TM1 modulates the strength of interactions between H8 and ER chaperones simply by making H8 more accessible for chaperone binding, which leads to delayed ER exit. Indeed, substituting the second hydrophobic amino acid in H8 with a hydrophilic residue, further destabilizing the hydrophobic network, resulted in more extensive mislocalization that appeared to be cytotoxic.
It has been proposed that H8 and TM1 (41, 42) along with TM4 and TM5 are involved in rhodopsin dimerization (43, 44). Synthetic peptides corresponding to TM1 and H8 sequences were reported to disturb the dimeric interface of rhodopsin and impeded rhodopsin transport from the ER/Golgi to the outer segment (45), although the specific mechanism by which these peptides operate are unclear because they include an ER retention signal that may lead to other interactions. Our analysis of the solved structures of rhodopsin dimers suggests that hydrophobic residues that we propose to form a hydrophobic network between H8 and TM1 are not involved in the dimer interface (Fig. 4) and thus mutations in these residues would not be expected to directly interfere with dimer formation. Interestingly, the proposed H8-H8 dimeric interface in mouse rhodopsin is made up of hydrogen bonds formed between R314 and C322 residues in the opposing rhodopsins. This raises further questions about the mechanism of rhodopsin interaction with ASAP1, which is proposed to bind the F313R314 motif during transport to the rod outer segment.
Experimental procedures
Cloning and mutagenesis
All expression constructs were built on the pEGFP-N1 vector (Clontech). The rhodopsin C-tail constructs were generated by overlapping primers strategy, where a primer corresponding to the C terminus of EGFP and the 44 C-terminal amino acids of either xRho or zRho were used to fuse EGFP to the xRho or zRho peptides without any intervening amino acids. The xRho-EGFP cDNA encoded Xenopus full-length rhodopsin fused in frame with EGFP as previously described (46). A single H was inserted between the C-terminal A of xRho and the N-terminal M of EGFP. Additionally, the eight C-terminal amino acids containing the VxPx motif of bRho were appended to the C terminus of EGFP. The promoter used in Xenopus transgenic experiments was a fragment containing nucleotides −503 to +41 from the XOP gene (46). The bRho-EGFP construct was developed in the Oprian lab (47) and received from David S. Williams (Jules Stein Eye Institute, University of California), and consists of the full length of bRho, followed by EGFP on the C terminus and the eight C-terminal amino acids of bRho, containing the VxPx motif, appended to the C terminus of EGFP. Expression of this construct is driven by the cytomegalovirus promoter. Site-directed mutagenesis with a single primer was used to generate mutant constructs.
Generation of transgenic frogs
X. laevis expressing transgenes in rods were created using the restriction enzyme-mediated integration approach (18, 48). Plasmids containing transgenes were linearized using XhoI restriction enzyme (New England Biolabs) and purified prior to integration into sperm nuclear DNA, also cut with XhoI. Sperm nuclei were then injected into WT eggs and dividing embryos were selected. Tadpoles were screened for EGFP expression in retinas using a fluorescence dissecting microscope. Positive tadpoles were kept on a 12 h light and 12 h dark cycle at 18 to 20 °C. The Association for Research in Vision and Ophthalmology's rules for the care and use of animals were followed in all animal handling and experimentation. The SUNY Upstate Medical University Committee on the Human Use of Animals approved this study.
Live-cell imaging of Xenopus retina
Fourteen-day-old tadpoles were euthanized by tricaine treatment and decapitation. Explant retinas were bathed in frog Ringer’s solution, consisting of (in mM): NaCl 111; KCl 2.5; CaCl2 1; MgCl2 1.6; Hepes 3; and D-glucose 10. Retinas remain viable in frog Ringer’s for 5 or more hours (49, 50, 51, 52), and all experiments were performed with 2 h of explant. Retinas were sliced and placed into an imaging chamber consisting of a 35 mm Petri dish into which a 5 mm hole was drilled, and a coverslip was attached with tacky wax (51, 53). The chamber was then sealed with another coverslip and placed onto the microscope stage. 3D confocal imaging was performed on a custom-built confocal and 2-photon microscope (53) with a 1.2 NA Plan-Apochromat water immersion objective (Nikon). Voxel sampling frequencies were 150 nm in x,y and 100 nm in z, thus satisfying the Nyquist sampling limit. Voxel dwell times were 5 μs, and the laser intensity was set to minimize autofluorescence to less than 1% of the weakest signal in the cells. All imaging was performed with identical microscope settings.
Quantification of EGFP distribution in photoreceptors
We quantified the EGFP intensity distribution along the long axis of rods using custom image segmentation routines written in MATLAB (51, 53, 54). Briefly, a spline line was drawn down the 3D center of the rod photoreceptor and fluorescence values were collected from voxels that this line intersected. To improve signal-to-noise, up to a 3 × 3 region of voxels neighboring the lines were averaged (see Fig. 1C). The axial fluorescence determined this way was plotted as a function of distance from the IS-OS junction, the position of which was determined by the dark line circumscribing the cell in the bright-field images as shown in Figure 1D. The degree of mislocalization to the inner segment was quantified by taking the ratio of the average spline line fluorescence within the inner segment (FIS) to that of the total of the florescence of the proximal 4 μm of the outer segment (FOS) and the inner segment fluorescence, which constitutes the mislocalization index (FIS/(FIS + FOS)). Constraining this analysis to the proximal base of the outer segment accounts for variability in transgene expression due to transient silencing, as described previously (51, 53, 55).
Structural modeling and hydrophobic interaction analysis of XRho
3D structural models of xRho and its mutants were built using Robetta online server (https://robetta.bakerlab.org/) (25). Robetta uses the RoseTTAfold prediction method for model building. The RoseTTAFold is a multidirectional tool that simultaneously considers patterns in protein sequences, amino acid interactions, and a possible 3D structure. Results give five predicted potential models and high confidence score >0.8 (where a score of 1.0 is good and 0.0 is bad) models were selected for further interaction analysis.
Intraprotein interactions were analyzed using the protein interaction calculator (http://pic.mbu.iisc.ernet.in/) (56) online web server. Several solved rhodopsin structures along with our modeled structures were separately uploaded to the server for intraprotein interaction analysis. Interactions between hydrophobic sidechains are identified using a distance cut-off of 4 Å between polar groups in the polar sidechains.
Cell culture and confocal microscopy of bRho constructs
The HEK293T cells were grown in Dulbecco's modified Eagle's medium high glucose culture medium with 10% fetal bovine serum, in an incubator at 95% O2/5% CO2 and 37 °C. Transfection was achieved with PEIpro transfection reagent (Polyplus). For transfection experiments 1 μg of plasmid was used per 9.6 cm2 well of a 6-well culture plate. Cells were seeded onto Fisherbrand No. 1, 25-mm cover glass (12-545-86) and allowed to reach confluence before imaging. Immediately prior to imaging, culture medium was replaced with a Hepes-based imaging medium, Dulbecco's modified Eagle's medium high glucose culture medium without phenyl red, maintained at 37 °C. Live confocal imaging of bRho-GFP and its mutant were performed on an LSM780 (Zeiss), equipped with a mammalian cell environmental chamber at 37 °C, using a Plan-Apochromat 63× /1.4 NA oil-immersion objective (Zeiss). The experimental conditions and image acquisition parameters were identical for all bRho constructs.
Quantifying bRho-EGFP distribution in HEK293 cells
Segmentation of plasma membrane and cytoplasmic signals in bRho-EGFP–expressing HEK293T cells was achieved using a custom MATLAB routine. Individual cells were segmented from neighboring cells using a polygon region of interest tool. The edge of the cell was then found using a Sobel operator and a binary mask was generated that covered the entire cell. The intensity values of the voxels under the mask were integrated to find the total cell fluorescence (Ftot). 200 nm of the edge of the mask was then eroded to exclude the plasma membrane region, yielding the cytoplasm mask. Fluorescence values of voxels beneath the cytoplasm mask were integrated to yield the total fluorescence within the cytoplasmic region (FCP). To find the plasma membrane region the cytoplasm mask was subtracted from the cell mask to yield a plasma membrane mask. The plasma membrane fluorescence (FPM) was then found by integrating the voxels beneath the plasma membrane mask. The cytoplasmic and plasma membrane localization indices were defined as FCP/Ftot and FPM/Ftot, respectively. The plasma membranes of cells that abutted neighboring cells that also expressed bRho-EGFP and its mutants appeared brighter than plasma membrane of cells that had no expressing neighbors. This was true of all the cells expressing the various mutants and for WT Rho-EGFP. We therefore did not attempt to separate plasma membrane signals between neighboring cells with the assumption that variability in plasma membrane signals introduced by this effect were uniform for all constructs expressed.
Statistical analysis and sampling
For Xenopus experiments, the results for each condition were based on confocal z-stacks of living retinal explants from at least three animals scanned on different experimental days. Rods where EGFP signal was found at the base of the outer segment were chosen for analysis to ensure that the XOP promoter was not in the silenced state, which would lead to an underestimation of the inner segment signal relative to the outer segment signal. We also selected cells where the axial dimension was aligned with the xy imaging plane, thus allowing more precise separation of the IS and OS compartments. We analyzed all the cells in a given imaging session that met these criteria. This led to variable numbers of cells for each construct, but not fewer than ten. For HEK293T cell experiments, at least five cells from at least three independent transient transfections of each construct were analyzed. For rods and HEK293T experiments, cells expressing constructs at levels spanning at least one decade, as judged by integrated cell fluorescence, were analyzed and any correlation between expression level and localization metrics were evaluated by linear regression analysis. In virtually all cases, except for the xRhoCT44-EGFP construct, there was no significant correlation.
One-way ANOVA and the Tukey-Kramer multiple comparison method were used to determine statistical significance of the mean of the mislocalization indices at p = 0.05. Statistical significance was also confirmed by notched box-whisker plots.
Data availability
All data are contained in this manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We are grateful for support from the Lions Clubs of Central New York, District 20-Y. We thank members of the Center for Vision Research for helpful discussions, and Carla Dias for technical assistance.
Author contributions
D. K. V., H. M., and P. D. C. conceptualization; D. K. V., H. M., and P. D. C. methodology; D. K. V., H. M., and P. D. C. validation; D. K. V., H. M., T. W., and P. D. C. formal analysis; D. K. V., H. M., T. W., and P. D. C. investigation; D. K. V., H. M., T. W., and P. D. C. data curation; D. K. V. and P. D. C. writing-original draft; D. K. V. and P. D. C. writing-review and editing; D. K. V. and P. D. C. visualization; P. D. C. supervision; P. D. C. project administration; P. D. C. funding acquisition.
Funding and additional information
This work was supported by National Institutes of Health grants R01-EY018421 and R01-EY028303 (to P. D. C.), S10OD023617 (to the State University of New York Upstate Medical University Proteomics Core). Dr Calvert is a recipient of a Stein Innovation Award, and the Upstate Medical University Department of Ophthalmology and Visual Sciences is a recipient of an unrestricted grant from Research to Prevent Blindness, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Kirill Martemyanov
Footnotes
Present address for Dipesh Kumar Verma: Department of Life Sciences and Biotechnology, Chhatrapati Shahu Ji Maharaj University, Kanpur, India.
References
- 1.Malhotra H., Barnes C.L., Calvert P.D. Functional compartmentalization of photoreceptor neurons. Pflugers Arch. 2021 doi: 10.1007/s00424-021-02558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes C.L., Malhotra H., Calvert P.D. Compartmentalization of photoreceptor sensory cilia. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.636737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tam B.M., Moritz O.L., Hurd L.B., Papermaster D.S. Identification of an outer segment targeting signal in the COOH terminus of rhodopsin using transgenic Xenopus laevis. J. Cell Biol. 2000;151:1369–1380. doi: 10.1083/jcb.151.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deretic D. A role for rhodopsin in a signal transduction cascade that regulates membrane trafficking and photoreceptor polarity. Vis. Res. 2006;46:4427–4433. doi: 10.1016/j.visres.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Wang J., Deretic D. The Arf and Rab11 effector FIP3 acts synergistically with ASAP1 to direct Rabin8 in ciliary receptor targeting. J. Cell Sci. 2015;128:1375–1385. doi: 10.1242/jcs.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemet I., Ropelewski P., Imanishi Y. Rhodopsin trafficking and mistrafficking: signals, molecular components, and mechanisms. Prog. Mol. Biol. Transl. Sci. 2015;132:39–71. doi: 10.1016/bs.pmbts.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Lodowski K.H., Lee R., Ropelewski P., Nemet I., Tian G., Imanishi Y. Signals governing the trafficking and mistrafficking of a ciliary GPCR. Rhodopsin J. Neurosci. 2013;33:13621–13638. doi: 10.1523/JNEUROSCI.1520-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbit K.C., Aanstad P., Singla V., Norman A.R., Stainier D.Y., Reiter J.F. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 9.Wang J., Morita Y., Mazelova J., Deretic D. The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting the. EMBO J. 2012;31:4057–4071. doi: 10.1038/emboj.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duvernay M.T., Dong C., Zhang X., Zhou F., Nichols C.D., Wu G. Anterograde trafficking of G protein-coupled receptors: function of the C-terminal F(X)6LL motif in export from the endoplasmic reticulum. Mol. Pharmacol. 2009;75:751–761. doi: 10.1124/mol.108.051623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thielen A., Oueslati M., Hermosilla R., Krause G., Oksche A., Rosenthal W., et al. The hydrophobic amino acid residues in the membrane-proximal C tail of the G protein-coupled vasopressin V2 receptor are necessary for transport-competent receptor folding. FEBS Lett. 2005;579:5227–5235. doi: 10.1016/j.febslet.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 12.Palczewski K., Kumasaka T., Hori T., Behnke C.A., Motoshima H., Fox B.A., et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 13.Okada T., Sugihara M., Bondar A.N., Elstner M., Entel P., Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J. Mol. Biol. 2004;342:571–583. doi: 10.1016/j.jmb.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 14.Okada T., Fujiyoshi Y., Silow M., Navarro J., Landau E.M., Shichida Y. Functional role of internal water molecules in rhodopsin revealed by x-ray crystallography. Proc. Natl. Acad. Sci. U. S. A. 2002;99:5982–5987. doi: 10.1073/pnas.082666399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J., Edwards P.C., Burghammer M., Villa C., Schertler G.F. Structure of bovine rhodopsin in a trigonal crystal form. J. Mol. Biol. 2004;343:1409–1438. doi: 10.1016/j.jmb.2004.08.090. [DOI] [PubMed] [Google Scholar]
- 16.Cherezov V., Rosenbaum D.M., Hanson M.A., Rasmussen S.G., Thian F.S., Kobilka T.S., et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang X., Peden A.A., van Eeden F.J.M., Malicki J.J. Identification of additional outer segment targeting signals in zebrafish rod opsin. J. Cell Sci. 2021;134 doi: 10.1242/jcs.254995. [DOI] [PubMed] [Google Scholar]
- 18.Knox B.E., Schlueter C., Sanger B.M., Green C.B., Besharse J.C. Transgene expression in Xenopus rods. FEBS Lett. 1998;423:117–121. doi: 10.1016/s0014-5793(98)00018-0. [DOI] [PubMed] [Google Scholar]
- 19.Karnik S.S., Ridge K.D., Bhattacharya S., Khorana H.G. Palmitoylation of bovine opsin and its cysteine mutants in COS cells. Proc. Natl. Acad. Sci. U. S. A. 1993;90:40–44. doi: 10.1073/pnas.90.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang S., Bahl K., Chou H.-T., Woodsmith J., Stelzl U., Walz T., et al. Near-atomic structures of the BBSome reveal the basis for BBSome activation and binding to GPCR cargoes. Elife. 2020;9 doi: 10.7554/eLife.55954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuffin L.J., Bryson K., Jones D.T. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16:404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- 22.Krause G., Hermosilla R., Oksche A., Rutz C., Rosenthal W., Schulein R. Molecular and conformational features of a transport-relevant domain in the C-terminal tail of the vasopressin V(2) receptor. Mol. Pharmacol. 2000;57:232–242. [PubMed] [Google Scholar]
- 23.Duvernay M.T., Dong C., Zhang X., Robitaille M., Hebert T.E., Wu G. A single conserved leucine residue on the first intracellular loop regulates ER export of G protein-coupled receptors. Traffic. 2009;10:552–566. doi: 10.1111/j.1600-0854.2009.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salom D., Lodowski D.T., Stenkamp R.E., Le Trong I., Golczak M., Jastrzebska B., et al. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D.E., Chivian D., Baker D. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 2004;32:W526–W531. doi: 10.1093/nar/gkh468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyte J., Doolittle R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 27.Sung C.H., Davenport C.M., Nathans J. Rhodopsin mutations responsible for autosomal dominant retinitis pigmentosa. Clustering of functional classes along the polypeptide chain. J. Biol. Chem. 1993;268:26645–26649. [PubMed] [Google Scholar]
- 28.Rajan R.S., Illing M.E., Bence N.F., Kopito R.R. Specificity in intracellular protein aggregation and inclusion body formation. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13060–13065. doi: 10.1073/pnas.181479798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu M.Y., Liu J., Mehrotra D., Liu Y., Guo Y., Baldera-Aguayo P.A., et al. Thermal stability of rhodopsin and progression of retinitis pigmentosa: comparison of S186W and D190N rhodopsin mutants. J. Biol. Chem. 2013;288:17698–17712. doi: 10.1074/jbc.M112.397257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sung C.H., Schneider B.G., Agarwal N., Papermaster D.S., Nathans J. Functional heterogeneity of mutant rhodopsins responsible for autosomal dominant retinitis pigmentosa. Proc. Natl. Acad. Sci. U. S. A. 1991;88:8840–8844. doi: 10.1073/pnas.88.19.8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasuda D., Okuno T., Yokomizo T., Hori T., Hirota N., Hashidate T., et al. Helix 8 of leukotriene B4 type-2 receptor is required for the folding to pass the quality control in the endoplasmic reticulum. FASEB J. 2009;23:1470–1481. doi: 10.1096/fj.08-125385. [DOI] [PubMed] [Google Scholar]
- 32.Sato T., Kawasaki T., Mine S., Matsumura H. Functional role of the C-terminal amphipathic helix 8 of olfactory receptors and other G protein-coupled receptors. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17111930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huynh J., Thomas W.G., Aguilar M.I., Pattenden L.K. Role of helix 8 in G protein-coupled receptors based on structure-function studies on the type 1 angiotensin receptor. Mol. Cell Endocrinol. 2009;302:118–127. doi: 10.1016/j.mce.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Deretic D., Lorentzen E., Fresquez T. The ins and outs of the Arf4-based ciliary membrane-targeting complex. Small GTPases. 2019;1:12. doi: 10.1080/21541248.2019.1616355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klink B.U., Zent E., Juneja P., Kuhlee A., Raunser S., Wittinghofer A. A Recombinant BBSome Core Complex and How it Interacts with Ciliary Cargo. Elife. 2017;6 doi: 10.7554/eLife.27434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abd-El-Barr M.M., Sykoudis K., Andrabi S., Eichers E.R., Pennesi M.E., Tan P.L., et al. Impaired photoreceptor protein transport and synaptic transmission in a mouse model of Bardet–Biedl syndrome. Vis. Res. 2007;47:3394–3407. doi: 10.1016/j.visres.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pretorius P.R., Baye L.M., Nishimura D.Y., Searby C.C., Bugge K., Yang B., et al. Identification and functional analysis of the vision-specific BBS3 (ARL6) long isoform. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang J., Promchan K., Jiang H., Awasthi P., Marshall H., Harned A., et al. Depletion of BBS protein LZTFL1 affects growth and causes retinal degeneration in mice. J. Genet. Genomics. 2016;43:381–391. doi: 10.1016/j.jgg.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bales K.L., Bentley M.R., Croyle M.J., Kesterson R.A., Yoder B.K., Gross A.K. BBSome component BBS5 is required for cone photoreceptor protein trafficking and outer segment maintenance. Invest. Ophthalmol. Vis. Sci. 2020;61:17. doi: 10.1167/iovs.61.10.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duvernay M.T., Zhou F., Wu G. A conserved motif for the transport of G protein-coupled receptors from the endoplasmic reticulum to the cell surface. J. Biol. Chem. 2004;279:30741–30750. doi: 10.1074/jbc.M313881200. [DOI] [PubMed] [Google Scholar]
- 41.Zhao D.Y., Pöge M., Morizumi T., Gulati S., Van Eps N., Zhang J., et al. Cryo-EM structure of the native rhodopsin dimer in nanodiscs. J. Biol. Chem. 2019;294:14215–14230. doi: 10.1074/jbc.RA119.010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knepp A.M., Periole X., Marrink S.J., Sakmar T.P., Huber T. Rhodopsin forms a dimer with cytoplasmic helix 8 contacts in native membranes. Biochemistry. 2012;51:1819–1821. doi: 10.1021/bi3001598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bayburt T.H., Leitz A.J., Xie G., Oprian D.D., Sligar S.G. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J. Biol. Chem. 2007;282:14875–14881. doi: 10.1074/jbc.M701433200. [DOI] [PubMed] [Google Scholar]
- 44.Liang Y., Fotiadis D., Filipek S., Saperstein D.A., Palczewski K., Engel A. Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J. Biol. Chem. 2003;278:21655–21662. doi: 10.1074/jbc.M302536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang T., Cao L.H., Kumar S., Enemchukwu N.O., Zhang N., Lambert A., et al. Dimerization of visual pigments in vivo. Proc. Natl. Acad. Sci. U. S. A. 2016;113:9093–9098. doi: 10.1073/pnas.1609018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mani S.S., Batni S., Whitaker L., Chen S., Engbretson G., Knox B.E. Xenopus rhodopsin promoter. Identification of immediate upstream sequences necessary for high level, rod-specific transcription. J. Biol. Chem. 2001;276:36557–36565. doi: 10.1074/jbc.M101685200. [DOI] [PubMed] [Google Scholar]
- 47.Jin S., McKee T.D., Oprian D.D. An improved rhodopsin/EGFP fusion protein for use in the generation of transgenic Xenopus laevis. FEBS Lett. 2003;542:142–146. doi: 10.1016/s0014-5793(03)00368-5. [DOI] [PubMed] [Google Scholar]
- 48.Kroll K.L., Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- 49.Maza N.A., Schiesser W.E., Calvert P.D. An intrinsic compartmentalization code for peripheral membrane proteins in photoreceptor neurons. J. Cell Biol. 2019;218:3753–3772. doi: 10.1083/jcb.201906024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Najafi M., Maza N.A., Calvert P.D. Steric volume exclusion sets soluble protein concentrations in photoreceptor sensory cilia. Proc. Natl. Acad. Sci. U. S. A. 2012;109:203–208. doi: 10.1073/pnas.1115109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peet J.A., Bragin A., Calvert P.D., Nikonov S.S., Mani S., Zhao X., et al. Quantification of the cytoplasmic spaces of living cells with EGFP reveals arrestin-EGFP to be in disequilibrium in dark adapted rod photoreceptors. J. Cell Sci. 2004;117:3049–3059. doi: 10.1242/jcs.01167. [DOI] [PubMed] [Google Scholar]
- 52.Calvert P.D., Govardovskii V.I., Arshavsky V.Y., Makino C.L. Two temporal phases of light adaptation in retinal rods. J. Gen. Physiol. 2002;119:129–145. doi: 10.1085/jgp.119.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calvert P.D., Peet J.A., Bragin A., Schiesser W.E., Pugh E.N., Jr. Fluorescence relaxation in 3D from diffraction-limited sources of PAGFP or sinks of EGFP created by multiphoton photoconversion. J. Microsc. 2007;225:49–71. doi: 10.1111/j.1365-2818.2007.01715.x. [DOI] [PubMed] [Google Scholar]
- 54.Calvert P.D., Schiesser W.E., Pugh E.N., Jr. Diffusion of a soluble protein, photoactivatable GFP, through a sensory cilium. J. Gen. Physiol. 2010;135:173–196. doi: 10.1085/jgp.200910322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Najafi M., Haeri M., Knox B.E., Schiesser W.E., Calvert P.D. Impact of signaling microcompartment geometry on GPCR dynamics in live retinal photoreceptors. J. Gen. Physiol. 2012;140:249–266. doi: 10.1085/jgp.201210818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tina K.G., Bhadra R., Srinivasan N. PIC: protein interactions calculator nucleic acids research. 2007;35:W473–W476. doi: 10.1093/nar/gkm423. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained in this manuscript.