Abstract
We have studied catabolite gene activator protein (CAP) activation at the araBAD promoter, pBAD, in the absence of DNA looping. We ruled out the two most plausible indirect activation mechanisms: CAP-induced folding of upstream DNA back upon RNA polymerase, and CAP-induced stabilization of AraC binding to DNA. Therefore, a direct CAP-RNA polymerase interaction seemed likely. We sought and found CAP mutants defective in transcription activation at pBAD that retained normal DNA binding affinity. Some mutations altered residues in the interval from positions 150 to 164 that includes CAP activating region 1 (AR1), which has been shown to contact RNA polymerase at a number of promoters. Unexpectedly, additional mutations were found that altered residues in the region between positions 46 and 68 and at position 133. This includes the region known as activating region 3 (AR3). Mutations from both groups also affect the araFGH and rhaBAD promoters.
The presence of multiple activators permits a promoter to respond to multiple environmental cues. The way in which several activators work together is an important question in the study of transcription regulation. The araBAD promoter in Escherichia coli is regulated by two transcription factors, AraC and catabolite gene activator protein (CAP) (13). The main activator protein, AraC, binds to two direct-repeat half-sites that partially overlap the −35 region of the promoter (8, 32). The second activator, CAP, has a binding site centered at position −93.5 (15). The mechanisms of transcription activation by these two proteins have been studied extensively (5, 10, 32, 38).
AraC protein is composed of a dimerization domain and a DNA binding domain (7). In the absence of arabinose, the dimeric AraC protein binds to the upstream araO2 half-site and the downstream araI1 half-site and forms a DNA loop to repress transcription (9, 26) (Fig. 1). The presence of arabinose induces conformational changes in AraC that lead it to bind to two adjacent half-sites, araI1 and araI2 (24, 26). When bound at araI1 and araI2, AraC helps RNA polymerase to bind to the araBAD promoter, pBAD, and also accelerates open-complex formation (38).
FIG. 1.
Regulation of ara pBAD. Upon induction of arabinose, AraC releases the upstream araO2 half-site and binds to the downstream araI1-araI2 site. From this position, AraC activates transcription with the aid of the CAP.
CAP is the sole activator at two classes of simple E. coli promoters. At class I promoters, where CAP binds at position −61.5 or further upstream, amino acids 156 to 164 of CAP have been shown to be essential for transcription activation (2, 12, 40). This activating region, activating region 1 (AR1), directly contacts the alpha subunit of RNA polymerase (10, 19). At class II promoters, where the CAP binding site is centered at position −41.5, AR1 and amino acids 19, 21, and 101, which constitute activating region 2 (AR2), were both found to interact with the alpha subunit of RNA polymerase (5, 29). Also, amino acids 52 to 58, a region known as activating region 3 (AR3), lie close to the sigma subunit of RNA polymerase (5, 21, 37). In wild-type CAP, AR3 plays little or no role in transcription activation; however, substitution of amino acid 52 can result in increased transcription activation, presumably by creating a new, nonnative interaction between AR3 and sigma (5).
The role of CAP in systems with multiple activators has also been studied. At some promoters where another activator protein is bound between CAP and RNA polymerase, the AR1 region of the upstream CAP has been shown to be involved in transcription activation (6, 23). Apparently, CAP can still contact RNA polymerase even though the two are separated by the third protein. CAP may also act as a structural protein by bending DNA about 90°. It has been argued that this sharp bend may facilitate the binding of upstream DNA to the backside of RNA polymerase (4). CAP-induced bending has been shown to modulate the location of binding of the primary activator MalT to trigger transcription activation in the malK promoter (33).
CAP activates ara pBAD in at least two ways. Previous studies have established that one role of CAP is to break the loop formed by AraC in the absence of arabinose, and thus CAP “activates” transcription by relieving repression (27). CAP also activates in a loop-independent way (15, 25, 36). When the upstream AraC half-site araO2 was deleted to prevent DNA looping, CAP still substantially activated pBAD (15). Although CAP activation requires the presence of AraC protein, sensitive in vitro studies did not detect any cooperative binding between CAP and AraC (16, 37a), and in a minicircle assay, CAP actually slightly destabilized AraC binding (27).
The loop-independent activation by CAP at ara pBAD can be explained if CAP directly contacts RNA polymerase. A possible determinant on CAP for this interaction is AR1, since it has been shown to contact RNA polymerase at a large number of promoters. A previous study with an AR1 mutation, H159L, of CAP however, did not detect any effect at pBAD (37), even though this mutation strongly disrupted the transcription activation of CAP at both class I and class II promoters. This finding left it unclear how CAP stimulates ara pBAD.
In this study, we first established that CAP activates the pBAD promoter when DNA looping is impossible. We also showed in in vivo experiments that CAP does not activate transcription by stabilizing AraC binding to the promoter. We then screened for CAP mutants defective in transcription activation and found mutants with amino acid changes in AR1 and in a region overlapping but not coincident with AR3. Our results suggest that CAP directly interacts with RNA polymerase to activate transcription at the araBAD promoter.
MATERIALS AND METHODS
General methods, culture media, and conditions.
General methods were used as described previously (34). For the β-galactosidase assay and in vivo footprinting, cells were grown in minimal medium, which includes M10 salts, 0.4% glycerol, 10 μg of thiamine per ml, 40 μg of leucine per ml, 0.2% Casamino Acids, and, when added, 0.2% arabinose or l-rhamnose. The β-galactosidase assay was performed as described previously (11). For screening CAP mutants, the indicator plates contained 25.5 mg of antibiotic medium 2 (Difco) per ml, 50 μg of tetrazolium per ml, and 1% lactose.
Strains and plasmids.
Strains XE64.2 (Δcrp39 strA thi) and XE82 (Δcrp39 strA thi lacPUV5-OCAP) were provided by R. H. Ebright (40). To assay promoter activity in an AraC− background, we used SH321 (ΔaraC-leu1022, araB+A+D+ Δlac74 galK Strr), and for the AraC+ background, we used SH322 [ara(CBAD)+ leu Δlac74 galK Strr] (14).
p10 and p10+CAP carry an ara pBAD fused to lacZ, with pBAD containing the AraC binding site araI1-araI1*, which is stronger than the wild-type araI1-araI2 site (32). The sequence of araI1*, is 5′-TAGCATTTTTATCCTGA-3′. p10 and p10+CAP were subsequently cloned into the pRS415 plasmid to drive lacZYA reporter genes (35), and they are named pXZ36 and pXZ51. pXZ23 is a derivative of p10+CAP with two upstream CAP binding sites separated by 4 bp, centered at positions −93.5 and −108.5, so that the two CAP molecules bind to opposite faces of the DNA.
pXZ59 contains a class I CAP-dependent promoter, CC+20pmelR, with its CAP binding site replaced with the ara pBAD CAP site (2). pXZ9 carries CCpmelR, which is a class II CAP-dependent promoter (2). Both were made by inserting synthesized DNA fragments containing the promoter sequences into pTAP4 plasmids to drive lacZ reporter genes (32).
pGBO21B expresses the AraC DNA binding domain (7). pGBO21B was digested with PstI and NcoI to delete the AraC gene, which was replaced with the wild-type CAP gene cloned by PCR from the chromosomal template. The new plasmid-expressing CAP protein is named pXZ29. Its sequence was verified by sequencing.
In vivo DMS footprinting.
Cells (10 ml) were grown at 37°C in 125-ml flasks to an optical density at 550 nm of 0.8 to 1.0. Dimethyl sulfate (DMS) (10 μl) was directly added to the flask, and the cell culture was shaken for 2 min at 37°C. The culture was transferred to an ice-cold centrifuge tube and centrifuged at 1,000 × g for 5 min at 4°C. Plasmid DNA was isolated from the cell pellet and dissolved in 70 μl of distilled H2O dH2O plus 10 μl of piperidine. The solution was incubated at 90°C for 30 min, extracted with 1 ml of 1-butanol, and centrifuged at 15,000 × g for 15 min. The plasmid pellet was rinsed with 70% ethanol, dried, and resuspended in 15 μl of distilled H2O. A PCR amplification was carried out in a 10-μl reaction mixture with only one 32P-labeled oligonucleotide primer. For p10, the primer was 5′-AATAGGCGTATCACGAGGCCC-3′. For p10+CAP, the primer was 5′-TTATTTGCACGGCGTCACACTTT-3′. The reaction mixture contained 7 μl of piperidine-treated plasmid, 0.5 U of Taq DNA polymerase, 3 ng of 32P-labeled oligonucleotide primer, 10 μM dATP, 10 μM dCTP, 10 μM dTTP, 20 μM dGTP, 10 mM MgCl2, and 50 mM Tris-HCl (pH 9). The cycle parameters were 94°C for 1 min, 60°C for 1 min, and 65°C for 1 min, repeated for 29 cycles. The reaction products were run on a 6% denaturing polyacrylamide sequencing gel.
Random mutagenesis and screening of CAP.
The DNA fragment containing the CAP gene was amplified by PCR with primers 5′-TCATCCGCCAAAACAGCC-3′ and 5′-AATTAATCATCCGGCTCG-3′. Mutations were generated due to the high error rate of Taq DNA polymerase. The PCR conditions were the same as those described by Zhou et al. (41), and the 100-μl PCR mixture contained 50 mM KCl, 20 mM Tris-HCl (pH 8.0), 1.5 mM MgCl2, 0.01% gelatin, 0.2 mM each deoxynucleoside triphosphate, 200 ng of each primer, 5 U of Taq DNA polymerase, and 5 ng of pXZ29 plasmid. The amplified DNA fragments were cut with PstI and NcoI and reinserted into pXZ29.
We electroporated competent XE82 cells containing pXZ51 plasmid with plasmids expressing mutagenized CAP and plated them on tetrazolium-lactose plates. As described in Results, only CAP positive-control (pc) mutants for ara pBAD yielded Lac− red colonies. We purified the plasmids from the red colonies and used them to retransform XE82 and XE64.2/pXZ51 to further verify their CAP pc phenotypes. We sequenced 20 plasmids carrying the mutant CAP gene, and 17 of them possessed only a single nucleotide change.
Protein purification and DNA binding.
Wild-type and mutant CAP proteins were purified by cyclic AMP (cAMP) affinity chromatography as described previously (39), yielding proteins more than 95% pure as judged from sodium dodecyl sulfate-acrylamide gel electrophoresis. The DNA binding affinities of these CAP proteins were measured by the DNA migration retardation assay (38). The 250-bp DNA fragments used in the assay contain ara pBAD, which was amplified from p10+CAP by PCR with the primers 5′-AATAGGCGTATCACGAGGCCC-3′ and 5′-GATGGGGAGTAAGCTTGGATTCCAATTGCAATCGC-3′. The DNA binding buffer contained 50 mM KCl, 25 mM sodium HEPES (pH 7.4), 2.5 mM MgCl2, 2.5 mM dithioerythritol, 100 μM cAMP, 100 μg of bovine serum albumin per ml, 0.1 mM potassium EDTA, 5% glycerol, and 20 μg of calf thymus DNA per ml. CAP was incubated with 0.03 nM labeled araBAD DNA fragments at 37°C for 10 min before the reaction products were subjected to electrophoresis.
RESULTS
CAP can activate the araBAD promoter without the involvement of upstream DNA.
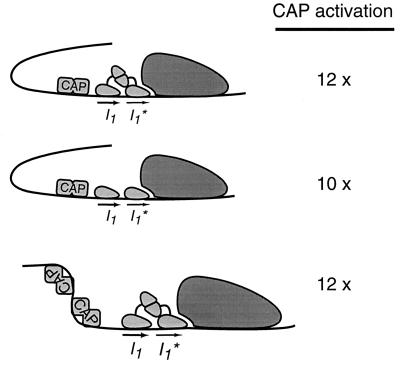
DNA upstream of the araBAD promoter participates in transcription repression by DNA looping (9). The loop is formed when AraC protein binds to the upstream araO2 and the downstream araI1 half-sites. CAP helps disrupt the loop to unlock the promoter (27). It is not known, however, how CAP activates transcription when the araO2 site has been deleted. One possibility is that AraC uses a hidden upstream binding site or nonspecific DNA and still forms a DNA loop that CAP helps to break. To rule out this possibility, we used just the DNA binding domain of AraC (7). This protein fragment does not dimerize and therefore cannot form a DNA loop. The domain can, however, bind to pBAD, with a special araI1* half-site replacing araI2. araI1* binds AraC considerably more tightly than araI2 binds AraC (32). As shown in Fig. 2, even in this case, CAP still stimulates the araBAD promoter. Apparently this stimulating activity of CAP is independent of AraC-induced DNA looping.
FIG. 2.
CAP activation at ara pBAD without the aid of upstream DNA. (Top) In the presence of wild-type AraC, CAP can activate ara pBAD-I1-I1* about 12-fold. (Middle) In the presence of the AraC DNA binding domain, CAP can also activate transcription from ara pBAD-I1-I1* about 10-fold. (Bottom) At ara pBAD-I1-I1*, with an additional CAP binding site to bend DNA away from RNA polymerase, CAP can still activate about 12-fold. The promoter activity was measured by the β-galactosidase assay in SH322 cells or in SH321 cells with the AraC DNA binding domain expressed from plasmid pGBO21B. Arabinose was not present. The transcription activation by CAP was calculated by comparing two promoters with or without CAP binding sites.
Conceivably, the role of CAP is just to bend DNA so that it will contact the back side of RNA polymerase. This upstream DNA-RNA polymerase interaction could stabilize the transcription complex and enhance the promoter activity. To investigate if this mechanism explains CAP activation at pBAD, we constructed a variation of the araBAD promoter. A second CAP site was placed upstream from the first and positioned so that it bends the DNA away from RNA polymerase. Again, as shown in Fig. 2, we did not see any reduction of CAP activation at this promoter. These experiments indicate that the mechanism of CAP activation at ara pBAD does not involve merely bending the DNA.
CAP does not affect AraC binding in the unlooped state.
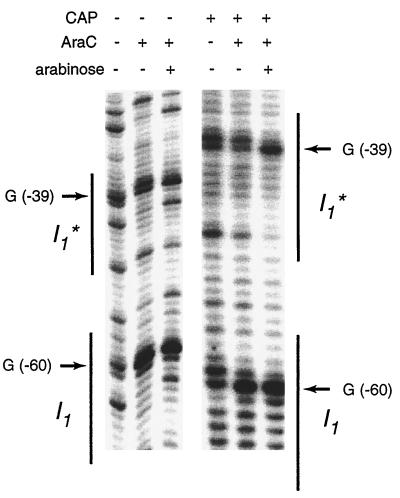
CAP does not significantly activate pBAD in the absence of AraC (data not shown). It is conceivable, then, that CAP may just stabilize AraC binding to DNA or force AraC to bend to reach a conformation from which it can activate transcription. Either way, CAP would influence the binding of AraC to DNA. To assess possible CAP-AraC interactions in vivo, we compared the amount of AraC binding in the presence and absence of CAP. We performed DMS footprinting at araBAD promoters with and without the CAP binding site. Neither promoter possessed the araO2 half-site, and thus CAP can stimulate them only directly, not by assisting in loop breaking. If CAP affects the amount of AraC binding, we should be able to detect a difference of AraC footprinting between these two promoters.
In the footprinting assay, cells in the exponential growth phase were treated with DMS. If AraC binds to DNA, two guanines in the AraC binding site become hypersensitive to DMS attack, and the extent of attack correlates well with the occupancy of the site by AraC protein (32). The left three lanes of Fig. 3 show the footprint of the araBAD promoter lacking the CAP binding site. When arabinose was added, AraC bound DNA and G(−39) and G(−60) became hypersensitive to DMS. The right three lanes show the footprint of the araBAD promoter that possesses the CAP binding site. In both the presence and absence of arabinose, the footprint by AraC was the same as on the promoter without the CAP binding site. AraC binding to DNA was not detectably altered by the presence of CAP. We also assayed the activities of the two promoters in vivo and found that the araBAD promoter with the CAP site was about 10 times more active than the one without CAP, even in the absence of arabinose. These results indicate that CAP does not activate transcription by affecting the amount of AraC binding to DNA.
FIG. 3.
In vivo DMS footprinting shows that AraC binding is not affected by CAP. The left three lanes show the DMS footprinting of plasmid p10, which has the ara pBAD-I1-I1* promoter without a CAP binding site. The right three lanes show the footprinting at p10+CAP plasmid, which has the araBAD-I1-I1* promoter with a CAP site. The footprinting was done in SH321 or SH322 cells.
Isolation of crp pc mutants for the araBAD promoter.
Having excluded other reasonable possibilities, we considered the possibility that CAP directly interacts with RNA polymerase through protein-protein interactions. If this were the case, we should be able to isolate mutants of CAP defective in the interaction. The desired mutants should still bind DNA normally, however, and could be called pc mutants.
The wild-type araBAD operon is not suitable for the isolation of pc mutants. CAP mutants could disable the arabinose transport operons and lower the arabinose concentration in vivo. This, in turn, would affect induction by AraC and indirectly influence the transcription activity of ara pBAD. Another complication is that the assay of wild-type araBAD operon activity involves the measurement of arabinose metabolism, which again relies on the arabinose transport operons. DNA looping at wild-type pBAD could also mask the CAP pc phenotype. To avoid these indirect effects, we based our selection on plasmid pXZ51 which carries pBAD-araI1-araI1* fused to lacZYA. This variant of the wild-type pBAD promoter possesses a normal basal level and can be activated by CAP to a high level even when AraC is not induced by arabinose (reference 32 and data not shown). This promoter lacks the upstream araO2 half-site, and thus CAP activates transcription independent of DNA looping. Hence, we completely bypassed DNA looping and the arabinose transporter genes to isolate CAP mutants specifically for ara pBAD. We further characterized our candidates by examining their behavior at two variant ara pBAD-araI1-araI1* promoters, one containing and one lacking a CAP binding site. This latter tests prevents possible confusion from any effects of the CAP mutants on AraC protein expression.
For the isolation of CAP pc mutants effective at the araBAD promoter, we used a screen similar to those used previously for the isolation of CAP pc mutants at the lac promoter (40). Plasmids expressing a mutagenized crp gene were transformed into XE82/pXZ51. This strain carries a chromosomal placUV5-OCAP promoter driving lacZYA, which can be repressed when CAP binds to a site downstream from the promoter and blocks RNA polymerase. DNA binding mutants of CAP do not block RNA polymerase and therefore do not prevent lacZYA expression. Wild-type CAP activates the ara pBAD promoter that is fused to lacZYA in the pXZ51 plasmid and gives Lac+ colonies. Only CAP pc mutants, which can bind to DNA but cannot activate ara pBAD-lacZYA, give Lac− colonies.
We used PCR to amplify DNA encoding CAP in about 30 independent reactions and inserted the mutagenized DNA into CAP-expressing plasmids. We screened 30,000 colonies, of which 270 were Lac−, and 20 of them yielded Lac− colonies upon retransformation. As shown in Table 1, these mutants fall into two groups. The first group includes mutants with mutations from residue 150 to 164 of CAP. This includes AR1 of CAP. Of these mutants, those with His-159→Pro, Gly-162→Ser, or Gly-162→Asp have been isolated previously as AR1 mutants (30). The second group of mutants includes those with changes in amino acids 46 to 68 and 133. These amino acids are in or near the antiparallel β-roll region of CAP that contains Lys-52. The amino acids overlap but are not coincident with AR3.
TABLE 1.
Properties of the CAP mutants with mutations at the ara pBAD promoter
| CAP mutanta | No. of isolates | Activation at ara pBAD-I1-I1*b | Activation at ara pBAD-I1-I2c | Repression at placUV5-OCAPd |
|---|---|---|---|---|
| Wild type | 5× | 5× | 7× | |
| -CAP | 1× | 1× | 1× | |
| Leu-150→Pro | 1 | 1.3× | 4× | |
| Ala-156→Gly | 1 | 4× | 5× | |
| His-159→Pro | 1 | 1.5× | 1× | 5× |
| Gly-162→Asp | 3 | 2× | 6× | |
| Gly-162→Ser | 2 | 1.5× | 7× | |
| Gln-164→Pro | 1 | 2× | 3× | |
| Ser-46→Pro | 1 | 2× | 5× | |
| Ile-51→Thr | 1 | 1× | 1× | 6× |
| Lys-52→Glu | 1 | 2× | 3× | 6× |
| Ile-60→Val | 1 | 2× | 6× | |
| Leu-64→Pro | 2 | 1.5× | 2× | 6× |
| Asp-68→Gly | 1 | 2× | 3× | 6× |
| Asn-133→Asp | 1 | 2× | 6× |
The first group contains AR1 mutations. The second group has mutations clustered around the AR3 region.
The CAP activation was calculated by comparing ara pBAD-I1-I1* promoter activity with and without a CAP binding site in the absence of arabinose in XE64.2 cells.
The CAP activation was calculated by comparing ara pBAD-I1-I2 promoter activity with and without a CAP binding site in the presence of arabinose in XE64.2 cells.
The repression of the placUV5-OCAP promoter by CAP was determined in XE82 cells. In the absence and presence of wild-type CAP, the β-galactosidase expression of placUV5-OCAP is 40 and 280 U, respectively.
Characterization of the CAP pc mutants.
The CAP mutants we isolated repress the placUV5-OCAP promoter as well as wild-type CAP (Table 1). This indicates that they are not defective in DNA binding, although it is possible that L150P and Q164P owe their reduced activation effects merely to weakened DNA binding. In vivo analysis shows that all these CAP mutants are defective in activating the ara pBAD promoter, in both the presence and absence of arabinose (Table 1). These results show that the CAP mutants we isolated meet the criteria of pc mutants for pBAD.
The first group of our mutants contains those previously found in the AR1 group. The mutants in this group that have been studied previously affected both class I and class II CAP-dependent promoters. Not surprisingly, our mutants were deficient in activating a lac-like class I promoter (data not shown) and CCpmelR (2), a class II promoter.
The mutants in our second group are moderately reduced in their ability to activate the lac-like promoter and the CCpmelR promoter. It has been previously shown that mutations in Lys-52 make CAP more active at class II promoters (2). Table 2 shows that our AR3 mutant, K52E, also activates the CCpmelR promoter fourfold more than wild-type CAP does.
TABLE 2.
Effects of CAP pc mutants at CCpmelR, araFGH, and rhaBAD promotersa
| CAP mutantb | Activation of:
|
||
|---|---|---|---|
| CCpmelR | araFGH | rhaBAD | |
| Wild type | 30× | 11× | 27× |
| Leu-150→Pro | 18× | 17× | |
| Ala-156→Gly | 23× | 1× | |
| His-159→Pro | 4× | 1× | 2× |
| Gly-162→Asp | 7× | 5× | |
| Gly-162→Ser | 7× | 1× | 2× |
| Gln-164→Pro | 10× | 1× | |
| Ser-46→Pro | 10× | 6× | |
| Ile-51→Thr | 10× | 1× | |
| Lys-52→Glu | 144× | 12× | 5× |
| Ile-60→Val | 30× | 6× | |
| Leu-64→Pro | 15× | 3× | 2× |
| Asp-68→Gly | 30× | 6× | |
| Asn-133→Asp | 35× | 7× | |
The CAP activation at these promoters was determined in XE64.2 cells by the β-galactosidase assay. CAP mutants were expressed from plasmid pXZ29.
See Table 1, footnote a.
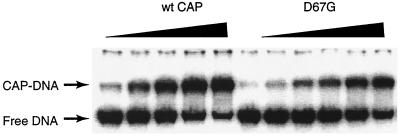
Since most of our second group of mutants have not been previously isolated, we purified three of them and the wild-type CAP protein for further analysis. Their DNA binding affinity to the araBAD promoter was measured by the DNA migration retardation assay. Two mutants bind to DNA as tightly as wild-type CAP does (Kd, 50 and 40 nM for L64P and D68G, respectively; Kd, 50 nM for the wild type) and K52E binds with about a threefold-lower affinity (Kd, 150 nM). We conclude that the DNA binding activities of our mutants are not substantially altered. Figure 4 shows the data for the wild type and the D67G mutant.
FIG. 4.
DNA migration retardation assay of CAP binding to DNA. The concentration of DNA fragments containing the ara pBAD-I1-I1* promoter was 0.03 nM. The concentrations of wild-type (wt) CAP protein from lanes 1 to 5 were 6.3, 12.5, 25, 50, and 100 nM. The concentrations of CAP mutant D67G from lanes 6 to 11 were 1.3, 2.5, 5, 10, 20, and 40 nM. The occupancy of CAP binding site increased with higher concentrations of CAP, and the equilibrium dissociation constant was calculated as shown in the text.
Do our pc mutations affect other promoters regulated by AraC or AraC family members? At the araFGH promoter, CAP binds at a site adjacent to RNA polymerase and AraC binds to the upstream site (17, 22). At the rhaBAD promoter, which is regulated by an AraC family member, RhaR binds immediately adjacent to RNA polymerase and CAP binds upstream from RhaR (11). As Table 2 shows, mutations in AR1 of crp affect both promoters. Interestingly, although the second group of CAP mutants, including the mutant with a mutation at Lys-52, have a large effect at the rhaBAD promoter, these mutations have much less of an effect on activation of the araFGH promoter.
DISCUSSION
The role of CAP as a coactivator at the araBAD promoter has been investigated in this study. We showed that CAP can stimulate transcription at this promoter in a loop-independent way that does not involve altering AraC DNA binding. We have identified two regions of CAP that are important for activation of the araBAD promoter and the related araFGH and rhaBAD promoters. In light of extensive work by others, our results suggest that CAP directly interacts with RNA polymerase at ara pBAD, ara pFGH, and rha pBAD.
At the araBAD promoter, the AraC protein binding site is centered at position −52.5 and CAP is centered at position −93.5. How the CAP protein manages to activate transcription from so far away is not clear. AraC protein represses pBAD transcription by forming a DNA loop, and previous experiments have shown that CAP can help open the repression loop and thereby stimulate promoter activity (15, 27), but this does not account for all the CAP activation effect.
An attractive model for CAP activation at ara pBAD is that CAP directly interacts with RNA polymerase. This view was cast in doubt, however, when it was reported that the AR1 mutation H159L did not reduce the CAP activation at ara pBAD (37). This mutation has been shown to disrupt a large number of promoters where CAP directly interacts with RNA polymerase (10). The confounding study was done with the wild-type araBAD operon, and DNA looping was possible (37). Hence, substantial CAP activation could have been seen merely as a result of opening the DNA loop. This may have obscured any direct effects of the AR1 mutation. On the other hand, the result did not exclude the possibility that other regions on CAP were responsible for interaction with RNA polymerase at ara pBAD.
We attempted to test the direct-interaction model by isolating CAP mutants defective in transcription activation at ara pBAD but not defective in DNA binding. Interestingly, one group of mutants we isolated had altered residues in or near the activating region one of CAP, AR1. Many lines of evidence have suggested that at class I and II promoters, where CAP is the sole activator, AR1 of CAP interacts with the C-terminal domain of the RNA polymerase alpha subunit (5, 10). CAP uses this interaction to recruit RNA polymerase to the promoter and increase its initial binding to DNA. The same mechanism probably applies at the araBAD promoter.
If CAP contacts the alpha subunit of RNA polymerase, how does it reach past the interposed AraC protein? If the intervening protein bends the DNA sharply, such an interaction may easily take place. Previous studies have shown that at some promoters with CAP bound around position −91 and another activator at −42, AR1 was also required for CAP to activate transcription (6, 23). Since the C-terminal domain of the alpha subunit is tethered to RNA polymerase through a flexible linker (3, 20), it is conceivable that this domain can reach over another protein to contact the AR1 of the upstream CAP. At ara pBAD, AraC-induced DNA bending may also help to move the upstream CAP closer to contact RNA polymerase.
The identification of the second group of crp pc mutants at the araBAD promoter was unexpected. The mutations clustered around Lys-52, which is contained in AR3 (5). A number of amino acid substitutions at Lys-52, including the one we isolated, can improve CAP activation at class II promoters (2). Also, a previous cross-linking study indicated that Lys-52 is positioned very close to the sigma subunit of RNA polymerase at these promoters (21). It was suggested that mutations at Lys-52 expose a nonnative activating region that can interact with the RNA polymerase sigma subunit to activate transcription at class II promoters (5). It is unclear, however, what the role of AR3 at ara pBAD is. Our AR3 mutation K52E reduced CAP activation at pBAD, which could mean that this mutation disrupted an existing activating region of CAP instead of revealing one. Recent studies have indicated that the two alpha subunits of RNA polymerase can each contact upstream activating elements (28). Possibly, one alpha subunit interacts with AR1 and another interacts with AR3 of CAP. It has also been proposed that at class II promoters, functional AR3 interacts with residues in the region of the sigma subunit from positions 590 to 600 (5). Previous studies identified sigma mutations at His-596, which made ara pBAD independent of CAP (18), suggesting a sigma-AraC interaction.
A number of the mutations we isolated in the AR3 region are buried inside the protein. These buried amino acids probably cannot contact another protein directly, yet they reduce the CAP activation at pBAD. Perhaps they induce conformational changes in CAP that disrupt the surface-exposed AR1 or AR3 or their accessibilities. We should also point out that residues in this area have been implicated in induction of CAP by cAMP (1). Recently, a second cAMP pocket was found to exist near the DNA binding domain of CAP (31). Its occupancy by cAMP or the effects of its occupancy could be affected by our mutations.
It is not surprising that the CAP AR1 mutants that are defective in transcription activation at ara pBAD also affect two related promoters, ara pFGH and rha pBAD. AR1 of CAP probably directly contacts RNA polymerase at these two promoters. Mutations near AR3 only slightly disturb CAP activation at pFGH, however, as might be expected since this is a class II CAP-dependent promoter that also contains upstream AraC binding sites, and AR3 and the surrounding region in wild-type CAP does not directly participate in transcription activation at class II promoters. At the araBAD and rhaBAD promoters, however, mutations in the AR3 region strongly reduce CAP activation. These two promoters both have another activator protein positioned between CAP and RNA polymerase, as opposed to class II CAP-dependent promoters, where CAP binds adjacent to RNA polymerase.
Our results indicate that CAP plays a direct role in transcription activation at ara pBAD. We have demonstrated that CAP can activate ara pBAD without altering DNA looping or AraC binding. A number of CAP pc mutants defective at pBAD were isolated, and they fell into the regions of CAP known to interact with RNA polymerase at many promoters. These lines of evidence suggest that direct CAP-RNA polymerase interactions also occur at ara pBAD.
ACKNOWLEDGMENTS
We thank Richard Ebright and Wei Niu for discussions and materials, Steve Busby, and Richard Gourse for comments, and members of our laboratory for ongoing discussions.
This work was supported by National Institutes of Health grant GM18277 to R.F.S.
REFERENCES
- 1.Aiba H, Nakamura T, Mitani H, Mori H. Mutations that alter the allosteric nature of cAMP receptor protein of Escherichia coli. EMBO J. 1985;4:3329–3332. doi: 10.1002/j.1460-2075.1985.tb04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell A, Gaston K, Williams R, Chapman K, Kolb A, Buc H, Minchin S, Williams J, Busby S. Mutations that alter the ability of the Escherichia coli cyclic AMP receptor protein to activate transcription. Nucleic Acids Res. 1990;18:7243–7250. doi: 10.1093/nar/18.24.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blatter E E, Ross W, Tang H, Gourse R L, Ebright R H. Domain organization of RNA polymerase alpha subunit: C-terminal 85 amino acids constitute a domain capable of dimerization and DNA binding. Cell. 1994;78:889–896. doi: 10.1016/s0092-8674(94)90682-3. [DOI] [PubMed] [Google Scholar]
- 4.Bracco L, Kotlarz D, Kolb A, Diekmann S, Buc H. Synthetic curved DNA sequences can act as transcriptional activators in Escherichia coli. EMBO J. 1989;8:4289–4296. doi: 10.1002/j.1460-2075.1989.tb08615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busby S, Ebright R. Transcription activation at class II CAP-dependent promoters. Mol Microbiol. 1997;23:853–859. doi: 10.1046/j.1365-2958.1997.2771641.x. [DOI] [PubMed] [Google Scholar]
- 6.Busby S, West D, Lawes M, Webster C, Ishihama A, Kolb A. Transcription activation by the Escherichia coli cyclic AMP receptor protein. J Mol Biol. 1994;241:341–352. doi: 10.1006/jmbi.1994.1511. [DOI] [PubMed] [Google Scholar]
- 7.Bustos S A, Schleif R F. Functional domains of the AraC protein. Proc Natl Acad Sci USA. 1993;90:5638–5642. doi: 10.1073/pnas.90.12.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carra J H, Schleif R F. Variation of half-site organization and DNA looping by AraC protein. EMBO J. 1993;12:35–44. doi: 10.1002/j.1460-2075.1993.tb05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn T M, Hahn S, Ogden S, Schleif R F. An operator at −280 base pairs that is required for repression of araBAD operon promoter: addition of DNA helical turns between the operator and promoter cyclically hinders repression. Proc Natl Acad Sci USA. 1984;81:5017–5020. doi: 10.1073/pnas.81.16.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebright R H. Transcription activation at class I CAP-dependent promoters. Mol Microbiol. 1993;8:797–802. doi: 10.1111/j.1365-2958.1993.tb01626.x. [DOI] [PubMed] [Google Scholar]
- 11.Egan S M, Schleif R F. A regulatory cascade in the induction of rhaBAD. J Mol Biol. 1993;234:87–98. doi: 10.1006/jmbi.1993.1565. [DOI] [PubMed] [Google Scholar]
- 12.Eschenlauer A C, Reznikoff W S. Escherichia coli catabolite gene activator protein mutants defective in positive control of lac operon transcription. J Bacteriol. 1991;173:5024–5029. doi: 10.1128/jb.173.16.5024-5029.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenblatt J, Schleif R. Arabinose C protein: regulation of the arabinose operon in vitro. Nat New Biol. 1971;233:166–170. doi: 10.1038/newbio233166a0. [DOI] [PubMed] [Google Scholar]
- 14.Hahn S, Dunn T, Schleif R. Upstream repression and CRP stimulation of the Escherichia colil-arabinose operon. J Mol Biol. 1984;180:61–72. doi: 10.1016/0022-2836(84)90430-3. [DOI] [PubMed] [Google Scholar]
- 15.Hahn S, Hendrickson W, Schleif R. Transcription of Escherichia coli ara in vitro. The cyclic AMP receptor protein requirement for pBAD induction that depends on the presence and orientation of the araO2 site. J Mol Biol. 1986;188:355–367. doi: 10.1016/0022-2836(86)90160-9. [DOI] [PubMed] [Google Scholar]
- 16.Hendrickson W, Schleif R F. Regulation of the Escherichia colil-arabinose operon studied by gel electrophoresis DNA binding assay. J Mol Biol. 1984;178:611–628. doi: 10.1016/0022-2836(84)90241-9. [DOI] [PubMed] [Google Scholar]
- 17.Hendrickson W, Stoner C, Schleif R. Characterization of the Escherichia coli araFGH and araJ promoters. J Mol Biol. 1990;215:497–510. doi: 10.1016/S0022-2836(05)80163-9. [DOI] [PubMed] [Google Scholar]
- 18.Hu J C, Gross C A. Mutations in the sigma subunit of E. coli RNA polymerase which affect positive control of transcription. Mol Gen Genet. 1985;199:7–13. doi: 10.1007/BF00327502. [DOI] [PubMed] [Google Scholar]
- 19.Igarashi K, Ishihama A. Bipartite functional map of the E. coli RNA polymerase alpha subunit: involvement of the C-terminal region in transcription activation by cAMP-CRP. Cell. 1991;65:1015–1022. doi: 10.1016/0092-8674(91)90553-b. [DOI] [PubMed] [Google Scholar]
- 20.Jeon Y, Yamazaki T, Otomo T, Ishihama A, Kyogoku Y. Flexible linker in the RNA polymerase alpha subunit facilitates the independent motion of the C-terminal activator contact domain. J Mol Biol. 1997;267:953–962. doi: 10.1006/jmbi.1997.0902. [DOI] [PubMed] [Google Scholar]
- 21.Jin R, Sharif K A, Krakow J S. Evidence for contact between the cyclic AMP receptor and sigma70 subunit of E. coli RNA polymerase. J Biol Chem. 1995;270:19213–19216. doi: 10.1074/jbc.270.33.19213. [DOI] [PubMed] [Google Scholar]
- 22.Johnson C M, Schleif R F. In vivo induction kinetics of the arabinose promoters in Escherichia coli. J Bacteriol. 1995;177:3438–3442. doi: 10.1128/jb.177.12.3438-3442.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joung J K, Le L U, Hochschild A. Synergistic activation of transcription by Escherichia coli cAMP receptor protein. Proc Natl Acad Sci USA. 1993;90:3083–3087. doi: 10.1073/pnas.90.7.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee N, Francklyn C, Hamilton E P. Arabinose-induced binding of AraC protein to araI2 activates the araBAD operon promoter. Proc Natl Acad Sci USA. 1987;84:8814–8818. doi: 10.1073/pnas.84.24.8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lichenstein H S, Hamilton E P, Lee N. Repression and catabolite gene activation in the araBAD operon. J Bacteriol. 1987;169:811–822. doi: 10.1128/jb.169.2.811-822.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobell R B, Schleif R F. DNA looping and unlooping by AraC protein. Science. 1990;250:528–532. doi: 10.1126/science.2237403. [DOI] [PubMed] [Google Scholar]
- 27.Lobell R B, Schleif R F. AraC-DNA looping: orientation and distance-dependent loop breaking by the cyclic AMP receptor protein. J Mol Biol. 1991;218:45–54. doi: 10.1016/0022-2836(91)90872-4. [DOI] [PubMed] [Google Scholar]
- 28.Murakami K, Kimura M, Owens J T, Meares C F, Ishihama A. The two alpha subunits of Escherichia coli RNA polymerase are asymmetrically arranged and contact different halves of DNA upstream element. Proc Natl Acad Sci USA. 1997;94:1709–1714. doi: 10.1073/pnas.94.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu W, Kim Y, Tau G, Heyduk T, Ebright R H. Transcription activation at class II CAP-dependent promoters: two interactions between CAP and RNA polymerase. Cell. 1996;87:1123–1134. doi: 10.1016/s0092-8674(00)81806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niu W, Zhou Y, Dong Q, Ebright Y W, Ebright R H. Characterization of the activating region of Escherichia coli catabolite gene activator protein (CAP). I. Saturation and alanine-scanning mutagenesis. J Mol Biol. 1994;243:595–602. doi: 10.1016/0022-2836(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 31.Passner J, Steitz T. The structure of a CAP-DNA complex having two cAMP molecules bound to each monomer. Proc Natl Acad Sci USA. 1997;94:2843–2847. doi: 10.1073/pnas.94.7.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeder T, Schleif R. AraC protein can activate transcription from only one position and when pointed in only one direction. J Mol Biol. 1993;231:205–218. doi: 10.1006/jmbi.1993.1276. [DOI] [PubMed] [Google Scholar]
- 33.Richet E, Sögaard-Andersen L. CRP induces the repositioning of MalT at the Escherichia coli malKp promoter primarily through DNA bending. EMBO J. 1994;13:4558–4567. doi: 10.1002/j.1460-2075.1994.tb06777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schleif R, Wensink P. Practical methods in molecular biology. New York, N.Y: Springer-Verlag; 1981. [Google Scholar]
- 35.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 36.Stoltzfus L, Wilcox G. Effect of mutations in the cyclic AMP receptor protein-binding site on araBAD and araC expression. J Bacteriol. 1989;171:1178–1184. doi: 10.1128/jb.171.2.1178-1184.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams R, Bell A, Sims G, Busby S. The role of two surface exposed loops in transcription activation by the Escherichia coli CRP and FNR proteins. Nucleic Acids Res. 1991;19:6705–6712. doi: 10.1093/nar/19.24.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Withey, J., and R. Schleif. Unpublished data.
- 38.Zhang X, Reeder T, Schleif R. Transcription activation parameters at ara pBAD. J Mol Biol. 1996;258:14–24. doi: 10.1006/jmbi.1996.0230. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X P, Gunasekera A, Ebright Y W, Ebright R H. Derivatives of CAP having no solvent-accessible cysteine residues, or having a unique solvent-accessible cysteine residue at amino acid 2 of the helix-turn-helix motif. J Biomol Struct Dyn. 1991;9:463–473. doi: 10.1080/07391102.1991.10507929. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Y, Zhang X, Ebright R H. Identification of the activating region of catabolite gene activator protein (CAP): isolation and characterization of mutants of CAP specifically defective in transcription activation. Proc Natl Acad Sci USA. 1993;90:6081–6085. doi: 10.1073/pnas.90.13.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y H, Zhang X P, Ebright R H. Random mutagenesis of gene-sized DNA molecules by use of PCR with Taq DNA polymerase. Nucleic Acids Res. 1991;19:6052. doi: 10.1093/nar/19.21.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]