Abstract
Background
Most labor-related problems can be attributed to the uterine myometrium muscle, as this irritable tissue must suppress its irritability potential during pregnancy. Unfortunately, fewer studies have investigated the causes of this lack of suppression in preterm labor.
Methods
We conducted a scoping narrative review using three online databases (PubMed, Scopus, and Science Direct).
Results
The review focused on ion channel functions in the myometrium, including sodium channels [Na K-ATPase, Na-activated K channels (Slo2), voltage-gated (SCN) Na+, Na+ leaky channels, nonselective (NALCN) channels], potassium channels [KATP (Kir6) channels, voltage-dependent K channels (Kv4, Kv7, and Kv11), twin-pore domain K channels (TASK, TREK), inward rectifier Kir7.1, Ca2+-activated K+ channels with large (KCNMA1, Slo1), small (KCNN1–3), intermediate (KCNN4) conductance], and calcium channels [L-Type and T-type Ca2+ channels, calcium-activated chloride channels (CaCC)], as well as hyperpolarization-activated cation channels. These channels' functions are associated with hormonal effects such as oxytocin, estrogen/progesterone, and local prostaglandins.
Conclusion
Electromechanical/hormonal activity and environmental autocrine factors can serve as the primary practical basis for premature uterine contractions in term/preterm labor. Our findings highlight the significance of.
-
1.
the amplitude rate of hyperpolarization and the frequency of contractions,
-
2.
changes in the estrogen/progesterone ratio,
-
3.
Prostaglandins E/F involvement in initiating potential spikes and the increase of intracytoplasmic Ca2+.
This narrative study highlights the range of hyperpolarization and the frequency of myometrium contractions as crucial factors. The synchronized complex progress of estrogen to progesterone ratio and prostaglandins plays a significant role in initiating potential spikes and increasing intracytoplasmic Ca2+, which further influences the contraction process during labor. Insights into myometrium physiology gained from this study may pave the way for much-needed new treatments to reduce problems associated with normal and preterm labor.
Keywords: Labor, Ion channels, Calcium, Placenta, Glucose
Abbreviations
- APs
action potentials
- β2AR
β2 adrenergic receptor
- BDNF
brain-derived neurotrophic factor
- cAMP
cyclic AMP
- CaCC
L-Type and T-type Ca2+ channels, calcium-activated- chloride channels
- EP
prostaglandin E receptors 1-4
- FP
prostaglandin F receptor
- IP3
inositol triphosphate
- IP3R
IP3 receptor
- LTCC
L-type calcium channel
- MD
muscle dystrophy
- MLCK
myosin light chain kinase oxytocin
- MLCP
Myosin light chain phosphatase
- Slo2
Na K-ATPase, Na-activated K channels
- OTR
oxytocin receptor
- PG
prostaglandins
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PIP3
phosphatidylinositol 3,4,5-thrisphosphate
- PLC
phospholipase C
- PKC
Protein kinase C
- ROCK
Rho kinase
- SERCA
sarcoplasmic/endoplasmic reticulum calcium ATPase
- NALCN
sodium (Na+) leak channel, nonselective
- STIM
stromal interaction molecule
- USMC
uterine smooth muscle contraction
- VGSCs
Voltage-gated Na + channels
1. Introduction
Controlling the timing of labor is a complex process that relies on the correct physiological interaction among the mother, the fetus, and the placenta. Labor is a physiological event accompanied by regular and painful uterine contractions, which coordinate with multiple paracrine/autocrine events, fetal hormonal changes, and overlapping maternal/fetal control mechanisms to initiate labor in women.
2. Labor
Labor can be categorized into three main phases: early labor, active labor, and transitional labor. In the initial phase, the uterus myometrium undergoes a transformation from a quiescent state to asynchronous contractions. This necessitates the formation of gap junctions between myofibrils to facilitate the transmission of the contractile signal. During this phase, the fetus contributes to the coordination by producing steroid hormones via the placenta, which occurs through mechanical distension. Additionally, the secretion of neuro-hypophysis hormones and other stimulators, such as prostaglandin, play a role in this process. In the secondary phase, the cervical connective tissue must be capable of dilation to allow for the delivery of the fetus from the uterus. These changes are accompanied by a shift from progesterone to estrogen dominance, increased responsiveness to oxytocin due to upregulation of myometrium oxytocin receptors, increased prostaglandin synthesis in the uterus, enhanced formation of myometrium gap junctions, decreased nitric oxide (NO) activity, and increased influx of calcium into myocytes with ATP-dependent myosin to actin, as well as increased endothelin levels leading to augmented uterine blood flow and myometrium activity [1]. Calcium plays a strategic key role in the process of muscle contraction, as it is required for every muscle activity.
2.1. Effect of the intracellular free Ca2+ concentrations in contraction of the myometrium
Upon depolarization of the plasma membrane of the myofibril, an influx of calcium ions through the L-type calcium channels of the sarcoendoplasmic reticulum (SR) triggers a significant release of calcium into the cytoplasm, which, in turn, stimulates myofibril contraction. The sarcoendoplasmic reticulum calcium ATPase (SERCA) pump plays a crucial role in regulating cellular calcium homeostasis. Following muscle contraction, this pump transports calcium ions from the cytosol back to the sarcoplasmic reticulum (SR). Maintaining cellular calcium homeostasis through SERCA is essential for muscle health and function; thus, dysfunction of SERCA can contribute to muscle pathogenesis. Studies have described its association with pathological conditions related to aging, neurodegeneration, and muscular dystrophy (MD), impairing intracellular calcium homeostasis and further contributing to muscle atrophy and weakness [2]. In the myometrium, the regulation of ion channel activity can influence intracellular free Ca2+ concentrations, making control of ion channel activity one of the approaches to controlling myometrial contractile activity.
2.2. Uterus, myometrium, and muscle fibers contraction
Calcium sensitization refers to the increased sensitivity of regulatory and contractile proteins to calcium, resulting in stronger contractions. Several proteins are recognized for their role in this sensitization process. Protein kinase C (PKC) and RhoA/Rho-kinase are two signaling pathways involved in initiating calcium sensitization [3]. Ras homology (RHO) proteins are small monomeric GTP-binding proteins that regulate actin polymerization and myosin phosphorylation in smooth muscle cells. During pregnancy, the mRNAs for RhoA, ROK-1, and ROK-2 are present in the non-gravid uterus and increase in expression. Inhibition of ROK prevents force development and increases relaxation without altering calcium levels. The RAS signaling pathway accompanies myosin light chain phosphatase (MLCP) inhibition, which controls myofibril contraction through non-calcium-dependent mechanisms [4]. Moreover, cAMP causes phosphorylation of myosin light chain kinase (MLCK) by the cAMP-dependent protein kinase, which reduces enzymatic activity by decreasing Ca2+/calmodulin-dependent myosin light chain kinase affinity. Additionally, several proteins regulate the balance of kinase or phosphatase activity, with MLCK and MLCP being prominent players in the machinery of smooth muscle contraction [5]. At present, there are several well-characterized agonists/antagonists for the contraction of uterine smooth muscle pathways, including Ca2+ channel blockers, oxytocin, progesterone, and Atosiban (an inhibitor of the oxytocin-mediated release of inositol trisphosphate from myofibrils) [6] (Fig. 1) [7].
Fig. 1.
Signaling pathways in uterine contractility: Ca2+ entry through voltage-gated Ca2+ channels (LTCC) and STIM/Orai, Members of the Store-operated calcium entry complex.
2) Ca2+ is released from the sarcoplasmic reticulum (SERCA) through the IP3R, increasing intracellular Ca2+ concentration and stimulating myosin phosphorylation. Myosin phosphorylation can also be triggered by hormones and tocolytic agents via G protein-coupled receptor-mediated pathways [7].
2.3. Excitation–contraction coupling in excitable cell of the myometrium
It is crucial to evaluate the excitation-contraction coupling (EC coupling) in the myometrium, as it holds the key to developing knowledge and advancing strategies to manage preterm labor. EC coupling is mainly achieved by maintaining high conductance of K+ and minimizing cell connections between myocytes and muscle bundles [8]. During pregnancy, irritability breaks should be controlled until full-term; otherwise, the cervix may lose control rapidly, leading to premature labor.
2.3.1. EC coupling in smooth muscle of the myometrium
The myometrium consists of three layers: a horizontal outer layer, followed by inner longitudinal fibers with a middle layer of blood vessels. These layers exhibit a phasic contractile pattern, classifying the myometrium as phasic smooth muscle. Phasic smooth muscle shows transient augmentations in force generation in response to potassium ion (K+) stimuli. This force generation is initiated by the characteristics of action potentials in the myofibrils and the differential expression patterns of contractile proteins. Contraction and relaxation in the myometrium result from cyclic depolarization and repolarization of myofibril membranes. Depolarization is driven by an inward current predominantly carried by Ca2+ ions and Na + ions, while repolarization involves an outward current through fast voltage-dependent K+ ions and slow Ca2+-activated components. The frequency and intensity of myometrium contractions are determined by the consistency and duration of action potentials in each myofibril [9]. The intracellular Ca2+ concentration [Ca2+]i serves as an essential trigger for uterine contractile machinery, and myometrium action potentials influence the shape of the calcium inward transient. The significance of EC coupling is evident from the experimental effects of calcium removal, which results in the rapid cessation of contraction by blocking Ca2+ L-type channels with Nifedipine in the external solution [10]. Various mechanisms related to the presence of this ion in uterine myometrium contraction have been identified, as outlined below.
2.3.2. The feed-forward loop for Ca2+ release in the myometrium
The increasing inward calcium ion can sensitize other Ca2+ channels to open through a complex feed-forward loop [11]. Ion channels are transmembrane proteins associated with gating properties that mediate many physiological functions. These channels can be either open or closed, with the transition between these two states facilitated by structural elements known as gates. Voltage-gated channels and ligand-gated channels activate through different processes, but both allow the movement of ions. Crystallography and mutational analyses provide detailed insights into the structure of these channels, aiding the understanding of the conversion of electrical signals generated by small ion currents across cell membranes, particularly in voltage-gated Ca2+, Na+, and K+ channels [12].
2.3.3. Activation voltage-gated channels or ion channels
The transduction of chemical signals into electrical signaling at the cell membrane is fundamental to cellular communication. The initiation of stimulation in myogenic smooth muscle and its propagation to the myometrium during preterm and term labor is a highly complex process involving several hormones and voltage-dependent channels. The influx of Ca2+ into myometrial myofibrils is a vital stimulus for uterine contractile activity. Important Ca2+ channels include 1) L-type and T-type Ca2+ channels, which undergo Ca2+ gating through the arrangement of voltage-sensing domains (the charged S4 segments) and the opening and closing of S6 gates [13]. These channels consist of a tetramer of membrane-bound domains with a positively charged S4 segment. The sliding-helix mechanism is a key factor responsible for positive gating alterations in the voltage sensor, leading to conformational changes in the pore module that opens the intracellular activation gate upon depolarization [12]. Initial structural details of K+ channels reveal potential coupling between voltage sensors and the pore region via movement of the S4 segment, which transmits the signal to the S6 helix, resulting in gate opening upon depolarization [14]. High-voltage-activated (HVA) Ca2+ channels, classified as R-type or E-type, are divided into two subfamilies: L-type and non-L-type channels [15].
2) Voltage-gated Na+ channels (VGSCs), Na-activated K+ channels (Slo2), voltage-gated (SCN) Na+ and Na+ leak channels, nonselective (NALCN) channels, the Na+/K+-ATPase, and hyperpolarization-activated cation channels. 3) KATP (Kir6) channels, voltage-dependent K+ channels (Kv4, Kv7, and Kv11), twin-pore domain K+ channels (TASK, TREK), inward rectifier Kir7.1, Ca2+-activated K+ channels with large (KCNMA1, Slo1), small (KCNN1-3), and intermediate (KCNN4) conductance [16].
The resting membrane potential during pregnancy gradually depolarizes from approximately −70 mV to −55 mV at parturition [17], approaching the voltage dependence of L-type channels that open at approximately −40 mV. These variations in membrane potential voltage may be one of the factors driving myometrium to increased contractility [18]. Oxytocin is another essential factor in mammalian labor that increases the entry of Ca2+ into uterine myometrial cells by enhancing the duration and frequency of action potentials [[19], [20]]. Voltage-gated calcium L-type channels play a crucial role in controlling insulin exocytosis in rodents and beta-cell lines.
Electrical signaling in excitable cells, such as muscles, neurons, and endocrine cells, involves generating and conducting action potentials by voltage-gated Na+ channels (VGSCs). An increase in inward sodium ion (Na+) flux causes membrane depolarization and leads to a more excitable and contractile uterine state by opening voltage-gated Ca2+ channels. Conversely, an increase in outward K+ flux results in more negative membrane potential, promoting quiescence. VGSCs play a critical role in many physiological processes by controlling action potentials in excitable tissues. In the uterus myometrium, Ferreira et al. (2021) proposed that sodium signaling through the interaction of the Na+-activated K+ channel (SLO2.1) and the non-selective Na+ leak channel (NALCN) allows these channels to functionally regulate the membrane potential of myometrium, influencing excitability and contractility. The combined action of these two channels (NALCN/SLO2.1) governs uterine excitability, and their reduction activates uterine contractility. Thus, the depolarization of the resting membrane potential of the myometrium is essential for the uterus to transition from a quiescent state to a contractile state (Fig. 2) [21].
Fig. 2.
Proposed model of NALCN/SLO2.1 with regulation of myometrium excitability. Comparison of two states quiescent and contractile [21].
A reduction in SLO2.1/NALCN activity induces membrane depolarization, leading to the entrance of Ca2+ through voltage-dependent Ca2+ channels, resulting in contraction. Their data demonstrate that NALCN and SLO2.1 are closely located in human MSMCs of the myometrial smooth muscle cell. In the inactive state, progesterone binding to its receptor increases NALCN expression and activity [21]. As a result, the sodium current via NALCN activates SLO2.1 channels, causing an efflux of K+ to maintain a hyperpolarized state in the myofibril. This keeps the voltage-dependent Ca2+ channels (VDCCs) closed, preventing uterine contractions in this relaxed state. However, during the myometrium's contractile state, estrogen binding to its receptor (ERα) inhibits NALCN expression [22], leading to reduced SLO2.1 activity. Consequently, the reduction in K+ efflux depolarizes the membrane potential, activating VDCCs, increasing intracellular Ca2+, and promoting uterine contractility. At the onset of labor, the binding of oxytocin (OXT) to its receptor (OTR) triggers the activation of phospholipase C (PLC), resulting in the formation of phosphatidylinositol 4,5-bisphosphate (PIP2) and inositol triphosphate (IP3). IP3 stimulates the release of Ca2+ from intracellular stores and activates protein kinase C (PKC) via PIP2, which inhibits SLO2.1 [23]. Inhibition of SLO2.1 further depolarizes the myometrial membrane, opening more VDCCs, increasing intracellular Ca2+, and ultimately activating myosin for uterine muscle contraction. In conclusion, these findings propose that, in addition to regulating the pacemaker, NALCN controls myometrium excitability by modulating the permeability of K+ ions and thus regulating the membrane potential of myometrial myofibrils [19], contributing to successful parturition [24].
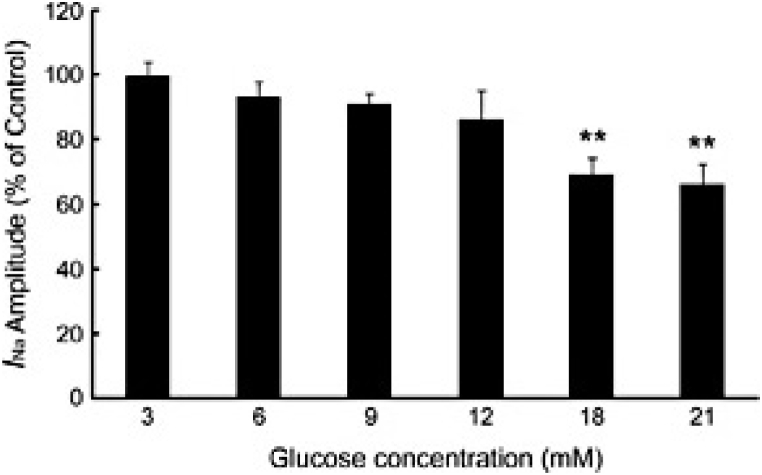
Chen et al., 2018, demonstrated the influence of glucose on the amplitude of sodium ion currents (INa) and the intrinsic kinetics of voltage-gated sodium channels (VGSCs) in the insulin-secreting β-cell line (INS-1) in rats, using whole-cell patch-clamp techniques. Their goal was to determine the role of extracellular glucose in VGSCs' function and its role in regulating insulin synthesis. They reported that increasing the extracellular glucose level reduced the amplitude of INa (Fig. 3) [25].
Fig. 3.
Glucose Inhibits INa in a Concentration-Dependent Manner. The Na+ current was reduced in a concentration-dependent manner by glucose: 100 % ± 3.85 % (n = 6) at 3 mM glucose, 93.39 % ± 4.81 % (n = 5) at 6 mM glucose, 91.17 % ± 3.09 % (n = 6) at 9 mM glucose, 86.22 % ± 8.85 % (n = 6) at 12 mM glucose, 69.32 % ± 5.29 % (n = 6) at 18 mM glucose, and 66.14 % ± 6.42 % (n = 7) at 21 mM glucose; *P < 0.05, **P < 0.01 [25].
The study reported that exposure to 18 mM glucose for only 1 min resulted in a concentration-dependent blockade of INa. Glucose regulates insulin secretion in INS-1 cells in a dose-dependent manner, partly through the inhibition of the INa channel. This suggests that VGSCs play a crucial role in modulating glucose homeostasis. Furthermore, the research revealed that increasing glucose levels lead to a decrease in the activity-dependent nature of INa [25]. The kinetics of the INa channel in INS-1 cells are ATP-dependent, as higher cytosolic ATP levels enhance the activity of INa, which may be vital in insulin secretion in response to increased blood glucose concentrations [26].
3. Metabolic support of term and preterm labor and gestation
Pregnancy and lactation are highly metabolically demanding states. Maternal glucose is a fundamental fuel source for the growing and developing fetus. Therefore, the systems responsible for regulating glucose homeostasis in the maternal body undergo significant adaptations during pregnancy. As part of these regulatory changes, insulin levels rise during pregnancy and decrease during lactation. In normal pregnancy, the plasma insulin level is inversely related to gestational age, as insulin sensitivity decreases as gestation progresses. Studies have shown that these changes are influenced by placental factors, progesterone, and estrogen, which, under physiological conditions, lead to a compensatory increase in insulin secretion to maintain normal glucose homeostasis.
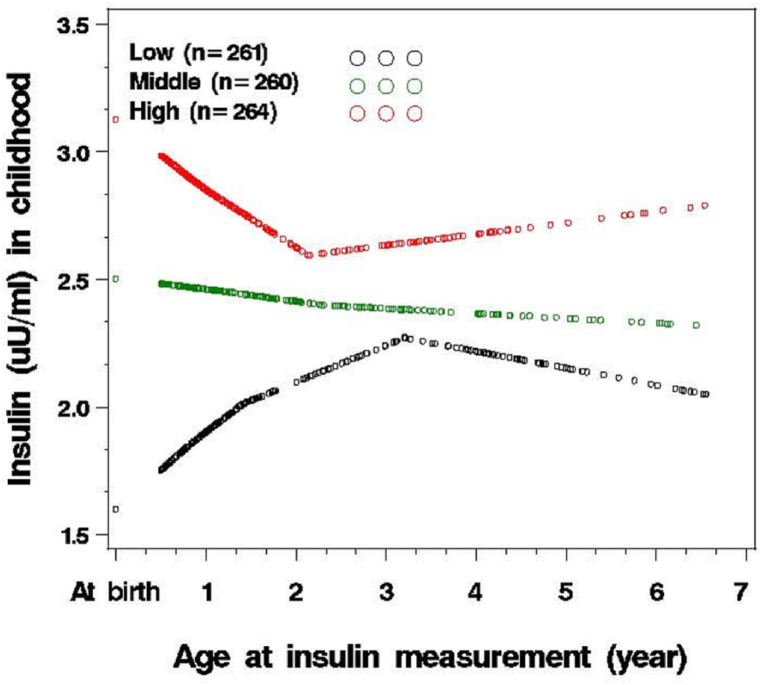
Wang et al., 2014, conducted a cohort study on preterm birth and plasma insulin levels at birth and early childhood. This prospective study included 1358 children born between 1998 and 2010, and they were monitored from 2005 to 2012 (from birth to age 6.5 years) at the Boston University Medical Center. The researchers aimed to investigate the hypothesis that preterm birth is associated with increased plasma insulin levels as an indirect predictor of insulin resistance (IR) at birth, and they also examined whether this elevation persists into early childhood (Fig. 4, Fig. 5) [27].
Fig. 4.
Relationship between plasma insulin levels at birth and gestational age, Stratified by Birthweight for gestational age Categories: Appropriate gestational age (AGA), small for gestational age (SGA), and large for gestational age (LGA) (n = 1117) [27].
Fig. 5.
Following of plasma insulin levels from birth to early childhood (n = 785) (27).
Their findings indicated an inverse association between gestational age and increased plasma insulin levels at birth and early childhood [27]. In a systematic review conducted by Yoshida-Montezuma et al., in 2022, preterm birth was significantly associated with childhood cardio-metabolic risk (CMR) [28].
The physiology of insulin resistance (IR) during pregnancy is intriguing and dynamic, as the degree of maternal IR observed during pregnancy is linked to the degree of glucose flux from the mother to the fetus (Fig. 6) [29,30]. Mild insulin resistance during pregnancy represents a physiological adaptation that prioritizes fetal growth, making pregnancy a period of unique metabolic plasticity for mothers. This adaptation ensures glucose availability in the maternal circulation for transmission to the fetus to support its growth. As a result, there are declines in maternal peripheral insulin sensitivity [31].
Fig. 6.
The filled line represents the mean daily insulin requests at various intervals in pregnant women with type 1 diabetes. The dashed line represents the pre-pregnancy insulin requirement, and the numbers indicate the percentage increase or reduction relative to the pre-pregnancy stages [29].
3.1. Increasing insulin secretion in pregnancy (peripheral & central functions)
The increased insulin secretion observed during pregnancy plays a dynamic role in both peripheral and central maternal tissues. The metabolic processes during pregnancy are linked to site-specific central insulin resistance. This resistance can be adaptive, allowing for increased peripheral insulin secretion without the adverse effects of increased central insulin functions, such as stimulating excessive glucose uptake into maternal tissues or inhibiting food intake [31].
One might wonder if this adaptation represents a central process for insulin resistance or is exclusive to the pregnancy period. Both animal and human studies have shown that insulin resistance directly affects pregnant women and their offspring [32]. Maternal hyperglycemia serves as the basis for fetal hyperglycemia and hyperinsulinemia, potentially leading to fetal macrosomia—a severe complication of maternal diabetes and obesity [33].
3.2. Insulin resistance and brain plasticity
In the central nervous system, brain insulin signaling plays a small role in glucose regulation, yet it significantly impacts the brain's metabolic health. Insulin signaling is crucial for mitochondrial activity, ATP production, and normal food intake. Consequently, brain insulin resistance (IR) contributes to obesity and may play a key role in neurodegeneration.
Increased obesity during reproductive age can lead to deteriorating physiological IR in women. This has a negative impact on the intrauterine environment, affecting perinatal planning and potentially causing metabolic dysfunction in offspring. A study by Spinelli et al., in 2020 demonstrated that metabolic diseases affect brain health and cognitive functions, disturbing them as well. The animal study showed that maternal high-fat diet (HFD) consumption induced insulin resistance across multiple generations, leading to synaptic plasticity and learning/memory impairments. Maternal tissues exposed to the HFD experience downregulation of brain-derived neurotrophic factor (BDNF) and insulin signaling. This, in turn, epigenetically inhibits BDNF expression in both the germline and hippocampus of the progeny. Consequently, maternal diet can have a multi-generational impact on the descendants' brain health through gamete mechanisms, putting them at risk for lifestyle-related issues [34].
Earlier studies have shown that insulin has an inhibitory role in endothelial, smooth muscle contraction. Thus in cases of insulin resistance, it can induce vasoconstriction. The first reports introduced that insulin significantly inhibits vascular endothelial-smooth muscle cell contraction through a nitric oxide synthase-dependent pathway in a culture medium [35].
During pregnancy, maternal vascular adaptation is crucial to increase the capacity of blood flow through the utero-placental unit, meeting the demands of the developing fetus. This adaptation is influenced by various growth factors and cytokines, playing a vital role in pregnancy [36].
Now, the question arises: are maternal vascular adaptation and IR only specific to normal pregnancy periods?
3.3. Vascular adaptation in pregnancy
The role of microvascular and macrovascular endothelial cells is crucial in maintaining vascular reactivity. Several diseases can alter endothelial function, such as hypertension, obesity, and diabetes mellitus. In gestational diabetes mellitus (GDM), dysfunction of both micro and macrovascular endothelial cells is well-documented, with severe consequences for fetal development. This condition is associated with increased l-arginine uptake through the human cationic amino acid transporter (hCAT-1) and nitric oxide synthase (NOs) by endothelial nitric oxide (eNOS). The process involves purinergic receptors and augmented umbilical veins, leading to increased expression and activity of hCAT-1 and eNOS. An elevated adenosine concentration in the umbilical vein blood in GDM reflects a defective metabolic state of the human placenta [30].
During a normal pregnancy, maternal metabolism changes to promote nutrient availability to the fetus, achieved through physiological insulin resistance (IR) while supporting maternal metabolic demands. However, a higher degree of IR in normal pregnancy can cause various adverse effects on both the mother and fetus, including preterm delivery, cesarean section, macrosomia, neonatal hypoglycemia, polyhydramnios, hypertensive disorders of pregnancy, fetal growth restriction, and other serious complications [[34], [35], [36], [37]]. IR increases with pre-pregnancy obesity and, when combined with pregnancy-induced IR, exposes the intrauterine environment to excess nutrients like glucose, lipids, and amino acids, metabolic disturbances, inflammation, and oxidative stress [38]. The elevated levels of placental and maternal hormones, including placental lactogenic hormone, growth hormone variant, progesterone, cortisol, and PRL, contribute to reduced insulin sensitivity, particularly during the third trimester of pregnancy [39,40].
In GDM, insulin sensitivity improves almost immediately after delivery but appears to worsen in the first 6 months postpartum, which can be a warning sign of diabetes [41]. Vascular remodeling of the maternal uterus during pregnancy is unique and essential for transmitting oxygen and nutrients for fetal growth. Placental hormones, such as the prolactin-growth hormone family, steroids, and neuropeptides, play critical roles in these physiological adaptations [40].
Raised blood sugar levels caused by GDM can increase the risk of premature birth. Findings suggest premature labor due to GDM can occur before the 24th week of pregnancy [42]. GDM also increases the risk of polyhydramnios, which can lead to preterm labor and premature birth. The prevalence rate of preterm labor in GDM is 30–40 %, and the approach to managing preterm labor is still a matter of ongoing discussion [43]. Fetal growth restraint is detected in ∼10 % of pregnancies and poses a significant challenge to the perinatal risk of morbidity and mortality [44]. Fetal growth restriction (FGR) is often linked to insufficient placental function and is also associated with the development of hypertension, coronary artery disease, diabetes, and metabolic syndrome. Consequently, it imposes a substantial social and economic burden on individuals and healthcare systems worldwide.
Intrauterine growth restriction (IUGR) impairs insulin secretion in both human and animal models of IUGR by disrupting β-cell function. A decrease in beta cell mass leads to changes in the progression of the cell cycle, accompanied by an increase in apoptosis and loss of neogenesis. This condition causes decreased islet vascularization and mitochondrial dysfunction, ultimately impairing β-cell function in the IUGR model of rodents. The results of the animal model of IUGR are similar to the results observed in the human model of insulin secretion [45].
4. Placenta: stress role of placenta in labor, fetus development and possible pathology
In 1937, Mossman made a significant statement regarding the mammalian placenta, describing it as the fusion of the fetal membrane to the uterine mucosa for physiological exchange. Consequently, placenta dysfunction can immediately affect pregnancy outcomes [45]. One of the most serious conditions during pregnancy is placenta hypoxia, which augments the risk of miscarriages and preterm labor. The detrimental effects of hypoxia are most pronounced in the middle to late stages of pregnancy.
During the early stages of normal pregnancy, the uterus typically has limited oxygen (O2) levels due to low blood circulation. This hypoxic microenvironment supports trophoblast stem cells or progenitor cells, maintains homeostasis, prevents DNA damage, and promotes selective differentiation.
Another crucial function of hypoxia is its impact on the immune and inflammatory processes. Reduced tissue oxygen levels alter the energy status of immune cells, leading to the suppression of immune responses. Hypoxia effectively regulates the innate and adaptive immune systems by controlling the proliferation, development, and effector function of immune cells, primarily through transcriptional modifications driven by hypoxia-inducible factor (HIF) [46,47].
Colson et al., 2021, investigated oxygen tension during primary placentation and trophoblast adaptations to hypoxia in the later stages of pregnancy. Additionally, they explored therapeutic approaches to reduce the negative effects of hypoxic stress in the placenta to mitigate fetal growth restriction (FGR) and its associated immediate and lifelong health consequences [48].
4.1. From the regulation of oxygen supply to the hypoxic stress in the placenta
During the first trimester of human pregnancy, the low-oxygen environment is a completely physiological process, but in the continuation of fetal growth, continuous hypoxia can lead to pregnancy complications [49]. In normal pregnancy, in the fallopian tube, fertilization occurs during 1–2 days of ovulation by following of the growth of the blastocyst in the uterine cavity through day 6 (Fig. 7) [50].
Fig. 7.
The graphic illustrates the growth of the placenta from blastocyst implantation to the remodeling of the spiral artery and villous formation within the span of 2–3 and 12 weeks. Various altered procedures are depicted, such as the proliferation of cytotrophoblasts (CTBs), differentiations of extravillous trophoblasts (EVTs), the formation of EVT plug in the spiral artery and Hofbauer cells (HBCs), along with the remolding of spiral arteries. Highlighted in the illustration are the fluctuations in oxygen levels that impact the villous area during the first trimester (2–3%) and second trimester (8–10 %), in contrast to the relatively constant oxygen levels in decidua (5–6%). Additional annotations point to the presence of uNK (uterine natural killer) cells and dMφ (decidua macrophages) in the placenta. This graphic is reprinted with permission from Ref. [50].
Around 10 − 12 weeks of pregnancy, the trophoblastic plugs are gradually removed, increasing the intervillous space (IVS) oxygenation from 20 to 50 mmHg [51]. This remodeling process is essential as the extravillous trophoblast (EVT) reduces resistance in the maternal spiral arteries, ensuring a steady blood flow for IVS perfusion and facilitating appropriate transport time for exchange [52,53]. Consequently, during the second trimester (>12 weeks), the placenta finally develops into the hemochorial stage [54].
A crucial objective in ensuring proper IVS perfusion is regulating oxygen supply within the first 12 weeks of the feto-placental unit. This regulation is necessary to mitigate harmful and teratogenic ROS production during embryogenesis and organogenesis [53]. Moreover, low oxygen tension plays a role in regulating the expression of chemokine ligands and receptors during cytotrophoblast (CTB) differentiation into invasive EVTs. For example, Schanz et al., 2014 observed a hypoxia-inducible factor HIF-dependent upregulation of CXCR4 associated with increased chemotaxis of first-trimester CTBs toward CXCL12 under hypoxic conditions. They proposed that this system is involved in placental development through the directed migration of cytotrophoblasts in the decidual compartment, ensuring an adequate supply for the growing fetus [54].
Sun et al., 2022, demonstrated that CXCL12 levels in the serum of miscarried women were significantly lower than in healthy women with early pregnancy. Additionally, the CXCL12 levels in normal non-pregnant women were significantly lower than in early pregnant women [55]. In a healthy pregnancy, the availability of glucose and oxygen is vital for fetal growth and health.
Glucose transport across the placenta occurs mainly through simplified diffusion facilitated by glucose transporter 1 (GLUT-1). Francois et al., 2017 reported differential expression of GLUT-1 between the placental microvillus and its basal membrane in women at high altitudes [56]. This finding highlights more complex regulatory interactions guiding trophoblast glucose transport in low-oxygen situations.
During early pregnancy, oxygen availability and hypoxia influence trophoblast differentiation and placental establishment. Later in pregnancy, the placental response to hypoxia includes changes in mitochondrial function, endoplasmic reticulum stress, hormone production, and angiogenic factor secretion, all dependent on the degree of hypoxia. Some of these responses are adaptive, while others may pose risks to placental support for fetal growth. Increasing the placental oxygen source or reducing oxidative stress effects could be potential emergent approaches for preventing/treating FGR [48]. However, glucose transport remains unaffected in the placentas of FGR pregnancies [57]. In FGR pregnancies, the expression of two major glucose transporter genes, GLUT-1 and GLUT-3, is altered in the placenta, likely due to an adaptive mechanism optimizing glucose supply in an adverse pregnancy environment [57,58].
In a study by Mathew et al., in 2017, the gene expression of GLUT1 and GLUT3 was detected in monochromic (MC) twin placentas after delivery. The study revealed that hypoxia is the leading cause of increased gene expression in the stem cells of placental mesenchymal cells [59]. Moreover, Xu et al., 2021 reported hypoxic conditions may damage trophoblast mitochondrial function in growth-restricted fetuses [60]. Consequently, numerous studies indicate that the expression of glucose transporters (GLUTs) in fetomaternal tissues may be influenced by conditions such as chronic hypoxia, preeclampsia, and intrahepatic cholestasis of pregnancy [61].
Mitochondrial function in mouse placenta under normal and hypoxic conditions can be studied using respirometry and molecular analysis.
4.2. Cellular respiration (mitochondrial)
In the placenta, mitochondria play a critical role as a cellular source of ATP during gestation [62]. In normal pregnancy, the mitochondrial respiration rate in the first trimester is lower than in the term. Early in pregnancy, when maternal blood flow to the placenta begins (around 10–12 weeks), there is a temporary reduction in mitochondrial respiration, followed by mitochondrial biogenesis after 12 weeks [63]. Therefore, a proper modulation between cellular mitochondrial respiration (respiration rate) and maternal blood flow to the placenta is necessary to maintain a steady state of oxygen tension. This can lead to proper fetal development and trophoblast adaptation to hypoxia in late pregnancy, reducing the negative effects of low oxygen stress in the placenta and preventing preterm labor.
4.3. Capacity of mitochondrial respiratory in the gestation placenta & hypoxia
Oxygen is essential for cellular function in aerobic organisms, with over 90 % of it used in mitochondria for energy production through oxidative phosphorylation (OXPHOS). Therefore, oxygen deficiency or hypoxia can pose a significant threat to cellular respiration. Hypoxia-inducible factors (HIFs) are responsible for the cellular response to low oxygen levels. They activate a transcriptional response that regulates the expression of hundreds of genes involved in adapting to reduced oxygen levels [64].
HIFs act as global transcriptional regulators and can control the expression of more than 1000 proteins by binding to hypoxic response elements (HRES) in gene regulatory regions. One of the crucial roles of HIFs is in facilitating mitochondrial function by regulating the metabolic switch from oxidative phosphorylation to glycolysis through signaling extracellular adenosine receptors [65]. HIFs are involved in various cellular processes during hypoxia, such as angiogenesis, cell migration/invasion, cellular metabolism, and immune cell function. The turnover of HIF-α, one of the subunits of HIF, is rapid, allowing it to respond quickly to changes in local oxygen levels [66].
In the human placenta, HIF-1α production varies with gestational age, with the highest expression occurring in early pregnancy (around 5 weeks). Studies in mice examining placental oxygen levels showed that HIF-1α protein levels are higher under severe hypoxia (2–3% oxygen) at 7–9 weeks of gestation. The peak of human HIF-1α mRNA transcriptional activity is observed between 14 and 18 weeks of gestation when re-oxygenation occurs [[67], [68], [69]]. HIF target genes are involved in glucose and lipid metabolism, reprogramming energy metabolism independent of insulin and reducing oxidative phosphorylation [66].
HIFs play a significant role in trophoblast cells under hypoxic conditions and are associated with the up-regulation of glucose transporters GLUT1 and GLUT3 [70]. Studies on pregnant mice subjected to hypoxic conditions showed that placental mitochondrial function adapts to support fetal growth, with moderate and severe hypoxia affecting the placenta differently [71].
Severe hypoxia stimulates the sympathetic nervous system and carotid chemoreceptors, leading to hyperventilation. Pregnant women residing at high altitudes (over 2500 m) with hypoxic conditions experience a decline in birth weight. Hypoxia during pregnancy can also reprogram energy metabolism, affecting weight gain, adiposity, dyslipidemia, and insulin resistance. Combining hypoxia with diet-induced insulin resistance harms the placenta and fetal outcome [72,73]. Lower birth weight is associated with infant mortality, morbidity, and an increased risk of cardiovascular and metabolic diseases later in life [74].
4.4. How mammalian cells sense oxygen level? Oxygen cellular sensing
-
1)
Hypoxia-Inducible Factors (HIFs) are the primary regulators in low oxygen conditions. They control gene expression that promotes cell survival and adaptation to reduced oxygen levels [75]. Under hypoxic conditions, the respiratory rate is suppressed, activating HIF-1, which regulates ATP generation as a metabolic source.
-
2)
Mitochondria Function changes in hypoxia can be done in different ways: such as alterations in the protein composition of the electron transport chain, changes in mitochondrial morphology by reducing mitochondrial mass, alterations in the respiration rate, changes in mitochondrial intermediate metabolism, and subsequent alterations in the capacity of ROS production. These changes disrupt mitochondrial biological functions, significantly affecting cell physiology [76].
-
3)
Oxidative Stress is induced by hypoxia, resulting in variations in metabolite levels and the generation of reactive oxygen species (ROS) by mitochondria. The electron transport chain (ETC) is the primary consumer of intracellular oxygen for ATP generation through oxidative phosphorylation. Therefore, changes in oxygen levels can impact the activity of the mitochondrial ETC. Intracellular activity under 0.3 % oxygen level becomes the rate-limiting step for ETC [77].
-
4)
electron transporter chain (ETC) activity and the tricarboxylic acid (TCA) cycle Prolonged hypoxia can reduce flux through the TCA cycle in mitochondria, decreasing metabolites necessary for aerobic cell respiration. This results in a reduction of ETC function [78,79].
Overall, the cellular response to reduced oxygen concentration is a critical stressor that affects the life of aerobic species and is a prominent feature of various pathological conditions, such as cancer, cardiovascular disorders, inflammation, and bacterial infections. Mammalian cells have evolved crucial adaptive mechanisms to manage hypoxia through various processes, including protein synthesis, carbon/lipid metabolism, energy metabolism, and mitochondrial respiration. These mechanisms are vital for suitable responses against hypoxic stress.
4.5. 3, 5. placental insufficiency & variable severities of hypoxia
Cellular metabolic conditions and survival during hypoxia depend significantly on compensatory changes that include.
-
1)
Increasing blood vessel density through angiogenesis and vascular regeneration, with a critical role played by the smooth muscle phenotype.
-
2)
Oxygen delivery.
-
3)
Metabolic adaptation to hypoxia [72].
Fetal hypoxia induces compensatory angiogenesis and vascular remodeling, although the exact mechanisms are not fully understood. Vascular endothelium, smooth muscle (SM), adventitia, sympathetic perivascular nerves (SPN), and the parenchyma are involved in these adaptive processes. Hypoxia increases the innervation of sympathetic nerves, leading to the release of noradrenaline (NA), neuropeptide-Y (NPY), and adenosine triphosphate (ATP), which have motor and trophic effects on cerebral arteries and impact smooth muscle phenotypes. The sympathetic nervous system (SNS) plays a crucial role in regulating vascular function, influenced by nitric oxide (NO), reactive oxygen species (ROS), endothelin (ET), and the renin-angiotensin system [[80], [81]].
HIFs are the primary regulators of cellular responses to hypoxia, inducing the expression of adaptive genes that increase oxygen supply and support anaerobic ATP generation. At the cellular level, ATP-consuming reactions are suppressed in hypoxia, and metabolism is altered to restore oxygen homeostasis. Sympathetic nerves express.
5. Parturition
The parturition cascade must be guided towards the onset of labor at term. The induction of labor at term is regulated by paracrine-autocrine factors and changes in hormone levels, working in coordination to promote uterine contractions (mechanical changes).
5.1. Uterus mechanical changes
Examining the frequency of myometrium contractions is essential as a method to diagnose preterm labor and assess patients at risk of it. Early models of wave propagation, such as the bioelectrical activity of the myometrium, have been proposed as a solitary mechanism for the increased force of contraction and its participation in contractions. In a retrospective analysis of 2355 term women, uterine contractions were invasively recorded using an intrauterine pressure catheter (IUPC) – the gold standard for such recordings – 30 min before parturition [82]. Young et al., in 2023 demonstrated that management decisions made earlier than 30 min before delivery are likely to have a direct impact on maternal and fetal outcomes. This is crucial since tachysystole has been associated with adverse neonatal outcomes. However, the duration of contractions, rest intervals, or baseline pressure (Fig. 8) did not show significant associations with adverse outcomes [83].
Fig. 8.
A & B display the peak onset time and peak duration as represented by the blue bars. The timing of peaks, contraction duration, and the rest period between contractions are all provided. Furthermore, the red arrow indicates the resting intrauterine pressure, also known as uterine tone. The peak force can also be accurately calculated using an IUPC, denoted by the black arrow [83].
The initiation of each uterine contraction stems from a localized contraction, which can cause a slight rise in intrauterine pressure. This increased pressure leads to a buildup of tension in the uterine wall, creating contractions in multiple regions and promoting intrauterine pressure through positive feedback synchronization. Each myofibril represents a region with an assigned action potential threshold, responsible for the rise in intrauterine pressure as governed by the Law of Laplace. A regional contraction occurs when the tension in a specific region surpasses its designated threshold [84].
Essentially, contractile activity is initiated by generating and propagating action potentials (APs) throughout the uterine myometrium. These APs consist of both simple and complex spikes. Studies on the human myometrium have revealed that simple spikes are formed by L-type calcium channels, transient sodium channels, and rapidly activating and inactivating calcium channels. On the other hand, complex APs are composed of simple spikes followed by a sustained plateau of depolarization, particularly pronounced in the inner layer and fundus segment of the human uterus during the third trimester and labor [85]. The duration of this spike plateau determines the length of the contraction.
Intercellular signaling in the pregnant myometrium must effectively control the properties of electrical excitability and contractile signals, along with the direct increase of intracellular calcium levels. Signal propagation through gap junctions leads to the synchronization of contraction waves. Despite several mathematical simulations of organ-level functioning being published, it remains unclear how the pregnant uterus synchronizes the kilogram of the myometrium with the frequent and simultaneous contractions observed during normal human labor [86].
To transition the uterine wall from a static state to dynamic coordinated muscle contractions, changes in the density and activity of ion channels, pumps, and gap junctions must occur, facilitating the extension of contraction activity throughout the myometrium myofibrils. Local and circulatory hormones induce these changes to prepare for labor. Young and Barendse suggested that the physiological process of the uterus is similar to the myogenic response of arteries, except that arterial contraction is tonic, while myometrium contraction is phasic [84].
5.2. Transcriptional control of parturition: connexin43 gene expression in the myometrium
Molecular transcription factors play a critical role in altering the expression of labor-associated genes. Myometrium cells begin to upregulate contraction-associated proteins (CAPs) toward the end of pregnancy. One well-studied labor-associated CAP is Gap junction alpha-1 (GJA1), also known as connexin 43 (CX43). CX43 exhibits a significant increase in myometrium between term non-laboring and active laboring in both rodent and primate models [87,88].
Adequate GJA1 protein levels are crucial for constructing gap junctions between adjacent smooth muscle cells (SMCs), facilitating cell-to-cell coupling, and allowing intercellular transmission of critical metabolites and ions to coordinate myometrium contractions. The variations in connexin composition influence channel properties such as ionic conductance, trans junctional voltage, and phosphorylation [89]. Additionally, changes in CX43 expression in myometrium and leiomyoma cells can affect the response to uterine steroid hormones [90].
Liu et al., 2019, reported the expression of CX43 in endometrial stromal cells in mice. Their findings revealed that CX43 gene expression in mouse uterine endometrial stromal cells significantly decreased during controlled ovarian hyperstimulation, and the improvement in gene expression occurred earlier, suggesting earlier decasualization [91].
According to Nadeem et al., in 2017, progesterone receptor A (PR-A) is the primary regulator of CX43 expression in gap junction construction for synchronization during labor. They found that P4, via PR-A/B, can regulate Cx43 translation, producing a Cx43-20K isoform that enables the transportation of the full-length Cx43 into the plasma membrane. This regulation occurs through the mTOR signaling pathway as a non-genomic mechanism [92].
Cell-to-cell coupling between uterine myocytes is required for synchronization during labor and normal parturition. In the myometrium, regulating CX43 construction and gap junction formation is a hormonal process. Cx43 protein construction occurs during pregnancy, but GJ formation occurs only before term labor and preterm labor in rodents [91] and humans [93]. In lower mammals' myometrium, CX43 and GJ formation expression has been definitively associated with plasma estrogen (E2) levels [88,94].
Towards the end of the term, a series of mechanical and endocrine signals are activated. Mechanical stretch occurs due to the growing fetus, applying tension on the uterine wall. Fetal-stimulated cortisol is released from the HPA axis as part of a series of feedback mechanisms that control the timing of labor. Consequently, the levels of other critical hormones, such as progesterone, estrogen, corticotropin-releasing hormone (CRH), and other uterotonic factors like prostaglandins and oxytocin, change [95].
These molecules amplify cytokine synthesis in the myofibrils, leading to an inflammatory myometrial situation. For example, IL-6 upregulates oxytocin receptors on myometrial cells to increase the oxytocin response and also stimulates oxytocin secretion in the myometrium during labor. IL-1β stimulates the signal transduction system NF-κB, increasing Connexin-2 expression, and subsequently, during labor, the expression of CX2 in the myometrium can stimulate PGE2 production [96]. IL-8 and TNF-ɑ stimulate the release of arachidonic acid, phospholipid metabolism, and increased prostaglandin production from the myometrium.
Myometrium inflammation plays a physiological role in transforming from an inactive to a contractile state in myofibrils at the onset of labor. However, compared to preterm labor, inflammation plays a pathological role. Thus, early delivery can occur in response to various triggers, including infection, over distension, and hemorrhage [97].
The upregulation of CX43 encourages the creation of a large number of gap junctions, leading to ionic coupling among myofibrils, which is crucial for coordinating myometrial contractility. During pregnancy, the number of gap junctions is low and must be expanded in both number and size during labor. However, these adherent cell-cell junctions are necessary for the uterus but insufficient for actual labor and delivery. Parturition involves a multifaceted combination of hormonal, inflammatory, and mechanical signals, which must initiate contraction-related genes in myofibrils. Sufficient expression of these contraction-related genes in myofibrils allows for the simultaneous generation of a strong force to expel the fetus from the uterus. Therefore, transcription of gap junctions is necessary to induce strong biomechanical contractions [98].
In humans, E2 and P4 levels in circulation are high during pregnancy, reaching a maximum at term. As a result, the hormonal control of CX43 expression in myofibrils and gap junction formation during parturition is closely related to the responsiveness of myometrium cells to E2/P4 [99].
5.3. Endocrinology of human gestation
During gestation and at the time of delivery, estrogens are secreted by the maternal-fetus-placental unit and the fetal adrenal cortex [100]. Estrogen can alter the shape of electrical waves from a simple spike to a complex spike, while oxytocin helps reduce the duration of contractions, ultimately facilitating the conclusion of pregnancy by increasing the plateau section of complex action potentials [101].
Animal studies have shown that the fetus plays a significant role in controlling the timing of labor. Mendelson et al. (2017) proposed a new pathway through which the fetus initiates labor by signaling to the mother when fetal lungs have reached sufficient growth for aerobic survival [102]. Two main factors initiate this signaling pathway.
-
1)
Fetal cortisol stimulates the placental enzyme cytochrome P450 17α-hydroxylase/17, 20-lyase (CYP17). This microsomal enzyme catalyzes two different activities, 17α-hydroxylase and 17, 20-lyase, both of which are necessary for the biosynthesis of adrenal and gonadal steroids, including the conversion of pregnenolone to the estradiol hormone [103].
-
2)
The altered estrogen/progesterone ratio up-regulates uterine prostaglandin (PG) synthesis and promotes labor. In humans, the placenta is not fully equipped for estrogen synthesis. Therefore, activation of the fetal hypothalamic-pituitary-adrenal (HPA) axis is necessary to increase the production of fetal pituitary adrenocorticotropin hormone (ACTH). This leads to abundant C19 estrogen precursor dehydroepiandrosterone sulfate (DHEAS) synthesis in the adrenal gland's intermediate (fetal) zone. The fetal liver converts DHEAS to 16-hydroxy DHEAS, which then transfers to the placenta and is further metabolized into estradiol (E2), estrone (E1), and estriol (E3) [104,105]. The action of estrogen is presumed to be paracrine-autocrine.
Up to seven weeks of pregnancy, the primary source of progesterone is the corpus luteum (CL). After seven to nine weeks, the placenta takes over this function because a dynamic balance between progesterone and estrogen is needed to regulate the uterus. In animals, the systemic cessation of progesterone is a critical component of the onset of labor. In humans, although there is no reduction in circulating progesterone at the onset of spontaneous labor, its physiologic activity is terminated at the progesterone receptor level. In vitro, studies have shown that progesterone inhibits the formation of myometrial gap junctions and reduces uterine contractility. Progesterone also stimulates uterine NO synthesis (a major factor for uterine inactivity) and down-regulates prostaglandins (E2 and F2a). Therefore, progesterone and estrogen establish a balance between maintaining pregnancy and initiating labor.
5.4. Stimulation NO synthetize, down-regulates prostaglandins etc
The fetal endocrine cascade is triggered by the activation of the fetal HPA axis, leading to an increase in estrogen and a decline in progesterone in maternal plasma. This cascade eventually leads to two processes: activation and stimulation. Activation involves expressing contraction-associated proteins (CAPs) such as gap junctions, connexin 43, and receptors for oxytocin and PGs (E2 and F2a). The stimulation process includes the activation of uterotonic agonists such as oxytocin and PGs. Oxytocin induces uterine contractions in two ways: directly by stimulating phospholipase C (PLC) and indirectly by releasing PGs (E2 and F2α) in fetal membranes through the activation of PLC, which, in turn, activates calcium channels and releases calcium from intracellular stores. By the time of labor, oxytocin gene expression increases three-to four-fold in the chorio-decidual tissues. In-vitro incubation studies have demonstrated that estradiol stimulates the synthesis of oxytocin mRNA, confirming the paracrine role of oxytocin and sex steroids within intrauterine tissues [106]. These communications can potentially lead to new approaches for inhibiting or treating preterm labor (Fig. 9).
Fig. 9.
The beginning of labor is controlled by the fetal genome through three pathways: mechanical, endocrine, and paracrine/autocrine signals, which are supported by the activity of the Hypothalamic-Pituitary-Adrenal axis, estrogen, and progesterone.
Gestation is a hyper estrogenic condition, with the placenta being the primary source of estrogen production. As gestational age progresses, the concentration of estrogen increases. However, the human placenta lacks the enzyme P450 17α-hydroxylase/17, 20-lyase (CYP17), which is necessary for converting progesterone to estradiol.
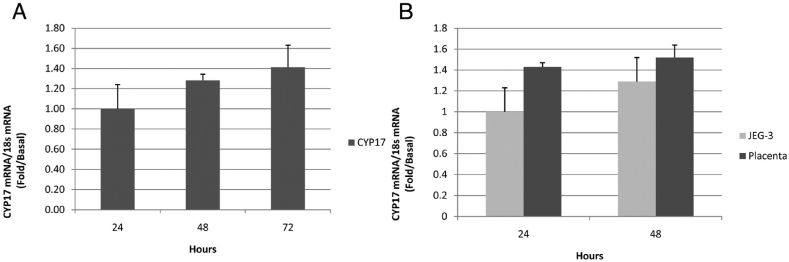
A study conducted by Escobar et al., in 2011 revealed that although the human placenta has minimal CYP17 expression, both CYP17 mRNA and its protein expression are present in the human trophoblast. This indicates that the human trophoblast can express CYP17 and produce androgens de novo. The findings of the study suggest that androgens significantly contribute to estrogen production during gestation. CYP17 is a bifunctional enzyme with two actions: 17α-hydroxylase and 17, 20-lyase. The 17α-hydroxylase action converts P4 to 17α-hydroxyprogesterone (17OHP) and then to androstenedione (A4) through the Δ4 pathway. On the other hand, the 17, 20-lyase action converts pregnenolone to 17α-hydroxypregnenolone (17OHPreg) and dehydroepiandrosterone (DHEA) through the Δ5 pathway. The first reaction is crucial for the corticosteroid pathway, regulated explicitly in the adrenal gland. The second reaction is responsible for sex hormone production (Fig. 10). CYP17 mRNA expression functions in human trophoblasts have been shown in Fig. 11[A (expression in CCP) and B (expression in PPC and JEG-3 cell lines) [106].
Fig. 10.
Steroidogenic pathway in the human trophoblast [106].
Fig. 11.
CYP17 mRNA expression in human trophoblasts. A: expression of the overtime in primary placental cells (PPC). RT-PCR was used to quantify CYP17 in these cells (1–3 d of culture). B: expression in PPC and JEG-3 cell lines were gotten from American Type Culture Collection (Manassas, VA) and were developed in the same media and conditions to PPC. RT-PCR was used to quantify CYP17 in both cell types [106].
They suggested that androgens play a significant role in estrogen creation during pregnancy. The changes in the myometrium during gestation are stimulated by estrogens. These changes include an increased creation of prostaglandins (E2/F2α) through the up-regulation of gene expression of its receptors (E-R). Primarily, PGE2 plays a critical role in ovulation, fertilization, and implantation processes. The meiotic maturation, cumulus development, and follicle rupture in the ovulatory cascade are linked to increasing intracellular cAMP levels [107,108].
-
3)
Oxytocin (OXT) and the increasing expression of its receptors (OXT-R) are mainly controlled by E2 through ESR1 in the placenta during the late phase of pregnancy [109,110]. Increased synthesis of gap junction formation and connexins, especially CX43, is a vital regulator of the cytotrophoblast-to-syncytiotrophoblast fusion pathway, confirming placental development [111]. Following this process, the up-regulation of the contractile enzyme (myosin light chain kinase) and calmodulin [112] occurs, and all these changes are associated with the synchronization of uterine contractions.
Mugisho et al., 2018 explained that hemichannels can produce feedback loops, reinforcing the open status of the hemichannel over time. This opinion is reliable, considering an autocrine feedback loop in the inflammation pathway facilitates ATP release as an inflammation amplifier. The Cx hemichannel-mediated ATP release in ischemia and inflammation holds special significance [113]. Therefore, hemichannels make available the signaling pathway for releasing autocrine/paracrine agents in this open status. However, this hemichannel status must be controlled because uncontrolled activation of hemichannels can disturb tissue homeostasis by allowing the transit of molecules and ions from the cell cytoplasm to the extracellular space. Consequently, it can disrupt gap junction transmission in certain types of cells [114].
5.5. Paracrine/autocrine factors of parturition: relaxin & endothelium‐derived NO‐mediated vasodilator pathways
Human labor involves a complex and dynamic biochemical dialogue between the maternal and fetoplacental unit, encompassing paracrine, autocrine, and endocrine events. Several factors, including Cyclooxygenase 2 (COX-2), Oxytocin (OT), Prostaglandin dehydrogenase (PGDH), 13, 14-dihydro-15-keto-PGE2 (PGEM), 13, 14-dihydro-15-keto-PGF2α (PGFM), Phospholipase A (PLA2), spontaneous rupture of the fetal membranes (SROM), 11β-hydroxysteroid dehydrogenase (11β-HSD), and 16-hydroxy-dehydroepiandrosterone sulfate (16-OH DHEAS), play significant roles in this process [115].
Relaxin, a 6-kDa polypeptide hormone from the insulin-like growth factor family (IGF), plays a crucial role in this complex system. It consists of a two-chain structure with two disulfide bridges. Initially synthesized in pre-pro relaxin form, it is cleaved into a signal peptide known as pro relaxin. Subsequently, C-peptide cleavage by exopeptidase enzymes yields the mature hormone. The relaxin family includes seven known peptides: relaxin 1, 2, 3, and INSL (insulin-like peptide) 3, 4, 5, and 6. Relaxin 1-3 serves as a pleiotropic hormone, influencing various phenotypic traits in the insulin-like growth factors (IGF) produced in the reproductive system, such as corpus luteum (CL), uterus, testis, prostate, and others. In females, it facilitates uterine contractility, and the endometrial thickening/angiogenesis process, while in males, it promotes spermatogenesis, spermatozoa motility, and the acrosome response [116]. The relaxin (RLN-2) gene in humans encodes a member of the relaxin subfamily and insulin superfamily of peptide hormones [117].
The production of relaxin from corpus luteum (CL), decidua, and trophoblasts plays a pivotal role in up-regulating nitric oxide synthase (NOs). Consequently, it increases NO and cyclic adenosine monophosphate (cAMP) while decreasing intracellular calcium ions, ultimately leading to the inhibition of myometrium contractions. Over the past few decades, relaxin has been recognized for its crucial role in the mother's adaptation during early pregnancy maintenance and in preparing the uterus for the parturition process (Fig. 12) [118]. During early pregnancy, relaxin regulates vital maternal hemodynamic adaptations through direct actions on the systemic vasculature. The mechanisms of relaxin's vascular actions occur primarily through endothelium‐derived NO‐mediated vasodilator pathways, enhancing arterial compliance in small resistance‐size arteries [119].
Fig. 12.
Cyclic AMP Pathway in Uterine Myofibrils. The activation of G protein membrane receptors (GPCRs) coupled to Gs initiates the activation of adenylyl cyclase (ADCY), which converts ATP to cAMP. The levels of cAMP are tightly regulated by phosphodiesterases (PDEs), with a particular emphasis on PDE4 isoforms. The cAMP signaling pathway can induce uterine relaxation through the phosphorylation and inhibition of myosin light chain kinase (MYLK) by a specific protein kinase (PRKA) [118].
Studies spanning several decades have consistently demonstrated a strong correlation between relaxin and vital metabolic pathways responsible for regulating metabolism in myocytes. During pregnancy, an excess of relaxin in the bloodstream, known as hyperrelaxinemia, has been linked to preterm delivery. Conversely, insufficient levels of relaxin during pregnancy could adversely affect glucose metabolism and increase insulin resistance (IR) [120].
According to reports, insulin reduces the intensity of contractions in both human and rat myometrium through various mechanisms. The relaxant effects of insulin on the myometrium can be attributed to cell hyperpolarization resulting from reduced calcium influx, as well as stimulated Na+ pump and inhibited K+ conductance. Additionally, insulin may influence uterine contractions by modifying the myometrium's response to different stimuli, including oxytocin [21].
6. Preterm labor
The induction of labor to initiate and establish the birthing process is a complex and dynamic phenomenon influenced by various factors, including endocrine, inflammatory, and mechanical aspects, alongside obstetric and pharmaceutical interventions. According to the World Health Organization (WHO) report in 2018, approximately 15 million women worldwide deliver their babies each year before completing the entire term of pregnancy (WHO 2018) [121]. This condition is known as preterm birth (PTB) and occurs before 37 weeks of gestation. Shockingly, around 28 % of global neonatal mortalities are linked to PTB, primarily caused by preterm delivery (PTL), with spontaneous PTL accounting for nearly 50 % of PTB cases. Tragically, complications arising from prenatal delivery claim the lives of approximately 1 million infants each year. PTB can be categorized into two groups: spontaneous PTB and provider-initiated PTB, both involving uterine contractions before the 37th week of gestation and the delivery of the baby via cesarean section [122]. Spontaneous PTB is a multifactorial condition arising from spontaneous PTL and the synchronous premature rupture of the membranes (PPROM). Some risk factors for spontaneous PTB include infections, inflammation, immunological disturbances, and vascular diseases [123]. Unfortunately, accurately predicting and identifying the underlying causes of preterm labor remains challenging, limiting the effective application of treatments. Surprisingly, about 60 % of preterm births are cryptic and considered idiopathic [124].
6.1. PTL & Pharmacotherapy
Tocolytic agents: The administration of tocolytic agents is the most common practice used to delay the PTL process. These agents help reduce uterine contractions, providing enough time for administering steroid therapy to aid in infant lung growth. Additionally, tocolytic administration allows for the safe transfer of the mother to a neonatal intensive care unit (NICU) or a specialized center with ample resources and educated staff to handle any potential complications.
The most commonly used tocolytic agents include B2 receptor agonists (such as Terbutaline), Ca2+ channel blockers (like Nifedipine), oxytocin receptor antagonists (Atosiban), non-steroidal anti-inflammatory drugs (NSAIDs) like Indomethacin, and myosin light chain inhibitors (such as magnesium sulfate) [125,126]. Corticosteroids play a crucial role in enhancing the survival of premature infants by allowing sufficient time for vital organ growth [127].
6.2. Preterm & risk factors
Recent treatment approaches for preterm birth (PTB) have increasingly focused on myometrium contractions. Systematic reviews and meta-analyses have indicated that several factors contribute to the risk of PTB. These include maternal age of 40 years or older, multifetal gestations, medical disorders like severe anemia [[128], [129], [130], [131], [132]], previous and family history of PTB, rupture of membranes (132), obesity, stress, sexual activity, placenta previa, gestational diabetes mellitus, hypertensive disorders complicating pregnancy, and reproductive anomalies [133].
In 2022, Raab et al. conducted a cohort study that revealed a connection between low dietary quality during early gestation and an increased likelihood of PTB. On the other hand, healthier dietary choices were found to be protective against PTB [134]. A healthy prenatal lifestyle focused on minimizing modifiable risk factors was associated with a decreased risk of PTB. Therefore, it is crucial to adopt modified lifestyle factors during preconception and early pregnancy to reduce the incidence of PTB.
7. Conclusion
The human myometrium is a therapeutic target for inducing labor and managing preterm labor. Numerous investigations have verified the need for a computational platform to facilitate further research on the ionic mechanism governing electrical and mechanical activities in uterine myofibrils, both in animal and human pregnancy models. Moreover, it is crucial to consider the unique role hormones play in this context. Specifically, the sodium (Na+) leak channel, nonselective (NALCN), as a Na+ leak current, holds significant importance in human uterine smooth muscle cells (USMC). Studies involving uterine mice lacking NALCN have demonstrated dysfunctional labor, highlighting the channel's relevance. Amazu et al. (2020) proposed that progesterone (P4) and estrogen (E2) regulate this Na channel. Their findings showed that E2 reduces NALCN mRNA expression (2.3-fold), while E2/P4 increases it (5.6-fold). Therefore, NALCN expression was not augmented in cells treated with P4 alone [which do not express progesterone receptor (PR)]; which supports the idea that P4 is necessary to regulate NALCN expression (A2, B2, C2). Therefore, they confirmed that E2/P4has more effect on this Na channels. This interaction between sodium channel and P4 through binding PR-A stimulates contraction, or progesterone receptor-B (PR-B), which induces quiescent state (Fig. 13) [22].
Fig. 13.
Schematic figure suggests model by NALCN/SLO2.1 complex for regulation of the myometrium excitability.
Fig. 13 is similar Fig. 2, but in this figure, the interaction between NALCN and PR-A/B is better shown in two states of Quiescent State [Hyperpolarization of VDCC (Closed) by Progestrone] and Contraction State [Depolarization of VDCC (open) by Oxytocin and estrogen].
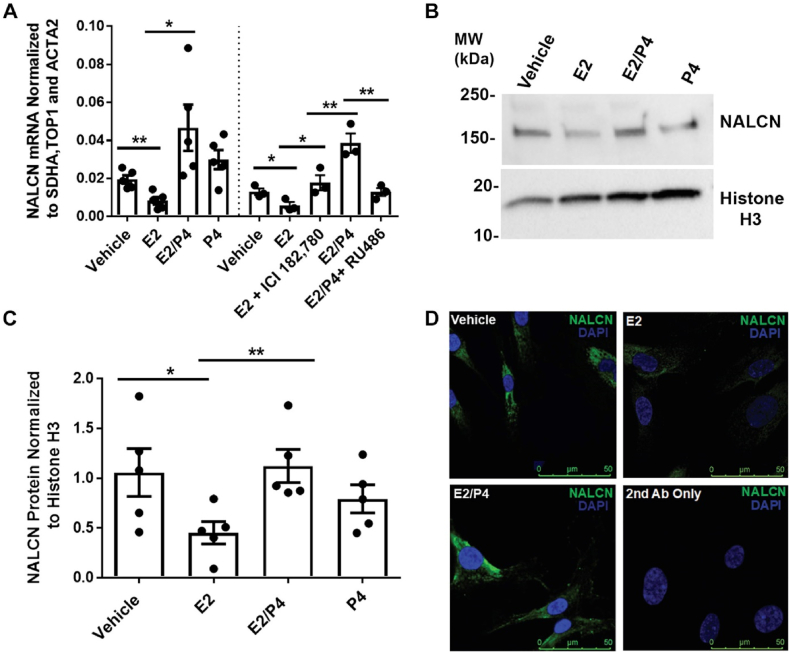
In the quiescent state, binding progesterone to PRA/B increases NALCN expression and its activity. Sodium influx via NALCN stimulates SLO2.1 channels, and increases K+ efflux to maintain the myometrium membrane in a hyperpolarized state. As a consequence, VDCCs are closed, and uterine contractions do not occur. In the contractile state, estrogen binding to ERα inhibits NALCN expression to lead for reducing SLO2.1 activity. The reduction of K+ efflux depolarizes the membrane for leading to VDCC activation, an increase in intracellular Ca2+ (SR), and uterine contractility [22]. The investigation of NALCN mRNA regulation by the two sex hormones E2 and P4and its protein expression is shown in Fig. 14 [A (quantification of quantitative real-time PCR of NALCN mRNA), B (Western blot of NALCN), C (NALCN signal was normalized to histone H3), D (immunofluorescence images of NALCN)] (Fig. 14) [22].
Fig. 14.
Demonstration of non-selective sodium leak channel (NALCN) mRNA regulation by the two sex hormones: estrogen (E2) and progesterone (P4) and its protein expression [22]. A: quantification of quantitative real-time PCR of NALCN mRNA in HM6ERMS2 treated with vehicle (n = 5), E2 (n = 5), E2/P4 (n = 5), P4 (n = 5), E2 and ICI 182,780 (ICI; n = 3), or E2/P4 and RU486 (n = 3). B: Western blot of NALCN in HM6ERMS2 whole-cell lysates. C: quantification of NALCN protein in the HM6ERMS2 whole-cell lysates (n = 5). D: illustrative immunofluorescence images of NALCN in HM6ERMS2 cells treated as showed (Scale bar: 50 μm) [22].
Ferreira et al., 2019 showed in labor state, binding oxytocin to OXTR to lead activity of PLC for creation PIP2, and production of inositol triphosphate (IP3). Then, IP3 stimulates the releasing Ca2+ from SR stores, and PIP2 activates (PKC) for SLO2.1 inhibition [23]. This inhibition leads to membrane depolarization for opening more VDCCs, and increasing intracellular Ca2+ for myosin activity to cause muscle contraction.The regulation of NALCN mRNA by E2/P4 and increasing protein expression (Fig. 10) confirms that human USMC labor is a cascade current like a domino that inevitably happens without interruption. Despite the importance of proper uterine smooth muscle function for women's health, the organ's electrical activity remains poorly understood. The findings of this narrative review reveal that the electrical events in the uterus include.
-
1)
Calcium ion influx through voltage-operated Ca2+ channels, which plays a fundamental role in regulating muscle contraction and hormone release during labor. Oxytocin, in particular, is crucial for increasing the entry of Ca2+ into uterine myometrium cells, leading to enhanced duration and frequency of action potentials (APs). This increase in calcium ions from the sarcoplasmic reticulum (SR) and extracellular space is necessary for the activation of voltage-gated L-type calcium channels, which in turn trigger voltage-gated Na+ channels (VGSCs) to generate and conduct APs in myofibrils. Both voltage-gated channels are essential for the proper physiology of uterine smooth muscle contraction (USMC), enabling strong force generation and invasive expansion in the uterine smooth muscle wall through synchronized contractions.
-
2)
Hormonal releasing processes: i) E2/P4 regulates NALCN mRNA by increasing protein expression in the USMC labor (Fig. 10). ii) Oxytocin binding to OTR activates second messenger PLC for production IP3. Then, IP3 stimulates Ca2+ release from SR and activates PKC via PIP2; which inhibits SLO2.1 [23]. SLO2.1 inhibition, depolarizes the myofibril membrane via opening more VDCCs for increasing Ca2+, and finally myosin activation happen for contraction in myometrium (Fig. 13).
-
3)
Glucose, as a maternal energy source, can influence the amplitude of electrical currents and the intrinsic kinetics of VGSC, as observed in insulin-secreting pancreatic β-cell lines (INS-1 cells). The glucose regulatory system is crucial for maintaining glucose homeostasis in the maternal body, and it undergoes significant adaptations during pregnancy to support insulin secretion. Hormones produced by the placenta during late pregnancy, such as estrogen, cortisol, and lactogenic hormones, may block insulin or cause insulin resistance (IR). Glucose, in a dose-dependent manner, regulates insulin secretion in INS-1 cells, partially inhibiting the INa channel.
-
4)
PGE/F (prostaglandins E and F) have a pivotal excitatory effect, followed by hyperpolarization related to the Na+/K+-ATPase pump. This hyperpolarization disrupts the synchronized contraction results in the electro-mechanical pattern of USMC, leading to limitations in the response to a single contraction and a decline in the frequency of subsequent contractions. The modulation of electric field hyperpolarization of the muscle membrane potential occurs rapidly in seconds under physiological conditions [135]. During labor, the amplitude of hyperpolarization declines, allowing the contraction frequency to rise [17]. This event is regulated by a cascade of events, including autoregulatory local responses (PGE/F), channels expression, connexin43 gene expression, and various autocrine/paracrine and endocrine hormones.
As described in the sections above, new studies may illuminate novel roles of voltage channels in USMC. Gaining a more comprehensive understanding of transcription factor-mediated regulatory pathways could pave the way for new therapeutic treatments to prevent preterm birth in at-risk pregnancies. Perhaps, in the future, a comprehensive quantitative electrophysiological approach, such as computer modeling or artificial intelligence, could elucidate the excitation-contraction coupling in the uterus.
Data availability
Sharing research data helps other researchers evaluate your findings, build on your work and to increase trust in your article. We encourage all our authors to make as much of their data publicly available as reasonably possible. Please note that your response to the following questions regarding the public data availability and the reasons for potentially not making data available will be available alongside your article upon publication.
Has data associated with your study been deposited into a publicly available repository?
Yes.
CRediT authorship contribution statement
Farideh Zafari Zangeneh: Writing – review & editing, Writing – original draft, Validation, Investigation, Conceptualization. Sedighe Hantoushzadeh: Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22259.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Garfield R.E., Saade G., Buhimschi C., Buhimschi I., Shi L., Shi S.Q., Chwalisz K. Control and assessment of the uterus and cervix during pregnancy and labour. Hum. Reprod. Update. 1998;4:673–695. doi: 10.1093/humupd/4.5.673. [DOI] [PubMed] [Google Scholar]
- 2.Xu H., Remmen H.V. The Sarco Endoplasmic Reticulum Calcium ATPase (SERCA) pump: a potential target for intervention in aging and skeletal muscle pathologies. Skeletal Muscle. 2021;11(25) doi: 10.1186/s13395-021-00280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anjum Irfan. Calcium sensitization mechanisms in detrusor smooth muscles. J. Basic Clin. Physiol. Pharmacol. 2018;29(3):227–235. doi: 10.1515/jbcpp-2017-0071. [DOI] [PubMed] [Google Scholar]
- 4.MacEvoy A, Sabir S. Physiology, Pregnancy Contractions. Copyright © 2023, StatPearls Publishing LLC. (http://creativecommons.org/licenses/by-nc-nd/4.0/). [PubMed]
- 5.Kiss A., Erdödi F., Lontay B. Myosin phosphatase: unexpected functions of a long-known enzyme. BBA-Mol. Cell Res. 2019;1866:2–15. doi: 10.1016/j.bbamcr.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Garfield L., Chin E. Pharmacology for preterm labor. J. Perinat. Neonatal Nurs. 2020;34:155–161. doi: 10.1097/JPN.0000000000000474. [DOI] [PubMed] [Google Scholar]
- 7.Malik M., Roh M., England S.K. Uterine contractions in rodent models and humans. Acta Physiology. 2021;231(4) doi: 10.1111/apha.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wray S. Insights into the uterus. Exp. Physiol. 2007;92:621–631. doi: 10.1113/expphysiol.2007.038125. [DOI] [PubMed] [Google Scholar]
- 9.Obayashi S., Word R.N. Encyclopedia of Hormones; 2003. Anatomic and Cellular Considerations of Uterine Contractility; pp. 540–545. [DOI] [Google Scholar]
- 10.Burdyga T., Borisova L., Burdyga A.T., Wray S. Temporal and spatial variations in spontaneous Ca events and mechanical activity in pregnant rat myometrium. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;144(Suppl 1) doi: 10.1016/j.ejogrb.2009.02.017. S25–S32. [DOI] [PubMed] [Google Scholar]
- 11.Wray S., Jones K., Kupittayanant S., Li Y., Matthew A., Monir-Bishty E., et al. Calcium signaling and uterine contractility. J Soc Gynecol Investig. 2003;10:252–264. doi: 10.1016/s1071-5576(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 12.Schneider T., Neumaier F., Hescheler J., Alpdogan S. Cav2.3 R-type calcium channels: from its discovery to pathogenic de novo CACNA1E variants: a historical perspective. Pflügers Archiv - European Journal of Physiology. 2020;472:811–816. doi: 10.1007/s00424-020-02395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hering S., Zangerl-Plessl E.M., Beyl S., Hohaus A., Andranovits S., Timin E.N. Calcium channel gating. Pflugers Arch. 2018;470:1291–1309. doi: 10.1007/s00424-018-2163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doyle D.A., Cabral J.M., Pfuetzner R.A., Kuo A.L., Gulbis J.M., Cohen S.L., Chait B.T., MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Reyes E., Schneider T. Calcium channels: structure, function, and classification. Drug Dev. Res. 1994;33:295–318. [Google Scholar]
- 16.Wray S., Arrowsmith S. Uterine excitability and ion channels and their changes with gestation and hormonal environment. Annu. Rev. Physiol. 2021;83:331–357. doi: 10.1146/annurev-physiol-032420-035509. [DOI] [PubMed] [Google Scholar]
- 17.Parkington H.C., Tonta M.A., Brennecke S.P., Coleman H.A. Contractile activity, membrane potential and cytoplasmic calcium in human uterine smooth muscle in the third trimester of pregnancy and during labor. Am. J. Obstet. Gynecol. 1999;181:1445–1451. doi: 10.1016/s0002-9378(99)70390-x. [DOI] [PubMed] [Google Scholar]
- 18.Arrowsmith S., Kendrick A., Hanley J.-A., Noble K., Wray S. Myometrial physiology – time to translate? Exprimental Physiology. 2013;99:495–502. doi: 10.1113/expphysiol.2013.076216. [DOI] [PubMed] [Google Scholar]
- 19.Nakao K., Inoue Y., Okabe K., Kawarabayashi T., Kitamura K. Oxytocin enhances action potentials in pregnant human myometrium—a study with microelectrodes. Am. J. Obstet. Gynecol. 1997;177:222–228. doi: 10.1016/s0002-9378(97)70465-4. [DOI] [PubMed] [Google Scholar]
- 20.Catterall W.A., Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol 590 (2012) 2577–2589.doi: 10.1113/jphysiol.2011.224204. Epub 2012 Apr 2. [DOI] [PMC free article] [PubMed]
- 21.Ferreira J.J., Chinwendu A., Puga-Molina lC., Ma X., England S.K., Santi C.M. SLO2.1/NALCN a sodium signaling complex that regulates uterine activity. iScience. 2021 doi: 10.1016/j.isci.2021.103210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amazu C., Ma X., Henkes C., Ferreira J.J., Santi C.M., England S.K. Progesterone and estrogen regulate NALCN expression in human myometrial smooth muscle cells. Am. J. Physiol. Endocrinol. Metab. 2020;318:E441–E452. doi: 10.1152/ajpendo.00320.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira J.J., Butler A., Stewart R., Gonzalez-Cota A.L., Lybaert P., Amazu C., Reinl E.L., Wakle-Prabagaran M., Salkoff L., England S.K., Santi C.M. Oxytocin can regulate myometrium smooth muscle excitability by inhibiting the Na(+) -activated K(+) channel,Slo2.1. J. Physiol. 2019;597:137–149. doi: 10.1113/JP276806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reinl E.L., Zhao P., Wu W., Ma X., Amazu C., Bok R., Hurt K.J., Wang Y., England S.K. Na+-leak channel, non-selective (NALCN) regulates myometrial excitability and facilitates successful parturition. Cell. Physiol. Biochem. 2018;48:503–515. doi: 10.1159/000491805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C., Wang S., Hu Q., Zeng L., Peng H., Liu C., Huang L., Song H., Li Y., Yao L.-H., Meng W. Woltage-gated Na+ channels are modulated by glucose and involved in regulating cellular insulin content of INS-1 cells. Cell. Physiol. Biochem. 2018;45:446–457. doi: 10.1159/000486921. [DOI] [PubMed] [Google Scholar]
- 26.Zou N., Wu X., Jin Y.-Y., He M.-Z., Wang X-x, Su L.-D., Rupnik M., Wu Z.-Y., Liang L., Shen Y. ATP regulates sodium channel kinetics in pancreatic islet beta cells. J. Membr. Biol. 2013;246(2):101–107. doi: 10.1007/s00232-012-9506-7. [DOI] [PubMed] [Google Scholar]
- 27.Wang G., Divall S., Radovick S., Paige D., Ning Y., Zhu Chen, et al. Preterm birth and random Plasma insulin levels at birth and in early childhood. JAMA. 2014;311(6):587–596. doi: 10.1001/jama.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida-Montezuma Y., Stone E., Iftikhar S., De Rubeis V., Andreacchi A., Keown-Stoneman C., Mbuagbaw L., Brown H.K., de Souza R.J., Anderson L.N. The association between late preterm birth and cardiometabolic conditions across the life course: a systematic review and meta-analysis. Pediatric and prenatal Epidemology. 2021 doi: 10.1111/ppe.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kampmann U., Knorr S., Fuglsang J., Ovesen P. Determinants of maternal insulin resistance during pregnancy: an Updated overview. J. Diabetes Res. 2019;2019 doi: 10.1155/2019/5320156. |Article ID 5320156 |. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skajaa G.Ø., Fuglsang J., Kampmann U., Ovesen P.G. Parity increases insulin requirements in pregnant women with type 1 diabetes. The Journal of Clinical Endocrinology & Metabolism. 2018;103(6):2302–2308. doi: 10.1210/jc.2018-00094. [DOI] [PubMed] [Google Scholar]
- 31.Salazar-Petres E.R., Sferruzzi-Perri A.M. Pregnancy-induced changes in β-cell function: what are the key players? J. Physiol. 2022;66(5):1089–1117. doi: 10.1113/JP281082. [DOI] [PubMed] [Google Scholar]
- 32.Hay W. Placental-fetal glucose exchange and fetal glucose metabolism. Transactions of the Agrawal R. Reni AR, Sharma S, Christensen C, Huang Y, Fisher SJ. Insulin action in the brain regulates both central and peripheral functions. Am J Physiol Endocrinol Metab. 2021;321:E156–E163. doi: 10.1152/ajpendo.00642.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahraman S., Dirice E., De Jesus D.F., Hu J., Kulkarni R.N. Maternal insulin resistance and transient hyperglycemia impact the metabolic and endocrine phenotypes of offspring. Am. J. Physiol. Endocrinol. Metab. 2014;307(10):E906–E918. doi: 10.1152/ajpendo.00210.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spinelli M., Fusco S., Mainardi M., Scala F., Natale F., Lapenta R., et al. Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat. Commun. 2017;8:2009. doi: 10.1038/s41467-017-02221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahn A.M., Husid A., Allen J.C., Seidel C.L., Song T. Insulin acutely inhibits cultured vascular smooth muscle cell contraction by a nitric oxide synthase-dependent pathway. Hypertension. 1997;30(4):928–933. doi: 10.1161/01.hyp.30.4.928. [DOI] [PubMed] [Google Scholar]
- 36.Boeldt D.S., Bird I.M. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 2017 Jan;232(1):R27–R44. doi: 10.1530/JOE-16-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guzmán-Gutiérrez E., Arroyo P., Salsoso R., Fuenzalida B., Sáez T., Leiva A., Pardo F., Sobrevia L. Role of insulin and adenosine in the human placenta microvascular and macrovascular endothelial cell dysfunction in gestational diabetes mellitus. Microcirculation. 2014;21:26–37. doi: 10.1111/micc.12077. [DOI] [PubMed] [Google Scholar]
- 38.Sun Y.Y., Juan J., Xu Q.Q., Su R.-N., Hirst J.E., Yang H.-X., et al. Increasing insulin resistance predicts adverse pregnancy outcomes in women with gestational diabetes mellitus. J. Diabetes. 2020;12(6):438–446. doi: 10.1111/1753-0407.13013. [DOI] [PubMed] [Google Scholar]
- 39.Benhalima K., Crombrugge P.V., Moyson C., Verhaeghe J., Vandeginste S., Verlaenen H., et al. Characteristics and pregnancy outcomes across gestational diabetes mellitus subtypes based on insulin resistance. Diabetologia. 2019;62(11):2118–2128. doi: 10.1007/s00125-019-4961-7. [DOI] [PubMed] [Google Scholar]
- 40.Mastrogiannis D.S., Spiliopoulos M., Mulla W., Homko C.J., et al. Insulin resistance: the possible link between gestational diabetes mellitus and hypertensive disorders of pregnancy. Curr Diab Rep. 2009;9(4):296–302. doi: 10.1007/s11892-009-0046-1. [DOI] [PubMed] [Google Scholar]
- 41.Longo S., Bollani L., Decembrino L., Comite A.D., Angelini M., Stronati M., et al. Short-term and long-term sequelae in intrauterine growth retardation (IUGR) J. Matern. Fetal Neonatal Med. 2013;26(3):222–225. doi: 10.3109/14767058.2012.715006. [DOI] [PubMed] [Google Scholar]
- 42.Hernandez T.L., Friedman J.E., Barbour L.A. Insulin resistance in pregnancy: implications for mother and offspring. Insulin Resistance. 2020:67–94. doi: 10.1007/978-3-030-25057-7_5. Part of the Contemporary Endocrinology book series (COE) [DOI] [Google Scholar]
- 43.Newborn D., Freemark M. Placental hormones and the control of maternal metabolism and fetal growth. Curr. Opin. Endocrinol. Diabetes Obes. 2011;18(6):409–416. doi: 10.1097/MED.0b013e32834c800d. [DOI] [PubMed] [Google Scholar]
- 44.Napso T., Yong H.E.J., Lopez-Tello J., Sferruzzi-Perri A. The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation. Front. Physiol. 2018;9:1091. doi: 10.3389/fphys.2018.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boehmer A.H., Limesand S.W., Rozance P.J. The impact of IUGR on pancreatic islet development and β-cell function. J. Endocrinol. 2017;235(2):R63–R76. doi: 10.1530/JOE-17-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor C.T., Colgan S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017;17:774–785. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao H., Wong R.J., Stevenson D.K. The impact of hypoxia in early pregnancy on placental cells. Int. J. Mol. Sci. 2021;22(18):9675. doi: 10.3390/ijms22189675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colson A., Sonveaux P., Debiève F., Sferruzzi-Perri A.N. Adaptations of the human placenta to hypoxia: opportunities for interventions in fetal growth restriction. Hum. Reprod. Update. 2021;27(3):531–569. doi: 10.1093/humupd/dmaa053. [DOI] [PubMed] [Google Scholar]
- 49.Aouache R., Biquard L., Vaiman D., MirallesF Oxidative stress in preeclampsia and placental diseases. Int. J. Mol. Sci. 2018;19:1496. doi: 10.3390/ijms19051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang C.W., Wakeland A.K., Parast M.M. Trophoblast lineage specification, differentiation and their regulation by oxygen tension. J. Endocrinol. 2018;236:R43–R56. doi: 10.1530/JOE-17-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jauniaux E., Watson A.L., Hempstock j, Bao Y.P., Skepper J.N., Burton G.J. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 2000;1576:2111–2112. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burton G.J., Woods A.W., Jauniaux E., Kingdom J.C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guibourdenche J., Fournier T., Malassiné A., Evain-Brion D. Development and hormonal functions of the human placenta. Folia Histochem. Cytobiol. 2009;47 doi: 10.2478/v10042-009-0110-3. S35–S40. [DOI] [PubMed] [Google Scholar]
- 54.Schanz A., Red-Horse K., Hess A.P., Baston-Bust D.M., Heiss C., Krussel J.S. Oxygen regulates human cytotrophoblast migration by controlling chemokine and receptor expression. Placenta. 2014;35:1089–1094. doi: 10.1016/j.placenta.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 55.Sun X.L., Zhe J.Z., Lin L.H., Hung Yu. Low expression levels of CXCL12, CXCR4, and CXCR 7 in peripheral blood and decidual tissues are associated with miscarriage in women. Immunological investigation J. 2022;51(7):2053–2065. doi: 10.1080/08820139.2022.2106871. [DOI] [PubMed] [Google Scholar]
- 56.Francois L.N., Gorczyca L., Du J., Bircsak K.M., Yen E., Wen X., Tu M.-J., Yu A.-M., Illsley N.P., Zamudio S., et al. Down-regulation of the placental BCRP/ABCG2 transporter in response to hypoxia signaling. Placenta. 2017;51:57–63. doi: 10.1016/j.placenta.2017.01.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jansson T., Ylven K., Wennergren M., Powell T.L. Glucose transport and system a activity in syncytiotrophoblast micro villous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002;23:392–399. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- 58.Stanirowski P.J., Szukiewicz D., Majewska A., Watroba M., Pyzlak M., Bomba-Opon D., Wielgos M. Differential expression of glucose transporter proteins GLUT-1, GLUT-3, GLUT-8 and GLUT-12 in the placenta of macrosomic, small-for-gestational-age and growth-restricted foetuses. J. Clin. Med. 2021;10:5833. doi: 10.3390/jcm10245833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mathew S.A., Chandravanshi B., Bhonde R. Hypoxia primed placental mesenchymal stem cells for wound healing. Life Sci. 2017;182:85–92. doi: 10.1016/j.lfs.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 60.Xu Y.-Y., Liu Y., Cui L., Wu W.-B., Quinn M.J., Menon R., Zhang H.-J. Hypoxic effects on the mitochondrial content and functions of the placenta in fetal growth restriction. Placenta. 2021;114:100–107. doi: 10.1016/j.placenta.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Sibiak R., Ozegowska K., Wender-Ozegowska E., Gutaj P., Mozdziak P., Kempisty B. Fetomaternal expression of glucose transporters (GLUTs)—biochemical, cellular and clinical aspects. Nutrients. 2022;14(10):2025. doi: 10.3390/nu14102025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kolahi K.S., Valent A.M., Thornburg K.L. Cytotrophoblast, not syncytiotrophoblast, dominates glycolysis and oxidative phosphorylation in human term placenta. Sci. Rep. 2017;7 doi: 10.1038/srep42941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holland O., Dekker Nitert M., Gallo L.A., Vejzovic M., Fisher J.J., Perkins A.V. Review: placental mitochondrial function and structure in gestational disorders. Placenta. 2017;54:2–9. doi: 10.1016/j.placenta.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 64.Koivunen P., Kietzmann T. Hypoxia-inducible factor Prolyl 4-Hydroxylases and metabolism. Trends Mol. Med. 2018;24:1021–1035. doi: 10.1016/j.molmed.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Downes N.L., Laham-Karam N., Kaikkonen M.U., Ylä-Herttuala S. Differential but complementary HIF1α and HIF2α transcriptional regulation. Mol. Ther. 2018;26:1735. doi: 10.1016/jymthe.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kierans S.J., Taylor C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J. Physiol. 2021;599:23–37. doi: 10.1113/JP280572. [DOI] [PubMed] [Google Scholar]
- 67.Zhao H., Wong R.J., Stevenson D.K. The impact of hypoxia in early pregnancy on placental cells. Int. J. Mol. Sci. 2021;22(18):9675. doi: 10.3390/ijms22189675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayashi M., Sakata M., Takeda T., Tahara M., Yamamoto T., Minekawa R., Isobe A., Tasaka K., Murata Y., Hypoxia up-regulates hypoxia-inducible factor-1alpha expression through RhoA activation in trophoblast cells, J. Clin. Endocrinol. Metab. 90(3) (2005) 1712–1719.doi: 10.1210/jc.2004-1547. Epub 2004 Dec 14. [DOI] [PubMed]
- 69.Chang Y.-L., Chang S.-D., Chao A.-S., Sieber M., Tsai C.-L., Chen P.J. Effect of Hypoxia on Glucose Transporter 1 and 3 Gene expressions in placental mesenchymal stem cells derived from growth-restricted fetuses, Genes (Basel). 2022;13(5):752. doi: 10.3390/genes13050752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kierans S.J., Taylor C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J Physiol. 2021;599(1):23–37. doi: 10.1113/JP280572. [DOI] [PubMed] [Google Scholar]
- 71.Higgins J.S., Vaughan O.R., Fernandez de Liger E., Fowden A.L., Sferruzzi-Perri A.N. Placental phenotype and resource allocation to fetal growth are modified by the timing and degree of hypoxia during mouse pregnancy. J Physiol. 2016;594:1341–1356. doi: 10.1113/JP271057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Desoye G, Demouzone SH. The human placenta in gestational diabetes mellitus the insulin and cytokine network. Diabetes Care; Suppl 2(Supplement 2):S120-S126. [DOI] [PubMed]
- 73.Adeoye O.O., Silpanisong J., Williams J.M., Pearce W.J. Role of the sympathetic autonomic nervous system in hypoxic remodeling of the fetal cerebral vasculature. J. Cardiovasc. Pharmacol. 2015;65(4):308–316. doi: 10.1097/FJC.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Määttä J., Sissala N., Dimova E.Y., Serpi R., Moor L.G., Koivunen P. Hypoxia causes reductions in birth weight by altering maternal glucose and lipid metabolism. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-31908-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.López-Barneo J., Ortega-Sáenz P., González-Rodríguez P., Fernández-Agüera M.C., Macías D., Pardal R., Gao L. Oxygen-sensing by arterial chemoreceptors: mechanisms and medical translation. Mol. Aspects Med. 2016;47–48:90–108. doi: 10.1016/j.mam.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 76.Lee P., Chandel N.S., Simon M.C. Cellular adaptation to hypoxia through HIFs and beyond. Nat. Rev. Mol. Cell Biol. 2020;21(5):268–283. doi: 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fuhrmanna D.C., Brüneab B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017;12:208–215. doi: 10.1016/j.redox.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilson D.F., Rumsey W.L., Green T.J., Vanderkooi J.M. The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J. Biol. Chem. 1988;263:2712–2718. [PubMed] [Google Scholar]
- 79.Wheaton W.W., Chandel N.S., Hypoxia. 2. Hypoxia regulates cellular metabolism, Am. J. Physiol-Cell Physiol. 300 (3) (2011) C385–C39. doi: 10.1152/ajpcell.00485.2010. Epub 2010 Dec 1. [DOI] [PMC free article] [PubMed]
- 80.Michiels C. Physiological and pathological responses to hypoxia. Am. J. Pathol. 2004 Jun;164(6):1875–1882. doi: 10.1016/S0002-9440(10)63747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bruno R.M., Ghiadoni L., Seravalle G., Dell'Oro R., Taddei S., Grassi G. Sympathetic regulation of vascular function in health and disease. Front. Physiol. 2012;3:284. doi: 10.3389/fphys.2012.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frey H.A., Tuuli M.G., Roehl K.A., Odibo A.O., Macones G.A., Cahill A.G. Can contraction patterns predict neonatal outcomes? J. Maternal-Fetal. Neonatal Medicine. 2014;27(14):1422–1427. doi: 10.3109/14767058.2013.866645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Young R.C., Marinescu P.S., Seligman N.S. Monitoring uterine contractions during labor: current challenges and future directions. Am. J. Obstet. Gynecol. 2023;228(5S):S1192–S1208. doi: 10.1016/j.ajog.2022.10.039. [DOI] [PubMed] [Google Scholar]
- 84.Young R.C., Barendse P. Linking myometrial Physiology to intrauterine pressure; how tissue-level contractions create uterine contractions of labor. PLoS Comput. Biol. 2014;10(10) doi: 10.1371/journal.pcbi.1003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shynlova O., Lee Y., Srikhajon K., Lye S.J. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod. Sci. 2013;20(2):154–167. doi: 10.1177/1933719112446084. [DOI] [PubMed] [Google Scholar]
- 86.Aslanidi O., Atia J., Benson A.P., van den Berg H.A., Blanks A.M., et al. Towards a computational reconstruction of the electrodynamics of premature and full term human labour. Prog. Biophys. Mol. Biol. 2011;107:183–192. doi: 10.1016/j.pbiomolbio.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 87.Garfield R.E., Rabideau S., Challis J.R., Daniel E.E. Hormonal control of gap junction formation in sheep myometrium during parturition. Biol. Reprod. 1979;21:999–1007. doi: 10.1095/biolreprod21.4.999. [DOI] [PubMed] [Google Scholar]
- 88.Lye S.J., Nicholson B.J., Mascarenhas M., MacKenzie L., Petrocelli T. Increased expression of connexin-43 in the rat myometrium during labor is associated with an increase in the plasma estrogen: progesterone ratio. Endocrinology. 1993;132:2380–2386. doi: 10.1210/endo.132.6.8389279. [DOI] [PubMed] [Google Scholar]
- 89.Kidder G.M., Winterhager E. Physiological roles of connexins in labour and lactation. Reproduction J. 2015;150(4):R129–R136. doi: 10.1530/REP-15-0134. [DOI] [PubMed] [Google Scholar]
- 90.Andersen J., Grine E., Eng C.L., Zhao K., Barbieri R.L., Chumas J.C., et al. Expression of connexin-43 in human myometrium and leiomyoma. Am. J. Obstet. Gynecol. 1993;169(5):1266–1276. doi: 10.1016/0002-9378(93)90293-r. [DOI] [PubMed] [Google Scholar]
- 91.Liu G., Tang Y., Han Y., Teng X. Effects of COH on the expression of connexin43 in endometrial stromal cells. Taiwan. J. Obstet. Gynecol. 2019;58(5):592–597. doi: 10.1016/j.tjog.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 92.Nadeem L., Shynlova O., Mesiano S., Lye S. Progesterone via its type-A receptor promote myometrial gap junction coupling. Sci. Rep. 2017;7:13357. doi: 10.1038/s41598-017-13488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roomans G.M., Kilarski W.M., Ciray N., Persson B.E., Bäckström T., Ulmsten U. Structural and functional studies of gap junctions in human myometrium. Eur. J. Morphol. 1993;31:55–59. [PubMed] [Google Scholar]
- 94.Mendelson C.R., Gao L., Montalbano A.P. Multifactorial regulation of myometrial contractility during pregnancy and parturition. Front. Endocrinol. 2019 doi: 10.3389/fendo.2019.00714. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Golightly E., Jabbour H.N., Norman J.E. Endocrine immune interactions in human parturition. Mol. Cell. Endocrinol. 2011;335:52–59. doi: 10.1016/j.mce.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 96.Terzidou V., Lee Y., Lindström T., Johnson M., Thornton S., Bennett P.R. Regulation of the human oxytocin receptor by nuclear Factor-κB and CCAAT/enhancer-binding protein-β. J. Clin. Endocrinol. Metabol. 2006;91:2317–2326. doi: 10.1210/jc.2005-2649. [DOI] [PubMed] [Google Scholar]
- 97.Sivarajasingam S.P., Imami N., Johnson M.R. Myometrial cytokines and their role in the onset of labor. J. Endocrinol. 2016;23(3):R101–R119. doi: 10.1530/JOE-16-0157. [DOI] [PubMed] [Google Scholar]
- 98.Khader N., Shckuka V.M., Shynlova O., Mitchell J.A. Transcriptional control of parturition: insights from gene regulation studies in the myometrium. Mol. Hum. Reprod. 2021;27(5):gaab024. doi: 10.1093/molehr/gaab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Merlino A.A., Welsh T.N., Tan H., Cannon V., Mercer B.M., Mesiano S., etal Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. The Journal of clinical endocrinology and metabolism. 2007;92:1927–1933. doi: 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
- 100.Kota S.K., Gayatri K., Jammula S., Krishna S.V.S., Meher L.K., Modi K.D. Endocrinology of parturition. Indian J Endocrinol Metab. 2013;17(1):50–59. doi: 10.4103/2230-8210.107841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Costa M.A. The endocrine function of human placenta: an overview. Reprod. Biomed. Online. 2016;32(1):14–43. doi: 10.1016/j.rbmo.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 102.Mendelson C.R., Montalbano A.P., Gaoa L. Fetal-to-Maternal signaling in the timing of birth. J. Steroid Biochem. Mol. Biol. 2017;170:19–27. doi: 10.1016/j.jsbmb.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schonemann M.D., Muench M.O., Tee M.K., Miller W.L., Mellon S.H. Expression of P450c17 in the human fetal nervous system. Endocrinology. 2012;153(5):2494–2505. doi: 10.1210/en.2011-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Flint A.P., Anderson A.B., Steele P.A., Turnbull A.C. The mechanism by which fetal cortisol controls the onset of parturition in the sheep. Biochem. Soc. Trans. 1975;3:1189–1194. [Google Scholar]
- 105.Schweigmann H., Sánchez-Guijo A., Ugele B., Hartmann K., Bergmann M., Pfarrer C., et al. Transport of the placental estriol precursor 16α-hydroxy-dehydroepiandrosterone sulfate (16α-OH-DHEAS) by stably transfected OAT4-, SOAT-, and NTCP-HEK293 cells. J. Steroid Biochem. Mol. Biol. 2014;143:259–265. doi: 10.1016/j.jsbmb.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 106.Escobar J.C., Patel S.S., Beshay V.E., Suzuki T., Carr B.R. The human placenta expresses CYP17 and generates androgens de novo. J. Clin. Endocrinol. Metab. 2011;96(5):1385–1392. doi: 10.1210/jc.2010-2504. [DOI] [PubMed] [Google Scholar]
- 107.Xu C., You X., Lu W., Sun Q., Ding X., Huang Y., Ni X. Prostaglandin F2α regulates the expression of uterine activation proteins via multiple signaling pathways in Reproduction. 2015;149(1):139–146. doi: 10.1530/REP-14-0479. [DOI] [PubMed] [Google Scholar]
- 108.Niringiyumukiza J.D., Cai H., Xiang W. Prostaglandin E2 involvement in mammalian female fertility: ovulation, fertilization, embryo development and early implantation. Reprod. Biol. Endocrinol. 2018;16(1):43. doi: 10.1186/s12958-018-0359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bale T.L., Dorsa D.M. Cloning, novel promoter sequence, and estrogen regulation of a rat oxytocin receptor gene. Endocrinology. 1997;138:1151–1158. doi: 10.1210/endo.138.3.4998. [DOI] [PubMed] [Google Scholar]
- 110.Kim S.-C., Lee J.-E., Kang S.S., Yang H.-S., Kim S.S., An B.-S. The regulation of oxytocin and oxytocin receptor in human placenta according to gestational age. J. Mol. Endocrinol. 2017;59(3):235–243. doi: 10.1530/JME-16-0223. [DOI] [PubMed] [Google Scholar]
- 111.Lye S.J., Nicholson B.J., Mascarenhas M., MacKenzie L., Petrocelli T. Increased expression of connexin-43 in the rat myometrium during labour is associated with an increase in the plasma estrogen: progesterone ratio. Endocrinology. 1993;132:2380–2386. doi: 10.1210/endo.132.6.8389279. [DOI] [PubMed] [Google Scholar]
- 112.Matsui K., Higashi K., Fukunaga K., Miyazaki K., Maeyama M., Miyamoto E. Hormone treatments and pregnancy alter myosin light chain kinase and calmodulin levels in rabbit myometrium. J. Endocrinol. 1983;97:11–19. doi: 10.1677/joe.0.0970011. [DOI] [PubMed] [Google Scholar]
- 113.oo Mugisho, Green C.R., Kho D.T., Zhang J., Graham E.S., Acosta M.L., Rupenthal I.D. The inflammasome pathway is amplified and perpetuated in an autocrine manner through connexin43 hemichannel mediated ATP release. Biochim. Biophys. Acta Gen. Subj. 2018;1862:385–393. doi: 10.1016/j.bbagen.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 114.Goldberg G.S., Valiunas V., Brink P.R. Selective permeability of gap junction channels. Biochim. Biophys. Acta. 2004;1662:96–101. doi: 10.1016/j.bbamem.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 115.Kota S.K., Gayatri K., Jammula S., Krishna S.V.S., Meher L.K., Modi K.D. Endocrinology of parturition. Indian J Endocrinol Metab. 2013;17(1):50–59. doi: 10.4103/2230-8210.107841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Herrera A.A., Bandin S.F., Penas D.R., Portolés M., Lletí E.R., Rivera M. A summary about relaxin and its reproductive function. Endocrinol Metab Int J. 2017;4(5):114–116. [Google Scholar]
- 117.Bathgate R.D., Halls M.L., van der Westhuizen E.T., Callander G.E., Kocan M., Summers R.J. Relaxin family peptides and their receptors. Physiol. Rev. 2013;93:405–480. doi: 10.1152/physrev.00001.2012. [DOI] [PubMed] [Google Scholar]
- 118.Yuan W., Lopez A. Cyclic AMP signaling pathways in the regulation of uterine relaxation. BMC Pregnancy Childbirth. 2007;7(Suppl 1):S10. doi: 10.1186/1471-2393-7-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leo C.H., Jelinic M., Ng H.H., Marshall S.A., Novak J., Tare M., Conard K.P., Parry L.G. Vascular actions of relaxin: nitric oxide and beyond. Br. J. Pharmacol. 2017;174(10):1002–1014. doi: 10.1111/bph.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goldsmith L.T., Weiss G. Relaxin in human pregnancy. Ann. N. Y. Acad. Sci. 2009 Apr;1160 doi: 10.1111/j.1749-6632.2008.03800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Preterm Birth - WHO. World Health Organization; 19 February 2018. [Google Scholar]
- 122.Blencowe H., Cousens S., Chou D., Oestergaard M., Say L., Moller A.B. Born too soon: the global epidemiology of 15 million preterm births. Reprod. Health. 2013;10(1):S2. doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Romero R., Espinoza J., Gonçalves L.F., Kusanovic J.P., Friel L.A., Nien J.K. Inflammation in preterm and term labour and delivery. Semin. Fetal Neonatal Med. 2006;11(5):317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haas D.M., Imperiale T.F., Kirkpatrick P.R., Klein R.W., Zollinger T.W., Golichowski A.M. Tocolytic therapy: a meta-analysis and decision analysis. Obstet. Gynecol. 2009;113(3):585–594. doi: 10.1097/AOG.0b013e318199924a. [DOI] [PubMed] [Google Scholar]
- 126.Muñoz-Pérez V.M., Ortiz M.I., Cariño-Cortés R., Fernández-Martínez E., Rocha-Zavaleta L., Bautista-Ávila M. Preterm birth, inflammation and infection: new alternative strategies for their prevention. Curr. Pharmaceut. Biotechnol. 2019;20(5):354–365. doi: 10.2174/1389201020666190408112013. [DOI] [PubMed] [Google Scholar]
- 127.Crane J., Armson A., Brunner M., De S.L.R., Farine D., Keenan-Lindsay L., Van Aerde J. Antenatal corticosteroid therapy for fetal maturation. J. Obstet. Gynaecol. Can. 2003;25(1):45–52. doi: 10.1016/s1701-2163(16)31081-7. [DOI] [PubMed] [Google Scholar]
- 128.Parks S., Hoffman M.K., Goudar S.S., Patel A., Saleem S., Ali S.A., et al. Maternal anaemia and maternal, fetal, and neonatal outcomes in a prospective cohort study in India and Pakistan. BJOG. 2019;126(6):737–743. doi: 10.1111/1471-0528.15585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chang Y.-K., Tseng Y.-T., Chen K.-T. The epidemiologic characteristics and associated risk factors of preterm birth from 2004 to 2013 in Taiwan. BMC Pregnancy Childbirth. 2020;20(201) doi: 10.1186/s12884-020-02903-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stephen G., Mgongo M., Hussein Hashim T., Katanga J., Stray-Pedersen B., Msuya S.E. Anaemia in pregnancy: prevalence, risk factors, and adverse perinatal outcomes in northern Tanzania. Anemia. 2018;2018 doi: 10.1155/2018/1846280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gurung A., Wrammert J., Sunny A.K., Gurung R., Rana N., Basaula Y.N., et al. Incidence, risk factors and consequences of preterm birth – findings from a multi-centric observational study for 14 months in Nepal. Arch. Publ. Health. 2020;78(64) doi: 10.1186/s13690-020-00446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Díaz-Rodríguez A., Feliz-Matos L., Matuk C.B.R. Risk factors associated with preterm birth in the Dominican Republic: a case-control study. BMJ Open. 2021;11(12) doi: 10.1136/bmjopen-2020-045399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang Y-P, Liu X-H, Gao S-H, Wang J-M, Gu Y-S, Zhang J-Y, et al. Risk factors for preterm birth in five maternal and child health hospitals in beijing, PLoS One 7(12): e52780. doi:10.1371/journal.pone.0052780. [DOI] [PMC free article] [PubMed]
- 134.Raab R., Hoffman J., Spies M., Geyer K., Meyer D., Günther J. Are pre- and early pregnancy lifestyle factors associated with the risk of preterm birth? A secondary cohort analysis of the cluster-randomised GeliS trial. BMC Pregnancy Childbirth. 2022;22(230) doi: 10.1186/s12884-022-04513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang L., Fang Z., Chen W. Quick and effective hyperpolarization of the membrane potential in intact smooth muscle cells of blood vessels by synchronization modulation electric field. J. Bioenerg. Biomembr. 2012;44:385–395. doi: 10.1007/s10863-012-9432-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sharing research data helps other researchers evaluate your findings, build on your work and to increase trust in your article. We encourage all our authors to make as much of their data publicly available as reasonably possible. Please note that your response to the following questions regarding the public data availability and the reasons for potentially not making data available will be available alongside your article upon publication.
Has data associated with your study been deposited into a publicly available repository?
Yes.