Abstract
Introduction
Fusariosis of the central nervous system (CNS) is extremely uncommon. Treatment and outcome data from previously published cases may provide some guidance in light of the ongoing fungal meningitis outbreak in 2023 involving Fusarium spp. in the United States and Mexico.
Methods
We reviewed the published literature describing cases of invasive fusariosis of the (CNS) that included data on patient demographic characteristics, treatment, and outcome.
Results
Twenty-six cases met inclusion criteria. The mean age was 36 years, 55% involved females, 60% had underlying hematologic malignancy, and another 16% were on immunosuppressants. The majority of infections were from Fusarium solani species complex. Overall 72% of patients died. The majority received monotherapy with amphotericin B, although some received voriconazole monotherapy or combination therapy with amphotericin B plus voriconazole with or without adjuvant surgery. Among the survivors, 3 received amphotericin B monotherapy, 2 voriconazole monotherapy, 1 combination therapy of both, and one surgery only.
Conclusion
The overall mortality rate in published cases of fusariosis of the CNS was high, although—unlike during the current outbreak—the preponderance of patients were severely immunocompromised. While historically the majority were treated with amphotericin B monotherapy, some recent patients were treated with voriconazole monotherapy or combination therapy with amphotericin B plus voriconazole. Current guidelines recommend monotherapy with voriconazole or lipid formulations of amphotericin B or combination of both for the treatment of invasive fusariosis, which is in line with the findings from our literature review and should be considered during the ongoing 2023 outbreak.
Keywords: Fusariosis meningitis, Fusarium solani, Fusarium spp., Fungal meningitis, Fungal meningitis outbreak
Introduction
Fusarium species are filamentous fungi ubiquitous in the environment and rarely cause invasive infection [1]. However, in many countries of the world, fusariosis ranks as the third most prevalent mold infection after aspergillosis and mucormycosis [1–4]. Risk factors for disease include profound or prolonged neutropenia, hematologic malignancy or allogeneic stem cell transplants [1, 5]. Fusariosis typically presents with fungal pneumonia, skin lesions and fungemia, while infections of the central nervous system (CNS) have been very rarely reported [6, 7]. Disseminated fusariosis has a fatality rate of 50–70% in immunocompromised individuals [8].
Despite the relative infrequency of this disease manifestation, an outbreak of Fusarium meningitis is currently ongoing [9]. Patients receiving epidural anesthesia in two clinics in Matamoros, Mexico have been identified at risk for Fusarium meningitis, with 10 confirmed cases, 9 deaths and another 22 probable or suspected cases in Matamoros involving United States (U.S.) cases, as of July 21, 2023 [10], with additional cases involving persons residing in Mexico. An additional outbreak occurred in late 2022 and early 2023 in Durango, Mexico, involving individuals receiving spinal anesthesia, including obstetric patients, with at least 79 cases of meningitis and 35 deaths with Fusarium spp. identified in a relevant proportion of cases as the causative organism [11]. The relationship, if any, between these locations and any potential common source remains unclear. While U.S. and Mexican Health Authorities continue their investigations, it is of paramount importance to review past cases in an attempt to guide current treatment regimens and ascertain prognostic information using prior reports of CNS fusariosis. We therefore reviewed the published literature for cases of CNS fusariosis to analyze treatment and outcome data.
Methods
We reviewed the published literature describing cases of invasive fusariosis involving the CNS, focusing on demographic characteristics of cases such as age and biologic sex, risk factors of disease such as immunosuppression or malignancy, location of infection, antifungal treatment, and outcome.
We searched PubMed, Google Scholar, and Web of Science using the keywords “Fusarium”, “fusariosis”, “CNS”, “brain”, and “central nervous system “ to select relevant clinical studies and case reports published in English between Jan 1, 1963 and July 20, 2023. We also searched citation lists of all relevant publications for additional references and contacted authors, when available, for additional case information.
Information on individual cases, namely age, sex, underlying diseases, extent of fusariosis, causative pathogen, treatment and outcomes was abstracted from the literature where available. Descriptive analysis was performed.
Results
Our literature review revealed 27 cases of CNS infections caused by Fusarium spp., although one case report was unevaluable as it lacked any clinical data [12]. Thus, this case review included 26 cases in total (Table 1).
Table 1.
Prior reports of Central Nervous System (CNS) or Meningeal Fusariosis
| #/Ref./Year published | Age | Sex | Risk Factors | Causative Species | Extent of Disease | Treatment | Outcome (day of death/follow up) |
|---|---|---|---|---|---|---|---|
| 1[31] / 2022 | 70 | NA | Relapsed AML receiving induction therapy | Fusarium spp. | Maxillary sinus with intraorbital extension and meningitis | L-AMB 8 mg/kg + VOR + intrathecal AMB | NA |
| 2[32] / 2022 | 32 | F | Prednisolone, methotrexate, adalimumab, rheumatoid arthritis | F. proliferatum | Brain mass with meningo-encephalitis | IV VOR (12 mo) + surgery | Alive (9 months follow-up) |
| 3[33] / 2022 | 6 | M | Tuberculous meningitis | F. falciforme | Multiple brain abscesses and meningitis | AMB 5 mg/kg + VOR + TERB + surgery | Deceased (1 month) |
| 4[34] / 2016 | 14 | F | Dexamethasone (4 mg, 3x/day for 14 days), Turberculous meningitis | Fusarium spp. | Meningitis | AMB (14 days) | Alive (25 days) |
| 5[13] / 2015 | 50 | F | DM and ALL with failed auto-HSCT, ponatinib and dexamethasone | F. solani | Nasal septum ulcer, 1 cm brain mass | VOR + ABLC 5 mg/kg + GCSF + surgery | Hospice/Deceased (1 month) |
| 6[35] / 2014 | 33 | F | None | Fusarium spp. | Brain mass | VOR + TER + AMB + surgery | Alive (18 months) |
| 7[36] / 2008 | 53 | F | Promyelocytic leukemia | F. oxysporum | Brain abscess | VOR | Alive (5 months) |
| 8[37] / 1998 | NA | NA | ALL | Fusarium spp. | Brain abscess | surgery | Alive (NA) |
| 9[38] / 1983 | 17 | F | Prednisone (60 mg/day) | Fusarium spp. | Brain abscess and meningitis | AMB + intrathecal AMB + surgery (burrhole aspiration) | Deceased (7 days) |
| 10[39] / 1974 | 2 | F | 60% burn | Fusarium spp. | Brain, skin, kidney | None (autopsy) | Deceased (42 days) |
| 11[40] / 1986 | 53 | M | Multiple myeloma | F. oxysporum | Skin, blood, brain | AMB | Deceased (30 days) |
| 12[41] / 1991 | 15 | M | ALL | Fusarium spp. | Leptomeningitis | AMB, 5FC, Miconazole + granulocyte transfusion | Deceased (NA) |
| 13[42] / 1996 | 6 | F | Aplastic anemia, allo BMT | Fusarium spp. | Skin, lung, brain | AMB | Deceased (NA) |
| 14[43] / 2003 | 76 | M | AML | F. solani | Brain abscess | ABLC 5 mg/kg then L-AMB 7.5 mg/kg, then VOR | Deceased but negative for Fusarium at autopsy (30 weeks) |
| 15[44] / 2009 | 55 | F | Lung transplant | Fusarium spp. | Brain abscess | None (autopsy) | Deceased (less than 7 days; not specified in report) |
| 16[45] / 2007 | NA | NA | NA | F. solani | Meningitis | AMB | Deceased (NA) |
| 17[46] /2013 | 23 | M | FSGS | Fusarium spp. | Brain and detection in blood and urine | None (autopsy) | Deceased (NA) |
| 18[47] / 1997 | 41 | M | AML, allo SCT, GVHD | Fusarium spp. | Brain, lung, skin | ABLC | Deceased (8 days) |
| 19[47] / 1997 | 71 | M | AML | Fusarium spp. | Brain, sinuses, skin | AMB | Deceased (4 days) |
| 20[47] / 1997 | 42 | M | CLL, allo SCT | Fusarium spp. | Brain, sinuses, lung, skin | L-AMB | Partial response / Deceased (85 days) |
| 21[6] / 2003 | 7 | F | aplastic anemia, allo SCT | Fusarium spp. | Stroke, lung, skin | AMB | Deceased (5 days) |
| 22[6] / 2003 | 42 | F | AML | Fusarium spp. | Cavernous thrombosis, lung, skin | AMB | Alive (NA) |
| 23[6] / 2003 | 49 | M | Multiple Myeloma, allo SCT, GVHD | F. solani | Brain, lung, skin | AMB, VOR | Deceased (41 days) |
| 24[6] / 2003 | 22 | M | AML, allo SCT, GVHD | Fusarium spp. | Brain, lung, skin | AMB | Deceased (11 days) |
| 25 [48] / 2017 | 65 | F | DM and cirrhosis | Fusarium spp. | Brain abscesses, meningitis | AMB then L-AMB | Deceased (3 months) |
| 26 [49] / 2015 | NA | NA | Chronic granulomatous disease | F. falciforme | Epidural abscess, meningitis, cervical myelitis | NA | Deceased (NA) |
ABLC: amphotericin B lipid complex; ALL: acute lymphocytic leukemia; alloSCT: allogeneic stem cell transplant; AMB: amphotericin B; AML: acute myeloid leukemia; BMT: bone marrow transplant; CLL: chronic lymphocytic leukemia; DM: diabetes mellitus; FSGS: focal segmental glomerulosclerosis; GCSF: granulocyte colony stimulating factor; GVHD: graft versus host disease; HSCT: hematopoietic stem cell transplant; L-AMB: liposomal amphotericin B; NA: not available; TERB: terbinafine; VOR: voriconazole
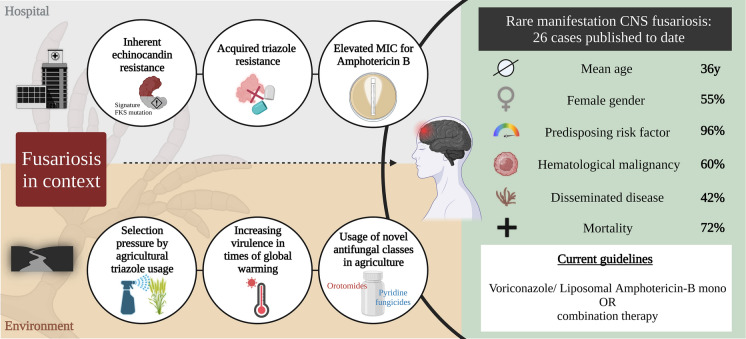
Mean age of cases with CNS fusariosis was 36 years (range 2–76 years, data available from n = 22), and 12/22 (55%) of cases occurred in females, with the remaining 10/22 cases (45%) occurring in males (data not available in 4 cases). Risk factors (or absence of any risk factors) were reported in 25/26 cases and hematological malignancies/diseases were observed most commonly (15/25 cases, 60%), while another 4 cases received immunosuppressive treatment for other conditions. Of note, in only one case was it reported that patients had no known risk factor for disease (Table 1; Fig. 1).
Fig. 1.
Interchange between fusariosis in the environment and the healthcare setting, and the problem of antifungal resistance. Key Takeaways from this review on CNS fusariosis and treatment recommendation
Clinical manifestations varied widely, including meningitis, leptomeningitis, meningoencephalitis, brain abscesses and masses, cavernous thrombosis and stroke. Of note, 11/26 cases (42%) developed CNS fusariosis as part of their disseminated disease which almost always involved the lungs and skin, with positive blood cultures obtained in a relevant proportion of cases. Of those 11 with disseminated disease including CNS fusariosis, 9/11 (82%) had underlying hematological malignancy. Fusarium solani species complex was the causative pathogen in the majority of cases with species identification, while Fusarium oxysporum and Fusarium proliferatum were also detected. Fungal biomarkers and other diagnostics were rarely utilized; only in one single case report serum galactomannan and beta-D-glucan were tested, both giving negative results [13].
In terms of treatment, 13/21 cases (62%; in three cases CNS fusariosis was only diagnosed at autopsy, one additional case received surgery only and in one no data was available) received amphotericin B, with more recent cases mostly receiving lipid formulations of amphotericin B. Also 10/21 cases occurred before voriconazole was approved for clinical use. In some cases intrathecal amphotericin B was added, or later stepdown to oral voriconazole performed. Five cases (24%) received combination therapy with a polyene plus voriconazole ± additional antifungal therapy (e.g. terbinafine), while two cases (10%) received voriconazole monotherapy (Table 1). Surgery was performed in a total of 6/22 (27%) of cases.
Overall 18/25 cases (72%) had a fatal outcome, while 7/25 (28%) survived (for one case no outcome data available). The survivors included patients without any known risk factors, and four patients with underlying hematological malignancy; three of the survivors received polyene monotherapy (one of them later stepping down to oral voriconazole), two received voriconazole monotherapy, while each one received antifungal combination therapy or surgery only. Information regarding neutrophil count recovery in the hematologic malignancy patients was not available.
Discussion
Invasive fusariosis, although rare, is one of the most common mold infections, typically affecting immunocompromised persons. While fusariosis is one of the most frequent invasive mold infections, cases involving the CNS have been rarely reported and have nearly exclusively occurred in patients with known risk factors prior to the recent Fusarium meningitis outbreak in Durango, Mexico, and the current ongoing outbreak involving Fusarium solani species complex among patients who underwent procedures involving epidural anesthesia at two clinics in the city of Matamoros, Tamaulipas, Mexico. F. solani has been detected in CSF in patients receiving follow-up care in both Mexico and the U.S. In addition, elevated levels of β-D-glucan have been detected in the CSF of at least 6 of these patients. In total, 212 residents in 25 U.S. states and jurisdictions who underwent epidural anesthesia at these two clinics are deemed to be at risk for Fusarium meningitis [14]. Thus, guidance on how best to diagnose and treat these infections is important.
While culture from CSF, CNS abscess formations or blood remains the diagnostic gold standard, fungal biomarker testing was reported in only 1/26 cases reviewed here. However, fungal biomarkers tested from serum and CSF, like galactomannan and β-D-glucan, can be positive in fusariosis [1] and aid in the diagnosis of this CNS invasive fungal infection that carries very high morbidity and mortality [15], as does panfungal PCR from CSF or tissue. Next generation sequencing from blood specimens, which has shown promise for other mold infections [16], may present an important diagnostic method in the future, allowing detection of the causative species.
We reviewed the literature on published cases of fusariosis involving the CNS, and identified a total of 26 cases published between 1974 and 2022. The majority of cases involved young patients with a mean age of 36 years. Fifty-five percent involved females and 60% had underlying hematologic malignancy, with another 16% on immunosuppressants. The majority of infections were caused by Fusarium solani species complex. Overall, 72% of patients died. While the majority received monotherapy with amphotericin B (also because a significant proportion of cases occurred before voriconazole was approved for clinical use), most of the newer cases received liposomal amphotericin B or voriconazole monotherapy, with some receiving combination therapy with amphotericin B plus voriconazole with or without adjuvant surgery.
Although the majority of patients involved in the current outbreak in Mexico and the U.S. likely lack many of the risk factors found in the published cases – particularly hematologic malignancy and immunosuppression – treatment and patient outcomes from the published cases reviewed here may be instructive. Among the survivors from the published cases, 3 received amphotericin B monotherapy, 2 voriconazole monotherapy, 1 combination therapy with amphotericin B plus voriconazole, and one surgery only. Treatment with voriconazole, lipid formulations of amphotericin B, or combination therapy with these two agents have been recently recommended as first line options [1]. While no MIC testing was performed in the vast majority of cases reviewed here, it is known that Fusarium spp. are intrinsically resistant to echinocandin therapy, and commonly have elevated MICs to both triazoles and polyenes [17, 18]. Past reports, in vitro susceptibility and murine models of infection suggest that combination therapy with liposomal amphotericin B and voriconazole should be used as first-line therapy [19], with the rationale to extend the spectrum of antifungal activity against these highly resistant pathogens. Intrathecal therapy may be used as adjunctive therapy, however consultation with those highly experienced with this treatment are recommended when considered for use. Data is insufficient to inform species-specific antifungal treatment recommendations [1]; although its clinical implications remain unclear [2], MIC testing should therefore be performed and may also guide therapy and novel antifungals obtained through compassionate use programs may also be needed in refractory cases, based on availability [20]. Novel antifungal compounds that are currently in clinical development, namely fosmanogepix or olorofim, have good CNS penetration and could also provide future treatment options for this deadly disease caused by Fusarium spp. that often show very high MICs against most available antifungals in vitro. [21–23].
Innate drug resistance in Fusarium spp. likely evolved in the environment as a survival mechanism. The presence of efflux pumps in Fusarium spp. have been shown to protect against phytoalexins, which are low molecular weight antimicrobial compounds produced by plants as a response to biotic and abiotic stresses, and the triazole fungicide tebuconazole [24, 25]. Thermotolerance in times of global warming may further contribute to increased virulence in some Fusarium spp., as shown for Fusarium graminearum species complex, a pathogen to wheat and other cereal crops which can result in yield losses up to 75%, particularly during warmer and humid years [26]. This increased virulence leading to increased threat of food supply may lead to increased usage of fungicides in agriculture, including not only azoles like tebuconazole, but because of increasing resistance also fungicides with new mode of action like iplufenoquin which shares its mode of action with a new antifungal olorofim [27], or the fungicide aminopyrifen, which shares its mode of action with fosmanogepix [28]. This broad use of fungicides with similar mechanisms of actions with the antifungals used to treat infections in humans may lead to a further increase in antifungal resistance in the future (Fig. 1).
The relative lack of fusariosis cases in immunocompetent hosts speaks to the opportunistic nature of this genus. However, the attack rate and accompanying morbidity and mortality of infection in those receiving spinal anesthesia as the source reminds us that virulence cannot be insinuated for all pathogens. Under the right circumstances, it is likely that most microbes have the potential for virulence under certain conditions [29]. For example, Exserohilum was not a known cause of human CNS infections until a fungal meningitis outbreak with this organism was described in 2013 [30].
Of note, the current fusariosis outbreak in Matamoros, Mexico involved medical tourism. The majority of those affected were from the U.S. and underwent procedures in Mexico that had shorter waiting times and were lower cost. The high cost of health care for many in the U.S. is undoubtedly linked to these two recent outbreaks, and individuals seeking low-cost health care abroad may continue to be at risk for poor outcomes in the future.
In conclusion, two recent outbreaks of fusariosis involving the CNS, including one that is ongoing in Matamoros, Mexico and the U.S., are related to receipt of epidural anesthesia in Mexico. It is unclear if these two outbreaks are related. Given the high morbidity and mortality associated with these cases of invasive fusariosis, it is important to understand which treatment regimens may be best to treat these infections. Current guidelines recommend first-line treatment with voriconazole monotherapy, lipid formulations of amphotericin B, or combination therapy with these two regimens [20]. This case series also found that the majority of survivors from fusariosis involving the CNS were treated with voriconazole or amphotericin B monotherapy, with an additional patient treated with combination therapy using these agents and another from surgery alone. The findings in this case series and current guidelines for the treatment of invasive fusariosis should be instructive in the treatment of these patients.
Funding
Open access funding provided by Medical University of Graz. No other funding obtained for this review.
Declarations
Conflict of interest
MH received research funding from Gilead, Astellas, MSD, IMMY, Mundipharma, Scynexis, F2G and Pfizer – all outside of the submitted work. All authors declare no conflict of interest. JDJ received research funding from Astellas, F2G, and Pfizer – all outside of the submitted work. MN received consulting fees from Knight, Pfizer, Abbvie, Abbott, Janssen, MSD, Astellas, Pharmalab, GSK, Takeda, AstraZeneca and Zodiac – all outside of the submitted work. GRT received research and consulting fees from Astellas, Amplyx, Cidara, F2G, Mayne, Melinta, Mundipharma, Scynexis, and served on the DSMB for Pfizer – all outside of the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shared First Author: Martin Hoenigl and Jeffrey D. Jenks.
Contributor Information
Martin Hoenigl, Email: hoeniglmartin@gmail.com.
George R. Thompson, III, Email: grthompson@ucdavis.edu.
References
- 1.Hoenigl M, Salmanton-García J, Walsh TJ, Nucci M, Neoh CF, Jenks JD, Lackner M, Sprute R, Al-Hatmi AMS, Bassetti M, Carlesse F, Freiberger T, Koehler P, Lehrnbecher T, Kumar A, Prattes J, Richardson M, Revankar S, Slavin MA, Stemler J, Spiess B, Taj-Aldeen SJ, Warris A, Woo PCY, Young JH, Albus K, Arenz D, Arsic-Arsenijevic V, Bouchara JP, Chinniah TR, Chowdhary A, de Hoog GS, Dimopoulos G, Duarte RF, Hamal P, Meis JF, Mfinanga S, Queiroz-Telles F, Patterson TF, Rahav G, Rogers TR, Rotstein C, Wahyuningsih R, Seidel D, Cornely OA. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European confederation of medical mycology in cooperation with the international society for human and animal mycology and the American society for microbiology. Lancet Infect Dis. 2021 doi: 10.1016/s1473-3099(20)30784-2. [DOI] [PubMed] [Google Scholar]
- 2.Nucci M, Jenks J, Thompson GR, Hoenigl M, Santos MCD, Forghieri F, Rico JC, Bonuomo V, López-Soria L, Lass-Flörl C, Candoni A, Garcia-Vidal C, Cattaneo C, Buil J, Rabagliati R, Roiz MP, Gudiol C, Fracchiolla N, Campos-Herrero MI, Delia M, Farina F, Fortun J, Nadali G, Sastre E, Colombo AL, Pérez Nadales E, Alastruey-Izquierdo A, Pagano L. Do high MICs predict the outcome in invasive fusariosis? J Antimicrob Chemother. 2020 doi: 10.1093/jac/dkaa516. [DOI] [PubMed] [Google Scholar]
- 3.Nucci M, Marr KA, Vehreschild MJ, de Souza CA, Velasco E, Cappellano P, Carlesse F, Queiroz-Telles F, Sheppard DC, Kindo A, Cesaro S, Hamerschlak N, Solza C, Heinz WJ, Schaller M, Atalla A, Arikan-Akdagli S, Bertz H, Galvao CC, Herbrecht R, Hoenigl M, Harter G, Hermansen NE, Josting A, Pagano L, Salles MJ, Mossad SB, Ogunc D, Pasqualotto AC, Araujo V, Troke PF, Lortholary O, Cornely OA, Anaissie E. Improvement in the outcome of invasive fusariosis in the last decade. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2013 doi: 10.1111/1469-0691.12409. [DOI] [PubMed] [Google Scholar]
- 4.Jenks JD, Reed SL, Seidel D, Koehler P, Cornely OA, Mehta SR, Hoenigl M. Rare mould infections caused by Mucorales, Lomentospora prolificans and Fusarium, in San Diego, CA: the role of antifungal combination therapy. Int J Antimicrob Agents. 2018;52:706–712. doi: 10.1016/j.ijantimicag.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nucci M, Shoham S, Abdala E, Hamerschlak N, Rico JC, Forghieri F, Nouer SA, Cappellano P, Solza C, Gonzaga Y, Nadali G, Nucci F, Colombo AL, Albuquerque AM, Queiroz-Telles Filho F, Lima CBL, Arrais-Rodrigues C, Rocha V, Marty FM. Outcomes of patients with invasive fusariosis who undergo further immunosuppressive treatments, Is there a role for secondary prophylaxis? Mycoses. 2019;62:413–417. doi: 10.1111/myc.12901. [DOI] [PubMed] [Google Scholar]
- 6.Nucci M, Anaissie EJ, Queiroz-Telles F, Martins CA, Trabasso P, Solza C, Mangini C, Simoes BP, Colombo AL, Vaz J, Levy CE, Costa S, Moreira VA, Oliveira JS, Paraguay N, Duboc G, Voltarelli JC, Maiolino A, Pasquini R, Souza CA. Outcome predictors of 84 patients with hematologic malignancies and Fusarium infection. Cancer. 2003;98:315–319. doi: 10.1002/cncr.11510. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy M, Rosengart A, Schuetz AN, Kontoyiannis DP, Walsh TJ. Mold infections of the central nervous system. N Engl J Med. 2014;371:150–160. doi: 10.1056/NEJMra1216008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashida MZ, Seque CA, Enokihara M, Porro AM. Disseminated fusariosis with cutaneous involvement in hematologic malignancies: report of six cases with high mortality rate. An Bras Dermatol. 2018;93:726–729. doi: 10.1590/abd1806-4841.20187476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Disease Outbreak News; Outbreak of suspected fungal meningitis associated with surgical procedures performed under spinal anaesthesia – the United States of America and Mexico. 2023. https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON470.
- 10.Centers for Disease Control and Prevention NCfEaZIDN, Division of Healthcare Quality Promotion (DHQP). Fungal meningitis outbreak associated with procedures performed under epidural anesthesia in Matamoros, Mexico. 2023. https://www.cdc.gov/hai/outbreaks/meningitis-epidural-anesthesia.html#anchor_1686761372360.
- 11.Organization. PAHOWH. 2022. Meningitis of unknown origin. December 10, 2022, Washington, D.C.: PAHO/WHO; 2022.
- 12.de Medeiros BC, de Medeiros CR, Werner B, Neto JZ, Loddo G, Pasquini R, Bleggi-Torres LF. Central nervous system infections following bone marrow transplantation: an autopsy report of 27 cases. J Hematother Stem Cell Res. 2000;9:535–540. doi: 10.1089/152581600419215. [DOI] [PubMed] [Google Scholar]
- 13.Garcia RR, Min Z, Narasimhan S, Bhanot N. Fusarium brain abscess: case report and literature review. Mycoses. 2015;58:22–26. doi: 10.1111/myc.12271. [DOI] [PubMed] [Google Scholar]
- 14.Center of Disease Control and Prevention (CDC). Important updates on outbreak of fungal meningitis in U.S. patients Who underwent surgical procedures under epidural anesthesia in Matamoros, Mexico. https://emergency.cdc.gov/han/2023/han00492.asp.
- 15.Forster J, Hoenigl M, Suerbaum S, Wagener J, Dichtl K. Serologic biomarkers in Candida and Aspergillus infections of the central nervous system: a comparison of galactomannan, mannan, and β-1,3-D-gucan testing from serum and cerebrospinal fluid. Mycoses. 2022 doi: 10.1111/myc.13451. [DOI] [PubMed] [Google Scholar]
- 16.Hoenigl M, Egger M, Price J, Krause R, Juergen Prattes P, White L. Metagenomic next-generation sequencing of plasma for diagnosis of COVID-19-associated pulmonary aspergillosis. J Clin Microbiol. 2023 doi: 10.1128/jcm.01859-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katiyar SK, Edlind TD. Role for Fks1 in the intrinsic echinocandin resistance of Fusarium solani as evidenced by hybrid expression in Saccharomyces cerevisiae. Antimicrob Agents Chemother. 2009;53:1772–1778. doi: 10.1128/AAC.00020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James JE, Lamping E, Santhanam J, Cannon RD. PDR transporter ABC1 is involved in the innate azole resistance of the human fungal pathogen Fusarium keratoplasticum. Front Microbiol. 2021;12:673206. doi: 10.3389/fmicb.2021.673206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruíz-Cendoya M, Mariné M, Guarro J. Combined therapy in treatment of murine infection by Fusarium solani. J Antimicrob Chemother. 2008;62:543–546. doi: 10.1093/jac/dkn215. [DOI] [PubMed] [Google Scholar]
- 20.Center for Disease Control and Prevention (CDC). Interim recommendations for diagnosing and managing suspected fungal meningitis associated with epidural anesthesia administered in Matamoros, Mexico. https://emergency.cdc.gov/coca/calls/2023/callinfo_060823.asp
- 21.Gebremariam T, Gu Y, Alkhazraji S, Youssef E, Shaw KJ, Ibrahim AS. The combination treatment of fosmanogepix and liposomal amphotericin B is superior to monotherapy in treating experimental invasive mold infections. Antimicrob Agents Chemother. 2022;66:e0038022. doi: 10.1128/aac.00380-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoenigl M, Sprute R, Egger M, Arastehfar A, Cornely OA, Krause R, Lass-Flörl C, Prattes J, Spec A, Thompson GR, 3rd, Wiederhold N, Jenks JD. The antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs. 2021 doi: 10.1007/s40265-021-01611-0:1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiederhold NP, Najvar LK, Jaramillo R, Olivo M, Birch M, Law D, Rex JH, Catano G, Patterson TF. The orotomide olorofim is efficacious in an experimental model of central nervous system coccidioidomycosis. Antimicrob Agents Chemother. 2018;62:10–1128. doi: 10.1128/AAC.00999-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellin P, King R, Urban M, Hammond-Kosack KE, Legrève A. The adaptation of Fusarium culmorum to DMI fungicides is mediated by major transcriptome modifications in response to azole fungicide, including the overexpression of a PDR transporter (FcABC1) Front Microbiol. 2018;9:1385. doi: 10.3389/fmicb.2018.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abou Ammar G, Tryono R, Döll K, Karlovsky P, Deising HB, Wirsel SG. Identification of ABC transporter genes of Fusarium graminearum with roles in azole tolerance and/or virulence. PLoS ONE. 2013;8:e79042. doi: 10.1371/journal.pone.0079042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leplat JFH, Abid M, Steinberg C. Survival of Fusarium graminearum, the causal agent of Fusarium head blight. A review. Agron Sustain Dev. 2013;33(1):97–111. doi: 10.1007/s13593-012-0098-5. [DOI] [Google Scholar]
- 27.Verweij PE, Arendrup MC, Alastruey-Izquierdo A, Gold JAW, Lockhart SR, Chiller T, White PL. Dual use of antifungals in medicine and agriculture: How do we help prevent resistance developing in human pathogens? Drug Resist Updates. 2022;65:100885. doi: 10.1016/j.drup.2022.100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatamoto M, Aizawa R, Kobayashi Y, Fujimura M. A novel fungicide aminopyrifen inhibits GWT-1 protein in glycosylphosphatidylinositol-anchor biosynthesis in Neurospora crassa. Pestic Biochem Physiol. 2019;156:1–8. doi: 10.1016/j.pestbp.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Pirofski LA, Casadevall A. Q and A: What is a pathogen? A question that begs the point. BMC Biol. 2012;10:6. doi: 10.1186/1741-7007-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiller TM, Roy M, Nguyen D, Guh A, Malani AN, Latham R, Peglow S, Kerkering T, Kaufman D, McFadden J, Collins J, Kainer M, Duwve J, Trump D, Blackmore C, Tan C, Cleveland AA, MacCannell T, Muehlenbachs A, Zaki SR, Brandt ME, Jernigan JA. Clinical findings for fungal infections caused by methylprednisolone injections. N Engl J Med. 2013;369:1610–1619. doi: 10.1056/NEJMoa1304879. [DOI] [PubMed] [Google Scholar]
- 31.Tawfiq RK, Ranganath N, Lehman V, Yin LX, Schuetz AN. Breakthrough invasive Fusarium orbital rhinosinusitis with meningitis. Mayo Clin Proc. 2022;97:1953–1955. doi: 10.1016/j.mayocp.2022.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Alavi Darazam I, Sharifi G, Jamali E, Khodavaisy S, Javandoust Gharehbagh F, Hakamifard A. Meningoencephalitis caused by Fusarium proliferatum: an unusual case. Infection. 2022;50:1023–1027. doi: 10.1007/s15010-022-01761-7. [DOI] [PubMed] [Google Scholar]
- 33.Karthigeyan M, Singh K, Kaur H, Salunke P, Pandey J, Nallasamy K. Multiple Fusarium brain abscesses in a young child. Childs Nerv Syst. 2022;38:1017–1021. doi: 10.1007/s00381-021-05320-7. [DOI] [PubMed] [Google Scholar]
- 34.Kaur R, Panda P. Fusarium co-infection in a patient of tubercular meningitis. J Neuroanaesthesiol Crit Care. 2016;03(03):249–251. doi: 10.4103/2348-0548.190076. [DOI] [Google Scholar]
- 35.Peterson A, Pham MH, Lee B, Commins D, Cadden J, Giannotta SL, Zada G. Intracranial fusarium fungal abscess in an immunocompetent patient: case report and review of the literature. J Neurol Surg Rep. 2014;75:e241–e245. doi: 10.1055/s-0034-1387182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anten S, Heddema ER, Visser O, Zweegman AS. Images in haematology. Cerebral fungal abscess in a patient with acute promyelocytic leukaemia. Br J Haematol. 2008;140:253. doi: 10.1111/j.1365-2141.2007.06813.x. [DOI] [PubMed] [Google Scholar]
- 37.Antunes NL, Hariharan S, DeAngelis LM. Brain abscesses in children with cancer. Med Pediatr Oncol. 1998;31:19–21. doi: 10.1002/(SICI)1096-911X(199807)31:1<19::AID-MPO4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 38.Steinberg GK, Britt RH, Enzmann DR, Finlay JL, Arvin AM. Fusarium brain abscess. Case report J Neurosurg. 1983;58:598–601. doi: 10.3171/jns.1983.58.4.0598. [DOI] [PubMed] [Google Scholar]
- 39.Abramowsky CR, Quinn D, Bradford WD, Conant NF. Systemic infection by fusarium in a burned child. The emergence of a saprophytic strain. J Pediatr. 1974;84:561–564. doi: 10.1016/S0022-3476(74)80681-5. [DOI] [PubMed] [Google Scholar]
- 40.Anaissie E, Kantarjian H, Jones P, Barlogie B, Luna M, Lopez-Berestein G, Bodey GP. Fusarium. A newly recognized fungal pathogen in immunosuppressed patients. Cancer. 1986;57:2141–2145. doi: 10.1002/1097-0142(19860601)57:11<2141::AID-CNCR2820571110>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 41.Agamanolis DP, Kalwinsky DK, Krill CE, Jr, Dasu S, Halasa B, Galloway PG. Fusarium meningoencephalitis in a child with acute leukemia. Neuropediatrics. 1991;22:110–112. doi: 10.1055/s-2008-1071428. [DOI] [PubMed] [Google Scholar]
- 42.Bleggi-Torres LF, de Medeiros BC, Neto JZ, Loddo G, Telles FQ, de Medeiros CR, Pasquini R. Disseminated Fusarium sp. infection affecting the brain of a child after bone marrow transplantation. Bone Marrow Transplant. 1996;18:1013–1015. [PubMed] [Google Scholar]
- 43.Vincent AL, Cabrero JE, Greene JN, Sandin RL. Successful voriconazole therapy of disseminated Fusarium solani in the brain of a neutropenic cancer patient. Cancer Control. 2003;10:414–419. doi: 10.1177/107327480301000511. [DOI] [PubMed] [Google Scholar]
- 44.Kleinschmidt-Demasters BK. Disseminated Fusarium infection with brain abscesses in a lung transplant recipient. Clin Neuropathol. 2009;28:417–421. doi: 10.5414/NPP28417. [DOI] [PubMed] [Google Scholar]
- 45.Njambi S, Huttova M, Kovac M, Freybergh PF, Bauer F, Muli JM. Fungal neuroinfections: rare disease but unacceptably high mortality. Neuro Endocrinol Lett. 2007;28(Suppl 2):25–26. [PubMed] [Google Scholar]
- 46.Muhammed M, Anagnostou T, Desalermos A, Kourkoumpetis TK, Carneiro HA, Glavis-Bloom J, Coleman JJ, Mylonakis E. Fusarium infection: report of 26 cases and review of 97 cases from the literature. Medicine (Baltimore) 2013;92:305–316. doi: 10.1097/MD.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boutati EI, Anaissie EJ. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years’ experience at a cancer center and implications for management. Blood. 1997;90:999–1008. doi: 10.1182/blood.V90.3.999. [DOI] [PubMed] [Google Scholar]
- 48.Chen YJ, Chou CL, Lai KJ, Lin YL. Fusarium brain abscess in a patient with diabetes mellitus and liver cirrhosis. Acta Neurol Taiwan. 2017;26(3):128–132. [PubMed] [Google Scholar]
- 49.Okura Y, Kawamura N, Okano M, Toita N, Takezaki S, Yamada M, Kobayashi I, Ariga T. Fusarium falciforme infection in a patient with chronic granulomatous disease: unique long-term course of epidural abscess. Pediatr Int. 2015;57:e4–e6. doi: 10.1111/ped.12458. [DOI] [PubMed] [Google Scholar]