Abstract
We present a diagnostically challenging case of intimal sarcoma of the pulmonary artery (PA) due to the histologic finding of a sclerosing appearance with no appreciable spindle/pleomorphic cell proliferation. Initial endarterectomy specimens were composed of sclerosing extracellular matrix with a few bland cells, some recanalization, and fibrin thrombi, impeding the confirmation of intimal sarcoma as these findings were also consistent with chronic thromboembolic pulmonary hypertension. However, the patient experienced recurrence 5 years later, and the second endarterectomy specimens revealed more firm and solid mass and the proliferation of atypical spindle/pleomorphic cells within a myxomatous matrix in the distal PA, leading to the definitive diagnosis of undifferentiated intimal sarcoma of the PA. The archival specimens from the endarterectomy confirmed intense MDM2 expression by immunohistochemistry, suggesting its role as a potential diagnostic marker for intimal sarcoma. This case highlights that prominent sclerosing extracellular matrix with very few atypical cells should raise the possibility of intimal sarcoma of the PA and that high index of suspicion, generous sampling, and ancillary tests are critical for accurate diagnosis. In this case, the tumor was incidentally removed by endarterectomy, resulting in 5 years of survival.
Keywords: chronic thromboembolic pulmonary hypertension, intimal sarcoma, pathology, pulmonary artery, pulmonary endarterectomy
CASE DESCRIPTION
A 60‐year‐old man presented with 1 year history of progressive dyspnea. Initial evaluation with contrast‐enhanced computed tomography (CT) revealed a significant filling defect in pulmonary arteries (PAs) (Figure 1A‐a), which was not resolved with thrombolytic therapy, and he was referred to our institution for suspicious chronic thromboembolic pulmonary hypertension (CTEPH). Notably, he had no history of deep vein thrombosis, acute pulmonary emboli, or thrombotic diathesis. The hemodynamic features, including a wide PA pulse pressure (96/13 mmHg) and pulmonary angiographical features such as the total occlusion in the left main PA (Figure 1B‐a,b), were not typical for CTEPH. The patient underwent pulmonary endarterectomy (PEA), and a markedly thickened, hard, whitish tissue was removed from bilateral PA tree, which extended retrogradely further down the PA trunk to end just above the pulmonary valve, was excised (Figure 1C). PEA led to marked improvement of clinical symptoms and CT (Figure 1A‐b) and angiographic findings (Figure 1B‐c,d). While these features were atypical for CTEPH, histologic examination revealed sclerosing extracellular matrix with a few bland cells, some recanalization, and fibrin thrombi without appreciable proliferation of atypical spindle/pleiomorphic cells (Figure 1D). Therefore, optimal anticoagulation therapy with close monitoring was continued. Recurrence of obstruction in some PA branches and mild pulmonary hypertension were observed during the first annual follow‐up visit. During the fifth year following PEA, dyspnea significantly worsened. Contrast‐enhanced CT demonstrated massive filling defects in the right main PA (Figure 1E‐a), with a mean PA pressure of 50 mmHg. Pulmonary angiography revealed filling detect and severe stenosis, which were not typical for CTEPH (Figure 1F). These findings raised the possibility of a tumor, but 18F‐fluoro‐2‐deoxyglucose positron emission tomography showed no uptake. The second PEA resulted in improved hemodynamics and CT findings (Figure 1E‐b). However, on gross examination the specimen collected from the right PA during the second PEA appeared as a firm and solid branching cast of the PA tree with a gelatinous mass in a distal tip (Figure 1G). Histologic findings demonstrated the proliferation of atypical spindle/pleiomorphic cells within a myxomatous matrix (Figure 1H), without specific differentiation determined by immunohistochemistry, leading to the definitive diagnosis of undifferentiated intimal sarcoma of the PA. Although the left PA appeared uninvolved by gross examination, tumor cells were found in the sampled specimens. Despite adjuvant chemotherapy, the patient died 6 months later.
Figure 1.
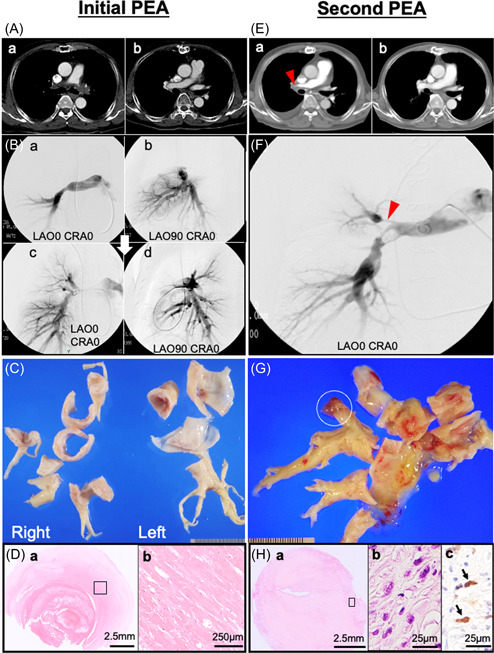
(A‐a) Computed tomography (CT) imaging before the first pulmonary endarterectomy (PEA) showing a filling defect in the main to bilateral pulmonary arteries. (B‐a, b) Pulmonary arteriography showing total occlusion of the left main pulmonary artery (PA) and multiple occlusions and narrowing of the right PA at the lobar level. (A‐b) CT and (B‐c, d) angiography after PEA showing improvement of distal perfusion in both pulmonary arteries. (C) Gross images of specimens obtained during the PEA showing a solid non‐mass‐forming lesion extending from the proximal PA to the sublobar branches of the pulmonary arteries. (D‐a) Microscopic image showing a fresh thrombus attached to the organized thrombus in the background of a sclerosing extracellular matrix (hematoxylin & eosin staining: H&E). (D‐b) High power view of the open square area of D‐a showing no tumor cells (H&E). (E‐a) CT imaging before the second PEA showing a filling defect in the proximal section of the right PA (red arrowhead). (E‐b) Improved contrast enhancement on CT after the second PEA. (F) Angiography showing occlusions and severe stenosis with a thread‐like appearance of the right PA (red arrowhead). (G) The PA mold specimen obtained during the second PEA shows a toffee‐like appearance with a gelatinous mass in a distal tip (white open circle). (H‐a) Microscopically, a myxomatous area containing proliferation of atypical spindle/pleomorphic cells (H&E). (H‐b) High power view of the open square area of H‐a showing atypical spindle cells (H‐b: H&E, H‐c: MDM2 immunohistochemical staining). The nuclei of spindle/pleomorphic cells positive for MDM2 (arrow).
The present case predated the availability of modern molecular markers; immunohistochemical examination of the specimens of the second PEA from the archival paraffin‐embedded/formalin‐fixed paraffin blocks confirmed intense nuclear immunostaining of MDM2, a potential diagnostic marker for intimal sarcoma (Figure 1H).
DISCUSSION
The prognosis of intimal sarcoma is very poor without surgical resection, with a 13% 1‐month mortality rate, and the median survival is less than 1 year in patients with incomplete resection. 1 , 2 Intimal sarcoma of the PA is occasionally misdiagnosed as CTEPH due to similarities in clinical and radiographic presentation. 3 , 4 In a pathologic study, PA intimal sarcoma was found in 1%−4% of PEA specimens obtained from patients with suspicious CTEPH. 5 While histologic evaluation is essential for diagnosis, intimal sarcoma can be diagnostically challenging due to the variety of histologic features and the presence of low‐grade tumor cells. 6 The gross descriptions of intimal sarcoma typically include an intraluminal branching mass attached to the arterial wall. 7 Sclerosing PA sarcoma is a rare type which results from fibrosis or hyalinization. 8 The most effective way of improving short‐term survival has been surgical intervention. 9 Improved survival with this disease can be accomplished with early diagnosis and radical surgical resection, possibly with adjuvant chemotherapy and radiation. 9 Today, immunohistochemical evidence of MDM2 overexpression in tumor cells may aid in diagnosing intimal sarcoma. 10 This case underwent PEA with 5 years‐survival. When the tumor is removed early phase, as completely as possible, the prognosis may be improved, indicating the significance of early diagnosis using histological evaluation. Therefore, given the potential for hidden malignancy in cases mimicking CTEPH, a high index of suspicion from clinical, hemodynamic, radiologic, and surgical findings is key to reach an accurate diagnosis. In cases with atypical presentation or findings, we propose detailed histologic assessment of all lesions with the help of molecular markers.
AUTHOR CONTRIBUTIONS
Conceptualization: Hatsue Ishibashi‐Ueda. Patient care: Morikazu Nishihira. Data curation: Keiko Ohta‐Ogo and Yoshihiko Ikeda. Drafting the article: Kisaki Amemiya and Morikazu Nishihira. Treatment decisions: Hiroaki Sasaki and Hitoshi Ogino. Supervision: Takeshi Ogo, Kinta Hatakeyama, and Hatsue Ishibashi‐Ueda.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The Institutional Research and Ethics Committee of the National Cerebral and Cardiovascular Center approved this study (R20075‐2). Data use was explained on our websites, and the 'opt‐out' approach for consent was approved.
ACKNOWLEDGMENTS
This study was supported in part by Grants‐in‐Aid for Scientific Research in Japan (grant no, 22K16122) from the Ministry of Education, Culture, Sport, Science, and Technology, Japan.
Amemiya K, Nishihira M, Ishibashi‐Ueda H, Ohta‐Ogo K, Ogo T, Ikeda Y, Hatakeyama K, Sasaki H, Ogino H. A 5‐year survivor of endarterectomy for sclerosing undifferentiated intimal sarcoma of the pulmonary artery: importance of clinical suspicion and careful histologic evaluation. Pulm Circ. 2023;13:e12315. 10.1002/pul2.12315
Kisaki Amemiya and Morikazu Nishihira contributed equally to this work.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
REFERENCES
- 1. Mussot S, Ghigna MR, Mercier O, Fabre D, Fadel E, Le Cesne A, Simonneau G, Dartevelle P. Retrospective institutional study of 31 patients treated for pulmonary artery sarcoma. Eur J Cardiothorac Surg. 2013;43(4):787–793. [DOI] [PubMed] [Google Scholar]
- 2. Grazioli V, Vistarini N, Morsolini M, Klersy C, Orlandoni G, Dore R, D'Armini AM. Surgical treatment of primary pulmonary artery sarcoma. J Thorac Cardiovasc Surg. 2014;148(1):113–118. [DOI] [PubMed] [Google Scholar]
- 3. Bandyopadhyay D, Panchabhai TS, Bajaj NS, Patil PD, Bunte MC. Primary pulmonary artery sarcoma: a close associate of pulmonary embolism‐20‐year observational analysis. J Thorac Dis. 2016;8(9):2592–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Srivali N, Yi ES, Ryu JH. Pulmonary artery sarcoma mimicking pulmonary embolism: a case series. QJM: Monthly J Associa Phy. 2017;110(5):283–286. [DOI] [PubMed] [Google Scholar]
- 5. Bernard J, Yi ES. Pulmonary thromboendarterectomy: a clinicopathologic study of 200 consecutive pulmonary thromboendarterectomy cases in one institution. Hum Pathol. 2007;38(6):871–877. [DOI] [PubMed] [Google Scholar]
- 6. Tavora F, Cresswell N, Li L, Ripple M, Fowler D, Burke A. Sudden coronary death caused by pathologic intimal thickening without atheromatous plaque formation. Cardiovasc Pathol. 2011;20(1):51–57. [DOI] [PubMed] [Google Scholar]
- 7. Burke AP, Virmani R. Sarcomas of the great vessels. A clinicopathologic study. Cancer. 1993;71(5):1761–1773. [DOI] [PubMed] [Google Scholar]
- 8. Tavora F, Miettinen M, Fanburg‐Smith J, Franks TJ, Burke A. Pulmonary artery sarcoma: a histologic and follow‐up study with emphasis on a subset of low‐grade myofibroblastic sarcomas with a good long‐term follow‐up. Am J Surg Pathol. 2008;32(12):1751–1761. [DOI] [PubMed] [Google Scholar]
- 9. Tuft C, Maheepala K, Raguparan A, Naeem A, Lodh S, Lindstrom S. Pulmonary artery sarcoma: an important mimic of pulmonary embolism—case reports and literature review. Respirol Case Rep. 2022;10(2):e0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koelsche C, Benhamida JK, Kommoss FKF, Stichel D, Jones DTW, Pfister SM, Heilig CE, Fröhling S, Stenzinger A, Buslei R, Mentzel T, Baumhoer D, Ladanyi M, Antonescu CR, Flucke U, Gorp J, Bode‐Lesniewska B, Deimling A, Mechtersheimer G. Intimal sarcomas and undifferentiated cardiac sarcomas carry mutually exclusive MDM2, MDM4, and CDK6 amplifications and share a common DNA methylation signature. Mod Pathol. 2021;34(12):2122–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.


