Abstract
Interspecies grafting is an economically relevant technique that allows beneficial shoot and root combinations from separate species to be combined. One hypothesis for the basis of graft compatibility revolves around taxonomic relatedness. To test how phylogenetic distance affects interspecific graft compatibility within the economically important Solanaceae subfamily, Solanoideae, we characterized the anatomical and biophysical integrity of graft junctions between four species: tomato (Solanum lycopersicum), eggplant (Solanum melongena), pepper (Capsicum annuum), and groundcherry (Physalis pubescens). We analyzed the survival, growth, integrity, and cellular composition of the graft junctions. Utilizing various techniques, we were able to quantitatively assess compatibility among the interspecific grafts. Even though most of our graft combinations could survive, we show that only intrageneric combinations between tomato and eggplant are compatible. Unlike incompatible grafts, the formation of substantial vascular reconnections between tomato and eggplant in the intrageneric heterografts likely contributed to biophysically stable grafts. Furthermore, we identified 10 graft combinations that show delayed incompatibility, providing a useful system to pursue deeper work into graft compatibility. This work provides new evidence that graft compatibility may be limited to intrageneric combinations within the Solanoideae subfamily. Further research amongst additional Solanaceous species can be used to test the extent to which our hypothesis applies to this family.
Keywords: Graft compatibility, interspecies interaction, pepper, Solanaceae, tomato, vascular reconnection
Within the economically important Solanaceae subfamily, Solanoideae, we demonstrate that graft compatibility is taxonomically limited, thus expanding our understanding of the evolutionary constraints that determine graft compatibility within the nightshade family.
Introduction
Grafting is an ancient agricultural practice, where distinct plant parts are combined into a single organism (Mudge et al., 2009). The root system is known as the stock and the grafted vegetative portion is known as the scion (indicated as scion:stock throughout this paper). The term graft compatibility refers to the capacity for a given scion and stock combination to regenerate and stably reconnect their non-vascular and vascular tissue within an anatomically unique region, referred to as the graft junction (Benda et al., 1960; Rasool et al., 2020). Despite our limited understanding regarding the determinants of graft compatibility, it has been noted for thousands of years that taxonomic relatedness is a good predictor of graft success (Pease, 1933; Mudge et al., 2009; Andrews and Marquez, 2010; Goldschmidt, 2014). For example, intraspecific grafts are generally more likely to be compatible than interspecific grafts (Mudge et al., 2009; Goldschmidt, 2014). In contrast, there are some families that exhibit wide graft compatibility, including many examples of intergeneric graft compatibility, such as Rosaceae where economically relevant species are routinely grafted together from seperate genera, such as apples (Malus domestica) and pears (Pyrus communis) (Westwood, 1993; Errea et al., 1994).
The Solanaceae or nightshade family is another such family that is often regarded as having wide graft compatibility (Westwood, 1993; Kawaguchi et al., 2008; Zeist et al., 2018). Recent work within the nightshade family highlights this broad compatibility, demonstrating that individuals from the Nicotiana and Petunia genera can be grafted with diverse species, including across family limits (Notaguchi et al., 2020; Kurotani et al., 2022). Whether the graft-compatible nature of Nicotianoideae applies to all of the Solanaceae remains unknown. Despite the abundance of economically important plants contained within the Solanoideae subfamily, little is known about the limits to interspecific graft compatibility, with a few notable exceptions (i.e. tomato:eggplant, eggplant:tomato, tomato:potato, and eggplant:potato; Romano and Paratore, 2001; Liu et al., 2009; Thompson and Morgan, n.d.). Previous work has shown that tomato and pepper are incompatible and fail to form vascular reconnections within the first week of grafting (Kawaguchi et al., 2008; Thomas et al., 2022). Despite this knowledge, the graft compatibility between pepper and other closely related crops remains unknown (Kawaguchi et al., 2008; Zeist et al., 2018; Thomas et al., 2022).
This work aims to determine the taxonomic limits that constrain intrafamily graft compatibility; we conducted a graft trial with four Solanoideae crops: tomato (Solanum lycopersicum var. M82), eggplant (Solanum melongena var. BARI-6), pepper (Capsicum annuum var. California Wonder), and groundcherry (Physalis pubescens). All four species belong to the Solanoideae subfamily, which is estimated to have diverged from Nicotianoideae 24 million years ago (ma; Fig. 1; Särkinen et al., 2013). Solanum, which contains over half of the species in Solanaceae, has an estimated divergence time from Capsicum and Physalinae of 19 ma (Frodin, 2004; Stern and Bohs, 2012; Särkinen et al., 2013). Soon after this divergence, Capsicum split into separate subclades ~18 ma (Särkinen et al., 2013). Eggplant and tomato, both members of Solanum, are the most closely related of the four crops in our study and are estimated to have diverged 14 ma (Särkinen et al., 2013).
Fig. 1.
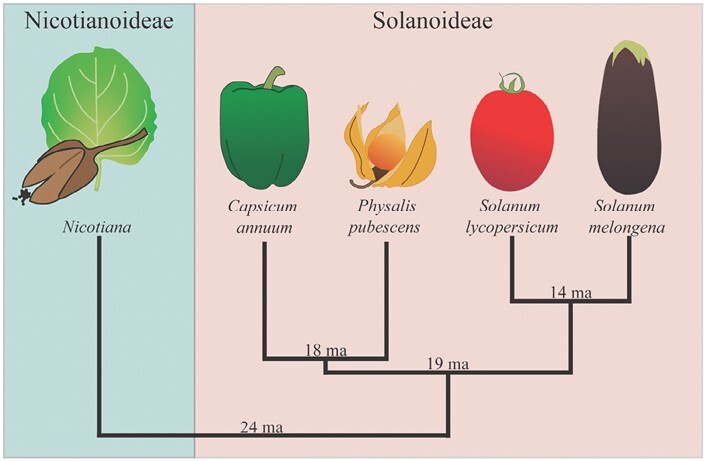
Phylogenetic relationship between Solanaceae subfamilies Nicotianoideae and Solanoideae. Nicotianoideae and Solanoideae are sister subfamilies within the Solanaceae. Three Solanoideae tribes are represented: Capsiceae (Capsicum annuum), Physaleae (Physalis pubescens), and Solaneae (Solanum lycopersicum and Solanum melongena). The estimated time of divergence is shown above the root of each branch (Särkinen et al., 2013).
Graft survival is often equated with graft compatibility. Our work within Solanoideae examines the anatomy of graft junctions and demonstrates that survival can occur in the absence of true compatibility. In order to test compatibility, we performed an extensive reciprocal graft trial, analyzed the vascular tissue in the graft junction, conducted stem stability tests, and measured lateral stem growth 30 d after grafting. By testing the graft junctions, we show that these four Solanoideae species, while broadly capable of surviving grafting, are generally not compatible, with the exception of intrageneric grafts between tomato and eggplant. To aid in the rapid identification of incompatible graft combinations, and avoid future conflation of survival and compatibility, we demonstrate that a simple bend test for biophysical stability within the junction can be used to diagnose delayed graft incompatibility.
Materials and methods
Plant materials and growth conditions
A total of 200 Capsicum annuum var. California Wonder and 200 Physalis pubescens seeds were stratified with 50% bleach for 30 s and then rinsed five times with sterile distilled water. The seeds were planted directly into LM-111 soil and kept on heat mats until grafted. A total of 100 Solanum lycopersicum var. M82 and 80 Solanum melongena var. BARI-6 seeds were bleach-stratified and placed into Phyatrays© in the dark for 3 d, then moved to the light for 3 d, and finally transferred into LM-111 soil on heat mats until grafted. All seedlings were grown in climate-controlled chambers set to 23 °C with 16:8 h day/night light cycles under F54T5/841/HO fluorescent bulbs (500–800 µmol m–2 s–1).
Graft conditions
Four-week-old pepper and groundcherry seedlings, and 2-week-old tomato and eggplant seedlings were used for grafting. This seedling germination timeline was optimized so the four species were of similar stem diameter (1.5–1.75 ± 3 mm) and hypocotyl length. The following grafts were performed between tomato (T), pepper (P), groundcherry (GC), and eggplant (E): T:T, P:P, GC:GC, T:P, P:T, T:GC, GC:T, P:GC, and GC:P (n=20) and E:E, T:E, E:T, P:E, E:P, GC:E, and E:GC (n=15). Any combination which included eggplant only had 15 graft replicates due to the low availability of the BARI-6 seed variety. Scion and stocks were joined with a slant graft below the cotyledons (Kubota et al., 2008). Grafts were held together with 1.5 mm silicon-top grafting clips (Johnny’s Selected Seeds, Albion, ME, USA). Grafted plants were generously watered, covered with plastic domes, and placed in the dark for 3 d. On day 4, plants were returned to light (500–800 µmol m–2 s–1). To test Solanoideae graft compatibility, survival of each graft combination was noted on 0, 5, 7, 10, 14, 21, and 30 days after grafting (DAG). Herbaceous grafts heal within the first week (Melnyk, 2017); observing survival up to 30 DAG allowed us to track how survival changes over time in response to physiological properties such as xylem connectivity.
Whole-plant imaging and stem phenotyping
Seedlings were imaged prior to grafting, immediately following grafting, and 30 DAG using a 12 megapixel wide-angle camera (Samsung, South Korea). The shoots of 30 DAG plants were imaged in their pots. The stem of the seedlings prior to grafting and the scion and stock directly above or below the graft junction were measured 30 DAG using digital calipers.
Bend test
Graft junction integrity was tested using the bend test (Thomas et al., 2022). Due to the variable nature of graft survival, biological replicates vary: T:T=42, P:P=29, E:E=40, GC:GC=12, T:E=19, E:T=20, T:P=14, P:T=7, T:GC=6, GC:T=6, P:GC=1, GC:P=15, P:E=18, and E:P=38. GC:E and E:GC had too few replicates to test. All graft combinations were used in the first technical replicate. A second technical replicate was conducted on low-surviving grafts: P:GC, GC:P. P:E, E:P, GC:E, and E:GC.
Tissue collection for confocal imaging
Graft junctions were harvested by cutting 2 cm above and below the cut site. Tissue was fixed and stained with propidium iodide as previously described (Thomas et al., 2022). Fully cleared graft junctions were imaged on a Zeiss LSM880 confocal microscope (Germany) using an argon laser 514 nm beam.
Statistical analysis of grafted plants
All statistical computations and graph generation were performed in R (R Core Team, 2021). Statistical significance of survival and stem integrity were calculated using Fisher’s exact test (Hervé, 2020). Two technical replicates of bend tests were conducted for low survival graft combinations (P:GC, GC:P, P:E, E:P, GC:E, and E:GC). A power analysis was conducted in G*Power using the first technical replicate as an a priori Fisher’s exact test with a power of 0.95 (Faul et al., 2007). The standard error was calculated for all proportions. All interval data were tested for normal distribution and homoscedasticity using Wilks–Shapiro test and Levene’s test from the CAR package (Fox and Weisberg, 2018). All data were found to be homoscedastic but non-parametric (Supplementary Table S5). ANOVA is robust enough to tolerate this violation. Tukey’s honest significant difference test with an adjusted P-value was used to determine pairwise significance. Plots were made in R using ggplot2 and dplyr (Wickham, 2011; R Core Team 2021).
Results
Solanoideae interspecies grafts have varying survival rates 30 days after grafting
We selected four Solanoideae crops (tomato, eggplant, pepper, and groundcherry) from three different tribes (Capsiceae, Physaleae, and Solaneae) to test the taxonomic limits of graft compatibility. These four species were chosen because of their economic importance, as well as their suitability as graft partners based on their similar stem compositions, stem diameters, and growth rates (Fig. 2; Supplementary Fig. S1; Supplementary Table S1).
Fig. 2.
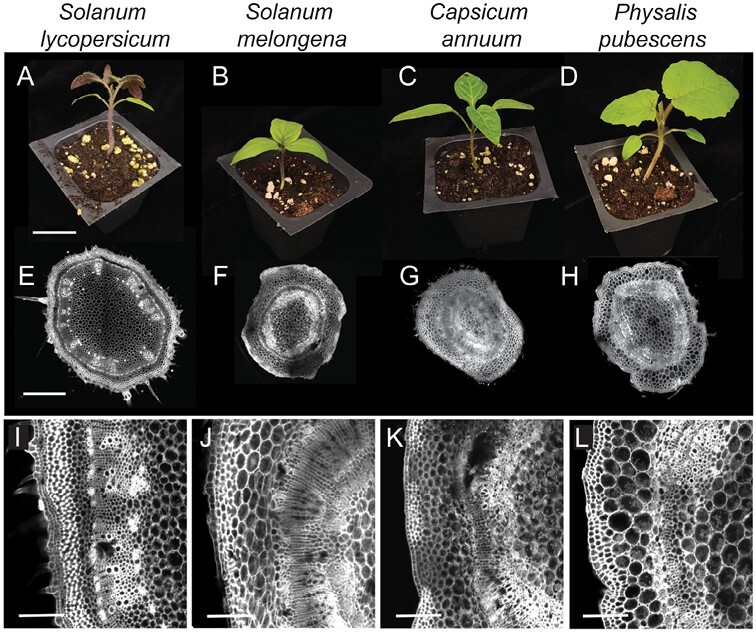
Solanoideae crops share similar stem anatomy. (A–D) Representative images of seedlings at the time of grafting. (E–H) Confocal micrographs show transverse stem sections taken from the location of grafting. (I–L) ×4 zoom of vascular cambium. Solanum lycopersicum (tomato; A, E, I), S. melongena (eggplant; B, F, J) Capsicum annuum (pepper; C, G, K), and Physalis pubescens (groundcherry; D, H, L) are all members of the Solanoideae subfamily. (E–L) were stained with propidium iodide and cleared in methyl salicylate prior to confocal imaging. Replication can be found in Supplementary Fig. S1. (A–D) Scale bar=2 cm, (E–H) scale bar=1 mm, (I–L) scale bar=250 um.
We analyzed the overall appearance of the graft combinations at 30 DAG and observed variable levels of health depending on the species combination. Self-grafts tended to look the healthiest (i.e. turgid and green) (Fig. 3A, F, K, P), while groundcherry heterografts generally appeared the least healthy (Fig. 3D, H, L–O). Despite this, at least one graft survived for each of the 16 combinations, superficially supporting the concept that broad graft compatibility amongst these four species is possible (Fig. 4A). Tomato, pepper, and groundcherry self-grafts survived at a high rate (75–95%), while eggplant self-grafts survived only 53% of the time (Fig. 4). Notably, this lower survival rate for self-grafted eggplant is consistent with previous work on eggplant grafting (Johnson and Miles, 2011). We observed that most heterograft combinations exhibited moderate rates of survival (40–60%), while eggplant and tomato heterografts had high survival rates [eggplant:tomato (87%), eggplant:pepper (80%)], and multiple combinations with ground cherry as a graft partner showed relatively low survival rates [pepper:groundcherry (20%), eggplant:groundcherry (20%), and groundcherry:eggplant (7%); Fig. 4]. Curiously, eggplant showed a strong sense of directionality in graft survival, where eggplant:pepper was one of the best-surviving heterografts (80%), while the reciprocal pepper:eggplant combination was one of the worst (27%). The exact role that graft orientation plays in survival and even compatibility remains unclear (Fig. 4).
Fig. 3.

Graft combinations across four Solanoideae species 30 DAG. (A–P) Representative images of each graft combination taken at 30 DAG. Tomato:tomato (A), eggplant:tomato (B), pepper:tomato (C), groundcherry:tomato (D), tomato:eggplant (E), eggplant:eggplant (F), pepper:eggplant (G), groundcherry:eggplant (H), tomato:pepper (I), eggplant:pepper (J), pepper:pepper (K), groundcherry:pepper (L), tomato:groundcherry (M), eggplant:groundcherry (N), pepper:groundcherry (O), groundcherry:groundcherry (P). The y-axis of the matrix displays the four stocks: Solanum lycopersicum (tomato), S. melongena (eggplant), Capsicum annuum (pepper), and Physalis pubescence (groundcherry). The x-axis also shows the scion species. The phylogenetic relationships of the species are drawn above the scions (Särkinen et al., 2013). Additional replication can be seen in Supplementary Fig. S3. All scale bars=5 cm.
Fig. 4.
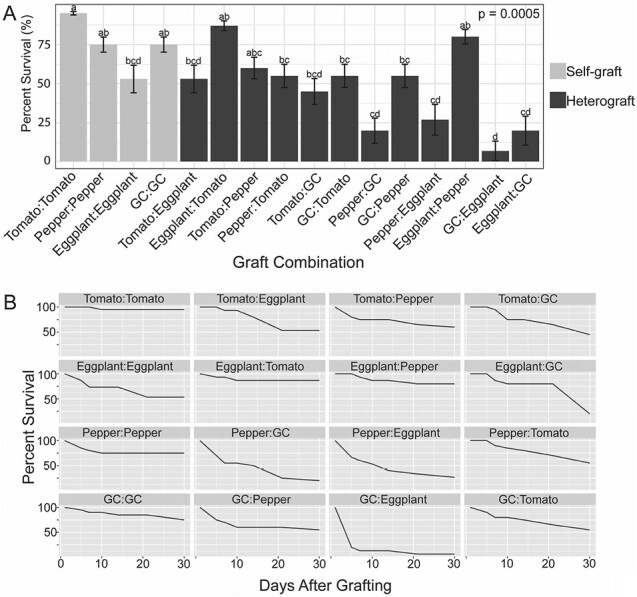
Survival rates for grafted plants 30 DAG. (A) Survival rates 30 DAG for all graft combinations. Light gray bars indicate self-graft combinations, and dark gray bars indicate heterograft combinations. GC=groundcherry. n=20 for all combinations except those containing eggplant, where n=15. Lower case letters above each bar indicate significant differences between the graft combinations based on pairwise comparisons using Fisher’s exact test, P<0.05. Error bars indicate the SE of proportion. (B) Survival rates over time for all graft combinations. Survival rates were calculated for each graft combination at 5, 7, 10, 14, 21, and 30 DAG. All graft combinations included at least 15 graft replicates; for a detailed description of bioreplication, see the Materials and methods.
All intergeneric grafts fail to form vascular reconnections and display delayed incompatibility
To test the qualitative biophysical integrity of the junctions formed in each graft combination, we performed bend tests (Fig. 5). The bend test is a simple field test that can be used to measure the integrity of the junction as a proxy for vascular reconnection (Thomas et al., 2022). A well-formed junction is considered to be the strongest part of the stem, as it is packed with lignified vasculature. If a bent stem snaps at the graft site, that is an indicator of poor vascular connectivity, and that plant fails the bend test. If the stem snaps anywhere outside of the junction, the plant passes the bend test. We were unable to break any self-grafted groundcherry junctions, while self-grafted tomato, pepper, and eggplant junctions broke 7, 28, and 5% of the time, respectively. Most heterografted junctions were easily broken (>97%), except for tomato:eggplant and eggplant:tomato which behaved similarly to self-grafts, and tomato:pepper which is known to show moderate survival despite being incompatible (Thomas et al., 2022). The three moderate graft combinations broke at the junction 37, 45, and 64% of the time, respectively (Fig. 5).
Fig. 5.
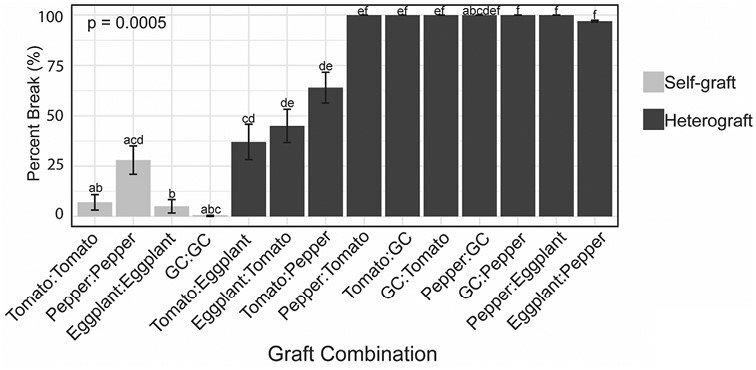
Tomato/eggplant heterografts exhibit strong graft compatibility based on the bend test. The percentage of plants that broke at the graft junction during the bend test. Light gray bars indicate self-grafted plants, and dark gray bars indicate heterografted plants. GC=groundcherry. GC:eggplant and eggplant:GC exhibited insufficient survival to conduct the bend test. Lower case letters above each bar indicate significant differences between graft combinations based on pairwise comparisons using Fisher’s exact test, P<0.05. Error bars indicate the SE of proportion. Replicate values dictated by survival of the graft combinations: T:T=42, P:P=29, E:E=40, GC:GC=12, T:E=19, E:T=20, T:P=14, P:T=7, T:GC=6, GC:T=6, P:GC=1, GC:P=15, P:E=18, E:P=38.
To examine the underlying anatomical basis for graft combinations that failed the bend test, we harvested graft junctions from all of our combinations and used confocal microscopy to examine the vascular anatomy of the graft junction. We have previously used 2D confocal microscopy taken from sections at the center of stems to deduce vascular strand redifferentiation in graft junctions (Frank et al., 2022, Preprint; Thomas et al., 2022). Again, groundcherry:eggplant grafts exhibited insufficient survival to collect replicates for image analysis. Our confocal micrographs of self-grafted tomato (Fig. 6A, E), eggplant (Fig. 6J, M), pepper (Fig. 6Q, U), and groundcherry (Fig. 6Z, AD) show distinct xylem bridges spanning the graft junction 30 DAG, providing a clear indication of vascular reconnection. Likewise, our tomato:eggplant (Fig. 6I, L) and eggplant:tomato (Fig. 6B, F) micrographs show well-formed vascular strands. In contrast, the other nine graft combinations that we imaged failed to form continuous vascular files (Fig. 6). This result is congruent with our bend tests that indicated graft incompatibility for all of the intergeneric combinations that we tested. Although we could not examine the vascular strands of eggplant:groundcherry grafts, the low survival rate for this combination clearly indicates that it is incompatible (Figs 4, 5). Thus, we have identified a collection of nine graft combinations that each exhibit varying survival, with definitive delayed graft incompatibility (Figs 5, 6).
Fig. 6.
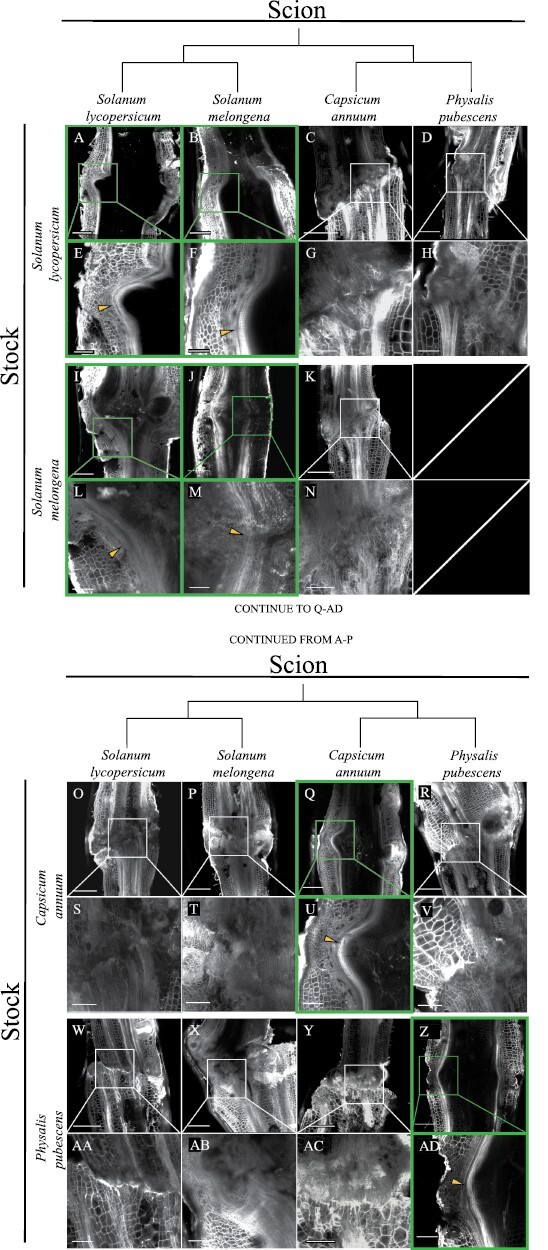
Graft compatibility based on anatomical vascular reconnection is restricted to intrageneric combinations. Confocal micrographs of all surviving graft combinations at 30 DAG. Full junctions for each combination are shown above ×3 magnified panels that provide anatomical detail. Tomato:tomato (A, E), eggplant:tomato (B, F), pepper:tomato (C, G), groundcherry:tomato (D, H), tomato:eggplant (I, M), eggplant:eggplant (J, N), pepper:eggplant (K, O), tomato:pepper (O, S), eggplant:pepper (P,T), pepper:pepper (Q, U), groundcherry:pepper (R, V), tomato:groundcherry (W, AA), eggplant:grounchcherry (X, AB), pepper:groundcherry (Y, AC), groundcherry:groundcherry (Z, AD). Groundcherry:eggplant grafts exhibited insufficient survival to be imaged with statistically relevant replication. The y-axis of the image matrix displays the four stocks: Solanum lycopersicum (tomato), S. melongena (eggplant), Capsicum annuum (pepper), and Physalis pubescence (groundcherry). The x-axis shows the scion genotypes. The phylogenetic relationship of the scions is shown along the x-axis above the scions (Särkinen et al., 2013). Panels outlined with green boxes indicate grafts with successful vascular bridges, and panels outlined with white boxes indicate grafts with failed vascular connections. Yellow arrows point to xylem bridges. Grafts were harvested, stained with propidium iodide, and cleared in methyl salicylate prior to imaging. (A–D, I–K, O–R, and W–Z) Scale bars=1 mm; (E–H, L–N, S–V, and AA–AD) scale bars=333 µm. Additional replicates are included in Supplementary Fig. S3.
To investigate whether the incompatible grafts in our study exhibit other measurable symptoms of graft failure, we measured scion and stock growth rates. Our data show significant differences in lateral growth between all graft combinations based on changes in stem diameter at 30 DAG (ANOVA: scion P<2e-16; stock P< 2e-16; Fig. 7A, B; Supplementary Tables S1–S3). We first wanted to examine the relationship between self- and heterografted stems. Using an ANOVA, we showed that there are significant differences in lateral growth between self- and heterografted stems, with self-grafts generally exhibiting healthier growth, where scions were 148% wider (ANOVA P<2.51e-11) and stocks were 91% wider than their heterografted counterparts (ANOVA P<1.16e-06; Fig. 7A, B; Supplementary Tables S1–S4).
Fig. 7.
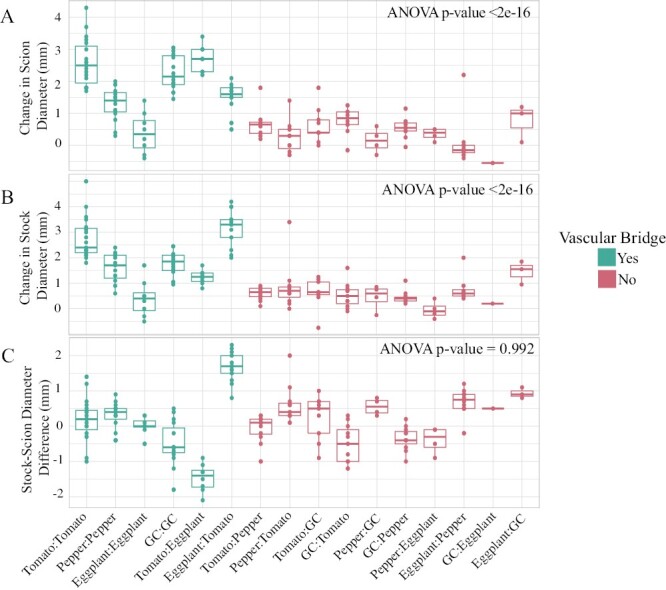
Stem growth is correlated with graft compatibility. (A) The change in scion diameter 30 DAG for all graft combinations. (B) The change in stock diameter 30 DAG for all graft combinations. (C) The difference between stock and scion diameter 30 DAG for all graft combinations. This differential growth was calculated by subtracting the scion diameter from the stock diameter of each graft combination 30 DAG. Teal indicates compatible grafts that formed successful vascular bridges (Fig. 6), and red indicates incompatible grafts that failed to form vascular reconnections. ANOVA was calculated between compatible and incompatible plants, P-value <0.05.
Since our anatomical and biophysical data indicate that eggplant:tomato and tomato:eggplant heterografts are compatible, we next tested if graft compatibility is more strongly correlated with increased lateral stem and stock growth post-grafting. We found a significant, pronounced difference in stem diameter, wherein compatible scions and stock were 392% (ANVOVA P<2e-16) and 213% (P<2e-16) wider than their incompatible counterparts, respectively (Fig. 7A, B). Therefore, the most significant variance that we detected in lateral growth was correlated with graft compatibility.
In woody crops, bulging scions are commonly noted as symptoms of graft incompatibility (Andrews and Marquez, 2010). To test differential growth between the scion and stock of individual plants, we looked at the diameter of the stock minus the diameter of the scion 30 DAG. While many of the heterografted combinations had varying diameters between the scion and stock (Fig. 7C), we found no significant relationship between junction bulging and graft incompatibility (ANOVA P=0.992; Supplementary Table S4).
Discussion
The Solanaceae family is often noted as a highly graftable group of plants, with eggplant, potato, and tobacco all capable of grafting with tomato (Dawson, 1942; Lee and Oda, 2010; Notaguchi et al., 2020). To explore this statement, we conducted an analysis using reciprocal grafts from four agronomically relevant crops: tomato (S. lycopersicum), pepper (C. annuum), eggplant (S. melongena), and groundcherry (P. pubescens; Figs 1, 2). All four of these species belong to the same subfamily, making these four crops more closely related than those used in previous studies looking at graft compatibility between Nicotianideae and Solanoideae (Notaguchi et al., 2015, 2020).
We performed reciprocal grafts amongst all four species, leading to the production of 16 graft combinations (Fig. 3). We observed a variety of survival rates at 30 DAG; self-grafted plants had high survival rates, except for eggplant which performed as previously predicted (Fig. 4; Johnson and Miles, 2011), while most heterografts survived at moderate rates (40–60% survival; Fig. 4). Interestingly, one of the highest surviving heterografted plants 30 DAG was eggplant:pepper. Further investigation into this graft showed that despite eggplant:pepper surviving in high numbers (80%), no vascular bridges were present in the graft junctions imaged, phenocopying the graft junction of many other incompatible graft combinations in Solanaceae such as tomato–pepper grafts (Fig. 6; Thomas et al., 2022).
The ability of non-vascular tissue to heal and sustain life, in the absence of vascular reconnection, is the definition of delayed incompatibility (Argles, 1937; Flaishman et al., 2008). The fact that plants without continuous vascular strands are able to survive raises a whole suite of interesting questions regarding the physiological requirements for sustained life, such as how plants are able to adequately sustain turgor pressure in the absence of continuous vascular connections between root and scion. Additional research into the role of diffusion across the graft junction, water transport through the symplast, and other unknown mechanisms is required to fully understand how delayed incompatible plants can survive.
All 16 graft combinations produced at least one surviving graft, while only six produced vascular bridges (Figs 4–6). Out of the six compatible grafts, four were self-grafts, and only tomato:eggplant and eggplant:tomato were compatible heterografts (Fig. 6). These findings demonstrate the importance of looking beyond graft survival to determine whether a given combination is truly compatible. Further testing, including quantification of graft junction biophysics and functional transport assays such as dye transport, are required for confident characterization of graft compatibility. Bend tests further bolstered our findings that although many of the graft combinations survived at rates similar to compatible grafts, the integrity of the stem was compromised due to reduced or absent vascular reconnections.
By selecting species from different genera, we were able to identify compatibility constraints within the Solanoideae subfamily. As we expected, we found that all self-grafts were compatible. However, the intrageneric grafts that we performed between tomato and eggplant were the only heterografts with true compatibility. While these two species did diverge around 14 ma, they are both members of the Solanum genus. In addition, potato (Solanum tuberosum) is also compatible with tomato and eggplant, further supporting intrageneric compatibility within Solanum (Thompson and Morgan, n.d.). All of the other intergeneric grafts that we tested, regardless of survival, were incompatible. Previous work investigating Solanaceae rootstocks that could be used for eggplant scion production showed that species outside of Solanum are incompatible with eggplant. We were able to extend this model for incompatibility, by identifying intergeneric graft limitations between Solanum, Capsicum, and Physalis (Ali et al., 1990).
While further graft studies are required to definitively state that intergeneric grafts are not possible within Solanoideae, our work demonstrates that phylogenetic constraints play a role in determining graft compatibility. The mechanisms underlying phylogenetic limitations to grafting have yet to be determined; however, we hypothesize that failed communication is responsible for this observed incompatibility. Specifically, the more evolutionarily distant two plants are from one another, the less likely they are to re-establish communication across the graft junction. This could be due to an inability to form secondary plasmodesmata between grafted rootstock and scion partners, as a consequence of either initial incompatible tissue responses creating a necrotic isolation layer or failed intercellular coordination between the graft partners. Alternatively, plasmodesmata may form, and evolutionarily diverged intercellular signaling components could be the cause of incompatibility; for example, diverged receptor–ligand pairs that can no longer interact may be encoded within the distinct genomes. Future studies investigating the role of intercellular signaling, plasmodesmata formation, as well as innate intergeneric immune responses will help to elucidate the precise mechanisms that define the evolutionary boundaries of graft compatibility, and inform the predictive selection and expansion of successful graft partners.
In this study, we show that intergeneric grafting with four species from the Solanoideae produces multiple instances of delayed incompatibility. We also demonstrate that comparing lateral growth of herbaceous stems can act as an early predictor for graft incompatibility. We utilized the bend test as a reliable, fast, and low-tech test for graft compatibility. Using this technique, we were able to show that heterografts between tomato and eggplant form truly compatible junctions, indicated by vascular reconnections that form biophysically stable grafts, making this combination an excellent choice for heterocompatible graft studies. Together, these compatible and incompatible graft combinations provide a useful toolkit to explore the underlying genetic mechanisms of graft compatibility. This work is based on four species and 16 graft combinations, for which we performed hundreds of grafts. Our research provides strong support that graft compatibility within the four species of Solanoideae tested is limited to intrageneric species combinations. It follows that drawing a conclusion about graft compatibility across the Solanoideae as a whole will require further studies with many more species. Future work with additional Solanaceous genera can be used to test whether intrageneric graft limitations exist broadly across the family.
Supplementary data
The following supplementary data are available at JXB online.
Table S1. Growth of grafted plants 0–30 DAG.
Table S2. Tukey multiple comparison of means of scion diameter 30 DAG.
Table S3. Tukey multiple comparison of means of stock diameter 30 DAG.
Table S4. ANOVA results.
Table S5. Levene’s and Wilks–Shapiro tests for ANOVA assumptions.
Fig. S1. Solanoideae species utilized in the graft trial.
Fig. S2. Solanoideae graft combinations 30 DAG.
Fig. S3. Propidium iodide-stained Solanoideae graft junctions 30 DAG.
Fig. S4. Tukey’s multiple comparison of means, Levene’s test, and Wilks–Shapiro tests.
Acknowledgements
The authors thank the Cornell Growth Chamber Facility and the Cornell Institute of Biotechnology Imaging Facility for their assistance.
Contributor Information
Hannah R Thomas, Cornell University, School of Integrative Plant Science, Ithaca, NY 14850, USA.
Alice Gevorgyan, Cornell University, School of Integrative Plant Science, Ithaca, NY 14850, USA.
Margaret H Frank, Cornell University, School of Integrative Plant Science, Ithaca, NY 14850, USA.
Madelaine Bartlett, University of Massachusetts Amherst, USA.
Author contributions
HRT and MHF: conceptualization and design; HRT and AG: gathering experimental data; HRT: data analysis; HRT, AG, and MHF: writing.
Conflict of interest
No conflict of interest declared.
Funding
This work was supported by the Frank Lab startup funds from Cornell University College of Agriculture and Life Sciences. MHF was supported by the National Science Foundation (NSF) [CAREER IOS-1942437]. HRT was supported by a United States Department of Agriculture National institute of Food and Agriculture (USDA AFRI-NIFA) Predoctoral Fellowship [2020-67011-31882]. AG utilized funding from the Cornell Institute for Digital Agriculture Research Innovation Fund. Image data were acquired through the Cornell Institute of Biotechnology’s Imaging Facility’s shared Zeiss LSM880 confocal/multiphoton microscope provided by the National Institute of Health (NIH) [S10OD018516].
Data availability
All data supporting the findings of this study are available within the paper and within its supplementary data published online.
References
- Ali M, Mohammad ALI, Fujieda K.. 1990. Cross compatibility between eggplant (Solanum melongena L.) and wild relatives. Journal of the Japanese Society for Horticultural Science 58, 977–984. [Google Scholar]
- Andrews PK, Marquez CS.. 2010. Graft incompatibility. Horticultural Reviews 15, 183–232. [Google Scholar]
- Argles GK. 1937. A review of the literature on stock–scion incompatibility in fruit trees: with particular reference to pome and stone fruits. East Malling, UK: Imperial Bureau of Fruit Production. [Google Scholar]
- Benda GTA, Hartmann HT, Kester DE.. 1960. Plant propagation: principles and practices. American Midland Naturalist 63, 253. [Google Scholar]
- Dawson RF. 1942. Accumulation of nicotine in reciprocal grafts of tomato and tobacco. American Journal of Botany 29, 66–71. [Google Scholar]
- Errea P, Felipe A, Herrero M.. 1994. Graft establishment between compatible and incompatible Prunus spp. Journal of Experimental Botany 45, 393–401. [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A.. 2007. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Flaishman MA, Loginovsky K, Golobowich S, Lev-Yadun S.. 2008. Arabidopsis thaliana as a model system for graft union development in homografts and heterografts. Journal of Plant Growth Regulation 27, 231–239. [Google Scholar]
- Fox J, Weisberg S.. 2018. An R companion to applied regression. Thousand Oaks, CA: SAGE Publications. [Google Scholar]
- Frank M, Komarov S, Wang Q, et al. 2022. Integrated PET and confocal imaging informs a functional timeline for the dynamic process of vascular reconnection during grafting. bioRxiv, 10.1101/2022.10.27.513862 [Preprint]. [DOI] [Google Scholar]
- Frodin DG. 2004. History and concepts of big plant genera. TAXON 53, 753–776. [Google Scholar]
- Goldschmidt EE. 2014. Plant grafting: new mechanisms, evolutionary implications. Frontiers in Plant Science 5, 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé M. 2020. Package ‘RVAideMemoire. https://CRAN.R-project.org/package=RVAideMemoire.
- Johnson SJ, Miles CA.. 2011. Effect of healing chamber design on the survival of grafted eggplant, tomato, and watermelon. HortTechnology 21, 752–758. [Google Scholar]
- Kawaguchi M, Taji A, Backhouse D, Oda M.. 2008. Anatomy and physiology of graft incompatibility in solanaceous plants. Journal of Horticultural Science and Biotechnology 83, 581–588. [Google Scholar]
- Kubota C, McClure MA, Kokalis-Burelle N, Bausher MG, Rosskopf EN.. 2008. Vegetable grafting: history, use, and current technology status in North America. HortScience 43, 1664–1669. [Google Scholar]
- Kurotani K, Huang C, Okayasu K, Susuki T, Ichihashi Y, Shirasu K, Higashiyama T, Niwa M, Notaguchi M.. 2022. Discovery of the interfamily grafting capacity of Petunia, a floricultural species. Horticulture Research 9, uhab056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-M, Oda M.. 2010. Grafting of herbaceous vegetable and ornamental crops. Horticultural Reviews 28, 61–124. [Google Scholar]
- Liu N, Zhou B, Zhao X, Lu B, Li Y, Hao J.. 2009. Grafting eggplant onto tomato rootstock to suppress Verticillium dahliae infection: the effect of root exudates. HortScience 44, 2058–2062. [Google Scholar]
- Melnyk CW. 2017. Plant grafting: insights into tissue regeneration. Regeneration 4, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge K, Janick J, Scofield S, Goldschmidt EE.. 2009. A history of grafting. Horticultural Reviews 35, 437–493. [Google Scholar]
- Notaguchi M, Higashiyama T, Suzuki T.. 2015. Identification of mRNAs that move over long distances using an RNA-Seq analysis of Arabidopsis/Nicotiana benthamiana heterografts. Plant and Cell Physiology 56, 311–321. [DOI] [PubMed] [Google Scholar]
- Notaguchi M, Kurotani K-I, Sato Y, et al. 2020. Cell–cell adhesion in plant grafting is facilitated by β-1,4-glucanases. Science 369, 698–702. [DOI] [PubMed] [Google Scholar]
- Pease AS. 1933. Notes on ancient grafting. Transactions and Proceedings of the American Philological Association 64, 66–76. [Google Scholar]
- Rasool A, Mansoor S, Bhat KM, Hassan GI, Baba TR, Alyemeni MN, Alsahli AA, El-Serehy HA, Paray BA, Ahmad P.. 2020. Mechanisms underlying graft union formation and rootstock scion interaction in horticultural plants. Frontiers in Plant Science 11, 590847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Romano D, Paratore A.. 2001. Effects of grafting on tomato and eggplant. Acta Horticulturae 559, 149–154. [Google Scholar]
- Särkinen T, Bohs L, Olmstead RG, Knapp S.. 2013. A phylogenetic framework for evolutionary study of the nightshades (Solanaceae): a dated 1000-tip tree. BMC Evolutionary Biology 13, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S, Bohs L.. 2012. An explosive innovation: phylogenetic relationships of Solanum section Gonatotrichum (Solanaceae). PhytoKeys (8), 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H, Van den Broeck L, Spurney R, Sozzani R, Frank M.. 2022. Gene regulatory networks for compatible versus incompatible grafts identify a role for SlWOX4 during junction formation. The Plant Cell 34, 535–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson and Morgan. n.d. TomTato® (Ketchup ‘n’ Fries™, Ketchup and Chips). https://www.thompson-morgan.com/p/tomtatoreg-improved-ketchup-n-friestrade-ketchup-and-chips/t69168TM [Google Scholar]
- Westwood MN. 1993. Temperate-zone pomology: physiology and culture. Portland, OR: Timber Press. [Google Scholar]
- Wickham H. 2011. ggplot2. Wiley Interdisciplinary Reviews: Computational Statistics 3, 180–185. [Google Scholar]
- Zeist AR, Giacobbo CL, da Silva Neto GF, Zeist RA, da R Dorneles K, de Resende JTV.. 2018. Compatibility of tomato cultivar Santa Cruz Kada grafted on different Solanaceae species and control of bacterial wilt. Horticultura Brasileira 36, 377–381. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and within its supplementary data published online.


