Abstract
Optimal antituberculosis therapy is essential for favorable clinical outcomes. Peak plasma concentrations of first-line antituberculosis drugs in infants with living HIV receiving WHO-recommended dosing were low compared with reference values for adults, supporting studies on increased doses of first-line TB drugs in infants.
Keywords: HRZE, Tuberculosis, HIV, pharmacokinetics, infants
First-line antituberculosis drug peak concentrations in infants with HIV were low compared with reference values for adults. The percent peak concentrations within adult reference values were 51%, 22%, 76%, and 6% for isoniazid, rifampicin, pyrazinamide, and ethambutol, respectively.
INTRODUCTION
Tuberculosis (TB) is a leading cause of death in children living with HIV. Of the 214 000 TB deaths among people living with HIV in 2021, children accounted for 10% [1]. Infants are at risk of developing severe forms of TB when compared with older children [1, 2]. Optimal TB treatment is crucial to avert TB-associated mortality in infants with HIV.
Due to dynamic alterations in metabolic capacity and body composition during growth, infants are susceptible to changes in the pharmacokinetics of drugs that in turn impact the determination of appropriate doses [3]. Pharmacokinetic studies show that children weighing <8 kg, who are dosed according to current WHO-recommended weight bands using fixed-dose combination (FDC) drugs, have lower plasma exposures of first-line TB drugs than adults [4, 5]. Furthermore, the presence of HIV infection is associated with lower exposures to rifampicin and ethambutol [6, 7]. In addition, infants living with HIV and TB are also highly vulnerable to opportunistic infections, and severe acute malnutrition that necessitate multidrug treatment with an associated risk of drug–drug interactions [8]. Antimycobacterial activity and treatment response observed in adults treated for TB are closely linked to plasma or serum drug exposures [9]. In the absence of target drug exposures for children, it is generally agreed that pediatric doses of TB drugs should result in similar exposures to those in adults [7].
Limited pharmacokinetic data of TB drugs are available for low-weight infants with HIV, particularly for infants weighing less than 4 kg [7]. We aimed to evaluate plasma concentrations of first-line TB drugs in infants below 1 year of age living with HIV who were hospitalized for severe pneumonia and received TB treatment during the EMPIRICAL trial.
METHOD
Study Population and Design
This was a single-arm pharmacokinetic substudy within the EMPIRICAL multicenter, open-label randomized controlled clinical trial (#NCT03915366) funded by EDCTP which aimed to assess the efficacy of empirical treatment with first-line TB treatment and/or valganciclovir for infants living with HIV who were admitted with severe pneumonia [8]. The main trial enrolled infants between 28 and 365 days old with confirmed HIV infection and severe pneumonia. All eligible infants received standard of care, including antibiotics, cotrimoxazole treatment with prednisolone, and antiretroviral treatment. They were randomized to receive no additional treatment, first-line TB treatment, valganciclovir for 15 days, or both (4 arms) [8]. Infants who received TB treatment either as part of trial randomization or who were diagnosed with TB post-randomization were enrolled from hospitals in Mozambique, Uganda, Zambia, and Zimbabwe.
Procedures
Pediatric isoniazid, rifampicin, and pyrazinamide FDC dispersible tablets (50/75/150 mg), ethambutol 100 mg dispersible tablets (intensive phase of TB treatment) and isoniazid and rifampicin FDC tablets (50/75 mg, continuation phase, all WHO-prequalified and from manufacturer Macleods) were administered once-daily according to WHO weight-band dosing [10]. The dosages are included in Supplementary File 1. A single blood sample was taken at 2 hours after dose administration (C2h) at day 30, 90, and 180 after enrollment in the main trial. Samples drawn outside the 1.5–2.5-hour timeframe after drug administration were excluded from the analysis. Food intake around dose administration and adherence 3 days prior to the pharmacokinetic visit were recorded. Isoniazid, acetyl-isoniazid, rifampicin, pyrazinamide, and ethambutol concentrations were determined using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method, as described in Supplementary File 2.
Statistical Analysis
The population geometric mean (GM, %coefficient of variation) C2h was determined for all drugs. We considered C2h to be a surrogate parameter for Cmax, and hence compared it to reference Cmax values. To compare with a similar population, although predominantly HIV-negative, we used Cmax data for children in the 4–7.9 kg weight band reported in an earlier intensive pharmacokinetic (PK) sampling study [5]. Additionally, the percentages of infants within the adult reference Cmax values were reported [9]. Spearman rank correlation and the Mann–Whitney U-test were used to assess bivariate associations between continuous (dose/kg, height, weight-for-length z-score [WLZ], weight-for-age z-score, age, and estimated glomerular filtration rate) and categorical (sex and acetylator status) covariates, respectively, and C2h values of TB drugs. Then, for covariates correlating with C2h at a significance level of P < .1, multiple linear regression on log-transformed data was conducted to test if associations held true after correcting for other covariates.
Ethics
The study protocol was approved by Investigation Ethics Committee of Medicines Hospital 12 de Octubre in Spain (#19/096) and by local ethics committees. The study consent documents were translated into local languages and all caregivers provided written informed consent.
RESULTS
Population
Forty-nine of 50 infants enrolled were included in the analysis (Table 1). One infant was excluded as the blood sample was drawn outside of the 1.5–2.5 hour range after dosing. All children were fed within 2 hours prior to or after dose administration.
Table 1.
Patient characteristics and pharmacokinetic results on study visit day 30 from all included infants (n = 49)
| Characteristic (study visit day 30; n = 49) | Value | |
|---|---|---|
| Sex (n) | ||
| Female | 21 (43%) | |
| Male | 28 (57%) | |
| Median (IQR) age (months) | 5.6 (4.4 to 9.1) | |
| Median (IQR) weight (kg) | 5.3 (4.8 to 6.2) | |
| Median (IQR) length (cm) | 61 (58 to 65) | |
| Weight bands (n) | ||
| <4.0 kg | 5 (10%) | |
| 4.0–7.9 kg | 41 (84%) | |
| 8.0–11.9 kg | 3 (6%) | |
| Median (IQR) WLZ | −1.80 (−2.60 to −0.35) | |
| Median (IQR) WAZ | −2.60 (−4.05 to −1.70) | |
| Median (IQR) eGFRa (mL/min/1.73 m2) |
105 (81 to 166) | |
| Acetylator status (n)b | ||
| Slow | 33 (67%) | |
| Intermediate/fast | 16 (33%) | |
| Confirmed TB (n)c | ||
| Yes | 8 (16%) | |
| No | 41 (84%) | |
| Median (IQR) drug dose (mg/kg) | ||
| Isoniazid | 9.6 (8.2 to 11.1) | |
| Rifampicin | 14.4 (12.3 to 16.7) | |
| Pyrazinamide | 28.8 (24.6 to 33.7) | |
| Ethambutol | 19.2 (16.4 to 22.5) | |
| ART regimen during PK sampling visit (n)d | ||
| DTG-based | 10 (21%) | |
| EFV-based | 1 (2%) | |
| LPVr-based | 12 (24%) | |
| NVP-based | 2 (4%) | |
| Triple NRTI | 16 (33%) | |
| None | 8 (16%) | |
| PK parameters (study visit day 30) | References | |
|---|---|---|
| Geometric mean (mg/L; %CV) C2h | Infant referencese | |
| Isoniazid | 2.8 (102) | 4.0 (2.3–4.7) |
| Rifampicin | 3.7 (161) | 4.9 (3.9–6.4) |
| Pyrazinamide | 22.3 (97) | 25.8 (22.3–31.8) |
| Ethambutol | 0.56 (101) | 1.2 (0.9–1.6) |
| Percentage of infants with C2h within adult Cmax references | Adult referencesf | |
| Isoniazid | 51% | 3–6 mg/L |
| Rifampicin | 22% | 8–24 mg/L |
| Pyrazinamide | 76% | 20–60 mg/L |
| Ethambutol | 6% | 2–6 mg/L |
aeGFR was calculated using the Schwartz equation for children under 1 year old: eGFR = 0.44 × length (cm)/creatinine (mg/dL).
bPhenotypic INH acetylator status was determined by calculating the metabolic ratio for C2h (acetyl-INH/INH). Infants having a metabolic ratio below 0.73 were considered slow acetylators and those with higher ratios intermediate/fast acetylators [11].
cDiagnosed by GeneXpert and/or TB urine lipoarabinomannan (TB-LAM).
dEight infants were ART naïve during the pharmacokinetic sampling visit. These were very sick infants at the time of recruitment and ART initiation was delayed due to the risk of IRIS (n = 4) or due to elevated liver enzymes (n = 4).
eThese reference values represent the median (IQR) Cmax for 16 infants weighing 4–7.9 kg that received drug dosages similar to our population reported by Chabala et al [5]. The majority of these infants (75%) ware HIV-negative.
fThese reference values represent the normal Cmax that can be expected in adults after the standard doses of TB drugs. They are based on data that were compiled from all available sources (both healthy volunteers and TB patients) [9].
Abbreviations: ART, antiretroviral treatment; CV, coefficient of variation; DTG, dolutegravir; EFV, efavirenz; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LPVr, ritonavir-boosted lopinavir; NRTI; nucleoside reverse transcriptase inhibitor; NVP, nevirapine; PK, pharmacokinetic; TB, tuberculosis; WAZ, weight-for-age z-score; WLZ, weight-for-length z-score.
Pharmacokinetics
Table 1 displays GM C2h values and individual C2h for isoniazid, rifampicin, pyrazinamide, and ethambutol are displayed in Figure 1, highlighting the large interpatient variability in exposure to each of the drugs. Individual C2h levels seemed comparable across the various weight bands (<4.0 kg; 4.0–7.9 kg; 8.0–11.9 kg) for all compounds, as displayed in Supplementary File 3. However, only few children were included in the <4.0 kg (n = 5) and 8.0–11.9 kg (n = 3) weight bands.
Figure 1.
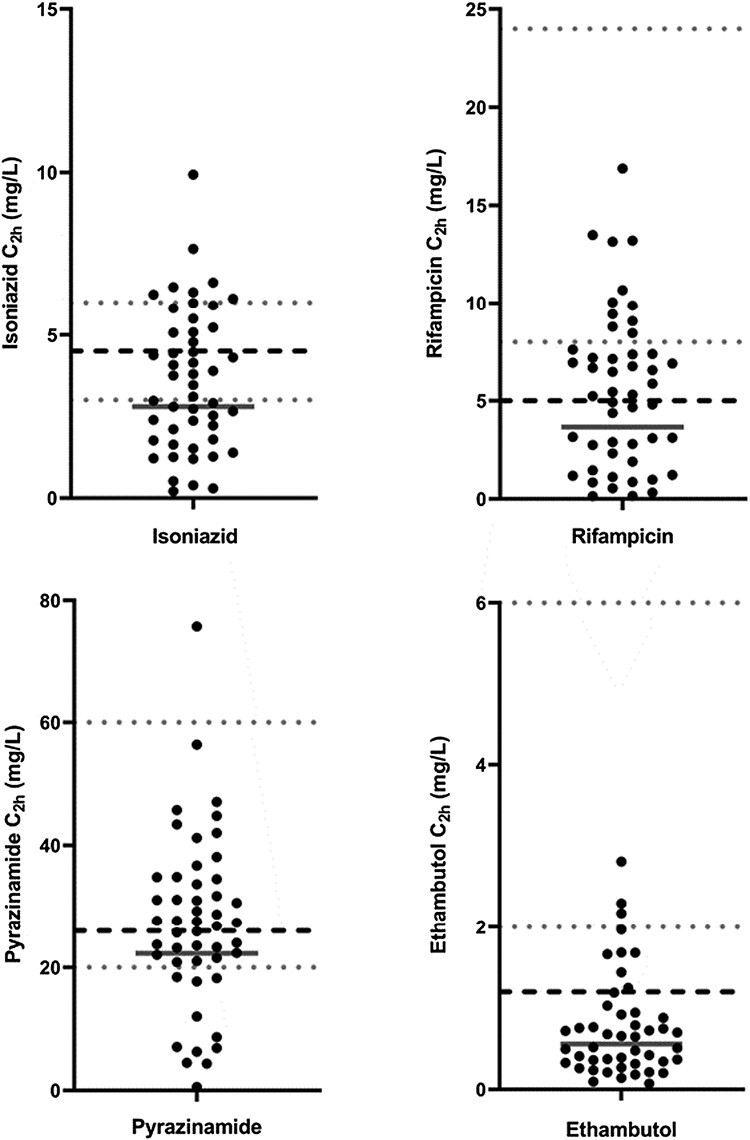
Individual TB drug concentrations on study visit day 30 at 2 hours after dose administration (C2h). The grey solid lines represent the geometric means of the individual C2h, the grey dotted lines represent the adult target Cmax ranges as reported by Alsultan et al. [9], and the black dashed lines represent the median Cmax for children within the 4-7.9 kg weight-band as reported by Chabala et al. [5]. Top left panel: isoniazid; top right panel: rifampicin; bottom left panel: pyrazinamide; bottom right panel: ethambutol.
The GM isoniazid C2h was 2.80 (102) mg/L at day 30 of the trial, with 51% of infants having a C2h within the adult Cmax reference range of 3–6 mg/L. Isoniazid C2h did not statistically differ per acetylator group, see Supplementary Files 5 and 6. Furthermore, isoniazid and rifampicin concentrations at visit days +90 and +180 were comparable to day +30 (Supplementary File 4). For rifampicin, the GM C2h was 3.7 (161) mg/L, with only 22% of infants falling within the adult reference range of 8–24 mg/L. Pyrazinamide GM C2h was 22.3 (97) mg/L, with 76% of infants within the adult reference range of 20–60 mg/L. Two infants had undetectable ethambutol concentrations after supervised drug intake and hence were excluded from the analysis. The GM ethambutol C2h was 0.56 (101) mg/L, with only 6% of infants within the adult reference range of 2–6 mg/L.
In the multivariable analysis, increasing WLZ was significantly but weakly associated with decreased rifampicin C2h and ethambutol concentrations were higher in females, details of the analyses are included in Supplementary File 6. None of the covariates showed a statistically significant effect on isoniazid and pyrazinamide C2h in the multivariable analysis.
DISCUSSION AND CONCLUSION
We found that GM C2h of rifampicin, isoniazid, and ethambutol values in infants living with HIV receiving TB treatment following WHO weight-band dosing were below the adult reference Cmax values, whereas the GM C2h of pyrazinamide fell within the lower end of the wide adult reference range. The findings were consistent over study visit day 30 after TB treatment initiation and 90, and 180 for the 2 drugs administered beyond 2 months, isoniazid and rifampicin. A dose corresponding with the 4–7.9 kg weight band provided appropriate TB drug exposure in 4 children weighing <4 kg. Of note, there is no formal dose recommendation for FDC tablets for children weighing <4 kg. An alarmingly high proportion of infants had a C2h below the adult reference window for rifampicin (78%) and ethambutol (94%). These findings are consistent with other studies that reported low C2h for first-line TB drugs in infants weighing <8 kg and children living with HIV, using the current WHO weight-band dosing [5–7].
Previous studies reported malnutrition to be associated with lower total TB drug levels [7]. Many (47%) infants in our study were malnourished, which may have contributed to the low C2h in our population. Conversely, we found a significant association between increased rifampicin C2h in children and low-WLZ in the multivariable analysis [7]. Of note, weight-for-length measurements in infants are challenging and an appropriate WLZ reference standard for infants below 6 months is less accurate, whilst 53% of our population was under 6 months old. Furthermore, we did not find a significant difference in isoniazid C2h for infants with different acetylator status. This may be explained by a more pronounced effect of acetylator status on isoniazid area under curve (AUC) compared with Cmax [11].
This study has several limitations. First, to limit the volume of blood to be drawn from the infants in the study, we drew a single PK sample and hence cannot provide full pharmacokinetic profiles. Secondly, while the C2h timepoint approximates Cmax, we may not have captured the Cmax for some children due to interpatient pharmacokinetic variability. Additionally, the actual time to Cmax (Tmax) for each drug may vary, with isoniazid and pyrazinamide often having a Tmax of slightly less than 2 hours in children while that of rifampicin and ethambutol is often slightly higher than 2 hours. Interpretation of C2h may further be complicated because of the fed state of our populations, which could result in delayed drug absorption [12]. Low C2h levels do not rule out the possibility of delayed absorption, regardless of fed state. Moreover, Cmax values are generally lower in patients who are fed compared with those who are fasted and may not correlate well with the total exposure (AUC) to the medications. To gather comprehensive data while minimizing the burden on infants, future studies in this population should consider employing limited-sampling strategies to predict AUC values or collecting single pharmacokinetic samples at different timepoints at each study visit to facilitate AUC prediction through population pharmacokinetic modeling. Lastly, we were unable to determine the relevance of the low drug concentrations in terms of efficacy, as the main trial efficacy outcomes were still blinded and confidential at the time of this analysis.
Our findings confirm low plasma concentrations of first-line TB drugs in a vulnerable population of infants with advanced HIV and a history of recent admission with severe pneumonia and were malnourished. These data support large clinical studies investigating increased doses of the first-line TB drugs in FDC and loose ethambutol in infants with HIV.
Supplementary Data
Supplementary materials are available at the Journal of The Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org).
EMPIRICAL clinical trial group:
Muhammad Sidat, Elias Manjate, Sónia Martins (Universidade Eduardo Mondlane Faculdade de Medicina, Maputo, Mozambique); Stella Langa, Natália Nipaco (Hospital Central de Maputo, Maputo, Mozambique); Sara Machava, Anastância Chirindza (Hospital Provincial de Matola, Matola, Mozambique); Luzidina Martins, Mércia Nhaca (Hospital Geral de Mavalane, Maputo, Mozambique); Dalila Rego, Dália Machel (Hospital Geral José Macamo, Maputo, Mozambique); Amir Seni, Kajal Chhanganlal, Belinda Macmillan, Aurora Mucarenga, Adelina Manheche (Hospital Central da Beira, Beira, Mozambique); Kusum J Nathoo, Moses Chitsamatanga, Ruth Marange, Shepherd Mudzingwa, Dorothy Murungu (University of Zimbabwe Clinical Research Centre); Idah Zulu, Perfect Shankalala, Mulima Mukubesa, Juliet Namwinwa, Chalwe Chibuye, Terence Chipoya, Bwalya Simunyola, John Tembo (University Teaching Hospital, Lusaka, Zambia); Muleya Inambao, Salome Chitondo, Wyclef Mumba, Endreen Mankushe, Henry Musukwa, Davies Sondashi (Arthur Davidson Children’s Hospital, Ndola, Zambia); Albert Kamugisha, Karen Econi (China Uganda Friendship Hospital Naguru, Kampala, Uganda); Andrew Kiggwe, Judith Beinomugisha, Sharafat Nkinzi, Lawrence Kakooza, Henriator Namisanvu, Nancy Lajara Mark, Josam Thembo Mwesige, Ivan Segawa, Joseph Ssessanga, Paul Mbavu (Makerere University Lung Institute, Kampala, Uganda); Bosco Kafufu (Infectious Diseases Institute Laboratory, Makerere University, Kampala, Uganda); Denis Nansera, Elizabeth Najjingo, Bashira T Mbabazi (Mbarara Regional Referral Hospital, Mbarara, Uganda); Abbas Lugemwa, Mariam Kasozi, Rogers Ankunda (Joint Clinical Research Centre, Regional Centre of Excellence, Mbarara, Uganda); Lilit Manukyan (Pediatric Unit for Research and Clinical Trials (UPIC), Hospital 12 de Octubre Health Research Institute (i + 12), Biomedical Foundation of Hospital Universitario 12 de Octubre (FIB-H12O), Madrid, Spain).
Acknowledgments
We thank all children and families who have participated in this study.
Contributor Information
Chishala Chabala, University of Zambia, School of Medicine, Lusaka, Zambia; University Teaching Hospital, Children’s Hospital, Lusaka, Zambia; HerpeZ, Lusaka, Zambia.
Tom G Jacobs, Department of Pharmacy, Radboudumc Institute for Medical Innovation (RIMI), Radboud University Medical Center, Nijmegen, The Netherlands.
Cinta Moraleda, Pediatric Unit for Research and Clinical Trials (UPIC), Hospital 12 de Octubre Health Research Institute (i+12), Biomedical Foundation of Hospital Universitario 12 de Octubre (FIB-H12O), Madrid, Spain.
John M Ndaferankhande, Malawi-Liverpool-Wellcome Trust Clinical Research Programme, Kamuzu University of Health Sciences, Blantyre, Malawi.
Vivian Mumbiro, University of Zimbabwe Clinical Research Centre, Harare, Zimbabwe.
Alfeu Passanduca, Universidade Eduardo Mondlane, Faculdade de Medicina, Maputo, Mozambique.
Natasha Namuziya, University Teaching Hospital, Children’s Hospital, Lusaka, Zambia.
Damalie Nalwanga, Department of Paediatrics and Child Health, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda.
Victor Musiime, Department of Paediatrics and Child Health, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda; Joint Clinical Research Centre, Kampala, Uganda.
Alvaro Ballesteros, Pediatric Unit for Research and Clinical Trials (UPIC), Hospital 12 de Octubre Health Research Institute (i+12), Biomedical Foundation of Hospital Universitario 12 de Octubre (FIB-H12O), Madrid, Spain.
Sara Domínguez-Rodríguez, Pediatric Unit for Research and Clinical Trials (UPIC), Hospital 12 de Octubre Health Research Institute (i+12), Biomedical Foundation of Hospital Universitario 12 de Octubre (FIB-H12O), Madrid, Spain.
Moses Chitsamatanga, University of Zimbabwe Clinical Research Centre, Harare, Zimbabwe.
Uneisse Cassia, Universidade Eduardo Mondlane, Faculdade de Medicina, Maputo, Mozambique.
Bwendo Nduna, Arthur Davidson Children’s Hospital, Ndola, Zambia.
Justina Bramugy, Centro de Investigação em Saúde de Manhiça, Maputo, Mozambique.
Jahit Sacarlal, Universidade Eduardo Mondlane, Faculdade de Medicina, Maputo, Mozambique.
Lola Madrid, Pediatric Unit for Research and Clinical Trials (UPIC), Hospital 12 de Octubre Health Research Institute (i+12), Biomedical Foundation of Hospital Universitario 12 de Octubre (FIB-H12O), Madrid, Spain; London School of Hygiene and Tropical Medicine (LMC), London, UK.
Kusum J Nathoo, University of Zimbabwe Clinical Research Centre, Harare, Zimbabwe.
Angela Colbers, Department of Pharmacy, Radboudumc Institute for Medical Innovation (RIMI), Radboud University Medical Center, Nijmegen, The Netherlands.
David M Burger, Department of Pharmacy, Radboudumc Institute for Medical Innovation (RIMI), Radboud University Medical Center, Nijmegen, The Netherlands.
Veronica Mulenga, University of Zambia, School of Medicine, Lusaka, Zambia; University Teaching Hospital, Children’s Hospital, Lusaka, Zambia.
W Chris Buck, Universidade Eduardo Mondlane, Faculdade de Medicina, Maputo, Mozambique; University of California Los Angeles, David Geffen School of Medicine, Los Angeles, California, USA.
Hilda A Mujuru, University of Zimbabwe Clinical Research Centre, Harare, Zimbabwe.
Lindsey H M te Brake, Department of Pharmacy, Radboudumc Institute for Medical Innovation (RIMI), Radboud University Medical Center, Nijmegen, The Netherlands.
Pablo Rojo, Pediatric Unit for Research and Clinical Trials (UPIC), Hospital 12 de Octubre Health Research Institute (i+12), Biomedical Foundation of Hospital Universitario 12 de Octubre (FIB-H12O), Madrid, Spain; Complutense University of Madrid, Madrid, Spain; Pediatric Service, Hospital Universitario 12 de Octubre, Servicio Madrileño de Salud (SERMAS), Madrid, Spain.
Alfredo Tagarro, Pediatric Unit for Research and Clinical Trials (UPIC), Hospital 12 de Octubre Health Research Institute (i+12), Biomedical Foundation of Hospital Universitario 12 de Octubre (FIB-H12O), Madrid, Spain; Pediatric Service, Infanta Sofia University Hospital, Servicio Madrileño de Salud (SERMAS), Madrid, Spain; Universidad Europea de Madrid, Madrid, Spain.
Rob E Aarnoutse, Department of Pharmacy, Radboudumc Institute for Medical Innovation (RIMI), Radboud University Medical Center, Nijmegen, The Netherlands.
EMPIRICAL clinical trial group:
Muhammad Sidat, Elias Manjate, Sónia Martins, Stella Langa, Natália Nipaco, Sara Machava, Anastância Chirindza, Luzidina Martins, Mércia Nhaca, Kusum J Nathoo, Moses Chitsamatanga, Ruth Marange, Shepherd Mudzingwa, Dorothy Murungu, Idah Zulu, Perfect Shankalala, Mulima Mukubesa, Juliet Namwinwa, Chalwe Chibuye, Terence Chipoya, Bwalya Simunyola, John Tembo, Muleya Inambao, Salome Chitondo, Wyclef Mumba, Endreen Mankushe, Henry Musukwa, Davies Sondashi, Albert Kamugisha, Karen Econi, Andrew Kiggwe, Judith Beinomugisha, Sharafat Nkinzi, Lawrence Kakooza, Henriator Namisanvu, Nancy Lajara Mark, Josam Thembo Mwesige, Ivan Segawa, Joseph Ssessanga, Paul Mbavu, Bosco Kafufu, Denis Nansera, Elizabeth Najjingo, Bashira T Mbabazi, Abbas Lugemwa, Mariam Kasozi, Rogers Ankunda, and Lilit Manukyan
Financial support . This project is part of the EDCTP2 programme supported by the European Union RIA2017MC-2013.
Potential conflicts of interest . The authors declare that they have no conflict of interest.
Data Availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
REFERENCES
- 1. UNAIDS. UNAIDS Data 2022. Geneva, Switzerland: Geneva Joint United Nations Programme on HIV/AIDS; 2022. [Google Scholar]
- 2. Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004; 8:392–402. [PubMed] [Google Scholar]
- 3. Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE.. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med 2003; 349:1157–67. [DOI] [PubMed] [Google Scholar]
- 4. Kwara A, Yang H, Martyn-Dickens C, et al. Adequacy of WHO weight-band dosing and fixed-dose combinations for the treatment of TB in children. Int J Tuberc Lung Dis 2023; 27:401–7. [DOI] [PubMed] [Google Scholar]
- 5. Chabala C, Turkova A, Hesseling AC, et al. Pharmacokinetics of first-line drugs in children with Tuberculosis, using World Health Organization-recommended weight band doses and formulations. Clin Infect Dis 2022; 74:1767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobs TG, Svensson EM, Musiime V, et al. ; WHO Paediatric Antiretroviral Working Group. Pharmacokinetics of antiretroviral and tuberculosis drugs in children with HIV/TB co-infection: a systematic review. J Antimicrob Chemother 2020; 75:3433–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gafar F, Wasmann RE, McIlleron HM, et al. Global estimates and determinants of antituberculosis drug pharmacokinetics in children and adolescents: a systematic review and individual patient data meta-analysis. Eur Respir J 2022; 61:2201596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rojo P, Moraleda C, Tagarro A, et al. Empirical treatment against cytomegalovirus and tuberculosis in HIV-infected infants with severe pneumonia: study protocol for a multicenter, open-label randomized controlled clinical trial. Trials 2022; 23:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alsultan A, Peloquin CA.. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 2014; 74:839–54. [DOI] [PubMed] [Google Scholar]
- 10. Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children. Geneva: World Health Organization Copyright© World Health Organization 2014; 2014. [PubMed] [Google Scholar]
- 11. Verhagen LM, Coenen MJ, López D, et al. Full-gene sequencing analysis of NAT2 and its relationship with isoniazid pharmacokinetics in Venezuelan children with tuberculosis. Pharmacogenomics 2014; 15:285–96. [DOI] [PubMed] [Google Scholar]
- 12. Saktiawati AM, Sturkenboom MG, Stienstra Y, et al. Impact of food on the pharmacokinetics of first-line anti-TB drugs in treatment-naive TB patients: a randomized cross-over trial. J Antimicrob Chemother 2016; 71:703–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.


