Abstract
Objective:
To evaluate the interrelationship among impaired renal function, brain pathology on imaging, and cognitive decline in a longitudinal poststroke cohort.
Methods:
The Tel Aviv Brain Acute Stroke Cohort study is a prospective cohort of mild-moderate ischemic stroke/TIA survivors without dementia who underwent a 3T MRI and were cognitively assessed at admission and for 24 months following stroke. Renal function was evaluated at admission by creatinine clearance (CCl) estimation. The volumes of ischemic lesions and preexisting white matter hyperintensities (WMH), brain atrophy, and microstructural changes of the normal-appearing white matter tissue were measured using previously validated methods.
Results:
Baseline data were available for 431 participants. Participants with a CCl <60 mL/min at baseline performed significantly worse in all cognitive tests over time (p = 0.001) than those with a CCl ≥60 mL/min and had larger WMH volume and cortical atrophy and smaller hippocampal volume (all p < 0.001). After 2 years, 15.5% of the participants were diagnosed with cognitive impairment. Multiple logistic regression analysis, controlling for traditional risk factors, suggested CCl <60 mL/min at baseline as a significant predictor for the development of cognitive impairment 2 years after the index stroke (odds ratio 2.01 [95% confidence interval 1.03–3.92], p = 0.041).
Conclusions:
Impaired renal function is associated with increased WMH volume and cortical atrophy, known biomarkers of the aging brain, and is a predictor for cognitive decline 2 years after stroke/TIA. Decreased renal function may be associated with cerebral small vessel disease underlying poststroke cognitive decline, suggesting a new target for early intervention.
Chronic kidney disease (CKD), affecting 13% of adults in the United States,1 is a known risk factor for cerebrovascular disease, including stroke, both ischemic and hemorrhagic, and carotid artery arteriosclerosis.2 Recent evidence shows that even mild renal impairment is associated with increased risk for cerebrovascular events3 and cognitive decline.4,5 Although patients with CKD often have other cardiovascular risk factors, it appears that the renal disease is an independent risk factor for cognitive decline.4 It is unknown, however, whether renal disease is linked to elevated risk for cognitive impairment in stroke patients.
In the present study, we sought to determine, in a large prospective cohort of patients with first-ever mild ischemic stroke or TIA, the interrelation among CKD, measured as reduced estimated glomerular filtration rate, imaging markers of cerebral small vessel disease (CSVD) and brain integrity, and cognitive performance 2 years following the index event.
METHODS
Study population.
Patients were eligible for the present study if they had mild to moderate first-ever acute ischemic stroke or TIA and were participating in the prospective cohort Tel Aviv Brain Acute Stroke Cohort study. Selection process for the study was previously described.6 Patients were excluded if they had stroke resulting from trauma or invasive procedures, hemorrhagic stroke, severe aphasia, or cognitive decline/dementia, or were unlikely to participate in follow-up or to be discharged from hospital. The neurologic assessment included verification of stroke etiology and NIH Stroke Scale (NIHSS).
Standard protocol approvals, registrations, and patient consents.
This study was registered as ClinicalTrials.gov NCT01926691. All participants signed informed consent forms, approved by the local ethics committee.
Estimation of renal function.
Baseline kidney function was estimated as creatinine clearance (CCl) using the Cockcroft-Gault7 formula: CCl = (140 − age) × (weight in kg)/(serum creatinine × 72) × (0.85 for women).
Participants were subdivided according to their renal function: not impaired if CCl ≥60 mL/min vs impaired or CKD if CCl <60 mL/min8 (defined by National Kidney Foundation guidelines9).
Baseline and follow-up cognitive assessments.
Cognitive impairment before the stroke was determined as Informant Questionnaire on Cognitive Decline in the Elderly10 score ≥3.3. Patients completed a baseline neuropsychologic assessment including the Montreal Cognitive Assessment (MoCA)11 and NeuroTrax computerized cognitive testing (NeuroTrax Corp., Bellaire, TX).12 These comprehensive neuropsychologic evaluations were repeated 6, 12, and 24 months following the event. The average of the 6 index scores (memory, executive functions, visuospatial perception, verbal function, attention, and motor skills) was computed as the global cognitive score. Data for each outcome parameter were normalized according to stratifications of age and education (≤12 years, >12 years) to give a distribution with a mean of 100 and SD of 15 (i.e., an IQ-style scale).
Criteria for cognitive impairment.
Patients with cognitive impairment were diagnosed with either mild cognitive impairment (MCI) or dementia. In order to diagnose MCI, the modified Petersen criteria13 were applied: the participant had to be impaired on at least 1 cognitive domain (≥1.5 SD) compared with age- and education-matched published norms on the MoCA score,14,15 to have no impairment of basic functional activities, and to not fulfill DSM-IV-TR criteria for dementia. The norms for NeuroTrax computerized cognitive testing were previously described.16,17
Participants with suspected cognitive impairment were referred to an experienced cognitive neurologist for assessment. Included were patients who could not complete the MoCA or whose NeuroTrax test had fallen by ≥1.5 SD in follow-up examinations, those with subjective cognitive complaints, and those who were suspected by a senior neurologist of having cognitive impairment.
Assessments were further reviewed by a consensus forum to determine whether the participant had dementia or MCI. The forum included the assessor, 3 senior neurologists specializing in memory disorders, and a neuropsychologist.
MRI analyses.
Acquisition of MRI was done using a 3T GE scanner (GE Signa EXCITE, Milwaukee, WI) within 7 days of stroke onset. Imaging parameters were described previously.6
Ischemic infarct identification.
A senior neuroradiologist determined the presence of an acute ischemic infarct based on diffusion-weighted imaging. Definitions of ischemic lesions as cortical, subcortical, or subtentorial infarct were as follows: any infarct that includes the cortex was defined as cortical infarct; cerebellar or brainstem infarction was defined as subtentorial infarct. An in-house method was used for the quantification of the ischemic and white matter lesion (WML) volumes.18
White matter hyperintensities (WMH) score.
WMH were identified on fluid-attenuated inversion recovery images and rated semiquantitatively based on a 4-point scale according to the periventricular score of Wahlund et al.19
Diffusion tensor imaging (DTI) analysis.
FMRIB Diffusion Toolbox, part of FMRIB Software Library, was used for calculation of the DTI maps (FSL; http://www.fmrib.ox.ac.uk/fsl/), and included eddy current and motion correction. We calculated 4 different maps: mean diffusivity (MD), fractional anisotropy (FA), and axial and radial diffusivities (Da and Dr).
Tissue segmentation.
Ischemic lesions, WML, and normal-appearing white matter (NAWM) identification and quantification were performed using multimodal view with an in-house semiautomatic method.18 Ischemic lesions and WML volumes were calculated across the whole brain. Mean MD, FA, Dr, and Da values were calculated for NAWM tissue.
Volumetric measure of the hippocampi.
3D T1-weighted spoiled gradient echo sequence (SPGR) axial images were used for the volumetric analysis of hippocampi. Analysis by FreeSurfer V5.1 image analysis suite (http://surfer.nmr.mgh.harvard.edu/) was performed for all brain segmentations based on intensity values and probabilistic atlas, as previously described.20–22 FreeSurfer analysis pipeline was performed with quality assurance and manual intervention of the data. For each participant, the right and left hippocampi volumes were extracted based on the automated segmentation.
Hippocampi MD analysis.
DTI analyses were performed using FreeSurfer (http://surfer.nmr.mgh.harvard.edu/). Before calculating DTI indices, motion and eddy current corrections were made. Then, all images were registered to the low b value (b = 0) image (T2-weighted) and a rigid transform was computed.23 The MD map was analyzed in register with the low b and the low b value and images were registered to each subject's anatomical volume. Hippocampi masks were eroded to avoid partial volume effects and MD values were averaged over all voxels.
Visualization of the association between renal function and hippocampi volumes.
In order to plot the effect of CCl on hippocampi volumes we used voxel-based morphometry analysis using statistical parametric mapping software (http://www.fil.ion.ucl.ac.uk/spm/SPM8; Wellcome Department of Imaging Neuroscience)24 running on MATLAB 2008a (MathWorks, Natick, MA). In brief, individual 3D T1-weighted SPGR images were segmented into white matter, gray matter (GM), and CSF images. Then, GM images were spatially normalized to Montreal Neurological Institute space and spatial smoothing of the normalized GM images was conducted by application of an 8 mm full width at half maximum Gaussian kernel. Correlations between hippocampi volumes and CCl values were assessed using bivariate 2-tailed Pearson correlation coefficient. The α error was set at 0.05 and Bonferroni correction for multiple comparisons was applied.
Depressive symptoms.
Depressive symptoms were assessed within 72 hours of admission, and again 6, 12, and 24 months later, using the 15-item Geriatric Depression Scale.25
Statistical analysis.
To assess the relationship between CCl and MRI measures as well as cognitive domains, 3 sets of multiple linear regression models were used. First, unadjusted regression (correlation) coefficients were calculated (model 1). Model 2 adjusted for age, sex, and education. Model 3 further accounted for cardiovascular risk factors: hypertension, diabetes, and ischemic heart disease.
As the variable hypertension correlated with the blood pressure values at admission, the variable diabetes mellitus correlated with the blood glucose and HbA1C levels, and the variable dyslipidemia correlated with cholesterol and low-density lipoprotein, we only included hypertension, diabetes mellitus, and dyslipidemia in the regression model to avoid colinearity.
Further, to determine univariate proportional hazard ratios for each risk factor, univariate logistic regression models were employed from index stroke to development of cognitive impairment 24 months poststroke as the dependent variable. Significant predictors for cognitive impairment were entered into a multivariate regression model; p < 0.05 was set for entry, p > 0.1 was set for removal.
Comparisons or distributions between categories were assessed using Student t test, the Mann-Whitney U test, or χ2 test, as appropriate. Associations between numeric variables were determined using the Spearman or the Pearson correlation analysis (coefficient estimate r).
A p value <0.05 was considered statistically significant for all analyses. We used SPSS/WIN (version 22.0; SPSS, Chicago, IL) software for all statistical analyses.
RESULTS
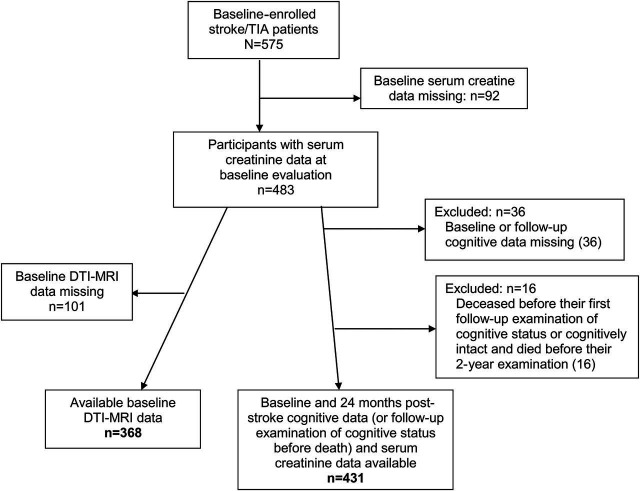
At the time of these analyses, 483 participants had a valid serum creatinine measure at baseline evaluation. Of them, cognitive assessments at baseline and 2 years poststroke were available for 431 participants. Brain MRI scans at baseline were available for 368 of them and thus neuroimaging analyses were obtained from this group only (figure 1). The average age of these 368 participants was 67 ± 9.9 years, similar to the overall cohort. Participants with MRI had significantly lower prevalence of diabetes (24.2% vs 35.8%), hypertension (56.9% vs 67.5%), and cardiac disease (12.1% vs 30.1%) than those who were not in the MRI study. A summary of baseline characteristics of the participants who had serum creatinine and cognitive assessments at baseline and at 2 years of follow-up (n = 431) are presented in table 1. Mean age of all study participants (n = 431) was 67.5 (±10.0) years; 60.6% were male; 124 (28.8%) were diagnosed with TIA.
Figure 1. Flowchart of patients included in this study.
DTI = diffusion tensor imaging.
Table 1.
Baseline characteristics of poststroke survivors (n = 431)

A total of 172 (172, 39.9%) participants had CCl <60 mL/min. Of them, 11 (2.6%) had CCl <30 mL/min, 1 had CCl <15 mL/min, and 259 (60.1%) had CCl >60 mL/min.
Stroke etiologies (based on Trial of Org 10,172 in Acute Stroke Treatment criteria26) were as follows: 181 lacunar stroke (59%), 43 cardioembolic stroke (14%), 27 large-artery atherosclerotic stroke (8.8%), 56 stroke of other or undetermined etiology (18.2%). No differences in baseline CCl values were observed across stroke subtypes or between stroke and TIA patients; the latter therefore were grouped together for further analyses.
CCl was lower in women and was related to older age, lower body mass index, lower hematocrit levels, higher high-density lipoprotein, ethnicity (Ashkenazi origin), higher physical activity and employment, dyslipidemia, smoking, and depression scores (table 1).
Association between renal function and MRI measurements.
Lower CCl was independently associated with more severe WMH, larger white matter lesion volume, worse global white matter microstructural integrity and lower brain parenchyma volumes, reflected as smaller intracranial volume (ICV), smaller hippocampal volume (normalized to ICV), thinner frontal cortex, larger CSF volume, and worse hippocampal integrity (table 2, figure 2). These findings remained significant adjusting further for age, sex, education, and vascular risk factors, including hypertension, diabetes, and cardiac disease (model 3).
Table 2.
Kidney function and imaging parameters or cognitive domains, unadjusted (model 1), adjusted for demographic factors (model 2), and adjusted for demographic and risk factors (model 3)
Figure 2. Statistical parametric maps of creatinine clearance (CCl)–related hippocampi volume.

(A) Glass brain representation and (B) statistical parametric maps (SPM) that were superimposed on a T1-weighted template showing positive association between higher levels of CCl and gray matter volume in the hippocampi (corrected for multiple comparison by false discovery rate [FDR] method for p < 0.05). For the left hippocampus, the x, y, and z Montreal Neurological Institute (MNI) coordinates for the peak association between CCl and gray matter volume were −22, −6, −22; = 32.6, pFDR < 0.05. For the right hippocampus, the x, y, and z MNI coordinates for the peak association between CCL and gray matter volume were 22, −6, −21; = 30.6, pFDR < 0.05. Colored bars represent t values; display threshold is set at t value 32. (C) Plotted along the y-axis are total hippocampal volume values in standardized (z score) units adjusted for age and total intracranial volume. Plotted along the x-axis are creatinine clearance values. *p < 0.001.
There was no association of CCl with stroke severity, as measured by NIHSS scoring at admission, lesion location, or infarct volume.
Association between renal function and cognitive performance.
Table 2 summarizes the associations between cognitive domains at baseline (hospital admission) as well as 24 months later and CCl. At baseline, lower CCl was associated with poor performance in executive functions, visuospatial and total cognitive scores, as well as in MoCA scores. Two years later, lower CCl was significantly associated with poor performance in executive functions, visuospatial, verbal function, MoCA, and total cognitive scores. This correlation remained significant after adjustment for age, sex, education, and vascular risk factors, including hypertension, diabetes, and cardiac disease (model 3).
No association was observed with memory and attention scores of the Neurotrax at both time points, but lower CCl was significantly associated with poor performance in the attention scores of the MoCA.
To support the causal role of white matter microstructural integrity (reflected as reduced NAWM FA) in the pathway connecting reduced CCl levels and decreased cognitive functions (r = 0.177, p = 0.004), we performed a partial correlation between CCl and global cognitive scores 2 years poststroke, adjusting for global FA, which resulted in a strong attenuation of the association (r = 0.154, p = 0.080). The same was observed for the role of hippocampal MD values in the pathway of reduced CCl levels and decreased cognitive function, also resulting in strong attenuation of the association (r = 0.093, p = 0.280).
Subanalysis of the CCl association with cognitive scores in the TIA group alone revealed that lower CCl was associated with lower executive function, memory, visuospatial, and language domains (p = 0.012, 0.002, 0.002, respectively), while in the stroke patients we found similar association with those domains, apart from memory, which was weaker.
Univariate and multivariate predictors of cognitive impairment.
During 2 years follow-up, 67 participants (15.5%) developed clinically significant cognitive impairment, as defined in the Methods. Of these, 11 patients (2.6%) developed dementia and 56 patients (13%) developed MCI. No significant differences in CCl values were observed between patients with MCI and dementia, and they were therefore grouped together as a cognitive impairment group.
Univariate and multivariate predictors for cognitive impairment in the 2-year follow-up period are shown in table 3. Univariate predictors included age (≥65 years), lower education (<12 years), hypertension, diabetes mellitus, antihypertensive medication, oral hypoglycemic agents, depression scores at admission, and CCI <60 mL/min.
Table 3.
Univariate and multivariate predictors of cognitive decline within 24 months from stroke
In multivariate analysis predicting cognitive impairment, predictors retained were age ≥65 years, <12 years of education, depression score at hospital admission, and CCl <60 mL/min (odds ratio 2.01, 95% confidence interval 1.03–3.92) (table 3).
The presence of new lesion in neuroimaging at admission, lesion location, or volume did not emerge as predictors of cognitive impairment in this cohort.
DISCUSSION
In this large prospective study, we found that impaired renal function at baseline was associated with decreased performance in almost all cognitive domains 2 years following mild ischemic stroke or TIA. A second finding from the current analysis is the association between impaired renal function and cortical atrophy, increased WMH volume, and abnormal microstructural integrity, known biomarkers of the aging brain and CSVD. Notably, these associations were independent of cardiovascular risk factors examined.
The pathophysiology leading to cognitive decline in patients with CKD is not understood. The striking association in our study between CKD and WMH, a radiologic marker of CSVD, may suggest that the latter is the missing link connecting renal impairment to cognitive decline. It is not clear, however, whether CKD is a direct contributor to CSVD or another manifestation of the same systemic pathology, such as endothelial dysfunction or disruption of microvascular autoregulation, affecting both organs. Several findings may support the former hypothesis. CKD was found to promote elevated levels of inflammatory and procoagulant mediators,27 suggested risk factors for WMH.27 Endothelial and smooth muscle dysfunction may also be derived from high level of uremic toxins among patients with CKD,28,29 possibly as a result of nitric oxide synthesis inhibition.30 An association between nitric oxide synthesis and WMH was also previously described.31 The strong attenuation in the association between CKD and cognitive performance, observed following adjustment for microstructural integrity of the white matter and cardiovascular risk factors, also supports the hypothesis that CKD is a key player and not just a biomarker for cognitive decline, promoting cerebral structural changes.
Another possibility is that both CKD and CSVD are endpoints of the same pathway resulting in microvascular damage. The brain and the kidneys have many common anatomic and vasoregulatory features as they are both low-resistance end organs exposed to high-volume blood flow (15% and 20% of resting cardiac output, respectively)32 and thus are susceptible to microvascular damage translating, in the brain, to loss of white matter integrity and cortical atrophy.
As renal function is easier to be assessed in life than CSVD, it may be used as a diagnostic marker for the identification of patients at risk for developing vascular dementia, being candidates for future implementation of prevention strategies or aggressive modifiable risk factors monitoring. Our results support CCI as a screening tool for the identification of a population at risk for developing vascular dementia.
The current study supports previous studies reporting an inverse association between renal function and cognitive decline in the elderly.2,3,33 This study aimed to examine this link exclusively in mild stroke patients, who are at increased risk for developing cognitive decline and dementia.34 Since most of our patients with CKD were slightly impaired in their kidney function (only 2.6% of the patients had CCl <30 mL/min, while 37.3% had CCl of 30–60 mL/min), we found that even minor impairment in kidney function doubled the risk for decline in almost all cognitive domains as well as in total cognitive scores 2 years following stroke, above and beyond vascular risk factors.
Of note, in patients with decreased renal function, we observed impairment in executive function and visuospatial scores already at hospital admission, followed by impairment in more domains after 2 years, but not with memory or attention scores. This could be explained by the sensitivity of executive functioning and processing speed to subtle, diffuse, and age- and vascular-related deterioration in white matter integrity. Indeed, previous studies have suggested that specific cognitive functions are more vulnerable to decreased renal functions, mainly executive cognitive function.35–37 We hypothesize that even mild stroke or TIA may accelerate subclinical cognitive decline, already affecting selective domains in patients with CKD, promoting conversion to dementia. The relation between longstanding renal disease and acute mild stroke deserves further study.
Strengths of the study are the systematic prospective follow-up, the availability of wide and comprehensive data on subject clinical status and macrostructural MRI changes, as well as the use of an extensive and validated computerized cognitive tool battery of multidomain cognitive tests and the use of a consensus forum of specialists for determining cognitive status. Indeed, previous studies assessing the prevalence of cognitive impairment in hemodialysis patients used short screening tests, such as the Mini-Mental State Examination (MMSE)38 and the 3 MS (an extension of the MMSE), with limited sensitivity for the diagnosis of vascular cognitive impairment in particular.39 The main limitations of our study are the absence of accurate neuropathologic correlation for renal disease or CSVD, the lack of a nonstroke control group, and not having enough power to analyze patients with very severe CKD (CCl <15 mL/min).
Decreased renal function is associated with radiologic markers of CSVD and loss of white matter integrity as well as hippocampal volume, and found to be a predictor for lower performance in cognitive tests 2 years following stroke/TIA.
Stroke patients may benefit from intervention to control renal function as patients with CKD may benefit from interventions to improve cognitive flexibility and performance.
ACKNOWLEDGMENT
The authors thank Prof. Amos D. Korczyn for performing assessments of participants suspected of having cognitive impairment.
GLOSSARY
- CCl
creatinine clearance
- CKD
chronic kidney disease
- CSVD
cerebral small vessel disease
- Da
axial diffusivity
- Dr
radial diffusivity
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- GM
gray matter
- ICV
intracranial volume
- MCI
mild cognitive impairment
- MD
mean diffusivity
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- NAWM
normal-appearing white matter
- NIHSS
NIH Stroke Scale
- SPGR
spoiled gradient echo
- WMH
white matter hyperintensities
- WML
white matter lesion
AUTHOR CONTRIBUTIONS
Dr. Auriel: study design, interpretation of results, manuscript writing. Dr. Kliper: volumetric analysis of neuroimaging data, interpretation of results. Dr. Shenhar-Tsarfaty: data ascertainment, interpretation of results. Dr. Molad: patient recruitment, data collection. Prof. Berliner: study design, interpretation of results. Prof. Shapira: study design, interpretation of results. Dr. Ben-Bashat: neuroimaging protocol designing, interpretation of results. Dr. Shopin: patient recruitment, data collection. Dr. Tene: data collection. Prof. Rosenberg: study design, interpretation of results. Prof. Bornstein: study design, interpretation of results. Dr. Ben Assayag: study design, interpretation of results, statistical analysis, manuscript writing.
STUDY FUNDING
This study is supported by grants 2011344 From the US–Israel Bi-national Science Foundation and grant RAG11482 from the American Federation for Aging Research. These funding agencies had no role in the conduct and publication of this study.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kurella Tamura M, Muntner P, Wadley V, et al. Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. Am J Kidney Dis 2011;58:756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol 2004;15:1904–1911. [DOI] [PubMed] [Google Scholar]
- 3.Buchman AS, Tanne D, Boyle PA, Shah RC, Leurgans SE, Bennett DA. Kidney function is associated with the rate of cognitive decline in the elderly. Neurology 2009;73:920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etgen T, Chonchol M, Forstl H, Sander D. Chronic kidney disease and cognitive impairment: a systematic review and meta-analysis. Am J Nephrol 2012;35:474–482. [DOI] [PubMed] [Google Scholar]
- 5.Kurella Tamura M, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis 2008;52:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben Assayag E, Korczyn AD, Giladi N, et al. Predictors for poststroke outcomes: the Tel Aviv Brain Acute Stroke Cohort (TABASCO) study protocol. Int J Stroke 2012;7:341–347. [DOI] [PubMed] [Google Scholar]
- 7.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089–2100. [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–147. [DOI] [PubMed] [Google Scholar]
- 10.Jorm AF, Christensen H, Korten AE, Jacomb PA, Henderson AS. Informant ratings of cognitive decline in old age: validation against change on cognitive tests over 7 to 8 years. Psychol Med 2000;30:981–985. [DOI] [PubMed] [Google Scholar]
- 11.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 12.Doniger GM, Dwolatzky T, Zucker DM, et al. Computerized cognitive testing battery identifies mild cognitive impairment and mild dementia even in the presence of depressive symptoms. Am J Alzheimers Dis Other Dement 2006;21:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnaiz E, Almkvist O, Ivnik RJ, et al. Mild cognitive impairment: a cross-national comparison. J Neurol Neurosurg Psychiatry 2004;75:1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pendlebury ST, Mariz J, Bull L, Mehta Z, Rothwell PM. MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards Neuropsychological Battery after TIA and stroke. Stroke 2012;43:464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivnik RJ, Haaland KY, Bieliauskas LA. American Board of Clinical Neuropsychology special presentation: The American Board of Clinical Neuropsychology (ABCN), 2000 update. Clin Neuropsychol 2000;14:261–268. [DOI] [PubMed] [Google Scholar]
- 16.Dwolatzky T, Whitehead V, Doniger GM, et al. Validity of the Mindstreams computerized cognitive battery for mild cognitive impairment. J Mol Neurosci 2004;24:33–44. [DOI] [PubMed] [Google Scholar]
- 17.Stuart M, Turman AB, Shaw J, Walsh N, Nguyen V. Effects of aging on vibration detection thresholds at various body regions. BMC Geriatr 2003;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artzi M, Aizenstein O, Jonas-Kimchi T, Myers V, Hallevi H, Ben Bashat D. FLAIR lesion segmentation: application in patients with brain tumors and acute ischemic stroke. Eur J Radiol 2013;82:1512–1518. [DOI] [PubMed] [Google Scholar]
- 19.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001;32:1318–1322. [DOI] [PubMed] [Google Scholar]
- 20.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I: segmentation and surface reconstruction. Neuroimage 1999;9:179–194. [DOI] [PubMed] [Google Scholar]
- 21.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 22.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage 1999;9:195–207. [DOI] [PubMed] [Google Scholar]
- 23.Fjell AM, Westlye LT, Greve DN, et al. The relationship between diffusion tensor imaging and volumetry as measures of white matter properties. Neuroimage 2008;42:1654–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage 2000;11:805–821. [DOI] [PubMed] [Google Scholar]
- 25.Sheikh JI, Yesavage JA. A knowledge assessment test for geriatric psychiatry. Hosp Community Psychiatry 1985;36:1160–1166. [DOI] [PubMed] [Google Scholar]
- 26.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 27.Khatri M, Wright CB, Nickolas TL, et al. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS). Stroke 2007;38:3121–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Brunet P. Vascular incompetence in dialysis patients: protein-bound uremic toxins and endothelial dysfunction. Semin Dial 2011;24:327–337. [DOI] [PubMed] [Google Scholar]
- 29.Zafeiropoulou K, Bita T, Polykratis A, Karabina S, Vlachojannis J, Katsoris P. Hemodialysis removes uremic toxins that alter the biological actions of endothelial cells. PLoS One 2012;7:e30975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linden E, Cai W, He JC, et al. Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clin J Am Soc Nephrol 2008;3:691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henskens LH, Kroon AA, van Boxtel MP, Hofman PA, de Leeuw PW. Associations of the angiotensin II type 1 receptor A1166C and the endothelial NO synthase G894T gene polymorphisms with silent subcortical white matter lesions in essential hypertension. Stroke 2005;36:1869–1873. [DOI] [PubMed] [Google Scholar]
- 32.Mogi M, Horiuchi M. Clinical interaction between brain and kidney in small vessel disease. Cardiol Res Pract 2011;2011:306189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the Health, Aging, and Body Composition Study. J Am Soc Nephrol 2005;16:2127–2133. [DOI] [PubMed] [Google Scholar]
- 34.Pasi M, Poggesi A, Salvadori E, Pantoni L. Post-stroke dementia and cognitive impairment. Front Neurol Neurosci 2012;30:65–69. [DOI] [PubMed] [Google Scholar]
- 35.Murea M, Hsu FC, Cox AJ, et al. Structural and functional assessment of the brain in European Americans with mild-to-moderate kidney disease: Diabetes Heart Study-MIND. Nephrol Dial Transpl 2015;30:1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slinin Y, Paudel ML, Ishani A, et al. Kidney function and cognitive performance and decline in older men. J Am Geriatr Soc 2008;56:2082–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zammit AR, Katz MJ, Lai JY, Zimmerman ME, Bitzer M, Lipton RB. Association between renal function and cognitive ability domains in the Einstein aging study: a cross-sectional analysis. J Gerontol A Biol Sci Med Sci 2015;70:764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 39.Godefroy O, Fickl A, Roussel M, et al. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke 2011;42:1712–1716. [DOI] [PubMed] [Google Scholar]