Abstract
Background
Milk carotenoids may support preterm infant health and neurodevelopment. Infants fed human milk often have higher blood and tissue carotenoid concentrations than infants fed carotenoid-containing infant formula (IF). Donor human milk (DHM) is a supplement to mother’s own milk, used to support preterm infant nutrition.
Objectives
We tested whether tissue and plasma β-carotene concentrations would be higher in preterm pigs fed pasteurized DHM versus premature IF.
Methods
This is a secondary analysis of samples collected from a study of the effects of enteral diet composition on necrotizing enterocolitis incidence. Preterm pigs received partial enteral feeding of either DHM (n = 7) or premature IF (n = 7) from 2 to 7 d of age. The diets provided similar β-carotene (32 nM), but DHM had higher lutein, zeaxanthin, and lycopene, whereas IF had higher total vitamin A. Plasma, liver, and jejunum carotenoid and vitamin A concentrations were measured by HPLC-PDA. Jejunal expression of 12 genes associated with carotenoid and lipid metabolism were measured.
Results
Liver β-carotene concentrations were higher in DHM- than IF-fed piglets (23 ± 4 compared with 16 ± 2 μg/g, respectively, P = 0.0024), whereas plasma and jejunal β-carotene concentrations were similar between diets. Liver vitamin A stores were higher in piglets fed IF than DHM (50.6 ± 10.1 compared with 30.9 ± 7.2 μg/g, respectively, P=0.0013); however, plasma vitamin A was similar between groups. Plasma, liver, and jejunum concentrations of lutein, zeaxanthin, and lycopene were higher with DHM than IF feeding. Relative to piglets fed DHM, jejunal low density lipoprotein receptor (Ldlr) expression was higher (61%, P = 0.018) and cluster determinant 36 (Cd36) expression (−27%, P = 0.034) was lower in IF-fed piglets.
Conclusions
Preterm pigs fed DHM accumulate more liver β-carotene than IF-fed pigs. Future studies should further investigate infant carotenoid bioactivity and bioavailability.
Keywords: carotenoids, absorption, bioavailability, infant nutrition, neonate, vitamin A
Introduction
Carotenoids are bioactive, lipophilic phytochemicals found throughout nature [1] and are predominantly acquired by humans from dietary fruits and vegetables. Young infants consume dietary carotenoids from human milk [2] or supplemented infant formula (IF) [[3], [4], [5]]. In early life, gestational carotenoid exposure and early dietary carotenoid intake are associated with neurocognitive, visual, and motor development and reduced inflammation [[5], [6], [7], [8], [9]]. Some carotenoids possessing an unsubstituted β-ionone ring, such as β-carotene, α-carotene, and β-cryptoxanthin, can be metabolized to vitamin A [10] to support vitamin A requirements for normal growth, vision, and immune function [11]. Other carotenoids, such as lycopene, lutein, and zeaxanthin, are not metabolized to vitamin A but function as antioxidants, anti-inflammatories, and bioactive signaling molecules [12].
Infants are born with relatively low plasma and tissue carotenoids, and preterm infants are born with even lower levels than term-born infants [13]. Human milk feeding is associated with greater blood and tissue carotenoid concentrations in infants compared with formula feeding [2,13,14]. For instance, infants fed human milk have blood lutein levels that are 4 times higher than those fed IF providing the same amounts of lutein [3]. Indeed, one study showed IF must be supplemented with 4 times more β-carotene to confer term infant plasma concentrations similar to those fed human milk [4]. However, carotenoid supplementation in formula is heterogeneous across products and brands [[15], [16], [17], [18]] and typically does not surpass mean carotenoid concentrations found in mature human milk [2]. Thus, formula-fed term and preterm infants might not benefit from the potential health benefits of carotenoids to the same degree as their human milk-fed counterparts.
The mechanisms that regulate the bioavailability and metabolism of carotenoids in human milk and IF are unclear, partly due to a lack of established experimental pediatric animal models. Neonatal pigs may provide a model of preterm infant carotenoid absorption and biodistribution from different dietary sources. The neonatal pig is an excellent model of infant gastrointestinal anatomy, physiology, and metabolism [[19], [20], [21]]. Previously, lutein concentrations were measurable in the tissues of piglets fed experimental lipid supplements, demonstrating enteral absorption of an intact carotenoid in piglets [22]. However, the degree to which neonatal pigs absorb carotenoids from human milk and IF, and whether the rates of bioaccumulation differ by milk type, as observed in infants, is unknown.
The purpose of this study was to first test the feasibility of preterm neonatal pigs as a model of preterm infant carotenoid nutrition by assessing carotenoid uptake and biodistribution from milk matrices. The second purpose was to compare carotenoid accumulation in tissues and plasma of piglets fed either donor human milk (DHM) or preterm IF, diets typically provided in a neonatal care setting. To pursue these goals, a secondary analysis of samples collected from a study where the aim was to compare how IF and DHM affect the incidence of necrotizing enterocolitis (NEC) in preterm piglets was conducted. Establishing a relevant animal model to study infant carotenoid absorption and biodistribution will facilitate physiologically-relevant mechanistic studies to define why carotenoids are more bioavailable in human milk than IF.
Materials and Methods
Study design and experimental model
The animal protocol was approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with the National Institutes of Health guidelines. The piglets were part of a larger study on the effect of neonatal nutrition on NEC risk and severity reported previously [23]. NEC is a common but serious condition found in preterm infants fed IF [24]. The specimens used for the current analyses came from piglets free of jejunal pathology. Pregnant mixed-breed sows were brought to the animal care facility and allowed to acclimate 1 wk before surgery. Preterm pigs were delivered through Cesarean section at 104 d of gestation (full term = 114 d) as described previously [25]. A few hours after birth, the piglets were surgically implanted with a jugular venous catheter (0.03” inner diameter, silastic) and an orogastric tube (6 French, Tygon) to facilitate parenteral nutrition and enteral feeding. The piglets received 50% of their total nutritional requirements through total parenteral nutrition (120 mL/kg×d, 410 kJ energy/kg×d), 12.5 g dextrose/(kg×d), 6.5 g amino acids/(kg×d), 2.5 g fat/(kg×d) (Intralipid 20%, Fresenius Kabi) at a rate of 5 mL/kg×h via the jugular venous catheter from day 1 until the end of the study on day 7. Beginning on day 2, every 4 h, the piglets were fed enterally, through their orogastric tube, the diets described below. Enteral feeding began at 20% of the total nutritional requirement (8 mL/kg×4h), progressed gradually (every 24 h) to 30%, 40%, and 50% (12, 16, and 20 mL/kg×4h) until day 5, and was maintained until day 7. Enteral feeds were provided to piglets randomly assigned to receive either premature IF (n = 7) (Enfamil Premature 24 Cal/fl oz High Protein Liquid Formula, Mead Johnson) or pasteurized DHM (n = 7) (Human Milk, Prolacta Bioscience). Maternal sow plasma collected at the time of Cesarean section was given intravenously to the piglets 6, 12, and 24 h after birth at a rate of 4, 5, and 7 mL/kg body weight, respectively.
Sample collection
Prior to euthanasia at day 7, jugular blood samples were collected, and plasma was prepared by centrifugation at 4000 × g for 15 min and frozen at −80°C for further analysis. Animals were euthanized (Beuthanasia-D, Merck), and tissue sections of the proximal jejunum were collected and frozen at −80°C for further analysis.
Experimental diets
The carotenoid composition of the diets is found in Table 1. The macronutrient distribution of IF was 14% of energy as protein, 45% as fat, and 42% as carbohydrate as provided by the manufacturer’s nutrition facts panel [27]. The DHM macronutrient distribution was 6% of energy as protein, 52% as fat, and 42% as carbohydrate.
TABLE 1.
Human milk and infant formula carotenoid and vitamin A concentrations1
| Nutrient | Experimental feeding |
|
|---|---|---|
| Donor human milk | Infant formula | |
| β-carotene (nM) | 32.1 ± 16.3 | 31.3 ± 7.1 |
| Lutein (nM) | 27.3 ± 3.3 | 7.8 ± 2.7 ∗ |
| Zeaxanthin (nM) | 20.2 ± 3.6 | 1.5 ± 0.4 ∗ |
| Lycopene (nM) | 18.5 ± 1.0 | – ∗ |
| Total preformed vitamin A2 (μM) | 2.07 ± 1.72 | 6.97 ± 0.70 ∗ |
| Theoretical total vitamin A activity3 (μM) |
2.13 ± 1.75 |
7.04 ± 0.71 ∗ |
| Retinol Activity Equivalents4 (μg/L) | 593 ± 493 | 1999 ± 200 ∗ |
∗ Indicates significant differences (P < 0.05).
The analyses were done in technical triplicates. Data are presented as means ± SD.
Sum of retinol and retinyl palmitate species detected.
Sum of total preformed vitamin A plus the theoretical yield of retinol from β-carotene of 2:1.
Sum of total preformed vitamin A in retinol equivalents plus β-carotene divided by 12, per the Institute of Medicine 2001 Dietary Reference Intake conversion factor for β-carotene from foods [26].
Body mass, growth rate measurement, and intestinal health assessment
Piglet body masses were recorded at birth and at the end of the study on day 7. Daily growth rate per kg mean body weight was calculated from final and starting body weights. The stomach, ileum, jejunum, and colon were assessed for gross signs of NEC as previously described [23].
Plasma and tissue carotenoid and vitamin A measurement
Carotenoids and vitamin A were extracted from DHM and IF as previously described [28]. Plasma (0.4–0.7 mL) carotenoids and vitamin A were extracted as previously described without saponification [28], and liver and jejunum carotenoids and total vitamin A were extracted with saponification as previously described from 100 μg samples [29]. Jejunum vitamin A was not analyzed due to the presence of co-eluting compounds that interfered with the quantitation. Echinenone was added to all samples as an internal recovery standard. Sample extracts were reconstituted in 1:1 methanol:methyl-tert-butyl ether, and all-trans-β-carotene, all-trans-α-carotene, all-trans- and 5-cis-lycopene, all-trans-lutein, and all-trans-zeaxanthin were quantified against authentic standards. Carotenoids were quantified by photodiode array detection at 452 nm, with the exception of lycopene, which was quantified at 472 nm. Retinol and retinyl palmitate were quantified against authentic standards by fluorescence (Dionex Fluorescence Detector, UltiMate 3000, ThermoFisher Scientific) with an excitation/emission of 325nm/471nm. Total preformed vitamin A in diets was calculated as the sum of retinol and retinyl palmitate; the theoretical total dietary vitamin A was calculated as the sum of total preformed vitamin A plus 2 times the concentration of β-carotene; and the retinol activity equivalents were calculated as the total preformed vitamin A plus one-twelfth of β-carotene, in accordance with the Institute of Medicine conversion factor for β-carotene to retinol from foods [26]. Authentic standards for echinenone, α-carotene, β-carotene, lycopene, lutein, zeaxanthin, retinol, and retinyl palmitate were obtained from Millipore-Sigma.
mRNA expression of genes associated with carotenoid and lipid transport and metabolism
Healthy jejunal mRNA expression of 12 target genes associated with carotenoid and lipid metabolism were measured by qRT-PCR and compared between treatment groups. RNA was extracted in triplicate from ground whole jejunum tissue per manufacturer’s protocol (RNA Purification Plus, Norgen Biotek Corp.). β-mercaptoethanol was added to the lysis solution to prevent nucleic acid degradation. The concentration of RNA was measured spectrophotometrically, and RNA quality was determined by high resolution automated electrophoresis (Bioanalyzer platform, Agilent Technologies) with RNA integrity numbers being deemed acceptable if >5.0. cDNA was synthesized from all RNA samples (High-Capacity cDNA Reverse Transcription Kit, Applied Biosystems), and double-distilled H2O was added to each cDNA sample to dilute samples to a concentration of 50 ng/μL.
Three potential housekeeping genes were tested, actin β (Actb), ribosomal protein L4 (Rpl4), and hypoxanthine phosphoribosyltransferase 1 (Hrpt1), using porcine gene expression assays (Taqman gene expression assays Ss03376563_uH, Ss03374067_g1, and Ss03388274_m1, respectively, ThermoFisher) according to the manufacturer’s instructions. Expression was measured by qRT-PCR (Applied Biosystems QuantStudio 6 Flex, ThermoFisher) according to the manufacturer’s directions. Housekeeping gene performance was compared by inspecting the within- and between-treatment group Ct stability, by comparing the mean within-group coefficient of variance of the Ct’s for each treatment group, and by testing for mean separation between treatment group Cts by 1-way analysis of variance (ANOVA). On this basis, Rpl4 was chosen as a suitable housekeeping gene (within-group coefficient of variance ≤1.4; P (between groups) = 0.32).
Expression of each target gene (scavenger receptor class B type 1 [Scarb1; Ss03391104_m1], low density lipoprotein receptor [Ldlr; Ss03376563_uH], cluster determinant 36 [Cd36], ATP binding cassette subfamily B member 1 [Abcb1; Ss03373435_m1], β-carotene oxygenase 1 [Bco1; Ss06942225_m1), β-carotene oxygenase 2 [Bco2; Ss06880622_m1], NPC1 like intracellular cholesterol transporter 1 [Npc1l1; Ss06874101_m1], ATP binding cassette subfamily A member 1 [Abca1; Ss04955209_m1], ATP binding cassette subfamily G member 5 [Abcg5; Ss03377267_u1], and ATP binding cassette subfamily G member 8 [Abcg8; Ss06906094_m1]) was measured using Taqman validated porcine gene expression assays as above.
Statistics
Group differences between body weights, growth rates, gene expression, and carotenoid and vitamin A concentrations were compared by 1-way ANOVA or nonparametric ANOVA (Kruskal-Wallis 1-way ANOVA on ranks with Tukey’s post hoc test) if the assumptions of normality (Shapiro-Wilk) or equal variance (Brown-Forsythe) were not met. Natural-log transformation was applied to jejunum carotenoid concentration data to normalize the data. Gene expression data were analyzed and interpreted using the ddCt method, relative to within-sample Rpl4 expression to yield dCt and then each sample relative to the DHM-fed group mean dCt to yield ddCt. Differences were interpreted as significant with a cutoff of α = 0.05. Statistical tests were performed using SigmaPlot 13 (Systat, Inpixon).
Results
Piglet body weights, growth rates, and gastrointestinal health
Body weights for DHM- and IF-fed piglets did not differ at birth (1010 ± 20 compared with 959 ± 24 g, respectively; P = 0.67) or at the end of the study (1228 ± 26 compared with 1150 ± 22 g, respectively; P=0.56). Growth rates were similar between DHM and IF-fed groups (27 ± 3 compared with 27 ± 19 g/(kg×d), respectively; P=0.27). None of the piglets analyzed in the current study had gross signs of NEC in the stomach, jejunum, or ileum. However, there was a higher incidence of colonic NEC in IF-fed piglets (86% incidence) than DHM-fed piglets (29% incidence).
Milk carotenoid and vitamin A composition
There was a respective descending concentration of β-carotene, lutein, zeaxanthin, and lycopene in both DHM and IF diets. The β-carotene concentration was similar between both DHM and IF, whereas lutein and zeaxanthin concentrations were higher in DHM compared with IF (Table 1). There was no lycopene detected in IF. There was a greater total preformed vitamin A concentration in the IF than DHM, as well as greater theoretical total vitamin A and retinol activity equivalents.
Plasma, liver, and jejunum carotenoid and vitamin A concentrations
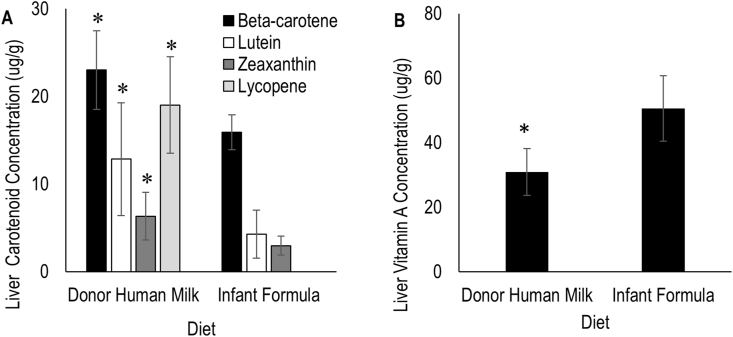
β-carotene, lutein, and zeaxanthin were detectable in both DHM- and IF-fed piglet livers, whereas lycopene was detectable only in DHM-fed piglet liver tissue. DHM-fed piglets had significantly higher liver concentrations of all carotenoids than IF-fed piglets (Figure 1). In both DHM- and IF-fed piglets, the most abundant liver carotenoid was β-carotene. The least abundant liver carotenoid was zeaxanthin for both diet groups. Notably, lycopene was the second most abundant carotenoid in DHM-fed piglets, whereas it was absent in IF-fed piglets. Additionally, DHM-fed piglets had lower liver retinol concentrations than in IF-fed piglets. Retinyl palmitate was undetectable in the livers in both DHM and IF, likely due to the saponification step of the extraction.
FIGURE 1.
Carotenoid (A) and vitamin A (B) concentrations in livers of piglets provided different milk diets for 5 d. n = 7 per group. Asterisk (∗) indicate significant differences in carotenoid and retinol concentrations between diet types, α = 0.05. Total vitamin A is equivalent to retinol concentration.
β-carotene and lutein were detected in both IF- and DHM-fed piglet plasma, whereas zeaxanthin and lycopene were only detectable in DHM-fed piglet plasma (Figure 2). The most abundant plasma carotenoid was lycopene in DHM-fed piglets and was β-carotene in IF-fed piglets. There was no significant difference found in the β-carotene, retinol, retinyl palmitate concentrations between DHM and IF piglet plasma (Figure 2).
FIGURE 2.
Carotenoid (A) and vitamin A (B) concentrations in plasma of piglets provided different milk diets for 5 d. n = 6 per group. Asterisk (∗) indicates significant differences in carotenoid concentrations between diet types, α = 0.05. Plasma vitamin A includes the combined concentrations of retinol and retinyl palmitate.
β-carotene, lutein, and zeaxanthin were detected in DHM- and IF-fed piglet jejunum tissues, whereas lycopene was absent in jejunum tissue of IF-fed piglets. Jejunum concentrations of lutein and zeaxanthin were higher in DHM-fed piglets, whereas β-carotene was similar between DHM- and IF-fed piglet jejunum tissues. The most abundant jejunum carotenoid was zeaxanthin in DHM-fed piglets and β-carotene in IF-fed piglets, whereas the least abundant carotenoid was β-carotene in the DHM-fed piglets and lutein in the IF-fed piglets (Figure 3).
FIGURE 3.
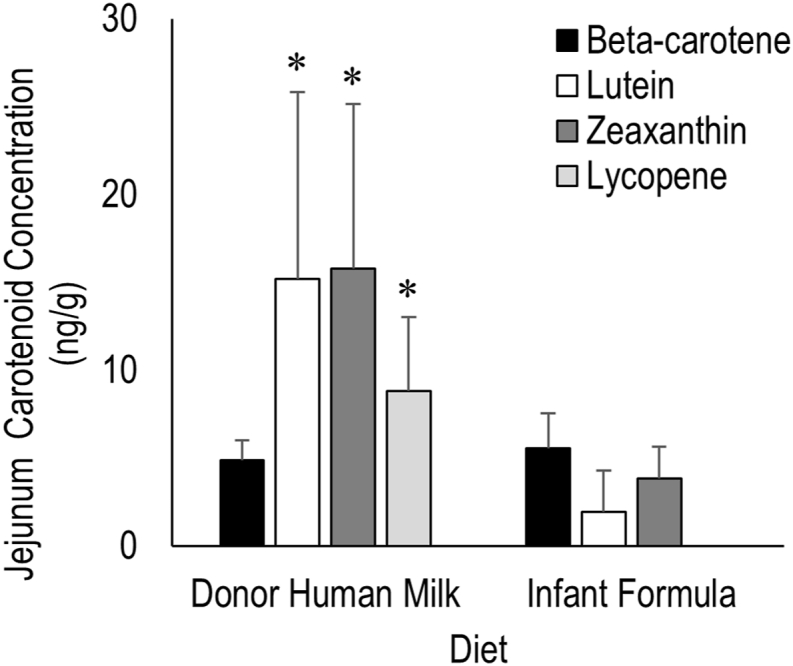
Carotenoid concentrations in jejunums of piglets provided different milk diets for 5 d. n = 7 per group. Asterisk (∗) indicates significant differences in carotenoid concentrations between diet types, α = 0.05.
Jejunal gene expression
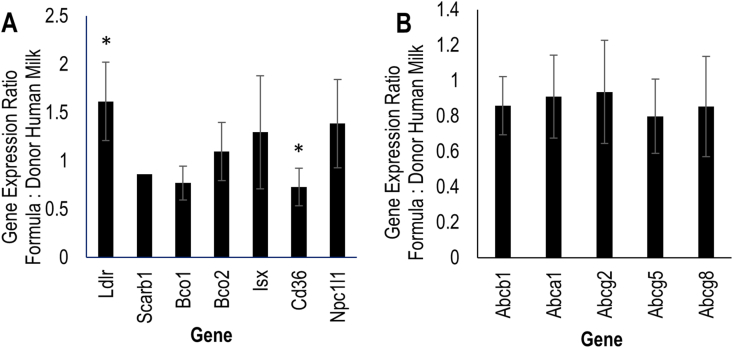
Expression of Ldlr was greater in formula-fed piglets (1.57 ± 0.39-fold, P = 0.018), whereas expression of Cd36 was lower with formula feeding (0.747 ± 0.20-fold, P = 0.034) than DHM-feeding (Figure 4). Expression of the other genes did not significantly differ by diet treatments.
FIGURE 4.
Jejunal expression in formula-fed piglets relative to donor human milk-fed piglets of genes (A) previously shown to be involved in intestinal carotenoid absorption and metabolism and (B) hypothesized to be involved in carotenoid uptake and efflux. Asterisk (∗) denotes significant upregulation or downregulation of formula-fed relative to donor human milk-fed group, α = 0.05.
Discussion
Human infants fed IF are reported to have lower plasma and tissue carotenoid concentrations than human milk-fed infants [[3], [4], [5]]. However, it is unknown whether this is due to differences in milk carotenoid contents and/or due to differences in the digestion, absorption, or metabolism of carotenoids in IF- and human milk. The preterm pig provides a novel model to study preterm infant carotenoid biodistribution and bioactivity. In this secondary analysis of specimens collected from a study of the effects of diet composition on NEC incidence, neonatal pigs absorbed and accumulated DHM and IF carotenoids in the plasma, jejunum, and liver. Further, DHM feeding was associated with higher concentrations of liver β-carotene than IF feeding. In addition, jejunum Cd36 and Ldlr expressions differ between IF and DHM, suggesting potential carotenoid and lipid metabolic differences by enteral feeding type.
Previously, it was unknown whether neonatal pigs absorb measurable amounts of intact carotenoids from milk diets. The plasma carotenoid concentrations in the piglets in the current study were lower than what is typically found in human infant serum [2]. However, this might be expected, as the feeding duration in the current study was only 5 d with only partial enteral feeding. It was previously reported that older pigs have a limited, but detectable, absorption of intact β-carotene into the plasma and liver [[30], [31], [32], [33]], and piglets fed a lutein-containing lipid supplement have greater liver lutein concentrations than controls [22]. In the current study, human milk-fed piglets demonstrated a general pattern of higher plasma carotenoid concentrations compared with IF-fed piglets, similar to that seen in human infants, though the concentrations were 2 to 3 orders of magnitude lower than that reported in human infants [[3], [4], [5]]. Specifically, a study of human infants fed either control formula, carotenoid-supplemented formula, or human milk for 56 d [4] resulted in a plasma carotenoid profile similar to the current study’s piglet plasma carotenoid concentrations, in which lycopene was the most abundant carotenoid in the plasma of human milk-fed infants and the current human milk-fed piglets [4,5]. These results are important and warrant further studies in preterm and term piglets to assess whether they may achieve similar blood carotenoid concentrations as human infants with longer-term feeding.
Carotenoid concentrations measured in plasma, liver, and jejunum were higher in piglets fed DHM compared with IF in all but 2 cases. In the plasma and jejunum samples, β-carotene was equal in concentration between both DHM-fed and IF-fed piglets. However, β-carotene accumulation in DHM-fed piglet liver tissue was higher than that in IF-fed piglets. Although there are limited data on infant tissue biodistribution of carotenoids by milk feeding type, infant skin carotenoid concentrations, measured noninvasively by resonance Raman spectroscopy, revealed that infants fed human milk had a higher overall skin carotenoid concentrations compared with infants fed formula [14]. Increasing carotenoid supplementation of IF can improve the bioequivalence with human milk feeding; however, formula must be supplemented to 2 to 5 times the concentrations in human milk to achieve similar plasma concentrations as human milk [4,5]. In vitro experimental studies demonstrated that carotenoids are more efficiently absorbed from human milk than from IF, but this effect is independent of carotenoid bioaccessibility, which is the ability of carotenoids to be released from the milk matrix [34].
The greater β-carotene concentrations in DHM-fed piglet livers, but not plasma or jejunum, could be due to plasma and jejunum reflecting more transient pools of β-carotene, whereas liver may serve as a more stable indicator of carotenoid stores [35,36]. Although there was no lycopene detected in IF-fed tissues, lycopene concentrations in DHM-piglet livers were comparable to that of β-carotene, the most abundant carotenoid, in DHM-fed piglet livers. This relative accumulation of liver lycopene compared to what was in the diet could be due to greater uptake from the plasma, slower release from the liver to the plasma, or slower breakdown or metabolic clearance in the liver. The major mammalian cleavage enzymes of carotenoids are Bco1 and Bco2 [12]. The efficiency of porcine Bco1 and Bco2 for cleavage of different carotenoids is unclear, though different animal species have different catalytic effiencies for carotenoids. For instance, human Bco1 catalyzes the central cleavage of carotenoids and has a similar cleavage efficiency of β-carotene and lycopene, whereas chicken Bco2 does not cleave lycopene, but ferret Bco2 does cleave lycopene [[37], [38], [39]]. The relative efficiency of pig carotenoid cleavage oxygenases may contribute to the accumulation of lycopene to β-carotene in tissues and plasma, relative to what is provided in the diet.
IF provided greater retinyl palmitate but similar retinol as the DHM. Plasma retinol and retinyl palmitate were similar in both diet groups. Liver retinol concentrations were lower in DHM-fed piglets than in IF-fed piglets, likely due to the lower intake of preformed dietary vitamin A (retinol + retinyl palmitate). The greater total vitamin A status of IF-fed piglets may have resulted in feedback-inhibition of carotenoid absorption, as has been described previously in other model systems [[40], [41], [42]], depressing tissue and plasma carotenoid concentrations.
Expression of Cd36 and Ldlr significantly differed between DHM- and IF feeding after only 5 d. CD36 mediates cellular carotenoid absorption [43,44], and therefore, the lower expression in the formula-fed piglets may provide a partial mechanistic explanation by which carotenoid absorption is lower in formula-fed than DHM-fed infants. LDLR plays a role in cholesterol homeostasis, and expression changes may indicate a shift in intestinal lipid metabolism resulting from different cholesterol contents in DHM compared with IF. Carotenoids share many uptake and metabolic pathways with cholesterol (reviewed in [45]); whether LDLR mediates carotenoid uptake directly or indirectly is currently unknown. However, several genes hypothesized to have greater variation between milk feeding types did not greatly differ. Although Scarb1, a gene involved in the transportation of nutrients such as lipids and provitamin A carotenoids, expression was lower in IF-fed piglets than DHM-fed piglets, the difference was not significant. Genes Abca1, Abcg5, and Abcg8 are all involved in intestinal cholesterol efflux into the bloodstream, but their expression did not differ by dietary treatments in the current study. Bco1 and Bco2 are carotenoid oxygenases involved in vitamin A production and generation of biologically active carotenoids, respectively, but their expression did not differ between groups. The current findings suggest that IF may cause changes in cell carotenoid absorption processes, via Ldlr and Cd36, to reduce carotenoid absorption. However, future mechanistic studies should investigate the effects of milk types on carotenoid absorptive and metabolic processes.
The current study had several strengths and limitations. Strengths included the clinically relevant animal model of premature infant nutrition and human IF and milk diets. Limitations were the short study duration and that there was a difference in the incidence of NEC between treatment groups, as is expected for this animal model, which could limit the applicability of the findings to a healthy infant population. The current study also lacked a control formula that was fully carotenoid- and vitamin A-matched to that of the DHM to directly compare milk matrix effects on carotenoid bioavailability from human milk and IF. In addition, many of the formula-fed piglets were experiencing NEC, which may increase systemic inflammation and immune responses, potentially altering carotenoid and vitamin A absorption and utilization.
In summary, carotenoids and vitamin A were detectable in piglets after short-term DHM and IF feeding, providing a feasible model of infant carotenoid bioaccumulation. Furthermore, even with short-term feeding, liver β-carotene differences between diets were apparent, mimicking differences observed in human infants fed different milk types [[3], [4], [5],14]. Our results suggest that the preterm pig is a promising animal model of human infant carotenoid metabolism. Future studies are warranted to define the mechanisms underlying differences in carotenoid bioaccumulation in human milk- and formula-fed piglets in early life to take a rational approach to improve formula carotenoid bioavailability.
Data availability
Data described in the manuscript will be made available upon request pending application and approval.
Author contributions
The authors’ responsibilities were as follows—NEM, ABH, BS, DGB: designed the research; NEM, JW, RS, BS, GG, DGB: conducted the research; NEM, JW, RS, BS, ABH, DGB: analyzed the data; NEM, JW, RS, DGB: wrote the paper; NEM: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Conflicts of interest
The authors report no conflicts of interest.
Funding
This project was supported in part by the USDA, Agricultural Research Service (cooperative agreements 3092-51000-059-NEW2S [NEM] and 3092-51000-060-01 [DGB]), and grants from the National Institutes of Health Grant (DK094616 [DGB] and K01 DK129408 [GG]). We are grateful to Prolacta Bioscience for donation of the human milk used in this study. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the USDA, the NIH, or Prolacta Bioscience.
References
- 1.Britton G., Liaaen-Jensen S., Pfander H., editors. Carotenoids. Birkhäuser Verlag; Basel, Boston: 1995. [Google Scholar]
- 2.Zaidi Y., Stroh R., Moran N.E. Systematic review of carotenoid concentrations in human milk and infant blood. Nutr. Rev. 2022;80(9):2029–2050. doi: 10.1093/nutrit/nuac018. [DOI] [PubMed] [Google Scholar]
- 3.Bettler J., Zimmer J.P., Neuringer M., DeRusso P.A. Serum lutein concentrations in healthy term infants fed human milk or infant formula with lutein. Eur. J. Nutr. 2010;49(1):45–51. doi: 10.1007/s00394-009-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackey A.D., Albrecht D., Oliver J., Williams T., Long A.C., Price P.T. Plasma carotenoid concentrations of infants are increased by feeding a milk-based infant formula supplemented with carotenoids. J. Sci. Food Agric. 2013;93(8):1945–1952. doi: 10.1002/jsfa.5996. [DOI] [PubMed] [Google Scholar]
- 5.Rubin L.P., Chan G.M., Barrett-Reis B.M., Fulton A.B., Hansen R.M., Ashmeade T.L., et al. Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. J. Perinatol. 2012;32(6):418–424. doi: 10.1038/jp.2011.87. [DOI] [PubMed] [Google Scholar]
- 6.Lai J.S., Cai S., Lee B.L., Godfrey K.M., Gluckman P.D., Shek L.P., et al. Higher maternal plasma β-cryptoxanthin concentration is associated with better cognitive and motor development in offspring at 2 years of age. Eur. J. Nutr. 2021;60(2):703–714. doi: 10.1007/s00394-020-02277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai J.S., Veetil V.O., Lanca C., Lee B.L., Godfrey K.M., Gluckman P.D., et al. Maternal lutein and zeaxanthin concentrations in relation to offspring visual acuity at 3 years of age: the GUSTO study. Nutrients. 2020;12(2):274. doi: 10.3390/nu12020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheatham C.L., Sheppard K.W. Synergistic effects of human milk nutrients in the support of infant recognition memory: an observational study. Nutrients. 2015;7(11):9079–9095. doi: 10.3390/nu7115452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmassani H.A., Switkowski K.M., Scott T.M., Johnson E.J., Rifas-Shiman S.L., Oken E., et al. Maternal intake of lutein and zeaxanthin during pregnancy is positively associated with offspring verbal intelligence and behavior regulation in mid-childhood in the Project Viva cohort. J. Nutr. 2021;151(3):615–627. doi: 10.1093/jn/nxaa348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanumihardjo S.A., Russell R.M., Stephensen C.B., Gannon B.M., Craft N.E., Haskell M.J., et al. Biomarkers of Nutrition for Development (BOND)-vitamin A review. J. Nutr. 2016;146(9):1816S–1848S. doi: 10.3945/jn.115.229708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowling J.E. Vitamin A: its many roles-from vision and synaptic plasticity to infant mortality. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2020;206(3):389–399. doi: 10.1007/s00359-020-01403-z. [DOI] [PubMed] [Google Scholar]
- 12.Eroglu A., Harrison E.H. Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids. J. Lipid Res. 2013;54(7):1719–1730. doi: 10.1194/jlr.R039537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernstein P.S., Sharifzadeh M., Liu A., Ermakov I., Nelson K., Sheng X., et al. Blue-light reflectance imaging of macular pigment in infants and children. Invest. Ophthalmol. Vis. Sci. 2013;54(6):4034–4040. doi: 10.1167/iovs.13-11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan G.M., Chan M.M., Gellermann W., Ermakov I., Ermakova M., Bhosale P., et al. Resonance Raman spectroscopy and the preterm infant carotenoid status. J. Pediatr. Gastroenterol. Nutr. 2013;56(5):556–559. doi: 10.1097/MPG.0b013e318282a8fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson C., Lyden E., Furtado J., Van Ormer M., Anderson-Berry A. A comparison of nutritional antioxidant content in breast milk, donor milk, and infant formulas. Nutrients. 2016;8(11):681. doi: 10.3390/nu8110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hostetler G.L. Determination of lutein and β-carotene in infant formula and adult nutritionals by ultra-high-performance liquid chromatography: single-laboratory validation, first action 2016.13. J. AOAC Int. 2017;100(3):768–781. doi: 10.5740/jaoacint.16-0386. [DOI] [PubMed] [Google Scholar]
- 17.Schimpf K.J., Thompson L.D., Pan S.J. Determination of carotenoids in infant, pediatric, and adult nutritionals by HPLC with UV-visible detection: single-laboratory validation, first action 2017.04. J. AOAC Int. 2018;101(1):264–276. doi: 10.5740/jaoacint.17-0287. [DOI] [PubMed] [Google Scholar]
- 18.Bohn T. Determinants and determination of carotenoid bioavailability from infant food formulas and adult nutritionals including liquid dairy products. J. AOAC Int. 2019;102(4):1044–1058. doi: 10.5740/jaoacint.19-0015. [DOI] [PubMed] [Google Scholar]
- 19.Sangild P.T., Thymann T., Schmidt M., Stoll B., Burrin D.G., Buddington R.K. Invited review: the preterm pig as a model in pediatric gastroenterology. J. Anim. Sci. 2013;91(10):4713–4729. doi: 10.2527/jas.2013-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burrin D., Sangild P.T., Stoll B., Thymann T., Buddington R., Marini J., et al. Translational advances in pediatric nutrition and gastroenterology: new insights from pig models. Annu. Rev. Anim. Biosci. 2020;8:321–354. doi: 10.1146/annurev-animal-020518-115142. [DOI] [PubMed] [Google Scholar]
- 21.Odle J., Lin X., Jacobi S.K., Kim S.W., Stahl C.H. The suckling piglet as an agrimedical model for the study of pediatric nutrition and metabolism. Annu. Rev. Anim. Biosci. 2014;2:419–444. doi: 10.1146/annurev-animal-022513-114158. [DOI] [PubMed] [Google Scholar]
- 22.Akinsulire O., Perides G., Anez-Bustillos L., Cluette-Brown J., Nedder A., Pollack E., et al. Early enteral administration of a complex lipid emulsion supplement prevents postnatal deficits in docosahexaenoic and arachidonic acids and increases tissue accretion of lipophilic nutrients in preterm piglets. JPEN J. Parenter. Enteral Nutr. 2020;44(1):69–79. doi: 10.1002/jpen.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melendez Hebib V., Taft D.H., Stoll B., Liu J., Call L., Guthrie G., et al. Probiotics and human milk differentially influence the gut microbiome and NEC incidence in preterm pigs. Nutrients. 2023;15(11):2585. doi: 10.3390/nu15112585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mϋller M.J., Paul T., Seeliger S. Necrotizing enterocolitis in premature infants and newborns. J. Neonatal Perinatal Med. 2016;9(3):233–242. doi: 10.3233/NPM-16915130. [DOI] [PubMed] [Google Scholar]
- 25.Ghoneim N., Bauchart-Thevret C., Oosterloo B., Stoll B., Kulkarni M., de Pipaon M.S., et al. Delayed initiation but not gradual advancement of enteral formula feeding reduces the incidence of necrotizing enterocolitis (NEC) in preterm pigs. PLOS ONE. 2014;9(9) doi: 10.1371/journal.pone.0106888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Institute of Medicine (US) National Academies Press; Washington, DC: 2001. Panel on Micronutrients. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc [Internet]https://www.ncbi.nlm.nih.gov/books/NBK222310/pdf/Bookshelf_NBK222310.pdf Available from: [PubMed] [Google Scholar]
- 27.Enfamil® Premature 24 Cal/fl oz HP | Mead Johnson HCP. 2023. https://hcp.meadjohnson.com/s/product/a4R4J000000PpQlUAK/enfamil-premature-24-calfl-oz-hp Internet]. [cited 3 April. Available from: [Google Scholar]
- 28.Moran N.E., Chang J., Stroh R., Zaidi Y., Hason N., Musaad S., et al. Noninvasive, reflection spectroscopy measurement of skin carotenoid score in infants is feasible and reliable. J. Nutr. 2022;152(12):2966–2977. doi: 10.1093/jn/nxac182. [DOI] [PubMed] [Google Scholar]
- 29.Moran N.E., Clinton S.K., Erdman J.W. Differential bioavailability, clearance, and tissue distribution of the acyclic tomato carotenoids lycopene and phytoene in Mongolian gerbils. J. Nutr. 2013;143(12):1920–1926. doi: 10.3945/jn.113.181461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schweigert F.J., Bathe K., Chen F., Büscher U., Dudenhausen J.W. Effect of the stage of lactation in humans on carotenoid levels in milk, blood plasma and plasma lipoprotein fractions. Eur. J. Nutr. 2004;43(1):39–44. doi: 10.1007/s00394-004-0439-5. [DOI] [PubMed] [Google Scholar]
- 31.Schweigert F.J., Rosival I., Rambeck W.A., Gropp J. Plasma transport and tissue distribution of [14C] beta-carotene and [3H]retinol administered orally to pigs. Int. J. Vitam. Nutr. Res. 1995;65(2):95–100. [PubMed] [Google Scholar]
- 32.Chew B.P., Wong T.S., Michal J.J., Standaert F.E., Heirman L.R. Kinetic characteristics of beta-carotene uptake after an injection of beta-carotene in pigs. J. Anim. Sci. 1991;69(12):4883–4891. doi: 10.2527/1991.69124883x. [DOI] [PubMed] [Google Scholar]
- 33.Chew B.P., Wong T.S., Michal J.J., Standaert F.E., Heirman L.R. Subcellular distribution of beta-carotene, retinol, and alpha-tocopherol in porcine lymphocytes after a single injection of beta-carotene. J. Anim. Sci. 1991;69(12):4892–4897. doi: 10.2527/1991.69124892x. [DOI] [PubMed] [Google Scholar]
- 34.Lipkie T.E., Banavara D., Shah B., Morrow A.L., McMahon R.J., Jouni Z.E., et al. Caco-2 accumulation of lutein is greater from human milk than from infant formula despite similar bioaccessibility. Mol. Nutr. Food Res. 2014;58(10):2014–2022. doi: 10.1002/mnfr.201400126. [DOI] [PubMed] [Google Scholar]
- 35.Schmitz H.H., Poor C.L., Gugger E.T., Erdman J.W. [11] Analysis of carotenoids in human and animal tissues. Methods Enzymol. 1993;214:102–116. doi: 10.1016/0076-6879(93)14058-Q. [DOI] [PubMed] [Google Scholar]
- 36.Schweigert F.J., Buchholz I., Schuhmacher A., Gropp J. Effect of dietary beta-carotene on the accumulation of beta-carotene and vitamin A in plasma and tissues of gilts. Reprod. Nutr. Dev. 2001;41(1):47–55. doi: 10.1051/rnd:2001111. [DOI] [PubMed] [Google Scholar]
- 37.dela Seña C., Narayanasamy S., Riedl K.M., Curley R.W., Schwartz S.J., Harrison E.H. Substrate specificity of purified recombinant human β-carotene 15,15′-oxygenase (BCO1) J. Biol. Chem. 2013;288(52):37094–37103. doi: 10.1074/jbc.M113.507160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.dela Seña C., Sun J., Narayanasamy S., Riedl K.M., Yuan Y., Curley R.W., et al. Substrate specificity of purified recombinant chicken β-carotene 9′,10′-oxygenase (BCO2) J. Biol. Chem. 2016;291(28):14609–14619. doi: 10.1074/jbc.M116.723684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu K.Q., Liu C., Ernst H., Krinsky N.I., Russell R.M., Wang X.D. The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J. Biol. Chem. 2006;281(28):19327–19338. doi: 10.1074/jbc.m512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Widjaja-Adhi M.A.K., Lobo G.P., Golczak M., Von Lintig J. A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Hum. Mol. Genet. 2015;24(11):3206–3219. doi: 10.1093/hmg/ddv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lobo G.P., Hessel S., Eichinger A., Noy N., Moise A.R., Wyss A., et al. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J. 2010;24(6):1656–1666. doi: 10.1096/fj.09-150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lobo G.P., Amengual J., Baus D., Shivdasani R.A., Taylor D., von Lintig J. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J. Biol. Chem. 2013;288(13):9017–9027. doi: 10.1074/jbc.M112.444240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borel P., Lietz G., Goncalves A., Szabo de Edelenyi F., Lecompte S., Curtis P., et al. CD36 and SR-BI are involved in cellular uptake of provitamin A carotenoids by Caco-2 and HEK cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans. J. Nutr. 2013;143(4):448–456. doi: 10.3945/jn.112.172734. [DOI] [PubMed] [Google Scholar]
- 44.Moussa M., Gouranton E., Gleize B., Yazidi C.E., Niot I., Besnard P., et al. CD36 is involved in lycopene and lutein uptake by adipocytes and adipose tissue cultures. Mol. Nutr. Food Res. 2011;55(4):578–584. doi: 10.1002/mnfr.201000399. [DOI] [PubMed] [Google Scholar]
- 45.Reboul E., Borel P. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog. Lipid Res. 2011;50(4):388–402. doi: 10.1016/j.plipres.2011.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript will be made available upon request pending application and approval.