Abstract
Background
The prognostic value of cardiac damage staging classifications across the haemodynamic spectrum of severe aortic stenosis (AS) remains unknown.
Aims
We aimed to investigate the prognostic impact of cardiac damage staging classifications in patients with high-gradient AS (HG-AS) and low-gradient AS (LG-AS) undergoing transcatheter aortic valve implantation (TAVI).
Methods
In a prospective TAVI registry, five-year mortality was evaluated for early stages of cardiac damage (stage 0, 1, or 2) and advanced stages of cardiac damage (stage 3 or 4) in patients with HG-AS, classical low-flow (LF) LG-AS, LF LG-AS with preserved ejection fraction (pEF), and normal-flow (NF) LG-AS.
Results
Among 2,090 patients undergoing TAVI, 1,045 patients had HG-AS, 337 patients had classical LF LG-AS, 394 patients had LF LG-AS with pEF, and 314 patients had NF LG-AS. The majority of patients with classical LF LG-AS exhibited advanced cardiac damage (73.6%), followed by LF LG-AS with pEF (55.6%), NF LG-AS (51.6%), and HG-AS (50.6%). Patients with advanced stage cardiac damage had significantly higher mortality after TAVI than those with early stage cardiac damage in all subtypes of AS (adjusted hazard ratio [HRadjusted] 1.66, 95% confidence interval [CI]: 1.34-2.06 for HG-AS; HRadjusted 1.49, 95% CI: 1.02-2.16 for classical LF LG-AS; HRadjusted 1.69, 95% CI: 1.22-2.35 for LF LG-AS with pEF; and HRadjusted 1.52, 95% CI: 1.04-2.32 for NF LG-AS).
Conclusions
Cardiac damage staging classifications stratified mortality after TAVI irrespective of AS subtype.
Introduction
Aortic stenosis (AS) is the prevailing valvular heart disease in high-income countries with ageing populations1. High-gradient AS (HG-AS) can be assumed to be severe irrespective of left ventricular (LV) function and flow condition, and current guidelines recommend aortic valve intervention for symptomatic HG-AS2,3. Low-gradient AS (LG-AS) is a unique entity, accounting for nearly 40% of patients with symptomatic, severe AS. The low-gradient state may represent a more advanced stage of severe AS than HG-AS due to impaired LV function or altered LV morphology caused by long-standing outflow obstruction; however, it may also be caused by other cardiac factors such as coronary artery disease, multivalvular heart disease, and atrial fibrillation (AF), resulting in a considerable heterogeneity of this entity4. Although current guidelines indicate that aortic valve replacement therapy is recommended or reasonable in patients with LG-AS and evidence of true stenosis2,3, the available evidence does not provide guidance on the indications nor the timing at an individual level for this heterogenic group of patients. As a result, patients with LG-AS are less likely to undergo aortic valve replacement therapy when compared to those with HG-AS, despite their poor prognosis5.
Recently, Généreux et al proposed a new staging classification to semiquantitatively assess the extent of extra-aortic valve cardiac damage6. Several studies demonstrated a strong prognostic impact of the staging classification in patients undergoing transcatheter aortic valve implantation (TAVI)7,8,9,10, and the concept may provide further insights into the indications and appropriate timing of TAVI for each AS subtype. In the present study, we aimed to investigate the extent of extra-aortic valve cardiac damage and its prognostic impact in patients with HG-AS and LG-AS who underwent TAVI.
Methods
STUDY DESIGN AND POPULATION
Between August 2007 and June 2022, consecutive AS patients undergoing TAVI at Bern University Hospital (Bern, Switzerland) were enrolled into an institutional prospective registry. The registry is part of the nationwide SwissTAVI Registry (ClinicalTrials.gov: NCT01368250)11. For the purpose of the present study, we excluded 1,405 patients with incomplete or unavailable baseline echocardiographic images to assess cardiac damage according to the staging classification proposed by Généreux and colleagues. After dividing patients into HG-AS and LG-AS groups, LG-AS was categorised into 3 subtypes according to LV systolic function and flow status. During this process, 19 patients without information on stroke volume index (SVI) and 1 patient with missing data on LV ejection fraction (LVEF) were excluded. The registry is approved by the Bern cantonal ethics committee, and patients provided written informed consent for participation.
CARDIAC DAMAGE STAGING CLASSIFICATION
The presence and extent of cardiac damage were evaluated prior to TAVI based on the modified staging scheme6,9,12. Patients were classified into the following stages: Stage 0 - no extra-aortic valve cardiac damage; Stage 1 - LV damage (LVEF <60%, LV mass index >95 g/m2 in women or >115 g/m2 in men, or LV diastolic dysfunction ≥grade II); Stage 2 - left atrial (LA) or mitral valve damage (LA volume index >34 ml/m2, mitral regurgitation ≥moderate, or presence of AF); Stage 3 - pulmonary vasculature or tricuspid valve damage (systolic pulmonary artery pressure [PAP] ≥60 mmHg, mean PAP ≥25 mmHg, or tricuspid regurgitation ≥moderate); and Stage 4 - right ventricular (RV) damage. PAP was obtained from either right heart catheterisation or echocardiographic measurements12. Patients were hierarchically classified into the most advanced stage if at least one of the criteria was met within that stage. Based on the considerable difference in prognostic impact13, we grouped these five stages into early stage disease (stage 0, 1, and 2) and advanced stage disease (stage 3 and 4) as previously validated14.
DEFINITION OF AS SUBTYPES
HG-AS was defined as an aortic valve area (AVA) ≤1.0 cm2 and aortic mean gradient (MG) ≥40 mmHg. Classical low-flow (LF) LG-AS was defined as an AVA <1 cm2, LVEF <50%, MG <40 mmHg, and SVI <35 ml/m2. LF LG-AS with preserved ejection fraction (EF) was defined as an AVA <1 cm2, LVEF ≥50%, MG <40 mmHg, and SVI <35 ml/m². Normal-flow (NF) LG-AS was defined as an AVA <1 cm2, LVEF ≥50%, MG <40 mmHg, and SVI >35 ml/m2. Aortic-valvular complex calcification was assessed using the end-systolic phase of computed tomography by a dedicated core lab, and the device landing zone calcium volume was quantified in the contrast images using a predefined Hounsfield unit threshold of 850, as previously validated15. Dobutamine stress echocardiography, which can be useful in the diagnosis of LG-AS, was not routinely performed.
ECHOCARDIOGRAPHY
Comprehensive transthoracic echocardiography was performed by a board-certified cardiologist and echocardiography specialist within 3 months before TAVI in accordance with the current American Society of Echocardiography guidelines16. Acquired images were independently re-evaluated by experienced imaging specialists in the Bern imaging core laboratory (Bern, Switzerland). Stroke volume was derived from the cross-sectional area of the LV outflow tract multiplied by the time-velocity integral of flow by pulsed-wave Doppler at that location. RV function was assessed as previously described, and RV dysfunction was documented in the presence of at least two of the following parameters: tricuspid annular plane systolic excursion <1.7 cm, tricuspid annular peak systolic velocity (S’) <9.5 cm/s and fractional area change <35%17.
DATA COLLECTION AND CLINICAL ENDPOINTS
Baseline clinical, procedural, and follow-up data were prospectively recorded in a dedicated database held at the Clinical Trials Unit of the University of Bern, Switzerland. All adverse events were systematically collected and adjudicated by a dedicated clinical event committee based on the Valve Academic Research Consortium definitions applicable at the time of the procedure18,19,20. The primary outcome of interest in the present study was all-cause mortality after TAVI.
STATISTICAL ANALYSIS
Categorical variables are represented as frequencies and percentages, and the differences between groups were evaluated with the Veni test or Fisher’s exact test. Continuous variables are presented as mean values±standard deviation and compared between groups using the F-test in an analysis of variance (ANOVA) or Kruskal-Wallis test in combination with pairwise Wilcoxon test with correction for multiple testing, as appropriate. Risk ratios with 95% confidence intervals (CIs) from Poisson regressions were provided where appropriate. Time-to-event curves were constructed using the Kaplan-Meier method; a comparison of cumulative event rates between these groups was performed by log-rank test. Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% CIs for the clinical outcomes. Age, sex, and Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM), baseline LVEF, baseline New York Heart Association (NYHA) Functional Class III or IV, estimated glomerular filtration rate, and the year of TAVI (the study period was divided into tertiles [1st: up to 20 May 2014; 2nd: 20 May 2014 to 12 August 2017; 3rd: 12 August 2017 to 30 June 2022]) were selected and introduced as covariates in multivariable Cox proportional hazards models. SPSS software version 23.0 (IBM) was used for statistical analysis. All statistical tests were 2-sided, and p-values<0.05 were considered significant.
Results
STUDY POPULATION AND BASELINE CHARACTERISTICS
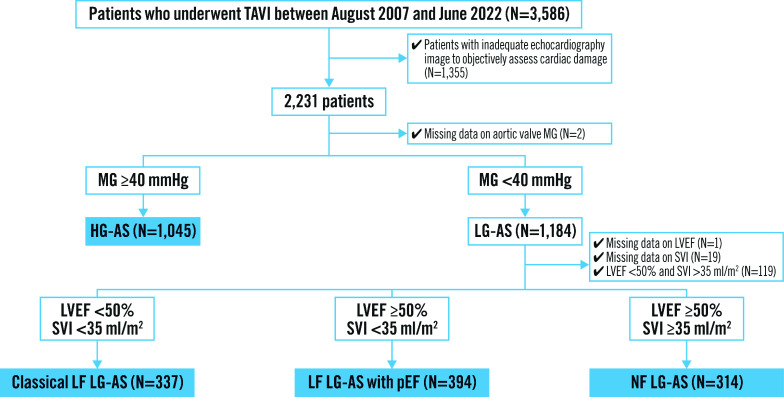
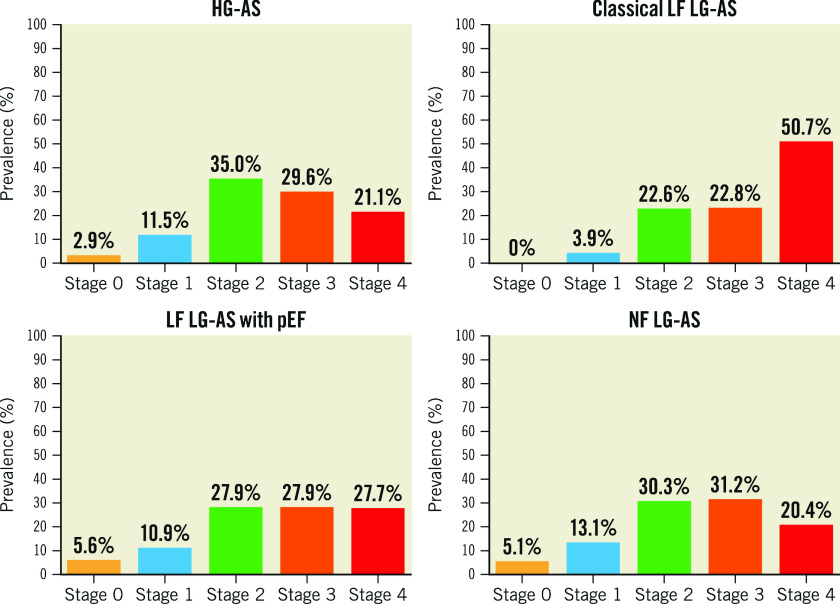
Among 3,586 consecutive patients undergoing TAVI between August 2007 and June 2022, 2,090 patients were included in the present analysis. Of these, 1,045 patients (50.0%) had HG-AS, 337 patients (16.1%) had classical LF LG-AS, 394 patients (18.9%) had LF LG-AS with preserved EF, and 314 patients (15.0%) had NF LG-AS (Figure 1). Baseline characteristics according to each AS subtype are shown in Table 1. The prevalence of cardiac damage stage across different subtypes of AS is summarised in Figure 2 and the Central illustration. An advanced stage of cardiac damage was most prevalent among patients with classical LF LG-AS (73.6%), followed by LF LG-AS with preserved EF (55.6%), NF LG-AS (51.6%), and HG-AS (50.6%).
Figure 1. Study flowchart.
AS: aortic stenosis; HG: high gradient; LF: low flow; LG: low gradient; LVEF: left ventricular ejection fraction; MG: mean gradient; NF: normal flow; pEF: preserved ejection fraction; SVI: stroke volume index; TAVI: transcatheter aortic valve implantation
Table 1. Baseline characteristics according to AS subtypes.
| HG-AS | Classical LF LG-AS | LF LG-AS with pEF | NF LG-AS | p-value | |
|---|---|---|---|---|---|
| N=1,045 | N=337 | N=394 | N=314 | ||
| Age, years | 82.4±5.9bd | 81.0±7.5a | 82.0±6.6 | 81.5±6.3a | 0.073 |
| Female | 565 (54.1)b | 109 (32.3)acd | 220 (55.8)b | 155 (49.4)b | <0.001 |
| Body mass index, kg/m² | 26.4±5.5c | 25.8±5.2c | 27.0±5.7abd | 25.9±5.1c | 0.018 |
| STS-PROM, % | 5.4±4.1b | 7.0±5.3acd | 5.1±3.2b | 5.1±3.5b | <0.001 |
| NYHA III or IV | 679 (65.0)b | 262 (78.0)acd | 273 (69.3)b | 197 (62.7)b | <0.001 |
| Concomitant diseases | |||||
| Hypertension | 882 (84.4)d | 289 (85.8) | 342 (86.8) | 280 (89.2)a | 0.177 |
| Diabetes mellitus | 256 (24.5)b | 113 (33.5)ad | 116 (29.4) | 76 (24.2)b | 0.004 |
| CKD (eGFR <60 ml/min/1.73 m2) | 720 (69.0) | 238 (70.6) | 267 (67.8) | 219 (70.0) | 0.847 |
| eGFR, ml/min/1.73 m2 | 52.5±21.7b | 50.2±22.1ac | 53.1±21.6b | 50.6±22.5 | 0.088 |
| COPD | 114 (10.9)c | 47 (14.0) | 61 (15.5)a | 43 (13.7) | 0.088 |
| Coronary artery disease | 580 (55.5)bd | 231 (68.5)ac | 240 (60.9)b | 194 (61.8)a | <0.001 |
| Atrial fibrillation | 311 (29.8)bc | 161 (47.8)ad | 167 (42.4)ad | 104 (33.1)bc | <0.001 |
| Previous history | |||||
| Previous myocardial infarction | 120 (11.5)b | 97 (28.8)acd | 51 (12.9)b | 40 (12.7)b | <0.001 |
| Previous cardiac surgery | 109 (10.4)bcd | 81 (24.0)ac | 64 (16.2)ab | 57 (18.2)a | <0.001 |
| Previous stroke | 115 (11.0)b | 52 (15.4)a | 49 (12.4) | 44 (14.0) | 0.141 |
| Previous permanent pacemaker implantation | 63 (6.0)bc | 49 (14.5)ad | 42 (10.7)a | 28 (8.9)b | <0.001 |
| Peripheral artery disease | 138 (13.2)b | 61 (18.1)a | 57 (14.5) | 43 (13.7) | 0.165 |
| Echocardiography | |||||
| Aortic valve area, cm² | 0.63±0.23bcd | 0.76±0.28ad | 0.77±0.26ad | 0.85±0.30abc | <0.001 |
| Mean aortic valve pressure gradient, mmHg | 53±13bcd | 24±9acd | 27±8ab | 28±9ab | <0.001 |
| Left ventricular ejection fraction, % | 57±13bcd | 32±11acd | 61±7abd | 63±7abc | <0.001 |
| Left ventricular mass index, g/m2 | 139±44bcd | 147±51acd | 121±41ab | 124±41ab | <0.001 |
| Left atrial volume index, ml/m2 | 43±16bcd | 49±23acd | 42±17ab | 42±24ab | <0.001 |
| Mitral regurgitation ≥moderate | 217 (21.0)b | 141 (42.7)acd | 80 (20.6)b | 59 (19.2)b | <0.001 |
| Tricuspid regurgitation ≥moderate | 161 (15.6)bc | 90 (27.4)ad | 84 (21.6)a | 52 (16.7)b | <0.001 |
| Systolic pulmonary artery pressure, mmHg | 46±18bd | 49±19acd | 46±18bd | 43±16abc | <0.001 |
| Tricuspid annular plane systolic excursion, mm | 21±5bc | 17±6acd | 19±5abd | 21±5bc | <0.001 |
| Stroke volume index, ml/m² | 34±12bcd | 25±6acd | 26±6abd | 45±12abc | <0.001 |
| Computed tomography | |||||
| Aortic valvular complex calcification, mm3 | 442.4±423.8bcd | 213.3±229.9a | 206.3±273.2a | 200.3±203.6a | <0.001 |
| Right heart catheterisation | |||||
| Mean pulmonary artery pressure, mmHg | 31±12bd | 34±12acd | 30±12bd | 28±9abc | <0.001 |
| Data are presented as n (%) or mean±standard deviation. ap-value<0.05 versus HG-AS. bp-value<0.05 versus classical LF LG-AS. cp-value<0.05 versus LF LG-AS with pEF. dp-value <0.05 versus NF LG-AS. AS: aortic stenosis; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; HG: high gradient; LF: low flow; LG: low gradient; NF: normal flow; NYHA: New York Heart Association; pEF: preserved ejection fraction; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality | |||||
Figure 2. Distribution of cardiac damage stage according to AS subtype.
AS: aortic stenosis; HG: high gradient; LF: low flow; LG: low gradient; NF: normal flow; pEF: preserved ejection fraction
Central illustration. 5-year all-cause mortality according to cardiac damage staging classification in each AS subtype.
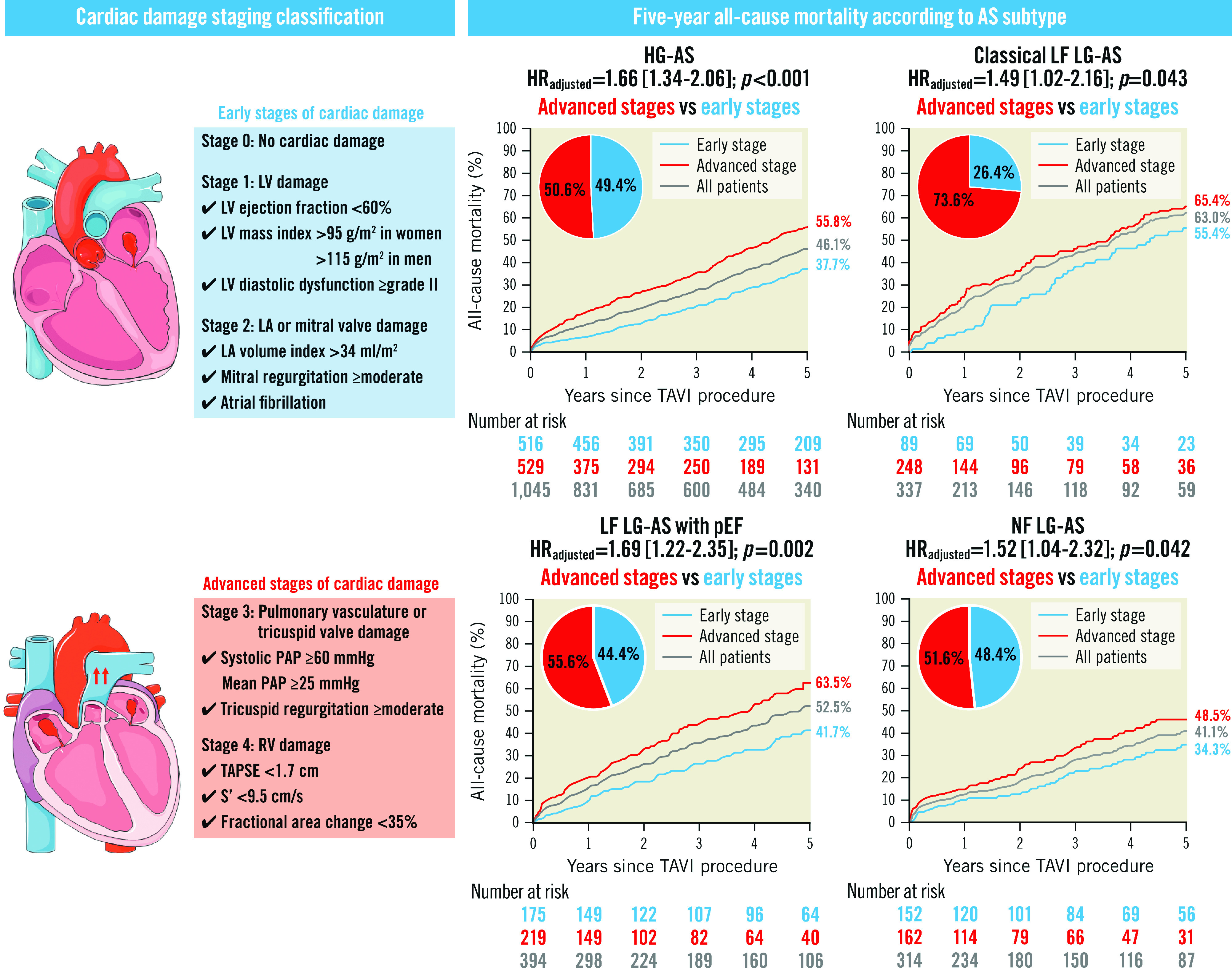
AS: aortic stenosis; HG: high gradient; HR: hazard ratio; LA: left atrial; LF: low flow; LG: low gradient; LV: left ventricular; NF: normal flow; PAP: pulmonary artery pressure; pEF: preserved ejection fraction; RV: right ventricular; S’: tricuspid annular peak systolic velocity; TAPSE: tricuspid annular plane systolic excursion; TAVI: transcatheter aortic valve implantation
In patients with HG-AS, approximately half (50.6%) had advanced cardiac damage. Patients with advanced stage cardiac damage had a higher surgical risk (STS-PROM: 6.2±4.7% vs 4.7±3.1%; p<0.001), worse heart failure symptoms (NYHA Functional Class III or IV: 68.6% vs 61.2%; p=0.012), and a lower LVEF (53±14% vs 61±10%; p<0.001) than those with early stage cardiac damage. Chronic kidney disease, AF, previous myocardial infarction, previous cardiac surgery, and previous permanent pacemaker implantation were more common in patients with advanced compared to early stage cardiac damage (73.5% vs 64.3%; p=0.001; 35.3% vs 24.0%; p<0.001; 14.2% vs 8.7%; p=0.006; 12.5% vs 8.3%; p=0.028; and 7.6% vs 4.5%; p=0.035, respectively) (Supplementary Table 1).
The majority of patients with classical LF LG-AS (73.6%) had advanced cardiac damage. Patients in this subgroup with advanced cardiac damage were comparable in terms of surgical risk and comorbidities to patients with early stage cardiac damage, except for the presence of AF (51.2% vs 38.2%; p=0.035). LVEF was lower in patients with advanced cardiac damage compared with those with early cardiac damage (31±12% vs 35±10%; p=0.004) (Supplementary Table 2).
LF LG-AS with preserved EF patients had advanced cardiac damage in 55.6% of cases. Patients in this particular group were found to have higher surgical risk (STS-PROM: 5.5±3.5% vs 4.7±2.6%; p=0.009), higher prevalence of AF (55.3% vs 26.3%; p<0.001), and a lower LVEF (60±7% vs 63±7%; p<0.001) compared to those with early stage cardiac damage (Supplementary Table 3).
In NF LG-AS patients, 162 (51.6%) were in an advanced stage of cardiac damage. They had worse heart failure symptoms (NYHA Functional Class III or IV: 69.1% vs 55.9%; p=0.016) and a lower LVEF (62±7% vs 64±7%; p=0.040) compared to patients with early stage cardiac damage. Along the same line, hypertension (93.8% vs 84.2%; p=0.006), coronary artery disease (68.5% vs 54.6%; p=0.011), and a history of cardiac surgery (22.8% vs 13.2%; p=0.026) were more common in patients with advanced stage cardiac damage than those with early stage damage (Supplementary Table 4).
The prevalence of AF was higher in the advanced stages than in the early stages of cardiac damage across all AS subtypes.
CLINICAL OUTCOMES
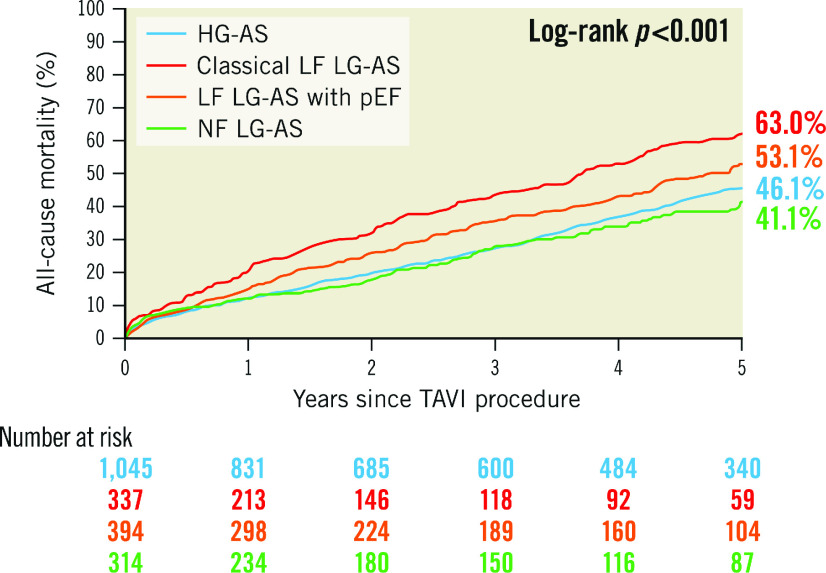
At a median follow-up of 1,095 (interquartile range 365-1,825) days after TAVI, mortality was highest in patients with classical LF LG-AS (63.0%), followed by LF LG-AS with preserved EF (53.1%), HG-AS (46.1%), and NF LG-AS (41.1%), reflecting the respective proportions of advanced cardiac damage (Figure 3). Adjusted HRs of all-cause and cardiovascular mortality in patients with advanced compared to early stage cardiac damage in each subtype of AS are summarised in the Central illustration and Table 2. The presence of advanced cardiac damage was independently associated with higher all-cause mortality, regardless of the AS subtype (HRadjusted 1.66, 95% CI: 1.34-2.06; p<0.001 for HG-AS; HRadjusted 1.49; 95% CI: 1.02-2.16; p=0.043 for classical LF LG-AS; HRadjusted 1.69; 95% CI: 1.22-2.35; p=0.002 for LF LG-AS with preserved LVEF; and HRadjusted 1.52; 95% CI: 1.04-2.32; p=0.042 for NF LG-AS, respectively). A detailed analysis differentiating four cardiac stages (stage 0 or 1, stage 2, stage 3, and stage 4) in each AS subtype is shown in Figure 4 and Supplementary Table 5.
Figure 3. Five-year all-cause mortality according to AS subtype.
AS: aortic stenosis; HG: high gradient; LF: low flow; LG: low gradient; NF: normal flow; pEF: preserved ejection fraction; TAVI: transcatheter aortic valve implantation
Table 2. Clinical outcomes according to cardiac damage in each AS subtype.
| All-cause mortality at 1 year | ||||
|---|---|---|---|---|
| HG-AS | Classical LF LG-AS | LF LG-AS with pEF | NF LG-AS | |
| Early stage | 35 (7.1) | 7 (9.5) | 17 (10.2) | 15 (11.1) |
| reference | reference | reference | reference | |
| Advanced stage | 89 (19.3) | 57 (28.6) | 42 (22.2) | 23 (16.9) |
| HR 2.39 [1.57-3.66] | HR 3.24 [1.46-7.19] | HR 1.65 [0.90-3.01] | HR 1.31 [0.67-2.58] | |
| p<0.001 | p=0.004 | p=0.105 | p=0.432 | |
| Cardiovascular mortality at 1 year | ||||
| Early stage | 21 (4.3) | 4 (5.4) | 11 (6.6) | 5 (3.7) |
| reference | reference | reference | reference | |
| Advanced stage | 59 (12.8) | 44 (22.1) | 31 (16.4) | 14 (10.3) |
| HR 2.47 [1.43-4.27] | HR 4.21 [1.49-11.86] | HR 1.78 [0.85-3.73] | HR 2.40 [0.84-6.87] | |
| p=0.001 | p=0.007 | p=0.129 | p=0.102 | |
| All-cause mortality at 5 years | ||||
| Early stage | 164 (37.7) | 37 (55.4) | 63 (41.7) | 41 (34.3) |
| reference | reference | reference | reference | |
| Advanced stage | 233 (55.8) | 114 (65.4) | 106 (63.5) | 55 (48.5) |
| HR 1.66 [1.34-2.06] | HR 1.49 [1.02-2.16] | HR 1.69 [1.22-2.35] | HR 1.52 [1.04-2.32] | |
| p<0.001 | p=0.043 | p=0.002 | p=0.042 | |
| Cardiovascular mortality at 5 years | ||||
| Early stage | 103 (26.7) | 26 (42.7) | 44 (32.7) | 25 (24.4) |
| reference | reference | reference | reference | |
| Advanced stage | 166 (45.4) | 91 (58.4) | 74 (51.6) | 38 (37.6) |
| HR 1.90 [1.45-2.47] | HR 1.60 [1.02-2.50] | HR 1.71 [1.15-2.53] | HR 1.59 [0.93-2.68] | |
| p<0.001 | p=0.040 | p=0.008 | p=0.105 | |
| Data are presented as n (%) or HR with 95% CI. HR was adjusted by age, sex, Society of Thoracic Surgeons Predicted Risk of Mortality, left ventricular ejection fraction, New York Heart Association Class III or IV, estimated glomerular filtration rate, and the year of transcatheter aortic valve implantation. AS: aortic stenosis; CI: confidence interval; HG: high gradient; HR: hazard ratio; LF: low flow; LG: low gradient; NF: normal flow; pEF: preserved ejection fraction | ||||
Figure 4. Five-year all-cause mortality according to four cardiac damage stages.
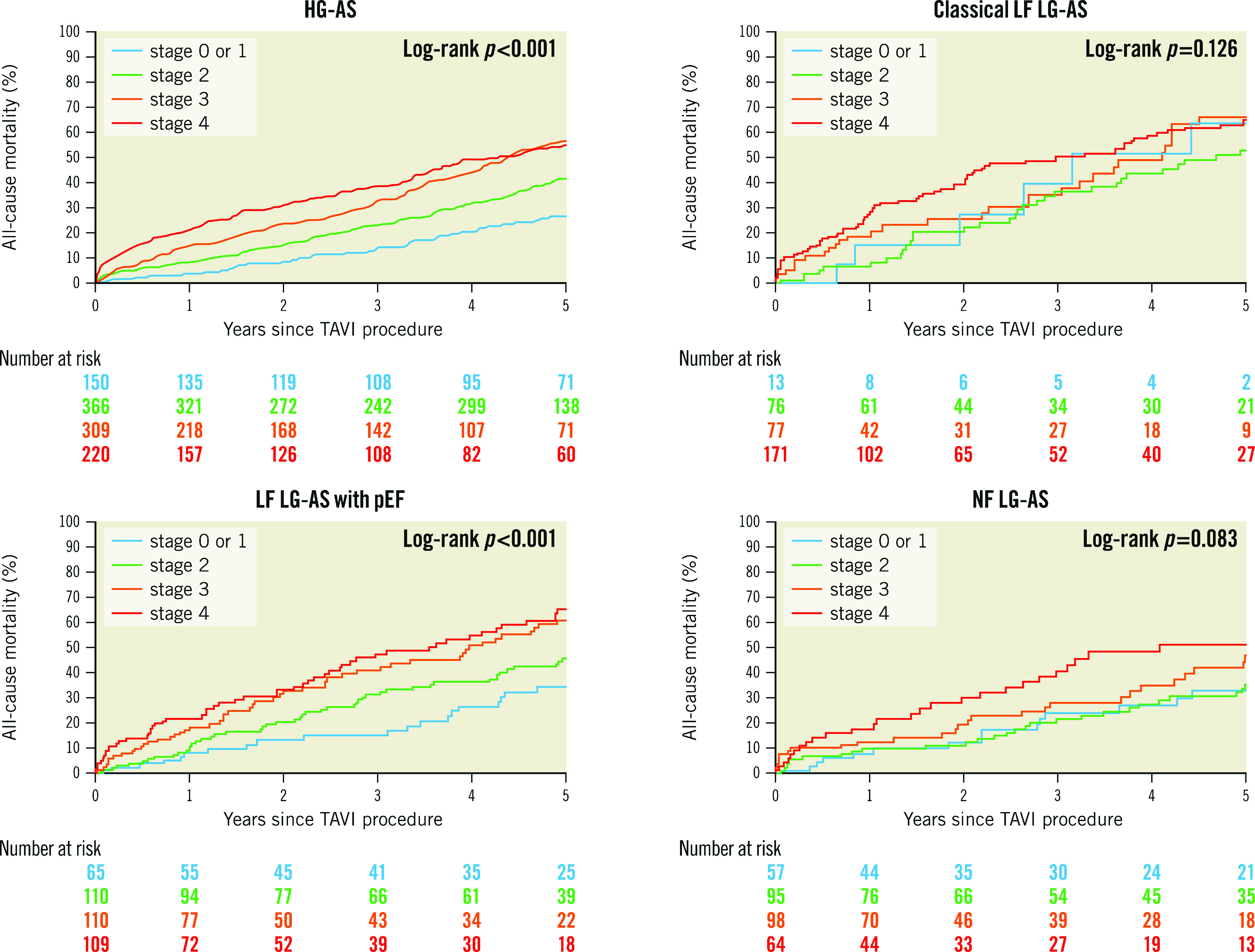
AS: aortic stenosis; HG: high gradient; LF: low flow; LG: low gradient; NF: normal flow; pEF: preserved ejection fraction; TAVI: transcatheter aortic valve implantation
Discussion
The main findings of the current study are as follows: 1) the majority of patients with classical LF LG-AS had evidence of advanced cardiac damage, followed by LF LG-AS with preserved EF, NF LG-AS, and HG-AS in descending order. 2) Mortality in both early and advanced stages was highest in patients with classical LF LG-AS, followed by LF LG-AS with preserved EF, HG-AS, and NF LG-AS. 3) Advanced cardiac damage conferred an increased mortality risk after TAVI irrespective of the AS subtype.
The cardiac damage staging classification characterising the extent of extra-aortic valve cardiac damage, as proposed by Généreux and colleagues, has important prognostic implications in patients undergoing TAVI6. The prognostic model was validated and refined in several populations, including symptomatic and asymptomatic AS patients7,8,9,10. In these studies, patients with LG-AS represented 20-30% of the total population, and this proportion increased with progressive stages of advanced cardiac damage. Recently, Snir et al reported that advanced cardiac damage was observed in 34.0% of classical LF LG-AS patients, 22.5% of LF LG-AS patients with preserved EF, 15.5% of NF LG-AS patients, and 14.0% of HG-AS patients21. Consistent with these results, the present study demonstrated that an advanced stage of cardiac damage was most common in classical LF LG-AS (73.6%), followed by LF LG-AS with preserved EF (55.6%), NF LG-AS (51.6%), and HG-AS (50.6%). The marked prevalence of advanced cardiac damage in our study, compared to the previous study, may be attributed, at least in part, to differences in the studied populations. Whereas the previous study was based on a national echo database in which only 20% of patients underwent aortic valve intervention, our study focused on elderly patients with symptomatic severe AS undergoing TAVI. Moreover, using an echocardiographic guideline-based definition of RV dysfunction may have enhanced the sensitivity in identifying patients with advanced cardiac damage in the present study7.
Previous studies have shown that patients with advanced cardiac stage were likely to be at higher surgical risk and have a higher prevalence of comorbidities7,8,9,10. In the present study, this observation was corroborated across the haemodynamic spectrum of AS. Furthermore, there was a higher proportion of women in advanced stages of cardiac damage across all AS subtypes, corroborating previous observations that women with AS tend to be underdiagnosed and undertreated22. We found that an advanced stage of cardiac damage conferred an approximately 1.5-fold increased risk of mortality across all AS subtypes, thus, underscoring the importance of timely aortic valve intervention before the development of secondary cardiac damage. However, a higher mortality was observed in classical LF LG-AS patients compared to other AS subtypes, even in the early stages.
Myocardial fibrosis, as indicated by the presence of late gadolinium enhancement, was more frequently observed in classical LF LG-AS23, implying that it is the most advanced AS phenotype; this is supported by our finding that more than 70% of patients with classical LF LG-AS are in an advanced stage of cardiac damage. However, considering that patients with classical LF LG-AS had larger AVA than those patients with HG-AS in the present study, this subtype is not necessarily a late presentation of AS. In classical LF LG-AS, the LF haemodynamic state is usually caused by impaired LV function due to afterload mismatch by chronic AS or concomitant cardiomyopathy, frequently as a result of coronary artery disease4. Indeed, in the present study, patients with classical LF LG-AS were more likely to be male and had the highest frequency of cardiovascular disease, chronic obstructive pulmonary disease, history of myocardial infarction and cardiac surgery, which may contribute to impaired LV systolic function. In the analysis from the PARTNER 2 trials, a lower proportion of patients with advanced cardiac damage had cumulative damage from earlier stages6. Nevertheless, this group showed that persistent cardiac damage after aortic intervention was associated with a worse prognosis24. These findings suggest that the development of cardiac damage is also driven by comorbid and/or underlying disease and highlight the importance of targeted-treatment strategies for the underlying disease in patients with severe AS referred for aortic valve intervention.
In contrast, patients with NF LG-AS featured the least extent of cardiac damage and experienced lower all-cause mortality compared with other AS subtypes, thus, supporting the notion that NF LG-AS represents only moderate to borderline severe AS3,25,26,27. Nevertheless, a recent study in 1,245 individuals reported that 17.5% of patients with moderate AS exhibited advanced cardiac damage and demonstrated a stepwise increase in long-term mortality according to cardiac damage stage28. Our findings corroborate these results by showing a significantly higher mortality in patients with advanced stages of cardiac damage compared to those in an earlier stage. Interestingly, however, patients with NF LG-AS had lower mortality than other AS subtypes, despite features of advanced cardiac damage. A previous study suggested that a proportion of patients exhibit regression of cardiac damage following TAVI24. It can therefore be hypothesised that advanced cardiac damage is reversible in patients with NF LG-AS and may improve after TAVI. An ongoing clinical trial will provide further insight into the clinical benefit of early intervention in patients with less severe AS and delineate the importance of cardiac damage in this population (PROGRESS; ClinicalTrials.gov: NCT04889872).
Limitations
The present analysis is a retrospective, observational, single-centre study with inherent limitations. First, more than 40% of the patients were excluded because of inadequate echocardiography for assessment of cardiac damage classification and AS subtype, which may have resulted in some degree of selection bias. As shown in Supplementary Table 6, patients included in the present analyses were older, had a higher surgical risk and a higher prevalence of comorbidities compared with those excluded from the present analysis. Second, as we did not routinely perform dobutamine stress echocardiography in patients with classical LF LG-AS, patients with pseudo-severe AS may have been included in this study. Furthermore, this study may have included some patients with moderate AS in the NF LG-AS group (AVA 0.85±0.30 cm²). However, all patients underwent intervention after thorough clinical assessment and interdisciplinary discussion within the Heart Team. Third, due to the small number of patients in each AS subtype, especially early stages, this study may have been underpowered to detect the smaller effect sizes of earlier cardiac damage stages. Furthermore, grouping cardiac damage into early and advanced stages is arbitrary. Finally, we did not investigate follow-up echocardiography after TAVI. Further studies on the evolution of cardiac damage and its impact on clinical outcomes in each AS subtype are needed.
Conclusions
Staging classifications of AS according to extra-aortic valve cardiac damage successfully stratified mortality after TAVI in all AS subtypes. It is essential to identify AS patients at an early stage of secondary cardiac damage and perform TAVI before progression to more advanced stages, irrespective of AS subtype. However, in patients with classical LF LG-AS, a conservative control group is mandatory for comparison to ultimately quantify the net benefit of TAVI in accordance with the staging classification of the extra-aortic valve cardiac damage.
Impact on daily practice
The staging system provided prognostic value across all AS subtypes. However, higher mortality was observed in classical LF LG-AS patients compared to other AS subtypes, even in the early stages. In this particular population, the risks and benefits of TAVI should be carefully weighed.
Supplementary data
Baseline characteristics of high-gradient aortic stenosis.
Baseline characteristics of classical low-flow low-gradient aortic stenosis.
Baseline characteristics of low-flow low-gradient aortic stenosis with preserved ejection fraction.
Baseline characteristics of normal-flow low-gradient aortic stenosis.
Clinical outcomes according to four cardiac stages in each AS subtype.
Comparison of baseline characteristics between included and excluded patients.
Acknowledgments
Conflict of interest statement
S. Windecker reports research and educational grants to the institution from Abbott, Amgen, AstraZeneca, Bristol-Myers Squibb, Bayer AG, Biotronik, Boston Scientific, Cardinal Health, CardioValve (Venus Medtech), CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Guerbet, InfraRedx, Johnson & Johnson, Medicure, Medtronic, Novartis, Polares, OrPha Suisse, Pfizer, Regeneron, Sanofi-Aventis, Sinomed, Terumo, and V-Wave. S. Windecker serves as an unpaid advisory board member and/or unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, Bristol-Myers Squibb, Boston Scientific, Biotronik, CardioValve (Venus Medtech), Edwards Lifesciences, MED Alliance, Medtronic, Novartis, Polares, Sinomed, V-Wave, and Xeltis, but has not received personal payments from pharmaceutical companies or device manufacturers. He is also member of the steering/executive committee group of several investigator-initiated trials that receive funding from industry without impact on his personal remuneration. S. Windecker is an unpaid member of the Pfizer Research Award selection committee in Switzerland and of the Women as One Awards Committee. He is a member of the Clinical Study Group of the Deutsches Zentrum für Herz-Kreislauf-Forschung and of the Advisory Board of the Australian Victorian Heart Institute. He is Chairperson of the ESC Congress Program Committee and Deputy Editor of JACC Cardiovascular Interventions. T. Pilgrim reports research grants to the institution from Edwards Lifesciences and Biotronik; and personal fees from Biotronik, Medtronic, Abbott, Edwards Lifesciences, and HighLife SAS. S. Stortecky reports research grants to the institution from Edwards Lifesciences, Medtronic, Abbott, and Boston Scientific; and personal fees from Boston Scientific, Teleflex, and BTG. F. Praz reports travel expenses from Abbott, Edwards Lifesciences, and Polares Medical. The other authors have no conflicts of interest to declare.
Abbreviations
- AS
aortic stenosis
- EF
ejection fraction
- HG
high gradient
- LF
low flow
- LG
low gradient
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- NF
normal flow
- TAVI
transcatheter aortic valve implantation
Contributor Information
Masaaki Nakase, Department of Cardiology, Inselspital, University of Bern, Bern, Switzerland.
Taishi Okuno, Department of Cardiology, Inselspital, University of Bern, Bern, Switzerland.
Daijiro Tomii, Department of Cardiology, Inselspital, University of Bern, Bern, Switzerland.
Bashir Alaour, Department of Cardiology, Inselspital, University of Bern, Bern, Switzerland.
Fabien Praz, Department of Cardiology, Inselspital, University of Bern, Bern, Switzerland.
Stefan Stortecky, Department of Cardiology, Inselspital, University of Bern, Bern, Switzerland.
Jonas Lanz, Department of Cardiology, Inselspital, University of Bern, Bern, Switzerland.
David Reineke, Department of Cardiovascular Surgery, Inselspital, University of Bern, Bern, Switzerland.
Stephan Windecker, Department of Cardiology, Inselspital, University of Bern, Bern, Switzerland.
Thomas Pilgrim, Department of Cardiology, Inselspital, University of Bern, Bern, Switzerland.
References
- Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, Pibarot P. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006. doi: 10.1038/nrdp.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM, 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis, De Paulis, Delgado V, Freemantle N, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. EuroIntervention. 2022;17:e1126–96. doi: 10.4244/EIJ-E-21-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel MA, Magne J, Pibarot P. Low-gradient aortic stenosis. Eur Heart J. 2016;37:2645–57. doi: 10.1093/eurheartj/ehw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Patel NK, Flannery LD, Selberg A, Kandanelly RR, Morrison FJ, Kim J, Tanguturi VK, Crousillat DR, Shaqdan AW, Inglessis I, Shah PB, Passeri JJ, Kaneko T, Jassar AS, Langer NB, Turchin A, Elmariah S. Trends in Utilization of Aortic Valve Replacement for Severe Aortic Stenosis. J Am Coll Cardiol. 2022;79:864–77. doi: 10.1016/j.jacc.2021.11.060. [DOI] [PubMed] [Google Scholar]
- Généreux P, Pibarot P, Redfors B, Mack MJ, Makkar RR, Jaber WA, Svensson LG, Kapadia S, Tuzcu EM, Thourani VH, Babaliaros V, Herrmann HC, Szeto WY, Cohen DJ, Lindman BR, McAndrew T, Alu MC, Douglas PS, Hahn RT, Kodali SK, Smith CR, Miller DC, Webb JG, Leon MB. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J. 2017;38:3351–8. doi: 10.1093/eurheartj/ehx381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T, Heg D, Lanz J, Stortecky S, Praz F, Windecker S, Pilgrim T. Staging cardiac damage associated with aortic stenosis in patients undergoing transcatheter aortic valve implantation. Int J Cardiol Heart Vasc. 2021;33:100768. doi: 10.1016/j.ijcha.2021.100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollema EM, Amanullah MR, Ng ACT, van der, Prevedello F, Sin YK, Prihadi EA, Marsan NA, Ding ZP, Généreux P, Pibarot P, Leon MB, Narula J, Ewe SH, Delgado V, Bax JJ. Staging Cardiac Damage in Patients With Symptomatic Aortic Valve Stenosis. J Am Coll Cardiol. 2019;74:538–49. doi: 10.1016/j.jacc.2019.05.048. [DOI] [PubMed] [Google Scholar]
- Tastet L, Tribouilloy C, Maréchaux S, Vollema EM, Delgado V, Salaun E, Shen M, Capoulade R, Clavel MA, Arsenault M, Bédard É, Bernier M, Beaudoin J, Narula J, Lancellotti P, Bax JJ, Généreux P, Pibarot P. Staging Cardiac Damage in Patients With Asymptomatic Aortic Valve Stenosis. J Am Coll Cardiol. 2019;74:550–63. doi: 10.1016/j.jacc.2019.04.065. [DOI] [PubMed] [Google Scholar]
- Fukui M, Gupta A, Abdelkarim I, Sharbaugh MS, Althouse AD, Elzomor H, Mulukutla S, Lee JS, Schindler JT, Gleason TG, Cavalcante JL. Association of Structural and Functional Cardiac Changes With Transcatheter Aortic Valve Replacement Outcomes in Patients With Aortic Stenosis. JAMA Cardiol. 2019;4:215–22. doi: 10.1001/jamacardio.2018.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stortecky S, Franzone A, Heg D, Tueller D, Noble S, Pilgrim T, Jeger R, Toggweiler S, Ferrari E, Nietlispach F, Taramasso M, Maisano F, Grünenfelder J, Muller O, Huber C, Roffi M, Carrel T, Wenaweser P, Windecker S. Temporal trends in adoption and outcomes of transcatheter aortic valve implantation: a SwissTAVI Registry analysis. Eur Heart J Qual Care Clin Outcomes. 2019;5:242–51. doi: 10.1093/ehjqcco/qcy048. [DOI] [PubMed] [Google Scholar]
- Okuno T, Heg D, Lanz J, Praz F, Brugger N, Stortecky S, Windecker S, Pilgrim T. Refined staging classification of cardiac damage associated with aortic stenosis and outcomes after transcatheter aortic valve implantation. Eur Heart J Qual Care Clin Outcomes. 2021;7:532–41. doi: 10.1093/ehjqcco/qcab041. [DOI] [PubMed] [Google Scholar]
- Tastet L, Généreux P, Bernard J, Pibarot P. The Role of Extravalvular Cardiac Damage Staging in Aortic Valve Disease Management. Can J Cardiol. 2021;37:1004–15. doi: 10.1016/j.cjca.2021.01.020. [DOI] [PubMed] [Google Scholar]
- Pellegrini C, Duesmann C, Rheude T, Berg A, Alvarez-Covarrubias HA, Trenkwalder T, Mayr NP, Schürmann F, Nicol P, Xhepa E, Joner M. The impact of extra-valvular cardiac damage on mid-term clinical outcome following transcatheter aortic valve replacement in patients with severe aortic stenosis. Front Cardiovasc Med. 2022;9:1039208. doi: 10.3389/fcvm.2022.1039208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T, Asami M, Heg D, Lanz J, Praz F, Hagemeyer D, Brugger N, Gräni C, Huber A, Spirito A, Räber L, Stortecky S, Windecker S, Pilgrim T. Impact of Left Ventricular Outflow Tract Calcification on Procedural Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2020;13:1789–99. doi: 10.1016/j.jcin.2020.04.015. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA, Velazquez EJ. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:1–64. doi: 10.1016/j.echo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, Krucoff MW, Mack M, Mehran R, Miller C, Morel MA, Petersen J, Popma JJ, Takkenberg JJ, Vahanian A, van Es, Vranckx P, Webb JG, Windecker S, Serruys PW. Standardized endpoint definitions for Transcatheter Aortic Valve Implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–69. doi: 10.1016/j.jacc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés-Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–54. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- VARC-3 WRITING COMMITTEE: Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, Bax JJ, Leipsic JA, Blanke P, Blackstone EH, Finn MT, Kapadia S, Linke A, Mack MJ, Makkar R, Mehran R, Popma JJ, Reardon M, Rodes-Cabau J, Van Mieghem NM, Webb JG, Cohen DJ, Leon MB. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J Am Coll Cardiol. 2021;77:2717–46. doi: 10.1016/j.jacc.2021.02.038. [DOI] [PubMed] [Google Scholar]
- Snir AD, Ng MK, Strange G, Playford D, Stewart S, Celermajer DS National Echo Database of Australia. Cardiac Damage Staging Classification Predicts Prognosis in All the Major Subtypes of Severe Aortic Stenosis: Insights from the National Echo Database Australia. J Am Soc Echocardiogr. 2021;34:1137–47.e13. doi: 10.1016/j.echo.2021.05.017. [DOI] [PubMed] [Google Scholar]
- Guzzetti E, Poulin A, Annabi MS, Zhang B, Kalavrouziotis D, Couture C, Dagenais F, Pibarot P, Clavel MA. Transvalvular Flow, Sex, and Survival After Valve Replacement Surgery in Patients With Severe Aortic Stenosis. J Am Coll Cardiol. 2020;75:1897–909. doi: 10.1016/j.jacc.2020.02.065. [DOI] [PubMed] [Google Scholar]
- Fukui M, Annabi MS, Rosa VEE, Ribeiro HB, Stanberry LI, Clavel MA, Rodés-Cabau J, Tarasoutchi F, Schelbert EB, Bergler-Klein J, Bartko PE, Dona C, Mascherbauer J, Dahou A, Rochitte CE, Pibarot P, Cavalcante JL. Comprehensive myocardial characterization using cardiac magnetic resonance associates with outcomes in low gradient severe aortic stenosis. Eur Heart J Cardiovasc Imaging. 2022;24:46–58. doi: 10.1093/ehjci/jeac089. [DOI] [PubMed] [Google Scholar]
- Généreux P, Pibarot P, Redfors B, Bax JJ, Zhao Y, Makkar RR, Kapadia S, Thourani VH, Mack MJ, Nazif TM, Lindman BR, Babaliaros V, Vincent F, Russo M, McCabe JM, Gillam LD, Alu MC, Hahn RT, Webb JG, Leon MB, Cohen DJ. Evolution and Prognostic Impact of Cardiac Damage After Aortic Valve Replacement. J Am Coll Cardiol. 2022;80:783–800. doi: 10.1016/j.jacc.2022.05.006. [DOI] [PubMed] [Google Scholar]
- Tribouilloy C, Rusinaru D, Maréchaux S, Castel AL, Debry N, Maizel J, Mentaverri R, Kamel S, Slama M, Lévy F. Low-gradient, low-flow severe aortic stenosis with preserved left ventricular ejection fraction: characteristics, outcome, and implications for surgery. J Am Coll Cardiol. 2015;65:55–66. doi: 10.1016/j.jacc.2014.09.080. [DOI] [PubMed] [Google Scholar]
- Chadha G, Bohbot Y, Lachambre P, Rusinaru D, Serbout S, Altes A, Pasquet A, Maréchaux S, Vanoverschelde JL, Tribouilloy C. Progression of Normal Flow Low Gradient "Severe" Aortic Stenosis With Preserved Left Ventricular Ejection Fraction. Am J Cardiol. 2020;128:151–8. doi: 10.1016/j.amjcard.2020.05.003. [DOI] [PubMed] [Google Scholar]
- Saeed S, Vamvakidou A, Seifert R, Khattar R, Li W, Senior R. The impact of aortic valve replacement on survival in patients with normal flow low gradient severe aortic stenosis: a propensity-matched comparison. Eur Heart J Cardiovasc Imaging. 2019;20:1094–101. doi: 10.1093/ehjci/jez191. [DOI] [PubMed] [Google Scholar]
- Amanullah MR, Pio SM, Ng ACT, Sin KYK, Marsan NA, Ding ZP, Leon MB, Généreux P, Delgado V, Ewe SH, Bax JJ. Prognostic Implications of Associated Cardiac Abnormalities Detected on Echocardiography in Patients With Moderate Aortic Stenosis. JACC Cardiovasc Imaging. 2021;14:1724–37. doi: 10.1016/j.jcmg.2021.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of high-gradient aortic stenosis.
Baseline characteristics of classical low-flow low-gradient aortic stenosis.
Baseline characteristics of low-flow low-gradient aortic stenosis with preserved ejection fraction.
Baseline characteristics of normal-flow low-gradient aortic stenosis.
Clinical outcomes according to four cardiac stages in each AS subtype.
Comparison of baseline characteristics between included and excluded patients.