Abstract
A homolog of the Staphylococcus aureus methicillin resistance gene mecA was recently shown to be ubiquitous in independent isolates of the animal species Staphylococcus sciuri. The mecA gene homolog and regions flanking it were cloned and sequenced from four strains of S. sciuri: strain K1 (ATCC 29062), a representative of S. sciuri subsp. sciuri; two strains (K3 and K8) representing S. sciuri subsp. rodentius; and strain K11, a representative of S. sciuri subsp. carnaticum. Strains K1 and K11 were susceptible to methicillin, while strains K3 and K8 showed heterogeneous resistance. The mecA genes of strains K1 and K11 and one of the two copies of mecA (mecA1) present in strain K3 had virtually identical DNA sequences in the mecA gene and were similar in genetic organization in the flanking regions. In contrast, the single copy of mecA in strain K8 and the second copy of mecA (mecA2) in strain K3 had mecA DNA sequences identical to that of S. aureus mecA, and the mecA region in these two strains was also similar to that of the same region in the S. aureus strain used for comparison. Interestingly, an open reading frame defining an N-terminal truncated polypeptide, NTORF101, with a high degree of homology to a DNA segment in the hypervariable region of methicillin-resistant S. aureus (and also similar to the Escherichia coli gene ugpQ) was also identified downstream of the mecA homolog of strain K11, representing S. sciuri subsp. carnaticum. The ugpQ-like gene is not present in methicillin-susceptible strains of S. aureus. The presence of such a ugpQ-like gene together with the homolog of mecA in strain K11 supports the speculation that these genetic elements may be evolutionary relatives and/or precursors of the genetic determinant of methicillin resistance in S. aureus.
It has been established that mecA and its product PBP2A are the central determinants of methicillin resistance (12, 13, 16, 17, 21, 29). The 2-kb mecA gene and flanking DNA are unique to methicillin-resistant strains of staphylococci and no equivalent locus exists in methicillin-susceptible bacteria (6, 19), indicating that the mec determinant was acquired by horizontal gene transfer.
A number of speculations concerning the origin of mecA have been proposed (1, 11, 20, 28). In a recent communication, we demonstrated the ubiquitous presence of a mecA-like gene in strains of the animal species Staphylococcus sciuri (8) and suggested that this species may harbor an evolutionary precursor of the structural gene of PBP2A of methicillin-resistant strains of staphylococci. Cloning and sequencing of the mecA homolog from S. sciuri K1 revealed an overall 88% similarity in amino acid sequence and 80% identity in DNA sequence to the mecA gene of methicillin-resistant S. aureus (MRSA). Comparison with sequence information available in the BLAST data bank indicated that the mecA homolog of S. sciuri was by far the most similar to mecA of all known genes (high score of 3,080, with the next-closest score, for the Enterococcus faecium PBP5 gene, being 300) (30).
The collection of 134 S. sciuri strains that reacted with the mecA DNA probe in the initial screen came from a wide variety of ecological sources, were genetically diverse, and contained members of three subspecies: S. sciuri subsp. sciuri, S. sciuri subsp. rodentius, and S. sciuri subsp. carnaticum (8). To test the possibility that one strain in this large and diverse collection contains a variant of mecA with even closer sequence similarity (>80% DNA sequence similarity) to the mecA of MRSA, we generated mecA gene fingerprints from 30 of the most diverse S. sciuri isolates in the collection and compared these for DNA sequence diversity of their respective mecA homologs. In addition, the mecA regions of two S. sciuri subsp. rodentius strains (K3 and K8) and strain K11 of S. sciuri subsp. carnaticum were cloned and sequenced to obtain more information on the genetic organization of the entire mecA region. As controls, appropriate sequence information was assembled for the mecA regions of the MRSA strains BMS-1 (2), BB270 (5), and BB589 (7), and additional sequencing was done on the native mecA homolog already identified in S. sciuri K1.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are described in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics or strain designation (origin)a | Origin or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | recA endA1 gyrA96 thi-1 hsdR17 supE44 relA1 φ80 dlacZΔM15 | Bethesda Research Laboratories |
| S. aureus | ||
| COL | Homogeneous Mcr | Rockefeller University collection |
| BB589 | 7 | |
| S. sciuri subsp. sciuri | K1 (eastern grey squirrel), K2 (beef tongue), K38 (Holstein cow), K47 (Palestine mole rat), K55 (pilot whale), K56 (Norway rat), K69 (adult human), K74 (horse), K105 (preadolescent child), K132 (howler monkey), K141 (California mouse), K144 (Jersey calf), K156 (hairy-footed hamster), KL056 (opossum), KL064 (southern flying squirrel) | 8 |
| S. sciuri subsp. rodentius | K3 and K4 (neonatal ward), K8, K9, K27, and K29 (Norway rat), K10 (European red squirrel), K26 (pilot whale) | |
| S. sciuri subsp. carnaticum | K11 (veal leg, sliced), K30 and K33 (Jersey cattle heifer), K31 (Jersey cattle calf), K61 (pilot whale) | |
| Plasmids | ||
| pBR322 | Cloning vector, Ampr Tcr | New England Biolabs |
| pGEM-3Z | Subcloning vector, Ampr | Promega Corp. |
| pSW-15 | pGEM-3Z/6.7-kb HindIII fragment from K11 containing K11 mecA | This study |
| pSW-16 | pGEM-3Z/5.8-kb HindIII fragment from K3 containing K3 mecA1 | This study |
| pSW-17 | pGEM-3Z/4.1-kb HindIII fragment from K3 containing K3 mecA2 and N-terminal mecR1 | This study |
| pSW-18 | pGEM-3Z/4.1-kb HindIII fragment from K8 containing K8 mecA and N-terminal mecR1 | This study |
| pSW-19 | pGEM-3Z/2.3-kb HindIII fragment from K3 containing C-terminal mecR1 and mecI | This study |
| pSW-20 | pGEM-3Z/1.5-kb HindIII fragment from K8 containing C-terminal mecR1 and mec1 | This study |
| pCP2 | pGEM-3Z/5.1-kb EcoRI fragment from K1 containing K1 mecA | 30 |
Mcr, methicillin resistance; Ampr, ampicillin resistance; Tcr, tetracycline resistance.
Media and growth conditions.
The bacterial strains of S. aureus and S. sciuri were grown in tryptic soy broth (Difco Laboratories, Detroit, Mich.) with aeration at 37°C. Luria-Bertani medium was used to propagate Escherichia coli DH5α, and ampicillin was added at the concentration of 100 μg/ml−1 for selection and maintenance of the plasmids listed in Table 1.
DNA methods and nucleotide sequencing.
All routine DNA manipulations were performed essentially as described in references 25 and 3. Restriction enzymes, calf intestine alkaline phosphatase, and T4 DNA ligase were purchased from New England Biolabs, Inc. (Beverly, Mass.), and used as recommended by the manufacturer. Southern analysis was performed with ECL random prime labeling and detection systems (Amersham Life Science, Arlington Heights, Ill.) as recommended by the manufacturer. DNA sequence was determined by the dideoxy-chain termination method (26) with an automated DNA sequencing system (model 377; PE/ABI). Nucleotide and derived amino acid sequences were analyzed with the Wisconsin Genetics Computer Group software.
Fingerprinting of the mecA gene.
A 1.3-kb internal fragment of the mecA gene was amplified from S. aureus COL and S. sciuri strains by PCR (24). The primers SAMECA165 (5′-CGATAATGGTGAAGTAGA-3′) and SAMECA1482 (5′-TATATCTTCACCAACACC-3′) used in the amplification were specific for the corresponding DNA sequence in both S. aureus mecA and S. sciuri K1 mecA; the amplified PCR fragment includes the internal mecA region starting at the 164th bp from the initiation codon and ending at the 531st bp from the termination codon (30). Chromosomal DNAs from S. aureus COL and S. sciuri strains were used as templates in amounts of 10 ng for each reaction. PCR amplification was performed in a DNA thermal cycler (Perkin-Elmer/Cetus) by using the Perkin-Elmer/Cetus PCR reagent kit according to the manufacturer’s instructions. The PCR program was as follows: 94°C for 5 min; 30 cycles of 94°C for 30 s, 53°C for 30 s, 72°C for 2 min, and 72°C for 5 min; 4°C, hold.
The PCR product was purified with the Wizard PCR Preps DNA purification system (Promega, Madison, Wis.), and then 10 ng of the PCR product was digested with MseI in a volume of 20 μl. Following the digestion, 4 μl of labeling mixture (10 mM Tris hydrochloride [pH 7.4], 10 mM MgCl2, 6 mM dithiothreitol, 0.25 mM dGTP, 0.25 mM dTTP, 0.25 mM dCTP, 10 μCi of [α-32P]dATP/μl, 0.4 U of Klenow fragment/μl) was added to the digests, the labeling sample was incubated at room temperature for 10 min, and 8 μl of loading buffer was used to stop the reaction. The plasmid pBR322 was digested with HpaII and labeled with [α-32P]dCTP by using a procedure similar to that described above to provide molecular size markers (31). Samples (2 to 4 μl) were applied to a 6% nondenaturing polyacrylamide gel, and electrophoresis was performed at 1,200 V for 2 h 20 min. The gel was transferred to Whatman filter paper, dried with a Gel Dryer (Bio-Rad model 583), and exposed for autoradiography using X-ray film (X-Omat; Kodak) at −70°C for 4 to 12 h.
RESULTS
Fingerprints of mecA from 30 strains of S. sciuri.
The fingerprints obtained by digesting the amplified mecA fragments of the 30 selected S. sciuri strains with MseI could be grouped into six different patterns (which differed in the number and molecular size of bands). The 16 S. sciuri subsp. sciuri isolates distributed across four of the six patterns (patterns I through IV), the 5 S. sciuri subsp. carnaticum strains fell into patterns I, III, and V, and 8 of the 10 S. sciuri subsp. rodentius strains shared fingerprint pattern V (Fig. 1). The first five fingerprint patterns turned out to be minor variants of pattern I shown by the already sequenced mecA in strain K1 (30).
FIG. 1.
MseI fingerprints of the mecA genes from a selected group of S. sciuri strains. Lanes 1 and 15, pBR322 digested with HpaII size markers; lanes 2 and 3, fingerprints of S. sciuri K1 and S. sciuri subsp. carnaticum K11 (pattern I); lanes 4 and 5, fingerprints of S. sciuri K47 and K105 (pattern II); lanes 6 and 7, fingerprints of S. sciuri K2 and S. sciuri subsp. carnaticum K61 (pattern III); lanes 8 and 9, fingerprints of S. sciuri K165 and KL064 (pattern IV); lanes 10 and 11, fingerprints of S. sciuri subsp. rodentius K3 (mecA1) and S. sciuri subsp. carnaticum K30 (pattern V); lanes 12 to 14, fingerprints of S. sciuri subsp. rodentius K3 (mecA2) and K8 and S. aureus COL (pattern VI).
The only substantially different mecA fingerprint (pattern VI) was detected in two strains belonging to S. sciuri subsp. rodentius. One of these, strain K3, carried two copies of mecA (8). One of the two mecA copies (mecA1) produced fingerprint pattern V; this copy is a variant of the mecA native to S. sciuri. The second copy of mecA (mecA2) showed the very different fingerprint pattern VI and required the use of pSW-17 plasmid DNA (containing K3 mecA2) as template. This second copy of mecA in strain K3 and the single copy of mecA found in the second S. sciuri subsp. rodentius strain, K8, were virtually identical in fingerprint pattern to the mecA from the MRSA strain COL (Fig. 1).
Cloning and DNA sequencing of the S. sciuri mecA genes and flanking regions.
Strain S. sciuri ATCC 29062 (K1), representing subspecies sciuri, two strains (K3 and K8) belonging to subspecies rodentius, and strain K11, belonging to subspecies carnaticum, were selected for further investigation. The mecA homologs were localized by HindIII restriction digestion and Southern blotting (8); the HindIII fragments containing the mecA region from strains K11 (6.7 kb), K3 (5.8 kb for mecA1 and 4.1 kb for mecA2), and K8 (4.1 kb) were each ligated into cloning vector pGEM-3Z to generate the recombinant plasmids pSW-15 (K11 mecA), pSW-16 (K3 mecA1), pSW-17 (K3 mecA2), and pSW-18 (K8 mecA). The upstream HindIII fragments of K3 mecA2 (2.3 kb) and K8 mecA (1.5 kb) were also cloned into pGEM-3Z to yield plasmids pSW-19 and pSW-20 since mecI and part of mecR1 were expected to be included in this area.
The DNA inserts in the plasmid constructs were sequenced through both strands by the strategy of primer walking. DNA sequences of the mecA regions were analyzed for open reading frames (ORFs), and the DNA sequences of pSW-19 and pSW-17 were assembled into a K3 mecA2 region. The sequences of pSW-18 and pSW-20 were similarly combined to generate the K8 mecA region. In addition, further sequencing was performed in the mecA region of S. sciuri K1 (30). The maps for the mecA regions of these strains are shown in Fig. 2.
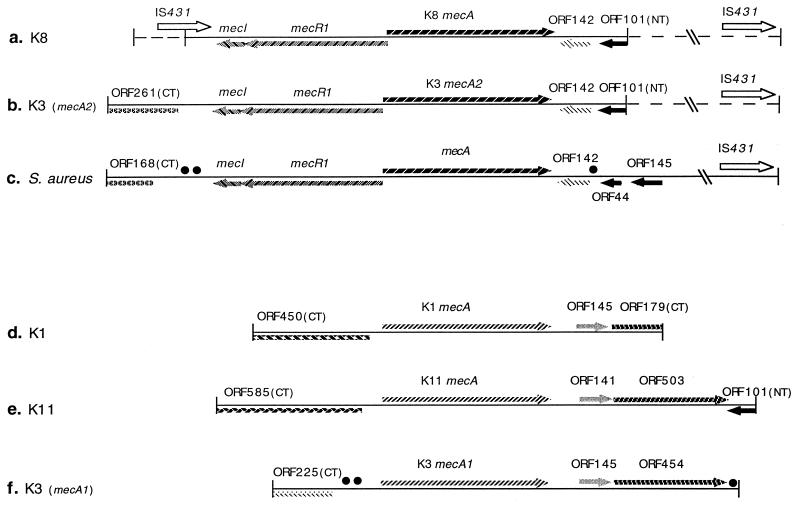
FIG. 2.
Genetic organization of the mecA regions of S. sciuri (K1), S. sciuri subsp. rodentius (K8 and K3), S. sciuri subsp. carnaticum (K11), and S. aureus. The S. aureus mecA region (c) was assembled by using DNA sequences from the database. The direction of gene transcription is shown by the arrows. Identical symbols are used to indicate sequences of significant similarity. Dashed symbols indicate regions determined by PCR analysis. Homologies between short DNA segments are indicated by • (near ORF454 of K3 and near ORF142 of S. aureus) or by •• (near CTORF225 of K3 and near CTORF168 of S. aureus). Accession numbers for these sequences are as follows: K8 (5,596 bp), Y13096; K3 mecA2 (6,368 bp), Y13095; K11 (6,684 bp), Y13094; K3 mecA1 (5,806 bp), Y13052; and K1 (5,068 bp), Y09223. To facilitate comparison, relevant DNA sequences of the mecA region of S. aureus are also provided: mecRI (1) (accession no. L14020), mecA (23) (accession no. X52593), hypervariable region (22) (accession no. X52594), and IS431 (4, 5) (accession no. X53818). These sequences were assembled to provide a map for the entire S. aureus mecA and flanking region (c [9,047 bp; accession no. Y14051]). Overlaps or gaps at junctions in this map were determined by sequencing the appropriate PCR products of S. aureus BB589 (7).
Similarities among the mecA genes.
K3 mecA1 and K11 mecA were similar to K1 mecA (94.5 and 98.2% for DNA sequence; 98.5 and 99.0% for amino acid sequence). In contrast, K3 mecA2 and K8 mecA were almost identical to S. aureus mecA (99.3 and 99.9% for DNA sequence; 96.6 and 96.9% for amino acid sequence). The similarity between S. aureus mecA and S. sciuri K1 mecA was 79.5% for DNA sequence and 87.8% for amino acid sequence (30).
Genetic organization of mecA of strain K8 and mecA2 of strain K3.
DNA sequences flanking the mecA genes in strains K8 and K3 showed close similarities to the corresponding areas surrounding mecA in the S. aureus strain used for comparison (Fig. 2). Intact mecI and mecR1, with 100% amino acid sequence similarity to the corresponding sequences in S. aureus, were identified at the 5′ ends of both K3 mecA2 (Fig. 2b) and K8 mecA (Fig. 2a). Similar genetic organization was also observed at the 3′ ends where ORF142s in K3 mecA2 and K8 mecA were more than 99% homologous to ORF142 located downstream of the S. aureus mecA (Fig. 2c; Table 2). These ORFs showed 81% similarity to a protein of unknown function from Bacillus subtilis (accession no. 1881333; Table 2). Further downstream of K8 mecA and K3 mecA2, a polypeptide, truncated by the HindIII cloning site in the N terminus and designated NTORF101, showed 58.3% similarity and 35.4% identity to the C-terminal part of glycerophosphosphoryl diester phosphodiesterase encoded by the E. coli ugpQ gene (14), and the 44 residues at the C-terminal part of NTORF101 were almost identical to ORF44 in the S. aureus mecA region (Fig. 2c). Using several oligonucleotides as primers, we examined the genetic organization beyond the 3′ HindIII restriction site by comparing the sizes of K3 and K8 PCR products with those of S. aureus BB589 PCR fragments. The results indicated that the 3′ flanks of K3 mecA2 and K8 mecA were similar to that of S. aureus and that this region contained the entire hypervariable region and IS431 sequence (Fig. 2a and b). In the K8 mecA region, an N-terminally truncated polypeptide (NTORF78) was identified at the 5′ end. NTORF78 was actually the C terminus of the putative transposase in IS431, and this IS431 was confirmed to be intact by PCR. At the 5′ end of the K3 mecA2 region, we identified a C-terminally truncated peptide, CTORF261, that showed 84% similarity to the xylose repressor of S. xylosus (27); amino acid residues 94 to 261 of this ORF were identical to ORF168 at the 5′ end of the S. aureus mecA region (Fig. 2b and c).
TABLE 2.
Amino acid sequence similarity of ORFs in S. sciuri mecA regions with polypeptides in the database
| ORF | Similarity with polypeptides in database (%) | Accession no. or reference |
|---|---|---|
| S. sciuri subsp. sciuri K1 mecA region | ||
| CTORF450 | Unknown-function protein of B. subtilis (75) | 1181242 |
| ORF145 | Unknown-function protein of A. cateoacelicus (55) | Y09102 |
| CTORF179 | Glutathione reductase (55) | 9 |
| S. sciuri subsp. carnaticum K11 mecA region | ||
| CTORF585 | CTORF450 of K1 mecA region (100) | Y09223 |
| ORF141 | ORF145 of K1 mecA region (91) | Y09223 |
| ORF503 | CTORF179 of K1 mecA region (99) | Y09223 |
| NTORF101 | C terminus of E. coli UgpQ (58) | 14 |
| S. sciuri subsp. rodentius K3 mecA1 region | ||
| CTORF225 | 4-Aminobutyrate aminotransferase of E. coli (50) | P50457 |
| ORF145 | ORF145 of K1 mecA region (98) | Y09223 |
| ORF454 | CTORF179 of K1 mecA region (99) | Y09223 |
| S. sciuri subsp. rodentius K8 mecA region | ||
| ORF142 | Unknown-function protein of B. subtilis (81) | 1881333 |
| NTORF101 | NTORF101 of K11 mecA region (98) | This study |
| S. sciuri subsp. rodentius K3 mecA2 region | ||
| CTORF261 | Xylose repressor of S. xylosus (84) | 27 |
| ORF142 | ORF142 of K8 mecA region (100) | This study |
| NTORF101 | NTORF101 of K11 mecA region (98) | This study |
Genetic organization of mecA of strain K11 and mecA1 of strain K3.
DNA sequences flanking the mecA homologs of strains K11 and K3 had several features in common with those also present flanking the native mecA already sequenced from S. sciuri K1 (Fig. 2; Table 2). CTORF585, located at the 5′ end of the K11 mecA region (in an orientation inverted with respect to mecA), showed significant amino acid sequence homology (75%) to a protein of unknown function from B. subtilis (14a [accession no. 1181242]) which was nearly identical to the corresponding part of CTORF450 in the S. sciuri K1 mecA region (Fig. 2d and e). The regions 3′ to K11 mecA, K3 mecA1, and K1 mecA were similar in genetic organization (Fig. 2d to f; Table 2); ORF141 of K11 showed 55% similarity to a protein of unknown function from Acinetobacter cateoacelicus (18a [accession no. Y09102]) and was also similar to ORF145 in the mecA regions of K3 (mecA1) and K1 (98 and 91% amino acid similarity, respectively). K11 ORF503 showed 55% homology to the glutathione reductase of Nicotiana tabacum (9) and was identical to the corresponding part of CTORF179 in the K1 mecA region and 92% similar to ORF454 in the mecA1 region of K3.
Together these observations document two distinct types of genetic organization in the mecA regions of these bacteria: (i) S. aureus-type organization shared by mecA from strain K8 and mecA2 of strain K3 and (ii) S. sciuri-type organization shared by mecA of strains K1 and K11 and mecA1 of strain K3. Nevertheless, some differences were also noted. For instance, CTORF225 located at the 5′ end of the K3 mecA1 region was absent from the K11 and K1 genes. CTORF225 showed 50% similarity to the 4-aminobutyrate aminotransferase of E. coli (17a [accession no. P50457]).
Similarities between the genetic organization of the S. aureus-type and S. sciuri-type mecA regions.
Interestingly, some similarities in genetic organization also existed across the two types of mecA regions. (i) An N-terminally truncated peptide (NTORF101) was found at the 3′ end of the S. sciuri-type mecA region in strain K11 (Fig. 2e); this was almost identical to the NTORF101s located in the S. aureus-type mecA regions of strains K3 and K8 and was also similar to ORF44 of the 3′ mecA flank of MRSA (Fig. 2a to c). (ii) At 135 bp upstream of K3 mecA1, a DNA fragment of 291 bp (nucleotides 893 to 1183 [Fig. 2f]) was 76.5% identical to a DNA segment downstream of mecI of S. aureus (nucleotides 938 to 1218 [Fig. 2c]). (iii) A DNA sequence of 47 bp downstream of ORF454 of K3 mecA1 (nucleotides 5565 to 5611 [Fig. 2f]) showed 81% identity to an area downstream of ORF44 in the S. aureus mecA region (nucleotides 6002 to 6048 [Fig. 2c]). (iv) The 306-bp DNA sequence (nucleotides 6378 to 6684 [Fig. 2e and 3b) at the 3′ end of K11 (corresponding to the coding area at the C terminus of K11 ORF503 and the entire region of K11 NTORF101) was 85% identical to a DNA segment in the S. aureus hypervariable region (nucleotides 6049 to 6355 [Fig. 2c and 3b]).
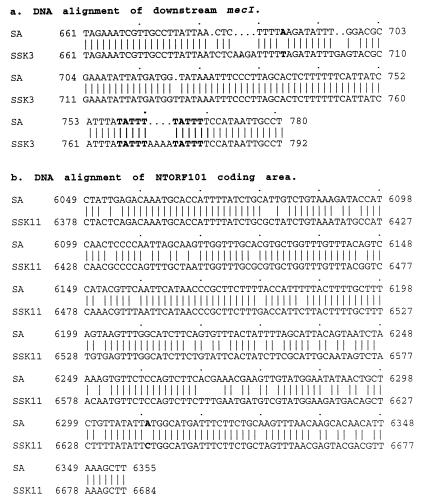
FIG. 3.
DNA sequence alignment of short segments in the area outside the mecA coding region. (a) DNA sequence at the N terminus of CTORF261 in K3 mecA2 compared with the corresponding region in S. aureus. Bold letters represent the direct repeat which is the likely position for IS insertion. (b) DNA sequence of NTORF101 in K11 compared with the intervening area between ORF142 and ORF145 in S. aureus. The nucleotide at position 6239 (C) in the S. aureus sequence was replaced with an A residue, creating an extra stop codon and causing interruption of the UgpQ-like polypeptide.
DISCUSSION
The S. sciuri K1 mecA homolog identified in our previous study is the gene most similar to the mecA of methicillin-resistant staphylococcal strains among all known genes (30). Moreover, the mecA homolog seems to be native to S. sciuri because of its ubiquitous presence in each of a large number of independent natural isolates (8), suggesting that S. sciuri mecA may be a close evolutionary relative of the mecA gene of S. aureus, which encodes PBP2A and is the central genetic determinant of β-lactam antibiotic resistance in that bacterium. A group of 30 S. sciuri strains selected for maximum genetic and ecological diversity was used to amplify and fingerprint the resident mecA homologs, which were then compared for sequence diversity and similarity to the S. aureus mecA gene. This method provides a convenient high-resolution technique for examination of genetic and sequence relatedness, and a difference of less than 5% in DNA sequence is detectable in the form of a different fingerprint pattern (18).
The results of this comparative fingerprinting study showed only a low degree of sequence diversity among the mecA homologs in spite of the broad genetic diversity of the strains examined, which included members of three different subspecies. In fact, the mecA homolog with the highest degree of relative similarity (79.5%) to the structural gene of PBP2A was still the previously sequenced mecA of S. sciuri K1.
Surprisingly, 2 of the 30 strains examined (K3 and K8, both belonging to the subspecies rodentius) contained mecA genes virtually identical to that of S. aureus. One of the strains (K3) contained two copies of mecA: one (mecA2) practically identical to the PBP2A determinant of S. aureus, and the other (mecA1) virtually identical to the mecA homolog already sequenced from S. sciuri K1, which appears to be, with minor variations, the dominant form of this gene in the species S. sciuri. S. sciuri strains carrying only this form of mecA are susceptible to methicillin, suggesting that this native or “silent” gene does not confer antibiotic resistance to the bacteria. The second strain, K8, seemed to have lost the native mecA homolog and carried only the MRSA type of mecA.
K3 mecA2 and K8 mecA were almost identical to the mecA of MRSA, both in DNA sequence and in genetic organization, and both strains showed heterogeneous methicillin resistance (8). These strains may have acquired a mecA gene from S. aureus or S. epidermidis. Strain K3 was a human-colonizing isolate recovered at a neonatal ward of an African hospital, and strain K8 was isolated from a rodent (8). The appearance of S. aureus mecA in these S. sciuri isolates suggests that avenues for genetic exchange between these two staphylococcal species exist. The apparent loss of the native mecA copy from K8 suggests that some genetic event such as deletion or exchange may have occurred in this strain following, perhaps, the transient coexistence of the two types of mecA genes similarly to the case of strain K3, which carries both the S. aureus-type and the native S. sciuri-type mecA.
The genetic organization at the 3′ ends of K3 mecA2 and K8 mecA was very similar to that of the so-called hypervariable region of S. aureus BB270 (22), and the entire IS431 was detected at both the 5′ and 3′ ends of K8 mecA and at the 3′ end of mecA2 of K3, supporting the proposal that these insertion sequence (IS) elements may be involved with the acquisition of mecA (5, 22). The organization at the 5′ part of the mecA genes in the S. sciuri subsp. rodentius strains showed two kinds of differences. One is that a second IS431 copy was located 191 nucleotides downstream of mecI in the K8 mecA region, i.e., in an area where in the case of most MRSA strains sequences of transposon Tn554 are located (10). Nevertheless, at least one MRSA isolate has been described in which a second copy of IS431 was found at a unique mecR1 deletion junction (20a).
The second difference involved CTORF261 in the K3 mecA2 region, which had 93 more N-terminal amino acid residues than CTORF168 in the S. aureus mecA region, while the rest of the amino acid sequences appeared to be identical. Nevertheless, DNA sequencing of this area revealed several deletions and one substitution within a stretch of 120 nucleotides (Fig. 3a), as well as evidence for rearrangement events possibly caused by IS or transposon insertion, which must have interrupted the translation of this polypeptide at the N terminus.
The genetic organization of the mecA regions in the three S. sciuri strains harboring native or silent mecA showed close similarities, as indicated in Fig. 2 and Table 2. The ORFs at the 3′ ends of all three mecA genes and also at the 5′ ends of the K11 and K1 genes were almost identical in both amino acid sequence and organization. Nevertheless, CTORF225 at the 5′ of K3 mecA1 was different from the CTORF585 of K11 and also from the CTORF450 of K1, which confirms that the subspecies carnaticum may be related more closely to the subspecies S. sciuri than to the subspecies rodentius (15). The polypeptides encoded by the ORFs at the 5′ end of the native S. sciuri mecA genes showed no homology to mecI and mecR1. It is conceivable that the mecA gene may have acquired the 5′ regulatory sequences of mecI-mecR1 by rearrangement of DNA at some later stage during the evolution of this region. It is interesting that the ORFs at the 5′ ends of S. sciuri mecA genes are located on the opposite DNA strand with respect to the direction of transcription of the genes. We do not know whether this arrangement may be advantageous for acquisition of the mecI-mecR1 sequence.
Two DNA fragments mapped in the area outside the K3 mecA1 region (Fig. 2) showed significant sequence similarities to two DNA segments also located outside the S. aureus mecA coding region at a location and in an orientation which were very similar in the S. sciuri and S. aureus strains. One may speculate that these short DNA homologs may represent remnants of DNA sequences in the area outside a hypothetical mecA precursor gene remaining after the precursor mecA and its flanks underwent the multiple and imprecise insertion, excision, and rearrangement events that eventually led to the emergence of the resistance determinant form of mecA.
It is also noteworthy that a 306-bp DNA sequence at the 3′ end of the native mecA in S. sciuri subsp. carnaticum K11 showed 85% identity to a segment of DNA in the S. aureus mecA hypervariable region (Fig. 3b). This finding is of considerable significance both because of the high degree of DNA sequence similarity and also because of the importance of the hypervariable region in the transposition of mec DNA. It is commonly accepted that the core sequence of mec DNA acquired originally by S. aureus probably included at least three regions: the mecA gene; 1 to 2 kb of 3′ sequence followed by a copy of the IS-like element IS431mec (IS257); and the 5′ regulatory sequence mecR1 and mecI (1). The DNA sequence located between mecA and IS431 was termed hypervariable because of its considerable length polymorphism among different staphylococcal isolates. Within this region was identified a polypeptide (ORF145) which was homologous to the N terminus of the glycerophosphoryl diester phosphodiesterase (UgpQ) of E. coli (except for the minimal direct repeat unit [dru] region) (22). As mentioned above, the 3′ flank of mecA and the DNA sequence in the hypervariable region are believed to be extraspecies (foreign) DNA for S. aureus. For these reasons, our finding of DNA sequences homologous to the hypervariable region in association with the native mecA homolog in S. sciuri provides further credence to the suggested evolutionary relationship between the native mecA of S. sciuri and the mecA region in S. aureus. To obtain more precise information on this important issue, we compared the DNA and deduced amino acid sequences of the 3′ region of mecA in strain K11 to the corresponding sequences in S. aureus BB270, which we have resequenced. We observed the following: (i) the similarity between the S. sciuri and S. aureus DNAs in this region was higher than in the mecA coding area (85 and 80% respectively [Fig. 3b]); (ii) the NTORF101 in strain K11 mecA was almost identical to the NTORF101s in the K3 mecA2 and K8 mecA regions, and the 44 C-terminal residues of NTORF101 were identical to ORF44 of the MRSA strain (Fig. 4d); and (iii) NTORF101 (58% similarity [Fig. 4c]) and ORF44 (65% [Fig. 4b]) were homologous to the C terminus of E. coli UgpQ to a degree similar to that of ORF145 (57% [Fig. 4a]) of the hypervariable region at the N terminus of E. coli UgpQ. By an alignment analysis using the Genetics Computer Group program, it could be clearly seen that ORF145 and ORF44 originally must have belonged to an intact UgpQ-like protein (Fig. 4a and b). Through comparison of the DNA sequences, it was possible to identify two point mutations in this sequence: one substitution at 45 nucleotides downstream of ORF145 (Fig. 3b) and one deletion or addition at a position very close to the stop codon of ORF145 (data not shown). These alterations created two extra stop codons and caused a frameshift by which the UgpQ-like polypeptide was truncated into a shorter ORF (Fig. 2c). The existence of a UgpQ-like protein in S. sciuri subsp. carnaticum and the high similarity of this peptide to the UgpQ-like peptide in the S. aureus hypervariable region further strengthen our speculation concerning the evolutionary origin of mecA. One may propose that the S. sciuri subsp. carnaticum mecA region which was the original source of the mec determinant became truncated, deleted, and mobilized, presumably by some IS or transposon. The coding sequence eventually emerging as the structural gene of methicillin resistance has undergone extensive modification under the selective pressure of antibiotics, while the linked UgpQ-like peptide may have been retained unchanged between the early and ultimate evolutionary stages of mecA because this protein performed a function not related to antibiotic resistance, and it has been retained as a conserved determinant throughout the evolution of the mec cluster.
FIG. 4.
Relationship of the E. coli UgpQ (ECUGPQ) and S. aureus hypervariable regions in ORF145 (SAORF145) and ORF44 (SAORF44) and NTORF101 of strain K11 (K11NTORF101). (a) ORF145 was 57% homologous to the N-terminal part of E. coli UgpQ. (b) ORF44 was 65% similar to the C-terminal part of UgpQ. (c) NTORF101 of strain K11 showed 58% similarity to the C terminus of E. coli UgpQ. (d) Multisequence alignment of S. aureus ORF44, K11 NTORF101, and K8 NTORF101, documenting a high degree of similarity.
We also analyzed two polypeptides, ORF141 and ORF503 in the intervening region between K11 mecA and NTORF101, and compared them with the mecA 3′ peptide ORF142. The amino acid sequence of ORF142 showed 43% homology with ORF141 and 50% similarity to the N-terminal portion of ORF503. These homologies are too low to represent an evolutionary relatedness between these ORFs and ORF142 located downstream of S. aureus mecA. It may be that the original component of this intervening region was already deleted and then the ORF142 fragment was added to the region.
ACKNOWLEDGMENTS
Partial support for this study was provided by the Lounsbery Foundation and the Aaron Diamond Foundation, by PRAXIS XXI grants 2/2.1/BIA/349/94 and 2/2.1/BIO/1154/95, and by the Fundação Calouste Gulbenkian.
REFERENCES
- 1.Archer G L, Niemeyer D M. Origin and evolution of DNA associated with resistance to methicillin in staphylococci. Trends Microbiol. 1994;2:343–347. doi: 10.1016/0966-842x(94)90608-4. [DOI] [PubMed] [Google Scholar]
- 2.Archer G L, Niemeyer D M, Thanassi J A, Pucci M J. Dissemination among staphylococci of DNA sequences associated with methicillin resistance. Antimicrob Agents Chemother. 1994;38:447–454. doi: 10.1128/aac.38.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Short protocols in molecular biology. New York, N.Y: Greene Publishing Associates and John Wiley; 1992. [Google Scholar]
- 4.Barberis-Maino L, Berger-Bächi B, Weber H, Beck W D, Kayser F H. IS431, a staphylococcal insertion sequence-like element related to IS26 from Proteus vulgaris. Gene. 1987;59:107–113. doi: 10.1016/0378-1119(87)90271-x. [DOI] [PubMed] [Google Scholar]
- 5.Barberis-Maino L, Ryffel C, Kayser F H, Berger-Bächi B. Complete nucleotide sequence of IS431 mec in Staphylococcus aureus. Nucleic Acids Res. 1990;18:5548. doi: 10.1093/nar/18.18.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck W D, Berger-Bächi B, Kayser F H. Additional DNA in methicillin-resistant Staphylococcus aureus and molecular cloning of mec-specific DNA. J Bacteriol. 1986;165:373–378. doi: 10.1128/jb.165.2.373-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger-Bächi B, Strassle A, Gustafson J E, Kayser F H. Mapping and characterization of multiple chromosomal factors involved in methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:1367–1373. doi: 10.1128/aac.36.7.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couto I, de Lencastre H, Severina E, Kloos W, Webster J, Santos Sanches I, Tomasz A. Ubiquitous presence of a mecA homologue in natural isolates of Staphylococcus sciuri. Microb Drug Resist. 1996;2:377–391. doi: 10.1089/mdr.1996.2.377. [DOI] [PubMed] [Google Scholar]
- 9.Creissen G P, Mullineaux P M. Cloning and characterisation of glutathione reductase cDNAs and identification of two genes encoding the tobacco enzyme. Planta. 1995;197:422–425. doi: 10.1007/BF00202667. [DOI] [PubMed] [Google Scholar]
- 10.Dubin D T, Chikramane S G, Inglis B, Matthews P R, Stewart P R. Physical mapping of the mec region of an Australian methicillin-resistant Staphylococcus aureuslineage and a closely related American strain. J Gen Microbiol. 1992;138:169–180. doi: 10.1099/00221287-138-1-169. [DOI] [PubMed] [Google Scholar]
- 11.El Kharrhoubi A, Jacques P, Piras G, Van Beeumen J, Coyette J, Ghuysen J M. The Enterococcus hirae R40 penicillin-binding protein 5 and the methicillin-resistant Staphylococcus aureuspenicillin-binding protein 2′ are similar. Biochem J. 1991;280:463–469. [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana R. Penicillin-binding proteins and the intrinsic resistance to β-lactams in Gram-positive cocci. J Antimicrob Chemother. 1985;16:412–416. doi: 10.1093/jac/16.4.412. [DOI] [PubMed] [Google Scholar]
- 13.Hartman B J, Tomasz A. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986;29:85–92. doi: 10.1128/aac.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasahara M, Makino K, Amemura M, Nakata A. Nucleotide sequence of the ugpQ gene encoding glycerophosphoryl diester phosphodiesterase of Escherichia coliK-12. Nucleic Acids Res. 1989;17:2845. doi: 10.1093/nar/17.7.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Kasahara, M., et al. Unpublished data.
- 15.Kloos W E, Ballard D N, Webster J A, Hubner R J, Tomasz A, Couto I, Sloan G, Dehart H P, Fiedler F, Schubert K, de Lencastre H, Sanches I S, Heath H E, Leblanc P A, Ljungh Å. Ribotype delineation and description of Staphylococcus sciurisubspecies and their potential as reservoirs of methicillin resistance and staphylolytic enzyme genes. Int J Syst Bacteriol. 1997;47:313–323. doi: 10.1099/00207713-47-2-313. [DOI] [PubMed] [Google Scholar]
- 16.Matsuhashi M, Song M D, Ishino F, Wachi, Doi M, Inoue M, Ubukata K, Yamashita N, Konno M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to β-lactam antibiotics in Staphylococcus aureus. J Bacteriol. 1986;167:975–980. doi: 10.1128/jb.167.3.975-980.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews P R, Reed K C, Stewart P R. The cloning of chromosomal DNA associated with methicillin and other resistances in Staphylococcus aureus. J Gen Microbiol. 1987;133:1919–1929. doi: 10.1099/00221287-133-7-1919. [DOI] [PubMed] [Google Scholar]
- 17a.Mori, H. 1996. Unpublished data.
- 18.Nei M, Li W H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Parche, P., and T. Hillen. 1996. Unpublished data.
- 19.Pattee P A. Staphylococcus aureus. Genet Maps. 1990;5:22–27. [Google Scholar]
- 20.Piras G, Raze D, El Kharroubi A, Hastir D, Englebert S, Coyette J, Ghuysen J M. Cloning and sequencing of the low-affinity penicillin-binding protein 3′-encoding gene of Enterococcus hiraeS185: modular design and structural organization of the protein. J Bacteriol. 1993;175:2844–2852. doi: 10.1128/jb.175.10.2844-2852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Pucci, M. J., and G. L. Archer. 1996. Unpublished data.
- 21.Reynolds P E, Fuller C. Methicillin-resistant strains of Staphylococcus aureus: presence of identical additional penicillin-binding protein in all strains examined. FEMS Microbiol Lett. 1986;33:251–254. [Google Scholar]
- 22.Ryffel C, Bucher R, Kayser F H, Berger-Bächi B. The Staphylococcus aureus mec determinant comprises an unusual cluster of direct repeats and codes for a gene product similar to the Escherichia coli sn-glycerophosphoryl diester phosphodiesterase. J Bacteriol. 1991;173:7416–7422. doi: 10.1128/jb.173.23.7416-7422.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryffel C, Tesch W, Birch-Machin I, Reynolds P E, Barberis-Maino L, Kayser F H, Berger-Bächi B. Sequence comparison of mecA genes isolated from methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. Gene. 1990;94:137–138. doi: 10.1016/0378-1119(90)90481-6. [DOI] [PubMed] [Google Scholar]
- 24.Saiki R K, Gelfant D H, Stoffel S, Scharf S J, Higuchi R, Horn R T, Mullis K B, Erlich H A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sizemore C, Buchner E, Rygus T, Witke C, Gotz F, Hillen W. Organization, promoter analysis and transcriptional regulation of the Staphylococcus xylosusxylose utilization operon. Mol Gen Genet. 1991;227:377–384. doi: 10.1007/BF00273926. [DOI] [PubMed] [Google Scholar]
- 28.Song M D, Wachi M, Doi M, Ishino F, Matsuhashi M. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureusby gene fusion. FEBS Lett. 1987;221:167–171. doi: 10.1016/0014-5793(87)80373-3. [DOI] [PubMed] [Google Scholar]
- 29.Tesch W, Strässle A, Berger-Bächi B, O’Hara D, Reynolds P, Kayser F H. Cloning and expression of methicillin resistance from Staphylococcus epidermidis in Staphylococcus carnosus. Antimicrob Agents Chemother. 1988;32:1494–1499. doi: 10.1128/aac.32.10.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu S, Piscitelli C, de Lencastre H, Tomasz A. Tracking the evolutionary origin of the methicillin resistance gene: cloning and sequencing of a homologue of mecA from a methicillin susceptible strain of Staphylococcus sciuri. Microb Drug Resist. 1996;2:435–441. doi: 10.1089/mdr.1996.2.435. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q, Jones D M, Sáez Nieto J A, Trallero E P, Spratt B G. Genetic diversity of penicillin-binding protein 2 genes of penicillin-resistant strains of Neisseria meningitidisrevealed by fingerprinting of amplified DNA. Antimicrob Agents Chemother. 1988;34:1523–1528. doi: 10.1128/aac.34.8.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]