Abstract
Diabetes is associated with increased susceptibility to Klebsiella pneumoniae and poor prognosis with infection. We demonstrate accelerated mortality in mice with streptozotocin-induced diabetes following tracheal instillation of K. pneumoniae. Diabetic mice recruited fewer granulocytes to the alveolar airspace and had reduced early production of CXCL1, CXCL2, IL-1β and TNF-α following tracheal instillation of K. pneumoniae-lipopolysaccharide. Additionally, TLR2 and TIRAP expression following K. pneumoniae-lipopolysaccharide exposure was decreased in hyperglycemic mice. These findings indicate that impaired innate sensing and failure to rapidly recruit granulocytes to the site of infection is a mechanism for diabetic susceptibility to respiratory K. pneumoniae infection.
Keywords: diabetes, Klebsiella pneumoniae, lung, pneumonia
1. Introduction
Klebsiella pneumoniae is one of the ESKAPE pathogens (also including Enterococcus faecium, Staphylococcus aureus, Acinetobacter baumanii, Pseudomonas aeruginosa, and Enterobacter species) responsible for the majority of hospital infections in the United States [1]. Resistance of K. pneumoniae to carbapenem antibiotics has spread to all regions of the world and in some countries carbapenem resistance is present in more than half of the patients treated for K. pneumoniae infections [2]. Klebsiella pneumoniae is also emerging as an agent of severe community-acquired infection including bacteremic pneumonia [3].
Comorbid diabetes mellitus is associated with increased risk for and severity of liver, urinary tract, head and neck, bloodstream and lung infections with K. pneumoniae [4–8]. The global prevalence of diabetes is increasing and will reach to over 590 million in 20 years. Approximately 80% of people with diabetes currently live in low- and middle-income countries with the greatest increases predicted to occur in these regions [9]. With an aging population and the rising prevalence of diabetes, particularly in Asian countries which have also become a source of antibiotic resistant K. pneumoniae strains [10], a better understanding of the diabetic host-K. pneumoniae interaction is essential. To that end, we investigated the impact of streptozotocin (STZ)-induced diabetes in mice on susceptibility to respiratory K. pneumoniae infection. Results suggest that diabetic vulnerability to K. pneumoniae stems from a reduced cytokine response to bacterial lipopolysaccharide (LPS) resulting in delayed granulocyte trafficking to the airspace.
2. Materials and methods
2.1. Animals and induction of hyperglycemia
Male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were used for studies conducted under protocols approved by the Institutional Animal Care and Use Committee and the Institutional Biosafety Committee of the University of Massachusetts Medical School. Hyperglcemia was induced by intraperitoneal injection of 150 m/kg body weight STZ (Sigma-Aldrich, St Louis, MO). At regular intervals, blood glucose levels were assessed in control and STZ-treated mice using a BD Logic glucometer (BD Biosciences, Franklin Lakes, NJ). Mice were considered hyperglycemic if their blood glucose was >300 mg/dL and euglycemic or control when blood glucose was <200 mg/dL.
2.2. Infection and bacterial burden
One hundred colony-forming units (CFU) of K. pneumoniae (strain 43816; ATCC, Manassas, VA) was delivered by tracheal instillation. Hyperglycemic (HG) mice were challenged 12 weeks after STZ treatment. After 48 h of infection, lungs were homogenized in PBS and serial dilutions were plated on nutrient agar plates. Colonies were counted after 4-6 h of incubation at 37°C.
2.3. Flow cytometry
Bronchoalveolar lavage (BAL) cells and lung homogenates were obtained as previously described [11] and analyzed by flow cytometry. Cells were treated with Fc blocking mAb then stained with anti-CD11b, anti-CD11c (BD Bioscience Pharmingen, San Diego, CA) and F4/80-APC (eBioscience, San Diego, CA). Cells were gated based on forward scatter/side scatter of light for different cell populations and the total and percentages of leukocytes and granulocytes (CD11bhiCD11c−), resident alveolar macrophages (CD11b−CD11c+), recruited macrophages (CD11b+CD11c−) and dendritic cells (CD11b+CD11c+) were enumerated. Results were analyzed using FlowJo version 7 (Tree Star, Ashland, OR).
2.4. Histopathology
Lungs were fixed with 4% paraformaldehyde and prepared for histology and stained with hematoxylin and eosin or myeloperoxidase (MPO; anti-MPO rabbit IgG, LifeSpan Biosciences, Seattle, WA) and DAPI for nucleus.
2.5. Response to lipopolysaccharide
Klebsiella pneumoniae LPS was purchased from Sigma-Aldrich (L1519, source strain ATCC 15380) and subjected to re-purification with two phenol-water extractions to remove contaminating lipoproteins [12]. Quality control was performed by verifying IL-8 inducing activity towards HEK293-huTLR4/MD-2 cells and lack of IL-8 induction in HEK293-huTLR2 cells [13]. Escherichia coli LPS was also purchased from Sigma-Aldrich (L2630, serotype O111:B4) and it was purified by phenol extraction. Following anesthesia with isoflurane, 0.05 μg of K. pneumoniae LPS or 2 mg/kg E. coli LPS in 50 μl of PBS was delivered to the lungs via the trachea. At 1 h and 2 h post LPS instillation, mice were sacrificed and lungs removed for histology and tissue homogenates prepared for ELISA or RNA purification.
2.6. Assessment of cytokine levels
Production of selected chemokines and cytokines (CXCL1, CXCL2, IL-1β TNF-α and IL-10) in control and hyperglycemic (HG) mice: 1 and 2 h after K. pneumoniae LPS challenge, 1 h after E. coli LPS challenge and 6 h after K. pneumoniae infection (~100 CFU) was determined by multiplex ELISA (R&D Systems, Minneapolis, MN). Absorbance was measured with a Multiskan Ascent microplate spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and the concentrations of the selected chemokines and cytokines in each sample were extrapolated using a standard curve.
2.7. Gene expression
Total RNA was isolated from lung tissue by using TRIzol® Reagent and Purelink® RNA mini kit from Ambion (Life Technologies, Grand Island, NY). Equal amounts of RNA were used and retro-transcribed with High Capacity Reverse Transcription Kit (Applied Biosystems, Life Technologies). Quantitative real-time polymerase chain reaction (qPCR) for CXCL1, CXCL2, IL-1β, TNF-α, IL-10, TLR2, TLR4 and TIRAP was performed at 60°C of annealing temperature using SYBR Green PCR mix (Applied Biosystems, Life Technologies) according to the manufacturer’s guidelines. TBP and GADPH were used as housekeeping genes and primer sequences were designed by Primer3 input (supplemental information). Results were calculated as fold change to the control group and relative to the reference genes of mice by the [delta] [delta] Ct method of Livak [14].
2.8. Bactericidal activity in neutrophils and alveolar macrophages
Neutrophils were isolated from the peritoneum 24 h after thioglycollate i.p. injection and using anti-Ly-6G microbeads from Miltenyi Biotec Inc. (San Diego, CA). Alveolar macrophages (AM) were obtained by bronchoalveolar lavage. One hundred thousand neutrophils or AM were seeded in a flat-bottom 96-well plate and infected with K. pneumoniae (MOI 1:10). After 30 min, media was washed and cells were lysed with 1% Triton-X at different time points: 0, 15, 30, 60 and 90 min (only 0 and 30 min for AM). Results were calculated as fold change in bacterial counts compared to time 0 for each mouse sample.
2.8. Data analysis
GraphPad Prism 6.0 was used to perform statistical analysis. When normality was confirmed, data were analyzed using a Student’s t-test. Statistical differences in the survival curves were analyzed by log-rank (Mantel Cox) test. A p value of <0.05 was considered to be statistically significant.
3. Results
3. 1. Hyperglycemia increases susceptibility to K. pneumoniae infection
Following 12 weeks of STZ-induced hyperglycemia, mice were infected with ~100 CFU K. pneumoniae by tracheal instillation. HG mice had a significant reduction in survival compared to control mice (Fig. 1A). Over half of all HG mice succumbed to infection 3 days after challenge. In contrast, the first control mouse succumbed on day 9. Mortality reached 100% for HG mice by day 14 post-infection, at which point survival in the control group was nearly 80%. While there was substantial individual variation in lung bacterial burden measured on day 2 of infection, mean CFU was nearly 1000-fold higher in HG mice compared to euglycemic controls (Fig. 1B).
Fig. 1.
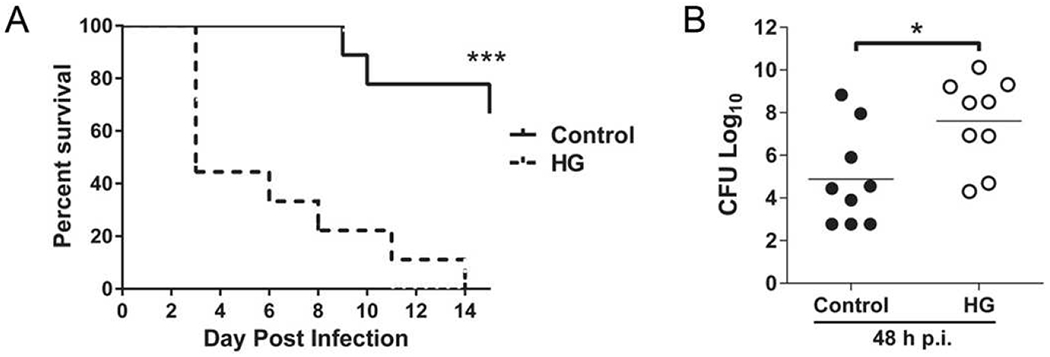
Higher susceptibility of hyperglycemic mice to K. pneumoniae infection. Mice were infected with ~100 CFU K. pneumoniae by tracheal instillation. (A) Survival curve of control and hyperglycemic (HG) mice demonstrating increased mortality of HG mice compared to controls. (B) Lung bacterial load was significantly higher in HG mice following 48 h of infection (n = 9). All experiments were repeated at least three times. Statistical differences were analyzed by Student’s t-test, *p < 0.05.
3.2. Reduced granulocyte recruitment to the site of pathology in hyperglycemic mice
The critical initial response to low dose K. pneumoniae infection involves the brisk recruitment of myeloid cells to the lung, leading to the clearance of the bacteria. To explore the basis for increased early mortality in HG mice, we analyzed myeloid cell recruitment to the whole lung and to airspace (BAL fluid) in response to tracheal instillation of highly purified K. pneumoniae LPS. Total cell numbers in both compartments were significantly reduced in the HG mice at 2 h post-challenge (Fig. 2A). From different populations assessed, only the number and proportion of granulocytes (predominantly neutrophils) were significantly decreased in the HG mice. These results were confirmed by the demonstration of reduced MPO signal, expressed in the granules of polymorphonuclear cells, in lungs from HG mice post LPS instillation (Fig. 2B). The proportion of other cell- populations was not altered, only resident alveolar macrophages show a slight but still significant increased percentage in the BAL (Supplemental Fig. 1).
Fig. 2.
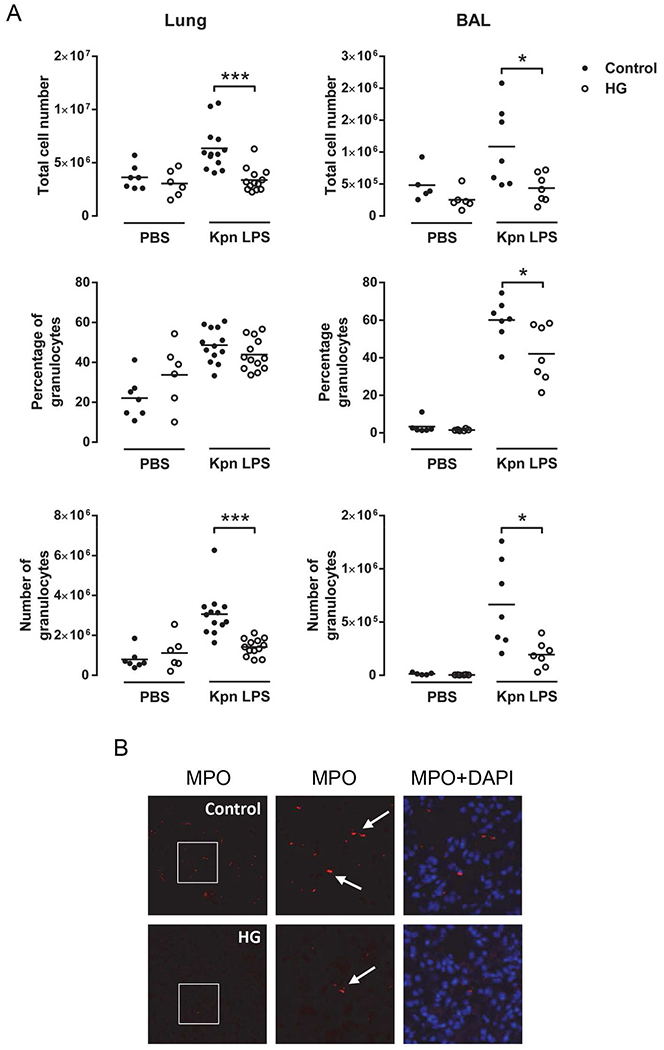
Reduced granulocyte recruitment following instillation of K. pneumoniae LPS in hyperglycemic mice. Mice were instilled with 0.05 μg LPS and after 2 h lung tissue was obtained. (A) Flow cytometry of isolated cells from lung homogenate (left) and BAL (right). Total cell number and percentage and cell number of granulocytes were quantified (n = 5-13). (B) Representative images of immunofluorescence staining for myeloperoxidase (MPO). All experiments were repeated at least twice. Statistical differences were analyzed by Student’s t-test, *p < 0.05 ***p < 0.001.
3.3. Hyperglycemia decreases the production and expression of cytokines in the lung
The chemokines CXCL1 and CXCL2, produced mainly by resident alveolar macrophages, are responsible for recruiting neutrophils. Production of these chemokines in the lung after 1 and 2 h of K. pneumoniae-LPS instillation was assessed by ELISA. At the 1 h post-instillation time point, HG mice produced significantly less CXCL1 and CXCL2 compared to controls (Fig. 3A, top). Measurement of CXCL1 mRNA expression confirmed the results obtained by ELISA (Fig. 3B). Inflammatory mediators such as IL-1β and TNF-α are induced when bacteria are recognized by pattern recognition molecules such as Toll-like receptors (TLRs) on macrophages. Interestingly, a ~50% reduction in IL-1β protein production was observed in HG mice 1 and 2 h after K. pneumoniae-LPS instillation (Fig. 3A). Reduced IL-1β mRNA expression in HG mice was confirmed by qPCR (Fig. 3B). The production of the anti-inflammatory cytokine IL-10 was also significantly reduced in lungs from HG mice 2 h after treatment (Fig. 3A).
Fig. 3.
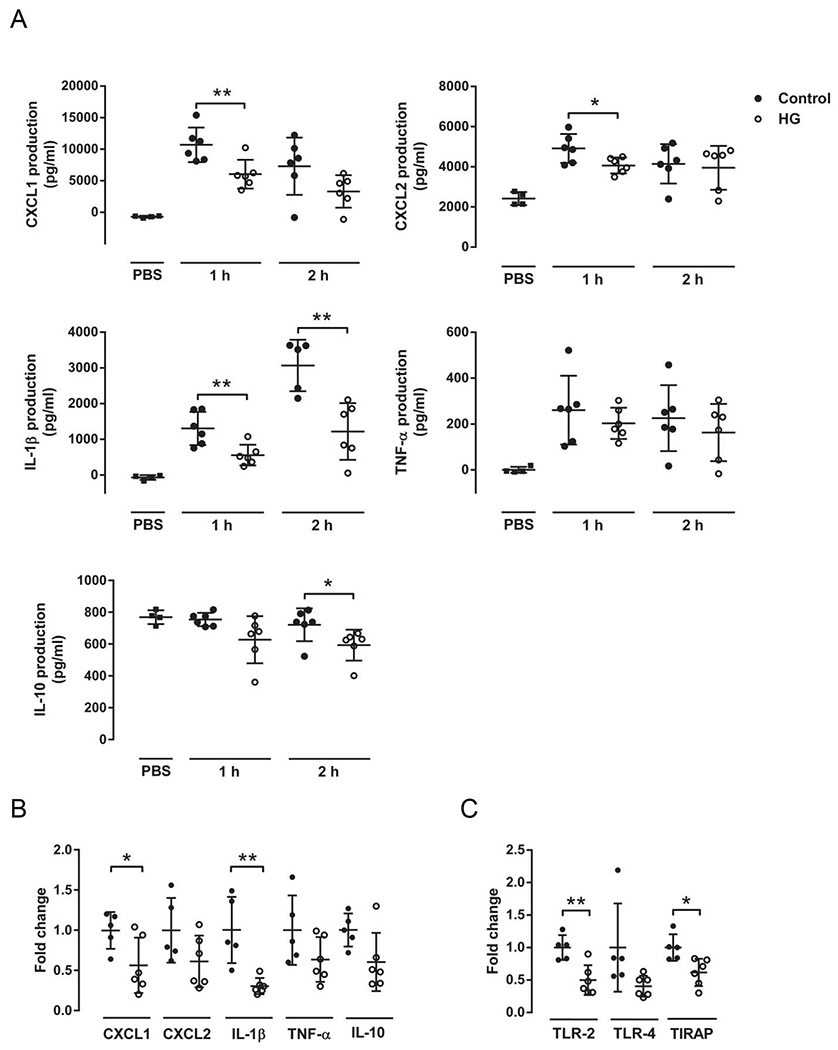
Reduced production and expression of cytokines in hyperglycemic mice instilled with K. pneumoniae LPS. (A) The levels of CXCL1, CXCL2, IL-1β, TNF-α and IL-10 in lung homogenates were lower in HG than control mice after 1 or 2 h instillation with PBS or 0.05 μg LPS (n = 6). (B) Gene expression of CXCL1, CXCL2, IL-1β, TNF-α and IL-10 in lung homogenates from control and HG mice after 1 h instillation with 0.05 μg LPS demonstrates a reduction in expression in HG mice (n = 6). (C) Gene expression of TLR2, TLR4 and TIRAP in lung homogenates from control and HG mice after 1 h instillation with K. pneumoniae LPS demonstrates a decrease in HG mice (n = 6). All experiments were repeated at least twice. Statistical differences were analyzed by Student’s t-test, *p < 0.05 **p < 0.01.
To investigate whether the expression of some components of the TLR cascade was altered in HG mice challenged with K. pneumoniae LPS, the levels of TLR2, TLR4 and TIRAP mRNA were assessed by qPCR. Expression of TLR2 and TIRAP was significantly reduced in the HG mice compared to controls, whereas there was no difference in TLR4 expression (Fig. 3C).
Changes in the gene expression and production of cytokines were specific for K. pneumoniae LPS. There were no significant differences in gene expression or cytokine production between control and HG mice instilled with E. coli LPS (Supplemental Fig. 2A). When mice were infected with live K. pneumoniae there was significant variability in CFU in the lung at different time points leading to low reproducibility. Although there was a decreased trend in the gene expression for cytokines in HG mice only IL-1β gene expression was significantly reduced 6 h following infection with ~100 CFU K. pneumoniae (Supplemental Fig. 2B).
3.4. Bactericidal activity in neutrophils and alveolar macrophages
Diabetic susceptibility could be a result of hyperglycemia-induced impairment of elicited neutrophil microbicidal activity. To test this, we infected neutrophils and AM and determine the bacterial growth after 15, 30, 60 and 90 min of infection, relating to the time 0 for each sample (Supplemental Fig. 3A).There was no difference between antibacterial activity between control and HG mice, indicating that the higher bacterial burden in hyperglycemia is probably linked to a defective TLR signaling and late neutrophils recruitment. Also, the analysis of the antibacterial activity of alveolar macrophages was not significantly different between control and HG mice after 30 min of infection (Supplemental Fig. 3B).
4. Discussion
Here we demonstrate that hyperglycemia in mice is associated with significantly higher mortality from lower respiratory infection with K. pneumoniae. An infecting dose lethal for 100% of HG mice was survived by nearly 80% of euglycemic controls. The early mortality of HG mice can be attributed to poor control of bacterial replication in the lung within the first 2 days after challenge. Successful defense against this virulent Gram-negative pathogen hinges on the rapid trafficking of neutrophils to the site of infection. That innate immune response is primarily triggered by LPS binding to TLR4 [15,16] with the induction of chemoattractant cytokines, of which CXCL1 may play a dominant regulatory role [17]. Hyperglycemic mice exhibited reduced granulocyte recruitment and a weaker CXCL1 mRNA and protein response to K. pneumoniae LPS than controls, demonstrating functional impairment of the TLR4 pathway. The expression of other cytokines linked to successful defense against K. pneumoniae and to TLR4 signaling (CXLC-2, IL-1β and TNF-α) was also impaired in HG mice. Other arms of the immune system that contribute to late control of K. pneumoniae infection might also be impacted by hyperglycemia but this could not be assessed in our study owing to the early mortality of HG mice.
Jeyaseelan et al. [18] reported that TIRAP−/− mice are highly susceptible K. pneumoniae infection and have reduced neutrophil accumulation in the lung, whereas TIRAP was not required for defense against Pseudomonas aeruginosa. The TIRAP-dependent protection was attributed to downstream induction of IL-1β and TNF-α, both of which were expressed at lower levels by HG compared to control mice in our study. Previous studies have linked the decreased IL-10 gene expression we observed, to disrupted TLR/MAPK signaling [19,20]. The lower expression of TIRAP by HG mice could be a contributing factor to their marked susceptibility to K. pneumoniae.
Diabetes is associated with increased susceptibility to K. pneumoniae and certain other important pathogens, notably Burkholderia pseudomallei and Mycobacterium tuberculosis [21,22]. Mice deficient in TLR4 control experimental B. pseudomallei infection comparably to wild type mice, while TLR2 deficiency confers enhanced rather than diminished protection [23]. However, impaired early cytokine production at the site of infection was demonstrated in mice with type 2 diabetes melioidosis comorbidity [24]. Host defense in tuberculosis hinges on a Th1 biased adaptive immune response but data from the HG model indicate that delayed priming of M. tuberculosis antigen-specific T cells in diabetic mice results from a delay in the innate immune response to initial infection of alveolar macrophages by inhaled bacilli [25]. While it is likely that chronic hyperglycemia interferes with a wide range of afferent and efferent immune host defense pathways, defective innate immune sensing is emerging as a common theme for the diabetic complication of susceptibility to infectious diseases.
Our data implicate a deficient cytokine response to the major pathogen-associated molecular pattern of K. pneumoniae resulting in delayed neutrophil granulocyte recruitment as a major immunological mechanism of diabetic susceptibility. An appropriate early response occurring within the first hour of infection is critical to prevent an overwhelming expansion of bacterial load. Since this response is impaired by diabetes, intervention strategies based on antibiotics given after K. pneumoniae infection in diabetics may not be adequate for a favorable outcome. With the expanding global epidemic of diabetes and the spread of multidrug-resistant K. pneumoniae, novel strategies for treatment and prevention are needed. The data presented here suggest that host-directed therapies to improve the early response to K. pneumoniae LPS could enhance protection against infection in the vulnerable diabetic population.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health grants HL081149 (to H.K.) and AI057588 (to E.L.) and by the Research Council of Norway (to E.L.). The authors thank Ms. Sara Montminy-Paquette for help with K. pneumoniae LPS purification and Michael Ethier for help with K. pneumoniae culture and infections.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None to declare.
References
- [1].Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 2009;48:1–12. [DOI] [PubMed] [Google Scholar]
- [2].WHO. Antimicrobial Resistance: Global Report on Surveillance 2014. http://www.who.int/drugresistance/documents/surveillancereport
- [3].Lin YT, Jeng YY, Chen TL, Fung CP. Bacteremic community-acquired pneumonia due to Klebsiella pneumoniae: clinical and microbiological characteristics in Taiwan, 2001-2008. BMC Infect Dis 2010;10:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ali AH, Smalligan RD, Ahmed M, Khasawneh FA. Pyogenic liver abscess and the emergence of Klebsiella as an etiology: a retrospective study. Int J Gen Med 2013;7:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].James R, Hijaz A. Lower urinary tract symptoms in women with diabetes mellitus: a current review. Curr Urol Rep 2014;15:440–3. [DOI] [PubMed] [Google Scholar]
- [6].Hidaka H, Yamaguchi T, Hasegawa J, Yano H, Kakuta R, Ozawa D, et al. Clinical and bacteriological influence of diabetes mellitus on deep neck infection: Systematic review and meta-analysis. Head Neck 2014. doi: 10.1002/hed.23776. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- [7].Stoeckle M, Kaech C, Trampuz A, Zimmerli W. The role of diabetes mellitus in patients with bloodstream infections. Swiss Med Wkly 2008;138:512–9. [DOI] [PubMed] [Google Scholar]
- [8].Wang JL, Chen KY, Fang CT, Hsueh PR, Yang PC, Chang SC. Changing bacteriology of adult community-acquired lung abscess in Taiwan: Klebsiella pneumoniae versus anaerobes. Clin Infect Dis 2005;40:915–22. [DOI] [PubMed] [Google Scholar]
- [9].International Diabetes Federation. IDF Diabetes Atlas, 6th edn. Brussels, Belgium: International Diabetes Federation, 2013. http://www.idf.org/diabetesatlas [Google Scholar]
- [10].Molton JS, Tambyah PA, Ang BS, Ling ML, Fisher DA. The global spread of healthcare-associated multidrug-resistant bacteria: a perspective from Asia. Clin Infect Dis 2013;56:1310–8. [DOI] [PubMed] [Google Scholar]
- [11].Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol 2007;37:518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hirschfeld M, Ma M, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol 2000;165:618–22. [DOI] [PubMed] [Google Scholar]
- [13].Montminy SW, Khan N, McGrath S, Walkowicz MJ, Conion JE, Fukase K, et al. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol 2006;7:1066–73. [DOI] [PubMed] [Google Scholar]
- [14].Livak JL, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and 2-ΔΔCT method. Methods 2001;25:402–04. [DOI] [PubMed] [Google Scholar]
- [15].Standiford LR, Standiford TJ, Newstead MJ, Zeng X, Ballinger MN, Kovach MA, et al. TLR4-dependent GM-CSF protects against lung injury in Gram-negative bacterial pneumonia. Am J Physiol Lung Cell Mol Physiol 2012;302:L447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wieland CW, van Lieshout MH, Hoogendijk AJ, van der Poll T. Host defence during Klebsiella pneumonia relies on haematopoietic-expressed Toll-like receptors 4 and 2. Eur Respir J 2011;37:848–57. [DOI] [PubMed] [Google Scholar]
- [17].Cai S, Batra S, Lira SA, Kolls JK, Jeyaseelan S. CXCL1 regulates pulmonary host defense to Klebsiella infection via CXCL2, CXCL5, NF-kappaB, and MAPKs. J Immunol 2010;185:6214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jeyaseelan S, Young SK, Yamamoto M, Arndt PG, Akira S, Kolls JK, et al. Toll/IL-1R domain-containing adaptor protein (TIRAP) is a critical mediator of antibacterial defense in the lung against Klebsiella pneumoniae but not Pseudomonas aeruginosa. J Immunol 2006;177:538–47. [DOI] [PubMed] [Google Scholar]
- [19].Teixeira-Coelho M, Guedes J, Ferreirinha P, Howes A, Pedrosa J, Rodrigues F, et al. Differential post-transcriptional regulation of IL-10 by TLR2 and TLR4-activated macrophages. Eur J Immunol 2014;44:856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Acorci-Valerio MJ, Bordon-Graciani AP, Dias-Melicio LA, de Assis Golim M, Nakaira-Takahagi E. de Campos Soares AMV Role of TLR2 and TLR4 in human neutrophil functions against Paracoccidioides brasiliensis. Scand J Immunol 2010;71:99–108. [DOI] [PubMed] [Google Scholar]
- [21].Hodgson K, Morris J, Bridson T, Govan B, Rush C, Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology 2015;144:171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol 2014;44:617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wiersinga WJ, Wieland CW, Dessing MC, Chantratita N, Cheng AC, Limmathurotsakul D, et al. Toll-like receptor 2 impairs host defense in gram-negative sepsis caused by Burkholderia pseudomallei (melioidosis). PLoS Med 2007;4:e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hodgson KA, Govan BL, Walduck AK, Ketheesan N, Morris JL. Impaired early cytokine responses at the site of infection in a murine model of type 2 diabetes and melioidosis comorbidity. Infect Immun 2013;81:470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vallerskog T, Martens GW, Kornfeld H. Diabetic Mice display a delayed adaptive Immune response to Mycobacterium tuberculosis. J Immunol 2010;184:6275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


