Graphical Abstract
METHODS
CLINICAL CONTEXT
Retroperitoneal soft tissue sarcomas (RPS) are a heterogeneous group of rare cancers, accounting for approximately 15% all diagnosed adult soft tissue sarcomas.1 Five-year survival is reported to be around 60% but varies by histologic type and tumor grade.1,2 Certain types, such as retroperitoneal grade 3 dedifferentiated liposarcoma (DDLPS) and leiomyosarcoma (LMS) are particularly recalcitrant, with 5-year recurrence rates around 80% following surgical extirpation.2,3 Nonetheless, histology-tailored en-bloc resection is the mainstay of therapy. Contrary to the neoadjuvant and perioperative treatment schemas that are increasingly utilized for truncal and extremity soft tissue sarcomas, the role of perioperative therapy remains controversial for RPS 4 despite recent efforts. For example, the STRASS trial evaluated the role of neoadjuvant radiotherapy in RPS and found that the role of radiation for patients with RPS was limited and linked to histologic type and malignancy grade.5 Specifically, while there was no benefit for high-grade RPS, radiotherapy did appear to have value for patients with grade 1–2 liposarcoma.
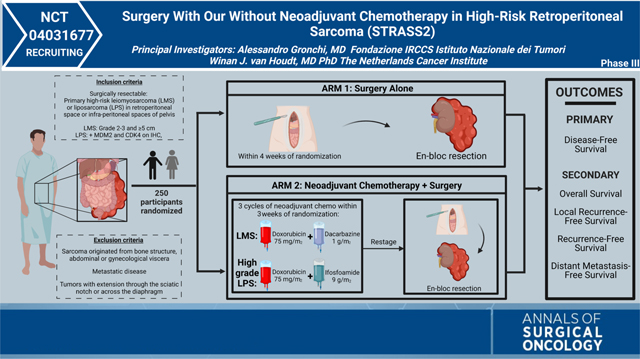
This trial, STRASS2, is sponsored by the European Organization for Research and Treatment of Cancer (EORTC) and is being led by principal investigators and surgeons Dr. Alessandro Gronchi from the Fondazione IRCCS Istituto Nazionale dei Tumori and Winan Van Houdt from the Netherlands Cancer Institute. It is a randomized phase III multinational trial taking place in 44 locations spanning 10 European countries, the United Kingdom, Canada, Australia, and New Zealand. Additionally, the trial recently obtained approval from the National Cancer Institute (NCI) and will be opening in the United States in late 2023 as EA7211. This trial marks a huge milestone for both EORTC and the NCI as both organizations look to foster transatlantic collaborations in the management and treatment of rare cancers such as sarcomas.
The trial is designed to assess whether histology-specific neoadjuvant chemotherapy improves disease-free survival for patients with retroperitoneal grade 3 dedifferentiated liposarcoma (DDLPS) and leiomyosarcoma (LMS), the 2 histologic types with the highest risk of metastatic spread, compared with surgical resection alone. Patients must not have undergone any prior treatment (no previous surgery, radiotherapy, or systemic therapy). Patients will be randomized 1:1 to either the standard arm of upfront en-bloc curative-intent surgery or the experimental arm of 3 cycles of histology-specific neoadjuvant chemotherapy followed by en-bloc curative-intent surgery. The results of this study have the potential to change the standard of care and improve survival rates for patients with retroperitoneal grade 3 DDLPS and LMS.
INVESTIGATOR INSIGHTS
The rationale for this trial is largely based on three important studies. The first relevant study is a large multicentric international retrospective study of more than 1000 patients with RPS, which demonstrated that the various histologic types exhibit different patterns of recurrence. Interestingly, retroperitoneal grade 3 DDLPS and LMS demonstrated an increased risk of distant recurrence compared with other histologies, which were much more commonly associated with local recurrences.2 The next relevant study is the STRASS trial, which was the first randomized study to investigate the role of preoperative radiotherapy for patients with localized primary RPS. The study showed no benefit for radiotherapy overall for patients with high-grade RPS; however, subgroup analysis showed that there may be a potential benefit for certain histologic types.5 Following the results of the STRASS trial, a propensity matched retrospective study (STREXIT) was published which assessed patients treated at the same institutions during the same time period as the STRASS trial. This study demonstrated that preoperative radiotherapy may be of value for patients with retroperitoneal well-differentiated or grade 1–2 DDLPS, while it did not benefit patients with LMS and grade 3 DDLPS.6 The explanation for this discrepancy is that the higher-grade and less differentiated tumors recur and succumb to distant disease and are therefore less susceptible to the potential benefit from locoregional radiation therapy.
With the STRASS2 trial, our intent is to leverage knowledge gained from prior work to improve outcomes for patients with grade 3 DDLPS and LMS through the use of neoadjuvant chemotherapy. For this trial, we chose to focus on the use of systemic treatment because the included histologic types demonstrate the highest risk of recurrence by distant metastasis. These histologic types stand to benefit more from a systemic therapy rather than a local therapy given their distant recurrence pattern;7 this key difference represents the rationale for this trial.
Specifically, the aim is to explore whether a multimodality treatment approach consisting of histology-specific neoadjuvant chemotherapy followed by standard surgery will improve survival. The specific chemotherapeutic regimens (anthracycline and ifosfamide for DDLPS, and anthracycline with dacarbazine for LMS) were chosen based on the results of a large multicenter retrospective study performed by the EORTC Soft Tissue and Bone Sarcoma Group which defined these regimens as first-line treatment for the selected histologic types.8 The results of STRASS2 have the potential to define a new standard of care for patients with high-risk retroperitoneal sarcomas. This trial highlights the importance of a histology-specific approach to patients with retroperitoneal sarcomas and emphasizes the importance of a multidisciplinary management approach. Regardless of the outcome of the trial, the STRASS2 is a distinct example of the application of a truly global trial. Hopefully this trial with help foster further international collaboration for the research and study of sarcomas as well as other ultra-rare diseases.
Supplementary Material
Footnotes
DISCLOSURE The authors declare they have no conflicts of interest and nothing to disclose.
SUPPLEMENTARY INFORMATION The online version contains supplementary material available at https://doi.org/10.1245/s10434-023-13500-9.
REFERENCES
- 1.Gronchi A, Miceli R, Shurell E, Eilber FC, Eilber FR, Anaya DA, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. 2013;31(13):1649–55. [DOI] [PubMed] [Google Scholar]
- 2.Gronchi A, Strauss DC, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the Multi-Institutional Collaborative RPS Working Group. Ann Surg. 2016;263(5):1002–9. [DOI] [PubMed] [Google Scholar]
- 3.Park JO, Qin LX, Prete FP, Antonescu C, Brennan MF, Singer S. Predicting outcome by growth rate of locally recurrent retroperitoneal liposarcoma: the one centimeter per month rule. Ann Surg. 2009;250(6):977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas DM, O’Sullivan B, Gronchi A. Current concepts and future perspectives in retroperitoneal soft-tissue sarcoma management. Expert Rev Anticancer Ther. 2009;9(8):1145–57. [DOI] [PubMed] [Google Scholar]
- 5.Bonvalot S, Gronchi A, Le Péchoux C, Swallow CJ, Strauss D, Meeus P, et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(10):1366–77. [DOI] [PubMed] [Google Scholar]
- 6.Callegaro D, Raut CP, Ajayi T, Strauss D, Bonvalot S, Ng D, et al. Preoperative radiotherapy in patients with primary retroperitoneal sarcoma: EORTC-62092 trial (STRASS) versus off-trial (STREXIT) results. Ann Surg. 2022. 10.1097/SLA.0000000000005492. [DOI] [PubMed] [Google Scholar]
- 7.Trans-Atlantic RPS Working Group. Management of recurrent retroperitoneal sarcoma (RPS) in the adult: a consensus approach from the Trans-Atlantic RPS Working Group. Ann Surg Oncol. 2016;23(11):3531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Ambrosio L, Touati N, Blay JY, Grignani G, Flippot R, Czarnecka AM, et al. Doxorubicin plus dacarbazine, doxorubicin plus ifosfamide, or doxorubicin alone as a first-line treatment for advanced leiomyosarcoma: a propensity score matching analysis from the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Cancer. 2020;126(11):2637–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


